Abstract
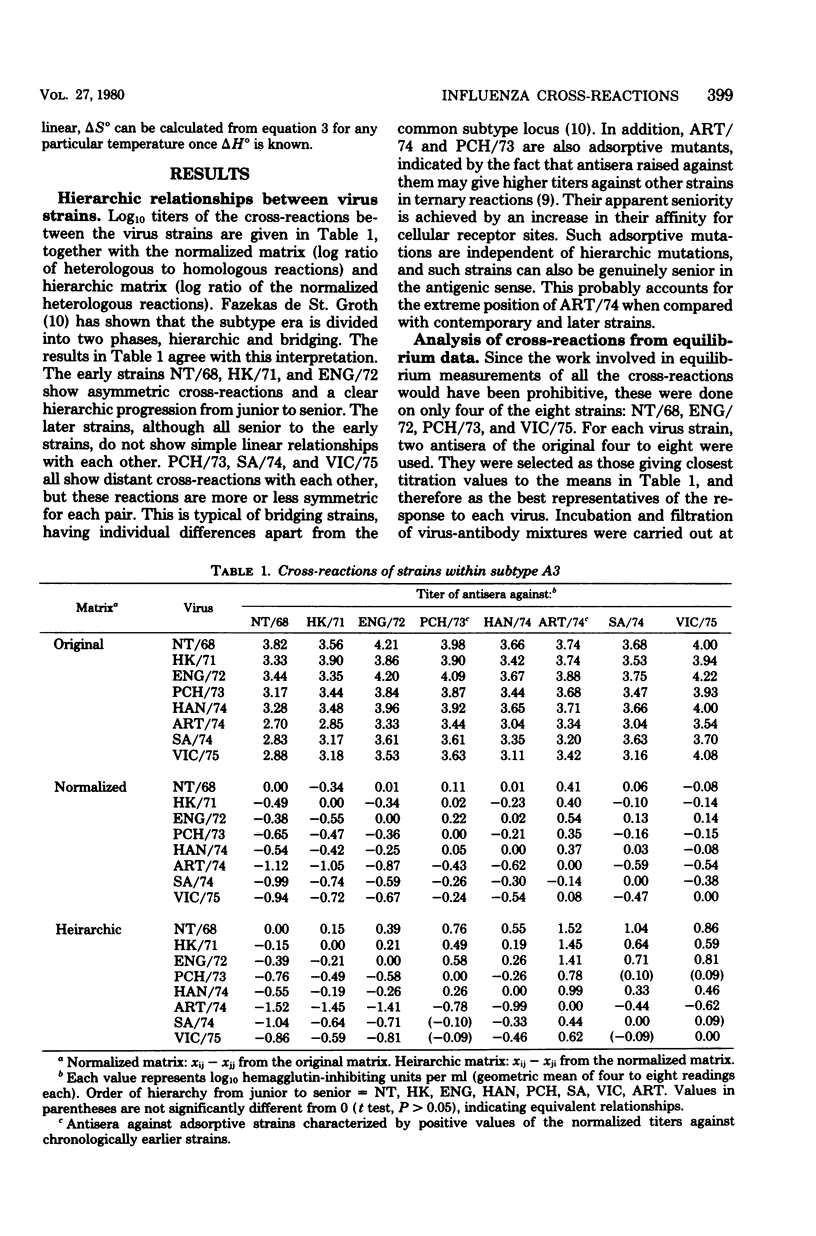
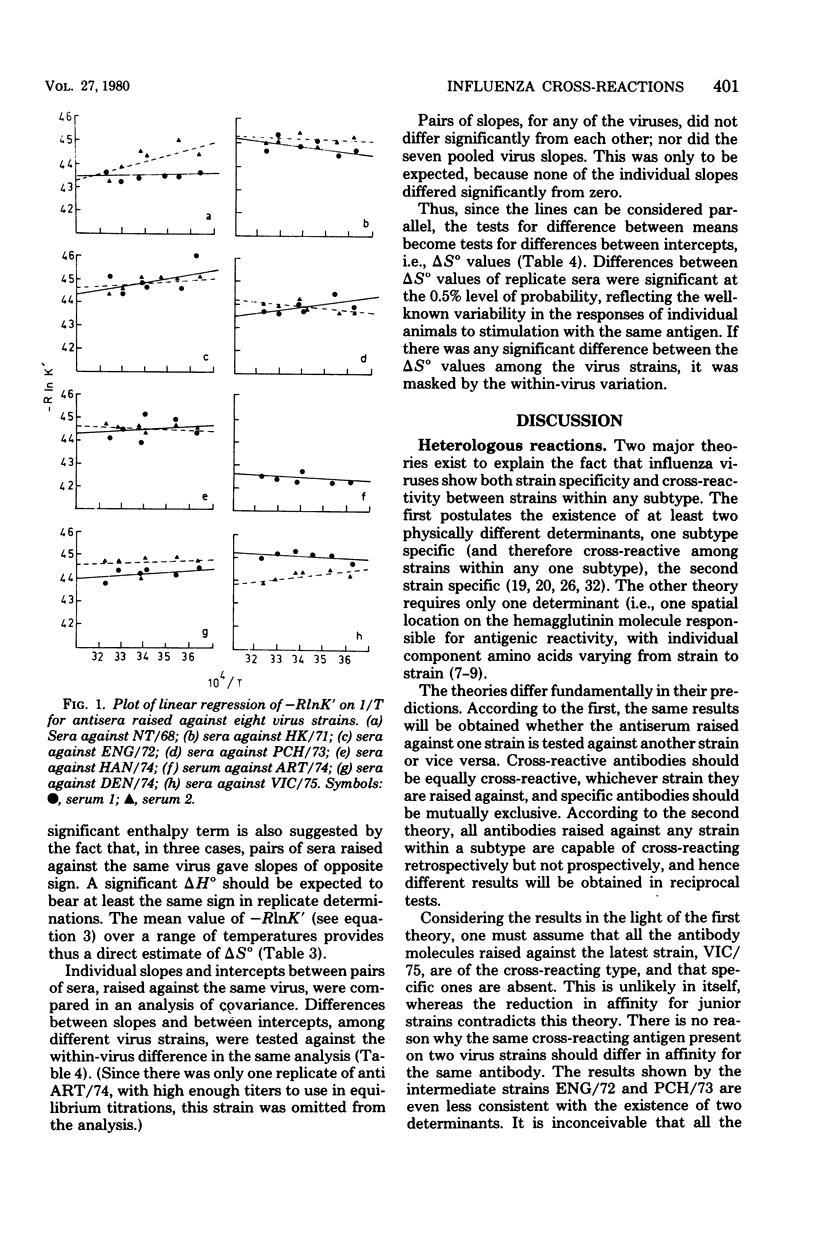
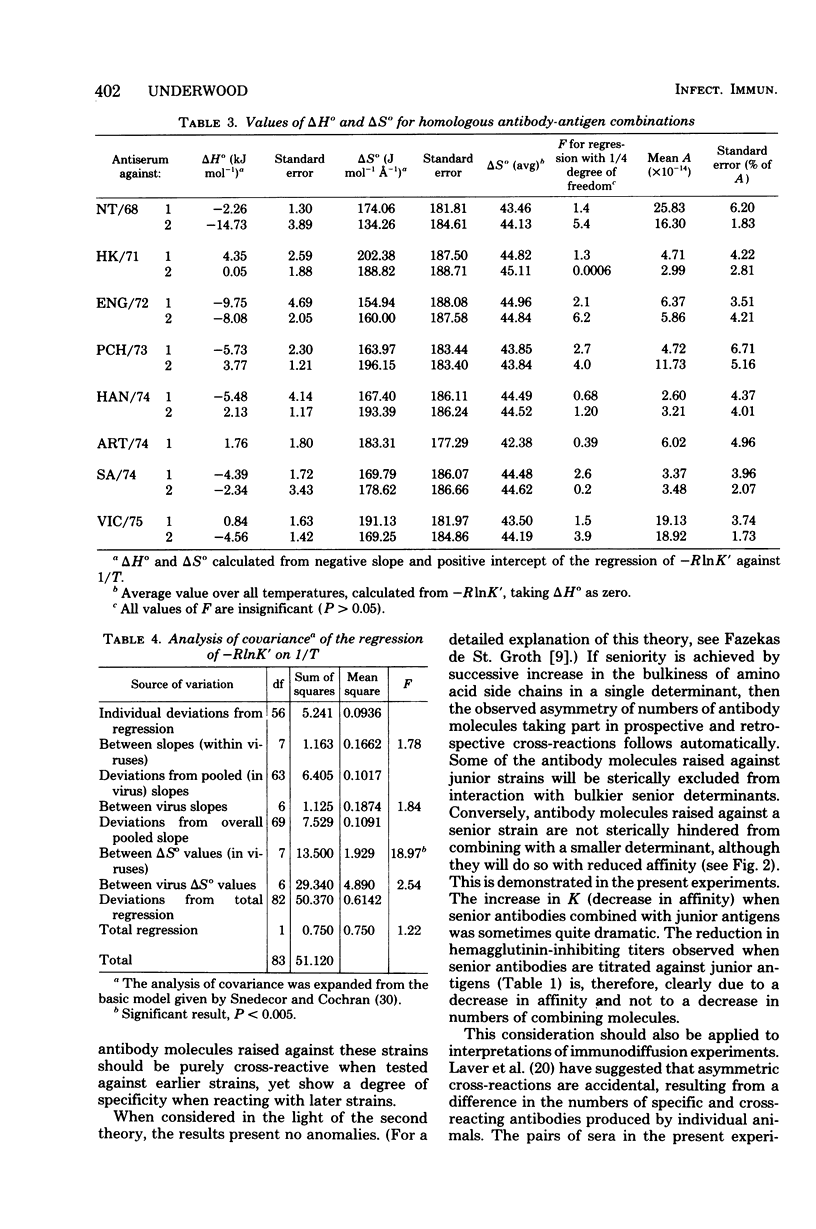
Reciprocal hemagglutination inhibition titrations were carried out with viruses and antisera of eight field strains of the A3 subtype of influenza A, covering the period from 1968 to 1975. The earlier strains (1968 through 1972) showed asymmetric cross-reactions, with antisera exhibiting more cross-reactions with antecedent strains than with subsequent ones. The later strains, although all were asymmetrically cross-reactive with earlier strains, tended to exhibit distant and variable cross-reactions with each other. The numbers and average affinities of antibody molecules capable of taking part in cross-reactions were calculated from equilibrium filtration experiments. It was found that all the antibody molecules in sera raised against the late strains could combine with earlier viruses, but with reduced affinity. Conversely, only a subset of the antibody molecules in sera raised against early strains could combine with later viruses. The results are discussed in the light of different