Abstract
Many survivors of the 2003 outbreak of severe acute respiratory syndrome (SARS) developed residual pulmonary fibrosis with increased severity seen in older patients. Autopsies of patients that died from SARS also showed fibrosis to varying extents. Pulmonary fibrosis can be occasionally seen as a consequence to several respiratory viral infections but is much more common after a SARS coronavirus (SARS-CoV) infection. Given the threat of future outbreaks of severe coronavirus disease, including Middle East respiratory syndrome (MERS), it is important to understand the mechanisms responsible for pulmonary fibrosis, so as to support the development of therapeutic countermeasures and mitigate sequelae of infection. In this article, we summarize pulmonary fibrotic changes observed after a SARS-CoV infection, discuss the extent to which other respiratory viruses induce fibrosis, describe available animal models to study the development of SARS-CoV induced fibrosis and review evidence that pulmonary fibrosis is caused by a hyperactive host response to lung injury mediated by epidermal growth factor receptor (EGFR) signaling. We summarize work from our group and others indicating that inhibiting EGFR signaling may prevent an excessive fibrotic response to SARS-CoV and other respiratory viral infections and propose directions for future research.
Keywords: Wound healing, SARS-CoV, EGFR, Fibrosis
Highlights
-
•
Patients who survived SARS coronavirus infection often developed pulmonary fibrosis.
-
•
Mouse models of SARS-CoV infection recapitulate fibrotic lesions seen in humans.
-
•
Epidermal growth factor receptor (EGFR) may modulate the wound healing response to SARS-CoV.
-
•
The EGFR pathway is a prime target for therapeutic interventions to reduce fibrosis after respiratory virus infection.
1. Introduction
Following the 2003 epidemic of severe acute respiratory syndrome (SARS), it was noticed that many patients who survived the severe illness developed residual pulmonary fibrosis, as shown by clinical findings and radiography. Varying degrees of fibrosis were also observed in autopsies of fatal cases. Although pulmonary fibrotic changes are occasionally observed as sequelae of other respiratory viral infections, they appear to be more common following SARS coronavirus (SARS-CoV) infection.
Given the threat of future outbreaks of severe coronavirus disease, including Middle East respiratory syndrome (MERS), it is important to understand the mechanisms responsible for pulmonary fibrosis, so as to support the development of therapeutic countermeasures and mitigate sequelae of infection. In this article, we summarize observations of pulmonary fibrosis during and after the SARS epidemic, note the extent to which fibrosis occurs after other pulmonary viral infections, describe efforts to recapitulate fibrotic changes in mouse models of SARS, and review evidence that the condition represents a hyperactive response to lung injury, driven by proinflammatory mediators acting through epidermal growth factor receptor (EGFR) signaling. We summarize work by our group and others indicating that inhibitors of EGFR may be useful in preventing an excessive fibrotic response in SARS and other respiratory viral infections, and indicate directions for future research.
2. SARS pathogenesis
Severe acute respiratory syndrome coronavirus (SARS-CoV) is a highly pathogenic respiratory virus. SARS patients initially present with mild disease often consisting of persistent high fever, chills, malaise, myalgia, headache and dry cough that progressed in severity over the following weeks (Lee et al., 2003). After an illness lasting 1–2 weeks, most patients resolve the infection, however about one-third develop severe pulmonary complications leading to acute lung injury and acute respiratory distress syndrome (ARDS), resulting in intubation and prolonged hospitalization (Tsui et al., 2003).
During the acute phase of SARS, lung damage results in edema, bronchiolar sloughing of ciliated epithelial cells and the deposition of hyaline-rich deposits at alveolar membranes, resulting in reduced gas exchange. During the next phase of infection (weeks 2–5), the lungs display signs of fibrosis, in which epithelial cells and alveolar spaces show fibrin deposition and infiltration of inflammatory cells and fibroblasts. During the final stage (weeks 6–8), pulmonary tissue becomes fibrotic with collagen deposits, and cellular proliferation is seen in alveoli and interstitial spaces (Cheung et al., 2004, Gu and Korteweg, 2007, Ketai et al., 2006). Radiographic features of patient's lungs varied greatly by individual however characteristic features were present in most patients including progression from unilateral focal air-space opacity to multifocal or bilateral consolidation in the later phases of disease. Computer tomography (CT) of patients revealed consolidation with interstitial thickening in predominantly peripheral and lower lobes of the lungs (Lee et al., 2003, Peiris et al., 2003).
Multiple autopsy studies showed that diffuse alveolar damage (DAD) with hyaline membrane formation and interstitial thickening were common features of SARS-CoV infected lungs (Chan et al., 2003). DAD occurs when there is trauma and injury to alveolar and bronchiolar epithelial cells that causes terminal small airways to be plugged with fluid and cellular debris, that can be seen both by pathological examination and radiological analyses (Nicholls et al., 2003, Tse et al., 2004). In patients lacking hyaline membrane formation, acute fibrinous pneumonia with organizing phase fibrin deposition was observed, resulting in reduced lung function. Acute lung injury, squamous metaphasia, multinucleated giant cells, and extensive cellular proliferation were seen in all cases (Mazzulli et al., 2004).
Autopsies of SARS patients also showed lung fibrosis in various stages of progression (Gu and Korteweg, 2007, Hwang et al., 2004, Tse et al., 2004). These observations are not unique to SARS, but common to many lung disorders (see below). Importantly, clinical findings showed that older SARS patients had an increased risk of fibrosis (Wu et al., 2016). The extent of fibrosis correlated with the severity and duration of illness (Hwang et al., 2004, Tse et al., 2004).
