Research Correspondence
The incidence of esophageal adenocarcinoma (EAC) has risen dramatically over the past four decades in the U.S. and much of the Western world.1 Most clinical guidelines recommend patients with Barrett's esophagus (BE) undergo endoscopic surveillance with tissue biopsy to grade the severity of precursor lesions and detect curable neoplasia.2 Additionally, techniques for endoscopic eradication treatment of BE, such as endoscopic mucosal resection (EMR) and radiofrequency ablation (RFA), have increasingly been employed to limit progression to EAC.3
The National Cancer Institute's (NCI) Cancer Intervention and Surveillance Modeling Network (CISNET) includes three esophageal cancer modeling groups who have independently developed population-based models for the natural history of BE and EAC;4 these models have been validated by calibration to NCI SEER data and by numerous comparative modeling exercises.5 The aim of the current study was to use the CISNET models to analyze perform a comparative modeling analysis to determine the effectiveness and cost-effectiveness of an RFA centered endoscopic eradication treatment strategy for management of a population of patients with BE. We sought to test and assess the impact of multiple strategies utilizing endoscopic eradication therapy on EAC incidence and mortality, and to estimate the number of surveillance endoscopies and treatments required to produce potential clinical benefits. In addition, we performed a cost-effectiveness analysis to assess the various strategies from a healthcare utilization perspective. Detailed technical profiles of each model are available on the NCI CISNET website and details regarding the methods are available in a downloadable pdf.6
In our base case analysis, the simulated cohort was comprised of males born in 1950 with BE diagnosed at age 60. Patients were tracked for EAC incidence and mortality until death by any cause or age 100. Endoscopic surveillance and eradication therapy were discontinued after age 80. Risk of progression to cancer was dependent on calendar year, birth cohort, age, and sex. Outcomes for each strategy analyzed included EAC incidence and mortality; total numbers of surveillance endoscopies and endoscopic eradicative treatments; numbers of treatments needed to avert one EAC death (NNT/death); unadjusted and quality-adjusted life years; number of complications from endoscopy and treatment; and total costs. The NNT/death was calculated as the total number of ablative treatments divided by the number EAC deaths averted by a given strategy. As many patients require multiple treatments, the total number of treatments required provides a better estimate of resource utilization than the number of patients that required treatment. Treatments included the total number of EMR and RFA treatments. Incremental results compared the NNT/death for a given strategy to the next-least invasive strategy by dividing the number of additional treatments by the additional EAC mortality reduction in the more invasive strategy. Cost-effectiveness analyses were conducted from a third party payer perspective. Quality of life (utility values) were derived from literature and used to convert absolute life-years of each strategy into quality-adjusted life-years (QALYs). We quantified the effectiveness of each strategy in terms of quality-adjusted life-years (QALYs), and associated costs, applying the conventional 3% discount rate to both. Incremental cost-effectiveness ratios (ICERs) were calculated between strategies as the ratio of incremental cost to incremental gain in quality-adjusted life-years. Comprehensive details of model inputs or parameter estimates including methods for derivations are available online.6