2.1. Follow-up of patients that have recovered from SARS-CoV infection
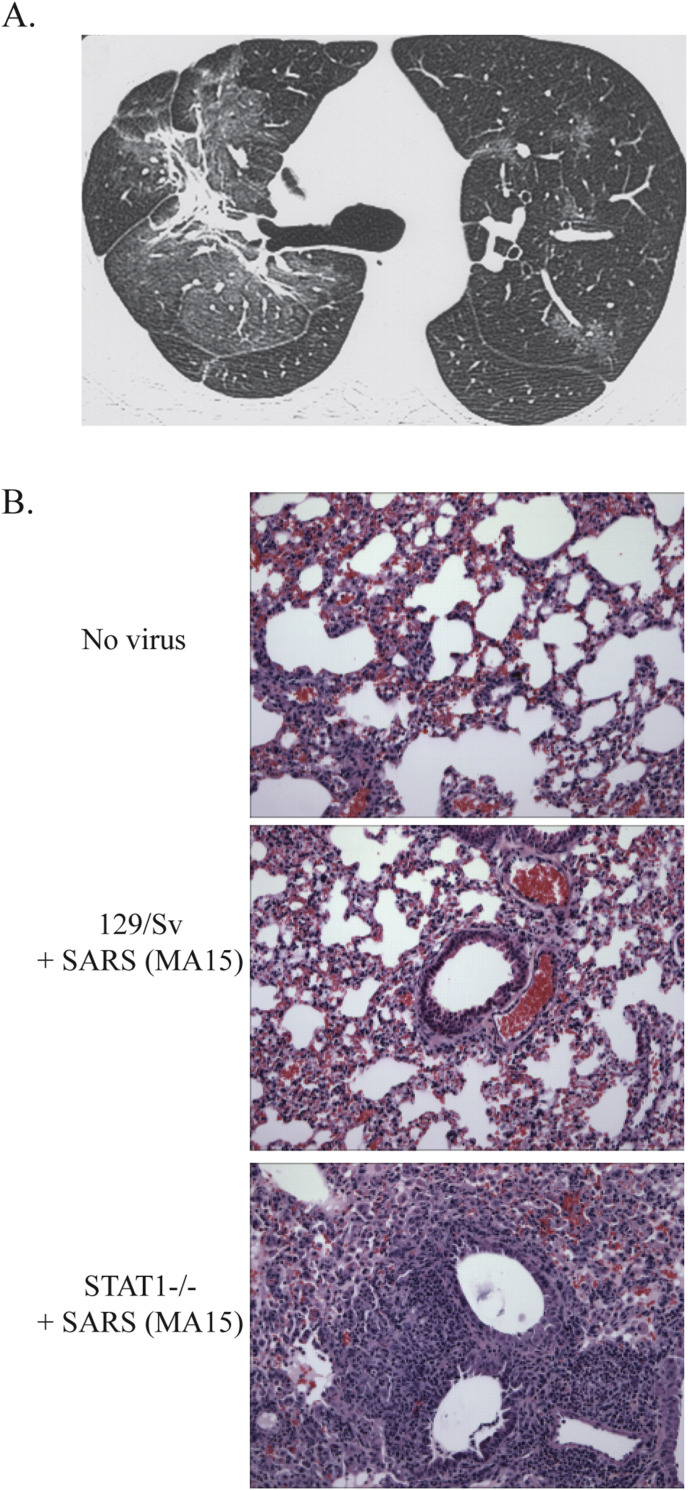
Several studies have been conducted on patients who have resolved SARS-CoV infection. Many studies have reported an increased incidence of fibrosis in patients, even after SARS-CoV had been cleared. In one study, 45% of patients showed a “ground-glass” appearance, an indication of fibrosis, by chest X-ray scores and high-resolution computerized tomography by one month after infection (Xie et al., 2005) (Fig. 1 A). Ground-glass opacification in the lungs describes regions that display a hazy attenuated signal under CT imaging, without obscuring normal bronchial and vascular structures. The differential diagnosis for ground-glass opacification can be an infection, pulmonary edema, interstitial thickening or fibrotic deposits (Collins and Stern, 1997). In a second study looking at the intermediate recovery periods of 3 and 6 months after infection, fibrotic features, including abnormal scoring of airspace opacity and reticular shadowing, were seen in 36% and 30% of the patients respectively (D. S. Hui et al., 2005a, Hui et al., 2005b). A one year follow-up study on 97 recovering SARS patients in Hong Kong showed that 27.8% of SARS survivors showed decreased lung function and increased lung fibrosis compared to a normal population (David S. Hui et al., 2005a, Hui et al., 2005b). Other follow-up studies have shown similar results (D. S. Hui et al., 2005a, Hui et al., 2005b, Ngai et al., 2010, Ngai et al., 2010). The molecular pathways responsible for the development of SARS-CoV induced fibrosis observed in recovered patients are not well understood. This gap in knowledge limits the development of novel therapies targeting the development of fibrosis or the repurposing of existing treatments that may be effective against SARS-CoV induced fibrosis after infection.
Fig. 1.
Pathologic features of SARS-CoV infection in humans and mice. A. Transverse thin-section CT scan in 36-year-old man at follow-up (obtained at day 43 after admission, 26 days since discharge) shows evidence of fibrosis. Large areas of ground-glass opacification are still present, both surrounding the areas of fibrosis and in other regions. (Permission for reuse from Antonio et al., Radiology 2003; 228:810–815.) B. H&E stained lungs from either PBS or SARS-CoV (MA15) inoculated mice in wildtype 129/Sv or 129/STAT1-/- mice at 9 days post-infection. Note the resolution of lung damage and inflammation in the infected 129/Sv mice while 129/STAT1-/- mice display extensive inflammation, fibrotic lesions surrounding airways and occlusion of alveolar space with proteinaceous fluid and a mixed inflammatory infiltrate.
3. Animal models of SARS
Non-human primate and small animal models are available to recreate various clinical aspects of SARS (Subbarao and Roberts, 2006). Among non-human primate models, cynomolgus and rhesus macaques, African green monkeys and common marmosets can all produced different levels of the clinical signs of disease seen in humans (Subbarao and Roberts, 2006). However, findings are often not consistent due to biological variability between animals.
Small animal models include ferrets, Syrian golden hamsters, and inbred mice. Ferrets and hamsters show virus replication in their lungs when infected intranasally with SARS-CoV. Hamsters show lung pathology (interstitial pneumonitis, pulmonary consolidation and diffuse alveolar damage) but conflicting symptoms are reported in ferrets (Subbarao and Roberts, 2006).
3.1. Mouse models to study SARS-CoV pathogenesis
A variety of mouse models for SARS-CoV have been developed that range from mild to severe disease depending on the viral strain and mouse background used. When the SARS-CoV (Urbani) strain is used to intranasally infect BALB/c, C57B/6, or 129/S strains of mice, there is viral replication in the lungs with no spread to other organs (Glass et al., 2004, Roberts et al., 2005, Subbarao et al., 2004). Infection is found to cause focal peribronchiolar and perivascular inflammation by 3 days post infection. Through 7 days post infection, SARS-CoV (Urbani) is largely cleared from lungs with minimal effects on weight loss or clinical symptoms of infection. Lung pathology over this time period is minimal as well, outside of the denuded bronchi around day 2 post infection. Minimally apparent peribronchiolar, perivascular or interstitial inflammation is noted in these infections.
A SARS-CoV strain with significant weight loss, clinical disease and lung pathology was created by blind passage of SARS-CoV (Urbani) in adult BALB/c mice. A mouse-adapted strain of SARS-CoV (called MA15, 15 passages before 100% mortality was produced) emerged which produced severe disease and death in young and old BALB/c mice (Frieman et al., 2012, Roberts et al., 2007). This virus carries 6 mutations in the genome: 4 in the replicase proteins and 2 in the structural proteins. In contrast to the SARS-CoV (Urbani) parent strain, the pulmonary pathology of mice infected with MA15 virus showed a rapid progression of inflammatory changes and more extensive damage to bronchiolar and alveolar epithelial cells. Intracellular MA15 antigens were highly prevalent in bronchiolar epithelium and alveolar pneumocytes with necrotic debris observed within the alveoli and the bronchiole lumen of mice (Roberts et al., 2007).
4. Molecular pathways involved in SARS-CoV pathogenesis
The MA15 strain of SARS-CoV has proved invaluable as a tool to understand host pathogen interactions in mouse models. Several knockout strains of mice infected with MA15 have identified immune factors that are critical for protection from SARS-CoV pathogenesis. To study the innate immune response to SARS-CoV, MyD88-/- mice were infected with MA15 which resulted in increased pulmonary inflammation and tissue damage with greater than 90% mortality by day 6 post-infection. In addition, MyD88−/− mice had significantly higher SARS-CoV viral loads in lung tissue throughout the course of infection demonstrating a critical role of the MyD88 innate immune signaling pathway for clearance of and protection from SARS-CoV (Sheahan et al., 2008). In addition to MyD88, TLR3 signaling through the TRIF adapter protein has been shown to regulate SARS-CoV pathogenesis as well again showing that deletion of either critical innate immune signaling molecules led to more severe disease (Totura et al., 2015). Recently, the use of a systems biology approach combining pathogenesis and transcription profiling identified the urokinase pathway as a key node in controlling lung damage and fibrin deposition during SARS-CoV infection (Gralinski et al., 2013). In these experiments, Serpine1, which regulates the deposition of fibrin after lung damage, is shown to have regulatory control over lung pathogenesis in the MA15 mouse model of SARS-CoV.
In 2004, Hogan et al. showed that mice deficient in the interferon-activated transcription factor Signal Transducer and Activator of Transcription 1 (STAT1) were much more susceptible to pathogenesis caused by SARS-CoV (Hogan et al., 2004). The authors attributed the findings to the role of STAT1 in interferon signaling. However, we have shown that the type I, II and III interferon pathways are largely dispensable for protection against SARS-CoV infection with mice deleted for the Type I IFN receptor, Type II IFN receptor or treated with IFN lambda neutralizing antibody displayed disease comparable with that seen in wildtype mice (Frieman et al., 2010). All were still permissive to SARS-CoV and displayed 10% weight loss during the first 4 days of the infection however they proceeded to regain weight and were able to reduce the levels of virus in their lungs through 9 days post infection. STAT1 knockout mice showed a higher propensity to develop fibrotic lesions compared to wild-type (WT) mice after SARS-CoV infection and showed increased pathogenesis even after SARS-CoV was cleared from lungs (Page et al., 2012) (Fig. 1B). Transcriptome analysis of SARS-CoV infected mouse lungs showed that STAT1 knockout mice developed a Th2 bias in their immune response (Zornetzer et al., 2010). The Th2 bias results in higher numbers of a subtype of macrophages in the lung, called alternatively activated macrophages (AAM), which in turn causes an overactive wound healing environment that induces pulmonary fibrosis (Page et al., 2012). AAMs are normally involved in clearing cell debris in damaged tissue after injury (Gordon, 2003). However in the study described in Page et al., AAMs are persistent in the lungs of Stat1 -/- mice where they become hyperactivated leading to increased fibrosis. This was similar to what was seen in a bleomycin induced fibrosis model where STAT1-/- mice developed higher rates of fibrosis after bleomycin treatment (Walters et al., 2005).
To summarize, the adverse pathogenic effects of SARS-CoV seen in human patients was modeled in STAT1-deficient mice where fibrosis evident in these mice by an overproliferation of fibroblasts and enhanced inflammatory response to infection. The signaling pathways resulting upstream or downstream of STAT1 and in which cell types are responsible for the host response to SARS-CoV seen in humans is still unknown.
5. Induction of fibrosis by other viruses
Induction of fibrosis is not unique to SARS-CoV. A recent meta-analysis of global idiopathic pulmonary fibrosis (IPF) rate finds that from the year 2000 onwards, a conservative incidence range of 3–9 cases per 100,000 per year for Europe and North America (Hutchinson et al., 2015). In the case of patients suffering from IPF, there is no known trigger for the onset of disease but viral infections are thought to be a co-factor (Naik and Moore, 2010, Vannella and Moore, 2008). Herpesviruses, such as Epstein-Barr virus (EBV) or human cytomegalovirus (HCMV), adenovirus, transfusion-transmitted virus (TTV, also known as Torque Teno Virus) and hepatitis C virus (HCV) have all been associated with IPF disease by detection of antibodies against viral proteins or viral gene products in lungs of IPF patients (Naik and Moore, 2010). Elevated serum levels of chemokines (such as transforming growth factor Beta 1 (TGF-β1)) and development of pulmonary fibrosis have also been reported in influenza A (H1N1) virus-infected patients (Wen et al., 2011). Irrespective of the etiology, pulmonary fibrosis has been shown to develop after apparent recovery from the infection. These studies only correlate the presence of a virus with IPF and it is unclear if and how the viruses directly trigger the disease or just create the conditions required for the development of pulmonary fibrosis caused by a secondary trigger (e.g. toxins, particulates or radiation) (Naik and Moore, 2010, Wynn and Ramalingam, 2012). This suggests that the some of the pathways induced by SARS-CoV infection that lead to the observed pulmonary fibrosis may be shared irrespective of the damage inducing factor.
In humans, acute viral infection often leads to the development of acute respiratory distress syndrome (ARDS), resulting from acute lung injury (Beigel et al., 2005). Histological analysis shows that 64% of ARDS patients may have pulmonary fibrosis (PF) during recovery (Martin et al., 1995). To demonstrate mechanistic correlation between viral infection, ARDS and PF, mouse models of viral infection has been utilized. Animal studies have produced additional evidence that viruses can trigger fibrosis. Murine gamma-herpesvirus 68 (MHV-68) infects mouse lungs and was shown to trigger pulmonary fibrosis in interferon (IFN)-γ receptor knockout mice (IFN-gammaR -/-) (Mora et al., 2005). Additionally, when an anti-viral drug cidofovir was used post infection, it resulted in protection from pulmonary fibrosis (Mora et al., 2007). A similar protective effect was seen in infections involving a mutant MHV-68 that was defective for reactivation from latency suggesting a connection between virus life cycle and lung fibrosis for this virus (Mora et al., 2007).
Respiratory syncytial virus (RSV) is the leading pediatric respiratory virus, resulting in high morbidity and mortality worldwide (Piedimonte and Perez, 2014). RSV infection causes significant acute lung injury with the potential for ARDS and other pulmonary complications (Piedimonte and Perez, 2014). In mouse models of RSV infection, it was shown that mouse airways that were pre-sensitized with ovalbumin (OVA), a common model for studying asthma and immune responses to lung infections in mice, were much more likely to develop fibrosis (Becnel et al., 2005). Another study showed RSV infection caused pulmonary fibrosis in C57Bl/6 mice and showed enhanced pathology in combination with cigarette smoke (Foronjy et al., 2014).
Taken together, these data indicate that there's a strong correlation between respiratory viral infections and the development of pulmonary fibrosis in humans. Animal models suggest that virus infection either alone on in combination with immune modulation could be triggers for the onset of fibrosis at least in some viruses. However, the molecular mechanisms following the viral infection that ultimately result in fibrosis have largely remained unexplored.
6. Current efforts to understand fibrosis induction by SARS-CoV
The normal wound healing response can be categorized into three distinct steps (Wilson and Wynn, 2009). The first step is the injury step during which disrupted epithelial and endothelial cells initiate an anti-fibrinolytic cascade that produces a temporary patch on the wound. The second step is the inflammation step where circulating neutrophils, macrophages and fibrocytes infiltrate to the site of injury. The recruited inflammatory cells secrete additional profibrotic cytokines such as IL-1, TNF, IL-13, and TGF-β. Macrophages and neutrophils also remove cell debris and eliminate pathogens. This in turn is followed by the proliferation and differentiation of fibroblasts into myofibroblasts, the key cell type that coordinates wound repair. Myofibroblasts secrete new ECM components on which tissue is rebuilt (Wynn, 2011). Finally, the third step involves resolution of the wound healing process, where myofibroblasts contract to reduce the size of the wound and its population numbers decrease by undergoing apoptosis.