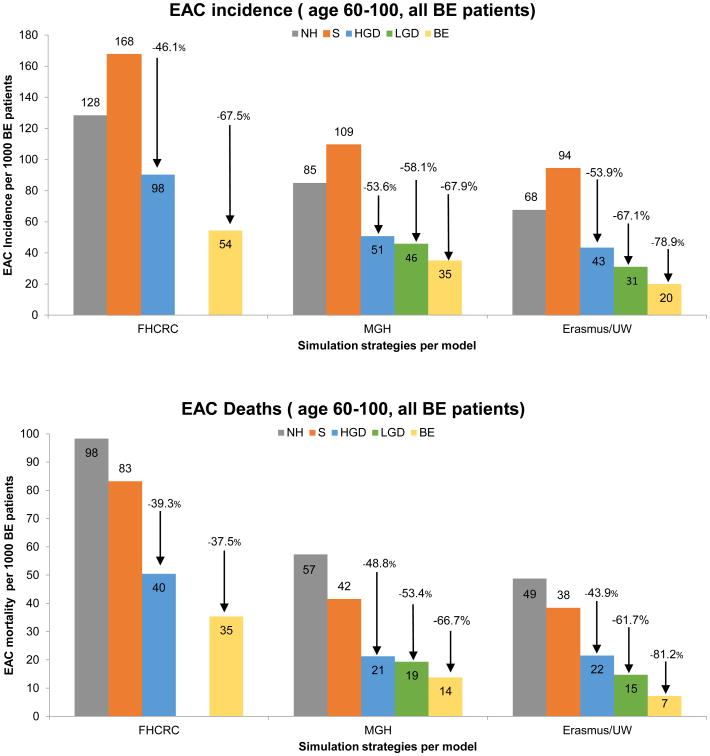
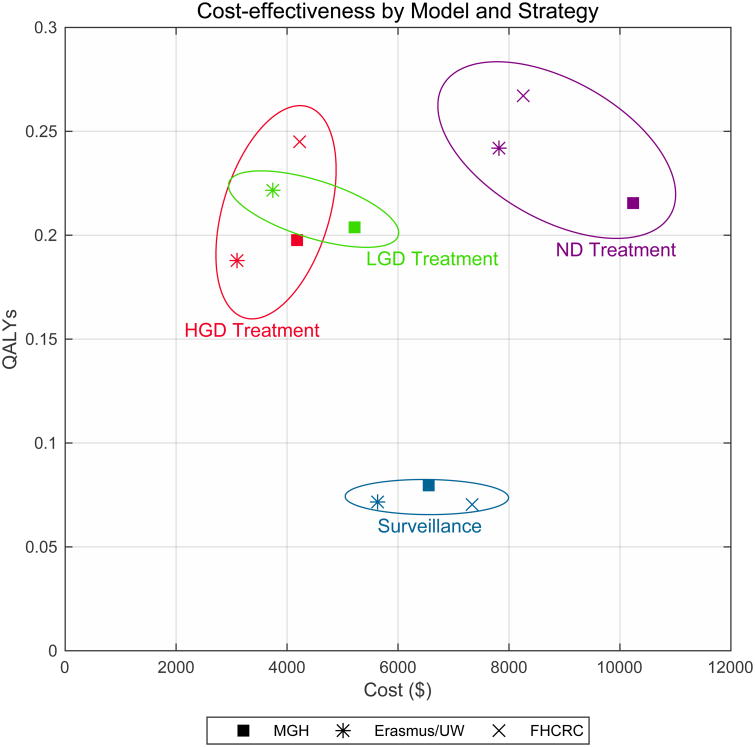
Table 1 summarizes the five strategies for the management of BE. Figure 1 presents the highlighted modeling results for the strategies for each of the three modeling groups with the endpoints of EAC incidence and mortality. The model analysis found that the impact of the different treatment strategies, measured relative to a baseline of surveillance alone, was consistent across all three models. HGD treatment resulted in an average decrease in EAC incidence of 51% (range 46%-54%) and an EAC mortality reduction of 44% (range 39%-49%). In terms of NNT/death, HGD treatment was the most efficient, with a mean of 44 (range 30-56). In this strategy, relatively few treatments were required to achieve a substantial reduction (range 39%-49%). In contrast, the incremental NNT/death for LGD compared to HGD treatment was 346 and 166 in the MGH and ERASMUS/UW models, respectively. The LGD treatment strategy (simulated by the MGH and ERASMUS/UW models only) resulted in a decrease in EAC incidence by 63% (range 58%-67%) and EAC mortality by 58% (range 53%-62%). Treating all BE patients at age 60 decreased the number of EAC cases by 71% (range 68%-79%) and the number of EAC deaths by 68% (range 58%-81%). This strategy was resource intensive, with NNT/death of 350 (range 253-518) compared to HGD treatment alone, and an ICER of $182,093-$422,256/QALY, which is above a $100,000/QALY willingness-to-pay threshold. Model predictions diverged on the cost-effectiveness of treatment for low grade dysplasia (LGD). Additional results of our modeling analyses including unadjusted life expectancy, costs per strategy, and sensitivity analyses on key parameter inputs and surveillance assumptions are available online.6
Table 1. Characteristics of simulated interventions on Barrett's esophagus patient cohort.
| Strategy | NDBE patients | LGD patients | HGD patients |
|---|---|---|---|
| Natural History (NH) | No intervention | No intervention | No intervention |
| Surveillance without RFA treatment (S Strategy) | Surveillance endoscopy with biopsies every 3 years | Surveillance endoscopy with biopsies every 6 months in the first year, thereafter every year | Surveillance endoscopy with biopsies every 3 months |
| BE surveillance with treatment for high-grade dysplasia only (HGD Strategy) | Surveillance endoscopy with biopsies every 3 years | Surveillance endoscopy with biopsies every year | RFA therapy followed by surveillance* |
| BE surveillance with treatment for all dysplasia (LGD Strategy) | Surveillance endoscopy with biopsies every 3 years | RFA therapy followed by surveillance* | RFA therapy followed by surveillance* |
| Treatment for all BE patients (BE Strategy) | RFA therapy followed by surveillance* | RFA therapy followed by surveillance* | RFA therapy followed by surveillance* |
Figure 1.