Analysis of lung biopsies from SARS-CoV infected people shows dysregulation of this normal wound healing response (Beijing Group of National Research Project for SARS, 2003). Pro-inflammatory cytokines IFN-γ, IL-6, TNF-α, IL-18, CXCL10, MCP1 and TGF-β were found to be highly upregulated in serum from SARS-CoV patients (Huang et al., 2005, Wong et al., 2004). TGF-β1 was also found to be upregulated in mouse models of SARS-CoV infection similar to what was observed in other respiratory virus infections (Baas et al., 2006, Rockx et al., 2009). This is significant due to the profibrotic nature of TGF-β1. The release of TGF-β from injured tissue promotes lung repair, which while normally lead to resolution of infection, in SARS-CoV infection it often leads to hyperactivation of the TGF-β pathway leading to the promotion of lung fibrosis. In animal models of TGF-β regulation, transgenic mice over-expressing TGF-β show produce severe pulmonary fibrosis in mice and rats (Sime et al., 1997). The TGF-β activation pathways leads to the production of fibrin, collagen and secreted proteases (Matrix metalloproteinases). TGF-β’s role in cellular proliferation is under investigation, however there is evidence it is a key factor in the epithelial–mesenchymal transition (EMT) seen in repaired tissue. TGF-β’s presence in the lungs is required to promote lung fibroblasts to differentiate into myofibroblasts that are important in repairing lung tissue. However, under high and sustained levels of TGF-β, persistence of the repair process results in further damage.
7. The role of epidermal growth factor receptor signaling in fibrotic disorders
We have previously published work demonstrating that in STAT1-/- mice infected with the mouse adapted strain of SARS-CoV (called MA15) there is significant lung damage that occurs during infection and proceeds at later time points to the development of fibrosis in the lungs of infected mice. Transcriptomic analysis of lungs undergoing MA15 infection found a significant time-dependent up-regulation of several pathways including M2 macrophage induction, chemokine dysregulation compared to other viral lung infections and the induction of wound healing genes. When analyzed, the wound healing pathway is led by the EGFR protein. Wound healing genes are regulated by EGFR signaling (Werner and Grose, 2003) which suggests that EGFR signaling may play a role in the development of fibrosis in SARS patients.
7.1. Overview of EGFR signaling
EGFR (named ErbB1 or human epidermal growth factor receptor 1 [HER1] in humans) is the prototypical member of a family of receptor tyrosine kinases known as the ErbB receptors. There are four known members in the ErbB family, the other three being HER2 (ErbB2/NEU), HER3 (ErbB3) and HER4 (ErbB4) (Jones and Rappoport, 2014). Ligand binding to an extracellular domain triggers conformational changes resulting in the dimerization of the receptors. The ErbB receptors all possess an intrinsic tyrosine kinase activity and can phosphorylate themselves. Specific tyrosine residues in the cytoplasmic tail are phosphorylated resulting in the activation of signaling to the MAPK, Akt and JNK pathways (Yarden and Shilo, 2007, Yarden and Sliwkowski, 2001).
Activation of EGFR signaling has a range of outcomes, such as the inhibition of apoptosis, increase in cell proliferation and migration, activation of the inflammatory response and increase in mucus production (Yarden and Sliwkowski, 2001). EGFR is expressed in tissues derived from epithelial, mesenchymal and neuronal origin (Yano et al., 2003). EGFR signaling is especially important in tissue that undergo extensive turnover of cells such as in the epithelial layers of the skin, lungs and gut. EGFR activation in skin keratinocytes leads to cell proliferation, migration and cell survival required for wound healing. In the lungs and gut, EGFR signaling plays a role in controlling cell turnover and mucus production.
The role of EGFR has been well studied in the context of cancer especially non-small cell lung cancer where mutations in EGFR are routinely found. Small molecule tyrosine kinase inhibitors (TKIs) like Gefitinib and Afatinib as well as monoclonal antibody based treatments like Cetuximab have been developed as chemotherapy agents to inhibit the activity of EGFR (Kato and Nishio, 2006, Lenz, 2006, Yano et al., 2003). However, since EGFR signaling also regulates wound healing and repair in normal tissue, it has also been associated with fibrotic disease in various organs.
8. The ligands of EGFR
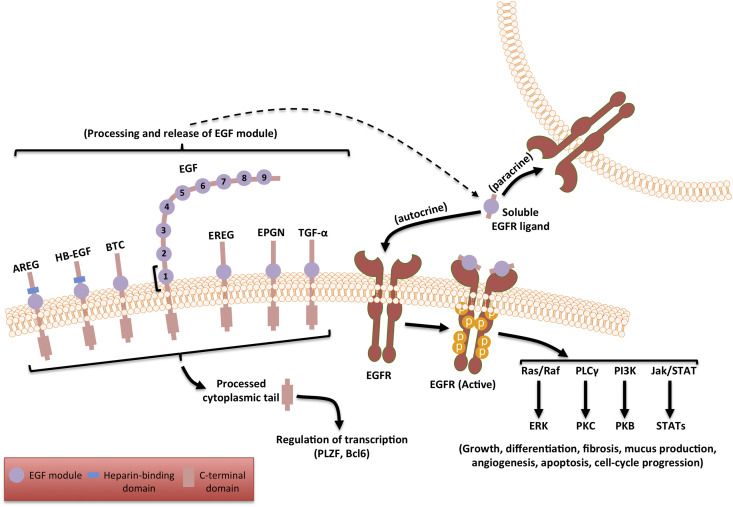
There are seven known identified ligands of EGFR (Schneider and Wolf, 2009) (Fig. 1): EGF, transforming growth factor alpha (TGF-α), amphiregulin (AR), epiregulin (EREG), heparin binding epidermal growth factor (HB-EGF), epithelial mitogen or epigen (EPGN) and betacellulin (BTC). All EGFR ligands are expressed as transmembrane precursor proteins that are cleaved by cell surface proteases upon injury or another signaling event and then released into the extracellular milieu as mature proteins, where they can bind the extracellular domain of EGFR.
Structurally, all the EGFR ligands possess at least one EGF module (Fig. 2 ), which is processed and released by the ‘a disintegrin and metalloproteinase’ (ADAM) family of metalloproteases (Seals and Courtneidge, 2003). The soluble fragment containing the EGF module serves as the activating ligand for EGFR (Schneider and Wolf, 2009). EGF has nine EGF modules but only the first one is released and serves as an EGFR ligand. All the other canonical ligands have a single EGF module. The C-terminal tails of the ligands are also known to function as potential transcriptional co-factors by translocating to the nucleus after proteolytic cleavage (Kinugasa et al., 2007, Nanba et al., 2003).
Fig. 2.
The seven known ligands of EGFR are in a membrane bound inactive form. The ADAM family of proteases are activated in response to tissue injury and cleave the pro-ligands to release the EGF module containing soluble ligand. The ligand binds to the receptor causing it to dimerize and autophosphorylate its C-terminal tail at specific tyrosine residues. The phosphorylated active form aggregates several adaptor proteins leading to the activation of multiple signaling cascades. A range of different outcomes are produced by the activation of these pathways some of which are listed in the schematic above.
In polarized human airway epithelial cells, the ligands are expressed in their pro-form in the apical membranes and the receptors are expressed in the basolateral surfaces of the cells (Vermeer et al., 2003). This spatial segregation of receptors from the ligand maintains the receptor in an inactive state during homeostasis. Upon injury, the epithelial barrier is compromised allowing airway surface liquid (ASL) containing small amounts of shed ligands to contact the basolateral surfaces of the epithelium and activate the receptor (Dempsey et al., 1997, Vermeer et al., 2003). This step initiates the wound healing response.
9. EGFR signaling and the induction of fibrosis
The role of EGFR signaling in fibrosis development is complex, with evidence for both a pro-fibrotic and anti-fibrotic role for EGFR signaling.
Cancer patients treated with tyrosine kinase inhibitors show an increased incidence of interstitial lung disease (ILD) which is often a precursor to pulmonary fibrosis (Kato and Nishio, 2006). Similar association with ILD were also seen in patients treated with an anti-EGFR monoclonal antibody, Panitumumab (Osawa et al., 2015, Yamada et al., 2013). Gefitinib also exacerbates pulmonary fibrosis induced by bleomycin in mice (Suzuki et al., 2003). These data suggest that inhibiting EGFR signaling increases the risk of pulmonary fibrosis, therefore suggesting that EGFR is anti-fibrotic in specific contexts.
However, TGF-β1 is known inducer of fibrosis and studies show it potently induces the expression of the EGFR ligand AR. Silencing AR by RNAi or using EGFR specific small molecule inhibitors such as AG1478 or Gefitinib, attenuated the fibrogenic effects of TGF-β1 (Zhou et al., 2012), contrasting with the bleomycin model of injury where Gefitinib made fibrosis worse (Suzuki et al., 2003) and where TGF-β1 protected mice from bleomycin injury (Tang et al., 2014). Furthermore, mice overexpressing the EGFR ligand TGF-α spontaneously develop lung fibrosis (Hardie et al., 1997, Hardie et al., 1996). Similar effects for other EGFR ligands are discussed in more detail in the section below. These studies seem to indicate that EGFR signaling is pro-fibrotic.
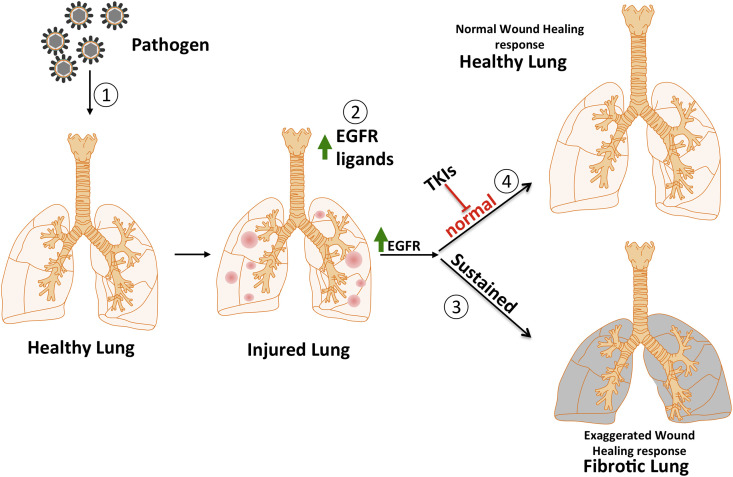
How can we reconcile these seemingly conflicting data? One possibility is that EGFR signaling has different outcomes in different species and for different fibrotic triggers. An alternate possibility is that fibrosis may be a result of dysregulation in the kinetics of EGFR signaling rather than simply the strength of the signal at a given time point. Deposition of fibrotic material is a normal component of the wound healing response, but it is unresolved wound healing that results in fibrotic disease (Adamson et al., 1988, Bitterman, 1992). The dysregulated control of either the upregulation of EGFR signaling, which is required for the initiation of normal wound healing, or downregulation of EGFR signaling, which is required for the resolution of the wound healing process, could result in fibrotic disease (Fig. 3 ).
Fig. 3.
The potential role of EGFR in fibrosis is illustrated above with the lung as an example. Physical injury or a pathogen (1) initiates the wound healing response by damaging healthy tissue, releasing EGFR ligands (2) and activating the EGFR pathway. A sustained activation of the EGFR pathway results in an exaggerated wound healing response leading to a fibrotic lung (3). The early use of tyrosine kinase inhibitors (4) could prevent the normal progress of wound healing and result in sustained injury and the development of fibrosis by alternate mechanisms.
10. Animal models available to study EGFR and fibrosis
The role of EGFR in fibrosis progression has been investigated using in vivo models, primarily mice. Mice lacking EGFR/HER1 die in utero (Miettinen et al., 1997). Models of EGFR transgenic mice with overactive EGFR mutations seen in human cancers have been made that demonstrate the rapid development of lung tumors depends on EGFR signaling (Politi et al., 2006). Mice containing EGFR mutations that lead to constitutive activation (DSK5 mice) show a skin related phenotype showing wavy hair, thick epidermis and darkened pigmentation (Fitch et al., 2003). Mice deficient in TGF-α, that lack EGFR signaling, are protected from chronic lung disease in models of lung damage (Madtes et al., 1999). Finally, in bleomycin induced fibrosis models in mice, the tyrosine kinase inhibitor Gefitinib is able to mitigate the onset of fibrosis (Ishii et al., 2006). Little is known about how alterations in EGFR signaling could be causing pulmonary fibrosis after a viral infection. However, by using these models, it has been demonstrated that EGFR regulation is a key pathway in the induction of damage induced pulmonary fibrosis.
A majority of the canonical EGFR ligands appear to play a role in promoting fibrosis in various organs (data summarized in Table 1 ). These data show that constitutive ubiquitous expression of some EGFR ligands, resulting in constant EGFR activation results in the activation of fibrosis. The inhibition of EGFR signaling by using TKIs or by co-expressing signaling defective EGFR mutants reverses the onset of fibrosis, at least in the case of TGF- α overexpressing mice (Hardie et al., 2008, Hardie et al., 1996).
Table 1.
Several groups have constructed transgenic mice expressing the known EGFR ligands. These mice are viable and show different fibrosis-related phenotypes as summarized above. Knockout mice are mostly viable except in the case of HB-EGF. The knockouts showed increased resistance to fibrosis in the case of TGF- α and AR and increased sensitivity to fibrosis in HB-EGF/BTC double-knockouts.