The upper part of the figure shows the EAC incidence per 1000 BE patients per model and strategy (no discounting). The lower part of the figure shows the EAC deaths per 1000 BE patients. The EAC incidence and mortality reductions are shown for the endoscopic eradicative treatment strategies compared to the strategy including only surveillance and no endoscopic eradicative treatment. The range in model estimates reflects differences in model structures and assumptions on BE prevalence and time to development of malignancy. Abbreviations: NH= Natural History; S=Surveillance; HGD=high-grade dysplasia; LGD=low grade dysplasia; BE=Barrett's esophagus without dysplasia
In conclusion, our results strongly confirm the current guidelines that endorse endoscopic eradication therapy for patients with high grade dysplasia.2 Our divergent results for LGD, highlights the need for a better understanding of the uncertainties surrounding the LGD health state.7 Benefits are predicted to be achieved for all BE endoscopic eradication strategies; however, the efficiency of eradication is substantially reduced if patients with LGD and no dysplasia are treated, and substantially more resources are required to avert a cancer death in these settings. These findings were consistent across all three CISNET esophageal cancer models and were robust to sensitivity analyses of RFA efficacy and durability. Our results add further support for endoscopic eradication therapy for BE patients with HGD, and suggest that strategies targeting less severe disease will require close scrutiny for cost-effectiveness. Efficiency of care would be greatly enhanced through improved methods to stratify risk of cancer in lesser forms of dysplasia and, therefore to better identify individuals who would benefit most from endoscopic therapy.
Supplementary Material
Acknowledgments
Funding statement: The study was supported by the National Institutes of Health/National Cancer Institute: U01 CA 199336 (CH, JI, GL); CA 152926 (CH, JI, GL); R01 CA 140574 (CH); and the National Science Foundation DGE-0718124: KC. The funding source had no role in the study design, conduct, and reporting.
Abbreviations
- BE
Barrett's Esophagus
- CE-D
Complete eradication of dysplasia
- CE-IM
Complete eradication of intestinal metaplasia
- EAC
Esophageal adenocarcinoma
- EET
Endoscopic Eradicative Therapy
- HGD
High grade dysplasia
- LGD
Low grade dysplasia
- ND
No dysplasia
- NNT/death
Number of treatments needed to prevent one death
- RFA
Radiofrequency ablation
Footnotes
Author Contributions: SK, CRH, KC, IL, WH, CK, MB, EL, EF, JI, CH: conception or design
SK, CRH, KC, WW, NS, SS, JR, IL, AA, WH, CK, MB, EL, ACT, EF, JI, CH: acquisition, analysis, or interpretation of data
SK, CRH, KC, WW, IL, AA, JI, CH: drafting of the manuscript
SK, CRH, KC, WW, NS, SS, JR, IL, WH, CK, MB, EL, AT, EF, JI, NN, SG, CH: critical revision of the manuscript for important intellectual content
SK, CRH, KC: statistical analysis EL, JI, CH: obtaining funding
AA, WW, JR: administrative, technical, or material support
IL, WH, CK, MB, EL, EF, JI, CH: supervision
Conflict of Interest Statement: Authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Gastroenterological A. Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 4.http://cisnet.cancer.gov/
- 5.Kong CY, McMahon PM, Gazelle GS. Calibration of Disease Simulation Model Using an Engineering Approach. Value in Health. 2009;12:521–529. doi: 10.1111/j.1524-4733.2008.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.http://www.mgh-ita.org/comprehensive_analysis_details.pdf.
- 7.Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and Management of Low-Grade Dysplasia in Barrett's Esophagus: Clinical Practice Updates Expert Review From the Clinical Guidelines Committee of the American Gastroenterological Association. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.09.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.