| Ligand | Phenotype in transgenic overexpression model | Phenotype in knockout mice |
|---|---|---|
| Betacellulin (BTC) | Increased post-natal mortality due to lung pathology (Schneider et al., 2005) | BTC-knockout mice show no phenotype but BTC/HB-EGF double-knockouts show cardiac fibrosis (Jackson et al., 2003) |
| Epidermal Growth Factor (EGF) | Defects in growth and spermatogenesis (Chan and Wong, 2000, Wong et al., 2000); No fibrotic defects reported | No fibrosis-related phenotype reported |
| Transforming Growth Factor alpha (TGF- α) | Spontaneous fibrosis 1 week after birth (Hardie et al., 1997) | Resistance to Bleomycin-induced fibrosis (Madtes et al., 1999) |
| Heparin Binding Epidermal Growth Factor (HB-EGF) | Pancreas specific overexpression resulted in pancreatic fibrosis (Means et al., 2003) | KO mice die shortly after birth; BTC/HB-EGF double-knockouts show cardiac fibrosis (Jackson et al., 2003); HB-EGF conditional knockout-induced liver fibrosis in a bile duct ligation model (Takemura et al., 2013) |
| Amphiregulin (AR) | Pancreas specific overexpression resulted in pancreatic fibrosis (Wagner et al., 2002); Role in liver fibrosis (Perugorria et al., 2008) | Knockout mice were significantly resistant to bleomycin-induced lung fibrosis (Ding et al., 2016) |
| Epiregulin (EREG) | No overexpression model; No role for fibrosis reported | No phenotype for fibrosis reported (Lee et al., 2004) |
| Epigen (EPGN) | Fibrosis in nerves and neurological defects (Dahlhoff et al., 2013a) | No phenotype for fibrosis reported (Dahlhoff et al., 2013b) |
11. EGFR inhibitors as therapeutics
The use of tyrosine kinase inhibitors like Erlotinib and its family members, are able to reverse or inhibit fibrosis development in a variety of animal models. TGF-β induction, a hallmark of many fibrotic diseases, drives expression of EGFR ligands which themselves lead to EGFR activation. Modulators of TGF-β signaling, induction and activation are in development. Upstream of TGF-β signaling, there are several FDA approved drugs (Losartan, Pirfenidone and Tranilast) that have effects at lowering TGF-β levels in the host. Several other small molecules, antibodies and siRNAs target TGF-β itself and are currently in clinical trials for a variety of fibrotic and cancer related diseases (Akhurst and Hata, 2012). EGFR activation induces the production of mucins (to assist in clearance of particles and debris) and IL-8 (a neutrophil recruiting chemokine) in addition to stimulating repair. Inhibitors of either overactive mucin production or antagonists of IL-8 could be useful in the modulation of a dysregulated EGFR response leading to resumption of control of host tissue repair. There are caveats for the use of tyrosine kinase inhibitors, specifically with respect to pulmonary toxicity. The use of Gefitinib has been reported to induce interstitial lung disease in patients treated for non-small cell lung cancer (NSCLC) (Kato and Nishio, 2006, Shi et al., 2014). TKIs are highly effective in treating EGFR positive cancers but their effect on normal tissue has not been well investigated.
12. Future directions
The development of severe lung disease leading to pulmonary fibrosis after SARS-CoV infection is a major complication of those who survived the SARS-CoV outbreak. Current research focuses on wound healing pathways that mediate tissue repair after injury, specifically the EGFR pathway. Specifically, we are studying how dysregulation of the EGFR pathway could lead to the development of pulmonary fibrosis in animal models of SARS-CoV. We hypothesize that targeted inhibition of EGFR, the proteins that activate EGFR or the proteins downstream of EGFR signaling could provide novel therapeutic interventions for pulmonary fibrosis patients. In addition, we believe that infections by other highly pathogenic respiratory viruses like MERS-CoV or future emerging respiratory viruses, could induce pulmonary fibrosis in patients. Understanding how the EGFR, wound healing and other pro-fibrotic pathways act after viral infection should lead to novel therapeutics in the future.
Acknowledgements
We thank Dr. Chris Coleman (UMB) for helpful suggestions and editing this review.
References
- Adamson I.Y., Young L., Bowden D.H. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am. J. Pathol. 1988;130:377–383. [PMC free article] [PubMed] [Google Scholar]
- Akhurst R.J., Hata A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio G.E., Wong K.T., Hui D.S., Wu A., Lee N., Yuen E.H., Leung C.B., Rainer T.H., Cameron P., Chung S.S., Sung J.J., Ahuja A.T. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003 Sep;228(3):810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- Baas T., Taubenberger J.K., Chong P.Y., Chui P., Katze M.G. SARS-CoV virus-host interactions and comparative etiologies of acute respiratory distress syndrome as determined by transcriptional and cytokine profiling of formalin-fixed paraffin-embedded tissues. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2006;26:309–317. doi: 10.1089/jir.2006.26.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becnel D., You D., Erskin J., Dimina D.M., Cormier S.A. A role for airway remodeling during respiratory syncytial virus infection. Respir. Res. 2005;6:122. doi: 10.1186/1465-9921-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Farrar J., Han A.M., Hayden F.G., Hyer R., de Jong M.D., Lochindarat S., Nguyen T.K.T., Nguyen T.H., Tran T.H., Nicoll A., Touch S., Yuen K.-Y. Writing committee of the world health organization (WHO) consultation on human influenza A/H5. N. Engl. J. Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. Avian influenza A (H5N1) infection in humans. [DOI] [PubMed] [Google Scholar]
- Beijing Group of National Research Project for SARS Dynamic changes in blood cytokine levels as clinical indicators in severe acute respiratory syndrome. Chin. Med. J. (Engl.) 2003;116:1283–1287. [PubMed] [Google Scholar]
- Bitterman P.B. Pathogenesis of fibrosis in acute lung injury. Am. J. Med. 1992;92:39S–43S. doi: 10.1016/0002-9343(92)90606-c. [DOI] [PubMed] [Google Scholar]
- Chan K., Zheng J., Mok Y., Li Y., Liu Y.-N., Chu C., Ip M. SARS: prognosis, outcome and sequelae. Respirology. 2003;8:S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.-Y., Wong R.W.-C. Expression of epidermal growth factor in transgenic mice causes growth retardation. J. Biol. Chem. 2000;275:38693–38698. doi: 10.1074/jbc.M004189200. [DOI] [PubMed] [Google Scholar]
- Cheung O.Y., Chan J.W.M., Ng C.K., Koo C.K. The spectrum of pathological changes in severe acute respiratory syndrome (SARS) Histopathology. 2004;45:119–124. doi: 10.1111/j.1365-2559.2004.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Stern E.J. Ground-glass opacity at CT: the ABCs. Am. J. Roentgenol. 1997;169:355–367. doi: 10.2214/ajr.169.2.9242736. [DOI] [PubMed] [Google Scholar]
- Dahlhoff M., Emrich D., Wolf E., Schneider M.R. Increased activation of the epidermal growth factor receptor in transgenic mice overexpressing epigen causes peripheral neuropathy. Biochim. Biophys. Acta. 2013;1832:2068–2076. doi: 10.1016/j.bbadis.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Dahlhoff M., Schäfer M., Wolf E., Schneider M.R. Genetic deletion of the EGFR ligand epigen does not affect mouse embryonic development and tissue homeostasis. Exp. Cell Res. 2013;319:529–535. doi: 10.1016/j.yexcr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Dempsey P.J., Meise K.S., Yoshitake Y., Nishikawa K., Coffey R.J. Apical enrichment of human EGF precursor in Madin-Darby canine kidney cells involves preferential basolateral ectodomain cleavage sensitive to a metalloprotease inhibitor. J. Cell Biol. 1997;138:747–758. doi: 10.1083/jcb.138.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Liu T., Wu Z., Hu B., Nakashima T., Ullenbruch M., Gonzalez De Los Santos F., Phan S.H. Bone marrow CD11c+ cell-derived amphiregulin promotes pulmonary fibrosis. J. Immunol. Balt. Md. 2016;1950(197):303–312. doi: 10.4049/jimmunol.1502479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch K.R., McGowan K.A., van Raamsdonk C.D., Fuchs H., Lee D., Puech A., Herault Y., Threadgill D.W., de Angelis M.H., Barsh G.S. Genetics of dark skin in mice. Genes Dev. 2003;17:214–228. doi: 10.1101/gad.1023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy R.F., Dabo A.J., Taggart C.C., Weldon S., Geraghty P. Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice. PLOS ONE. 2014;9:e90567. doi: 10.1371/journal.pone.0090567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M., Yount B., Agnihothram S., Page C., Donaldson E., Roberts A., Vogel L., Woodruff B., Scorpio D., Subbarao K., Baric R.S. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J. Virol. 2012;86:884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M.B., Chen J., Morrison T.E., Whitmore A., Funkhouser W., Ward J.M., Lamirande E.W., Roberts A., Heise M., Subbarao K., Baric R.S. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I, II and III interferon receptor independent mechanism. PLoS Pathog. 2010;6:e1000849. doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. Balt. Md. 2004;1950(173):4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gralinski L.E., Bankhead A., Jeng S., Menachery V.D., Proll S., Belisle S.E., Matzke M., Webb-Robertson B.-J.M., Luna M.L., Shukla A.K., Ferris M.T., Bolles M., Chang J., Aicher L., Waters K.M., Smith R.D., Metz T.O., Law G.L., Katze M.G., McWeeney S., Baric R.S. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4 doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie W.D., Bruno M.D., Huelsman K.M., Iwamoto H.S., Carrigan P.E., Leikauf G.D., Whitsett J.A., Korfhagen T.R. Postnatal lung function and morphology in transgenic mice expressing transforming growth factor-alpha. Am. J. Pathol. 1997;151:1075–1083. [PMC free article] [PubMed] [Google Scholar]
- Hardie W.D., Davidson C., Ikegami M., Leikauf G.D., Le Cras T.D., Prestridge A., Whitsett J.A., Korfhagen T.R. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-alpha-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1217–L1225. doi: 10.1152/ajplung.00020.2008. [DOI] [PubMed] [Google Scholar]
- Hardie W.D., Kerlakian C.B., Bruno M.D., Huelsman K.M., Wert S.E., Glasser S.W., Whitsett J.A., Korfhagen T.R. Reversal of lung lesions in transgenic transforming growth factor alpha mice by expression of mutant epidermal growth factor receptor. Am. J. Respir. Cell Mol. Biol. 1996;15:499–508. doi: 10.1165/ajrcmb.15.4.8879184. [DOI] [PubMed] [Google Scholar]
- Hogan R.J., Gao G., Rowe T., Bell P., Flieder D., Paragas J., Kobinger G.P., Wivel N.A., Crystal R.G., Boyer J., Feldmann H., Voss T.G., Wilson J.M. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires Stat1. J. Virol. 2004;78:11416–11421. doi: 10.1128/JVI.78.20.11416-11421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.-J., Su I.-J., Theron M., Wu Y.-C., Lai S.-K., Liu C.-C., Lei H.-Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Joynt G.M., Wong K.T., Gomersall C.D., Li T.S., Antonio G., Ko F.W., Chan M.C., Chan D.P., Tong M.W., Rainer T.H., Ahuja A.T., Cockram C.S., Sung J.J.Y. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Wong K.T., Ko F.W., Tam L.S., Chan D.P., Woo J., Sung J.J.Y. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J., Fogarty A., Hubbard R., McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur. Respir. J. 2015;46:795–806. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2004;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Fujimoto S., Fukuda T. Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am. J. Respir. Crit. Care Med. 2006;174:550–556. doi: 10.1164/rccm.200509-1534OC. [DOI] [PubMed] [Google Scholar]
- Jackson L.F., Qiu T.H., Sunnarborg S.W., Chang A., Zhang C., Patterson C., Lee D.C. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Rappoport J.Z. Interdependent epidermal growth factor receptor signalling and trafficking. Int. J. Biochem. Cell Biol. 2014;51:23–28. doi: 10.1016/j.biocel.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Kato T., Nishio K. Clinical aspects of epidermal growth factor receptor inhibitors: benefit and risk. Respirol. Carlt. Vic. 2006;11:693–698. doi: 10.1111/j.1440-1843.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- Ketai L., Paul N.S., Wong K.T. Radiology of severe acute respiratory syndrome (SARS): the emerging pathologic-radiologic correlates of an emerging disease. J. Thorac. Imaging. 2006;21:276–283. doi: 10.1097/01.rti.0000213581.14225.f1. [DOI] [PubMed] [Google Scholar]
- Kinugasa Y., Hieda M., Hori M., Higashiyama S. The carboxyl-terminal fragment of pro-HB-EGF reverses Bcl6-mediated gene repression. J. Biol. Chem. 2007;282:14797–14806. doi: 10.1074/jbc.M611036200. [DOI] [PubMed] [Google Scholar]
- Lee D., Pearsall R.S., Das S., Dey S.K., Godfrey V.L., Threadgill D.W. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol. Cell. Biol. 2004;24:8907–8916. doi: 10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lenz H.-J. Anti-EGFR mechanism of action: antitumor effect and underlying cause of adverse events. Oncol. Willist. Park N. 2006;20:5–13. [PubMed] [Google Scholar]
- Madtes D.K., Elston A.L., Hackman R.C., Dunn A.R., Clark J.G. Transforming growth factor-alpha deficiency reduces pulmonary fibrosis in transgenic mice. Am. J. Respir. Cell Mol. Biol. 1999;20:924–934. doi: 10.1165/ajrcmb.20.5.3526. [DOI] [PubMed] [Google Scholar]
- Martin C., Papazian L., Payan M.J., Saux P., Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107:196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- Mazzulli T., Farcas G.A., Poutanen S.M., Willey B.M., Low D.E., Butany J., Asa S.L., Kain K.C. Severe acute respiratory syndrome-associated coronavirus in lung tissue. Emerg. Infect. Dis. 2004;10:20–24. doi: 10.3201/eid1001.030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A.L., Ray K.C., Singh A.B., Washington M.K., Whitehead R.H., Harris R.C., Jr., Wright C.V.E., Coffey R.J., Jr., Leach S.D. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology. 2003;124:1020–1036. doi: 10.1053/gast.2003.50150. [DOI] [PubMed] [Google Scholar]
- Miettinen P.J., Warburton D., Bu D., Zhao J.-S., Berger J.E., Minoo P., Koivisto T., Allen L., Dobbs L., Werb Z., Derynck R. Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev. Biol. 1997;186:224–236. doi: 10.1006/dbio.1997.8593. [DOI] [PubMed] [Google Scholar]
- Mora A.L., Torres-González E., Rojas M., Xu J., Ritzenthaler J., Speck S.H., Roman J., Brigham K., Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am. J. Respir. Crit. Care Med. 2007;175:1139–1150. doi: 10.1164/rccm.200610-1426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A.L., Woods C.R., Garcia A., Xu J., Rojas M., Speck S.H., Roman J., Brigham K.L., Stecenko A.A. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L711–L721. doi: 10.1152/ajplung.00007.2005. [DOI] [PubMed] [Google Scholar]
- Naik P.K., Moore B.B. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev. Respir. Med. 2010;4:759–771. doi: 10.1586/ers.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba D., Mammoto A., Hashimoto K., Higashiyama S. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. J. Cell Biol. 2003;163:489–502. doi: 10.1083/jcb.200303017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai J.C., Ko F.W., Ng S.S., To K.-W., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J., Dong X.-P., Jiang G., Peiris M. SARS: clinical virology and pathogenesis. Respirol. Carlt. Vic. 2003;8(Suppl):S6–S8. doi: 10.1046/j.1440-1843.2003.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M., Kudoh S., Sakai F., Endo M., Hamaguchi T., Ogino Y., Yoneoka M., Sakaguchi M., Nishimoto H., Gemma A. Clinical features and risk factors of panitumumab-induced interstitial lung disease: a postmarketing all-case surveillance study. Int. J. Clin. Oncol. 2015;20:1063–1071. doi: 10.1007/s10147-015-0834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page C., Goicochea L., Matthews K., Zhang Y., Klover P., Holtzman M.J., Hennighausen L., Frieman M. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus infection. J. Virol. 2012;86:13334–13349. doi: 10.1128/JVI.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M., Law K.I., Tang B.S.F., Hon T.Y.W., Chan C.S., Chan K.H., Ng J.S.C., Zheng B.J., Ng W.L., Lai R.W.M., Guan Y., Yuen K.Y., HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet Lond. Engl. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugorria M.J., Latasa M.U., Nicou A., Cartagena-Lirola H., Castillo J., Goñi S., Vespasiani-Gentilucci U., Zagami M.G., Lotersztajn S., Prieto J., Berasain C., Avila M.A. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatol. Balt. Md. 2008;48:1251–1261. doi: 10.1002/hep.22437. [DOI] [PubMed] [Google Scholar]
- Piedimonte G., Perez M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 2014;35:519–530. doi: 10.1542/pir.35-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K., Zakowski M.F., Fan P.-D., Schonfeld E.A., Pao W., Varmus H.E. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor orto down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L., Zaki S.R., Baric R., Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Paddock C., Vogel L., Butler E., Zaki S., Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 2005;79:5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., Dyer M.D., Teal T.H., Proll S., van den Brand J., Baric R., Katze M.G. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.R., Dahlhoff M., Herbach N., Renner-Mueller I., Dalke C., Puk O., Graw J., Wanke R., Wolf E. Betacellulin overexpression in transgenic mice causes disproportionate growth, pulmonary hemorrhage syndrome, and complex eye pathology. Endocrinology. 2005;146:5237–5246. doi: 10.1210/en.2005-0418. [DOI] [PubMed] [Google Scholar]
- Schneider M.R., Wolf E. The epidermal growth factor receptor ligands at a glance. J. Cell. Physiol. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Courtneidge S.A. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Sheahan T., Morrison T.E., Funkhouser W., Uematsu S., Akira S., Baric R.S., Heise M.T. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4:e1000240. doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Tang J., Tong L., Liu Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer Amst. Neth. 2014;83:231–239. doi: 10.1016/j.lungcan.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Sime P.J., Xing Z., Graham F.L., Csaky K.G., Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K., Packard M., Shieh W.-J., Zaki S., Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., Roberts A. Is there an ideal animal model for SARS? Trends Microbiol. 2006;14:299–303. doi: 10.1016/j.tim.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Aoshiba K., Yokohori N., Nagai A. Epidermal growth factor receptor tyrosine kinase inhibition augments a murine model of pulmonary fibrosis. Cancer Res. 2003;63:5054–5059. [PubMed] [Google Scholar]
- Takemura T., Yoshida Y., Kiso S., Kizu T., Furuta K., Ezaki H., Hamano M., Egawa M., Chatani N., Kamada Y., Imai Y., Higashiyama S., Iwamoto R., Mekada E., Takehara T. Conditional loss of heparin-binding EGF-like growth factor results in enhanced liver fibrosis after bile duct ligation in mice. Biochem. Biophys. Res. Commun. 2013;437:185–191. doi: 10.1016/j.bbrc.2013.05.097. [DOI] [PubMed] [Google Scholar]
- Tang Y.-J., Xiao J., Huang X.R., Zhang Y., Yang C., Meng X.-M., Feng Y.-L., Wang X.-J., Hui D.S.C., Yu C.-M., Lan H.Y. Latent transforming growth factor-β1 protects against bleomycin-induced lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2014;51:761–771. doi: 10.1165/rcmb.2013-0423OC. [DOI] [PubMed] [Google Scholar]
- Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T., Baric R.S. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015;6:e00638. doi: 10.1128/mBio.00638-15. 00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse G.M.-K., To K.-F., Chan P.K.-S., Lo A.W.I., Ng K.-C., Wu A., Lee N., Wong H.-C., Mak S.-M., Chan K.-F., Hui D.S.C., Sung J.J.-Y., Ng H.-K. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J. Clin. Pathol. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui P.T., Kwok M.L., Yuen H., Lai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic Correlates1. Emerg. Infect. Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannella K.M., Moore B.B. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenes. Tissue Repair. 2008;1:2. doi: 10.1186/1755-1536-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer P.D., Einwalter L.A., Moninger T.O., Rokhlina T., Kern J.A., Zabner J., Welsh M.J. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- Wagner M., Weber C.K., Bressau F., Greten F.R., Stagge V., Ebert M., Leach S.D., Adler G., Schmid R.M. Transgenic overexpression of amphiregulin induces a mitogenic response selectively in pancreatic duct cells. Gastroenterology. 2002;122:1898–1912. doi: 10.1053/gast.2002.33594. [DOI] [PubMed] [Google Scholar]
- Walters D.M., Antao-Menezes A., Ingram J.L., Rice A.B., Nyska A., Tani Y., Kleeberger S.R., Bonner J.C. Susceptibility of signal transducer and activator of transcription-1-deficient mice to pulmonary fibrogenesis. Am. J. Pathol. 2005;167:1221–1229. doi: 10.1016/S0002-9440(10)61210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Deng B.C., Zhou Y., Wang Y., Cui W., Wang W., Liu P. Immunological features in patients with pneumonitis due to influenza A H1N1 infection. J. Investig. Allergol. Clin. Immunol. 2011;21:44–50. [PubMed] [Google Scholar]
- Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Wynn T.A. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.W.-C., Kwan R.W.-P., Mak P.H.-S., Mak K.K.-L., Sham M.-H., Chan S.-Y. Overexpression of epidermal growth factor induced hypospermatogenesis in transgenic mice. J. Biol. Chem. 2000;275:18297–18301. doi: 10.1074/jbc.M001965200. [DOI] [PubMed] [Google Scholar]
- Wu X., Dong D., Ma D. Thin-section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS) Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016;22:2793–2799. doi: 10.12659/MSM.896985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Liu Y., Xiao Y., Tian Q., Fan B., Zhao H., Chen W. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127:2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Moriwaki T., Matsuda K., Yamamoto Y., Sugaya A., Akutsu D., Murashita T., Endo S., Hyodo I. Panitumumab-induced interstitial lung disease in a case of metastatic colorectal cancer. Onkologie. 2013;36:209–212. doi: 10.1159/000349959. [DOI] [PubMed] [Google Scholar]
- Yano S., Kondo K., Yamaguchi M., Richmond G., Hutchison M., Wakeling A., Averbuch S., Wadsworth P. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2003;23:3639–3650. [PubMed] [Google Scholar]
- Yarden Y., Shilo B.-Z. SnapShot: EGFR signaling pathway. Cell. 2007;131 doi: 10.1016/j.cell.2007.11.013. 1018.e1-1018.e2. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Lee J.-Y., Lee C.-M., Cho W.-K., Kang M.-J., Koff J.L., Yoon P.-O., Chae J., Park H.-O., Elias J.A., Lee C.G. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-β-induced pulmonary fibrosis. J. Biol. Chem. 2012;287:41991–42000. doi: 10.1074/jbc.M112.356824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornetzer G.A., Frieman M.B., Rosenzweig E., Korth M.J., Page C., Baric R.S., Katze M.G. Transcriptomic analysis reveals a mechanism for a prefibrotic phenotype in STAT1 knockout mice during severe acute respiratory syndrome coronavirus infection. J. Virol. 2010;84:11297–11309. doi: 10.1128/JVI.01130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]