theories concerning the nature and number of antigenic determinants on the hemagglutinin molecule. They support the existence of a single antigenic area to which all antibody molecules are directed, with differing affinities, rather than the existence of both “common” and “specific” determinants. Thermodynamic measurements on the homologous antigen-antibody reactions indicated that combination was mostly entropy driven. This suggested hydrophobic interaction as the mechanism of combination, i.e., that the complementary regions of antigen and antibody were made up largely or entirely of amino acids with hydrophobic side chains. There was no statistical difference in the magnitude of the entropy term (i.e., the average firmness of binding) among the different virus strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- CAIRNS H. J. F., EDNEY M., FAZEKAS DE ST GROTH S. Quantitative aspects of influenza virus multiplication. J Immunol. 1952 Aug;69(2):155–181. [PubMed] [Google Scholar]
- DE ST GROTH S. F., WITHELL J., LAFFERTY K. J. An improved assay method for neutralizing antibodies against influenza viruses. J Hyg (Lond) 1958 Sep;56(3):415–426. doi: 10.1017/s0022172400037906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher J., Verhagen W. Investigations on strain-specific and common antigen antihemagglutinin antibodies oriented to the influenza virus H3N2 strains A/Hong Kong/1/68 and A/Port Chalmers/1/73. Infect Immun. 1978 Sep;21(3):792–797. doi: 10.1128/iai.21.3.792-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAZEKAS DE ST. GROTH S., GRAHAM D. M. The production of incomplete virus particles among influenza strains: experiments in eggs. Br J Exp Pathol. 1954 Feb;35(1):60–74. [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth Evolution and hierarchy of influenza viruses. Arch Environ Health. 1970 Sep;21(3):293–303. doi: 10.1080/00039896.1970.10667241. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth New criteria for the selection of influenza vaccine strains. Bull World Health Organ. 1969;41(3):651–657. [PMC free article] [PubMed] [Google Scholar]
- Gerhard W. The analysis of the monoclonal immune response to influenza virus. II. The antigenicity of the viral hemagglutinin. J Exp Med. 1976 Oct 1;144(4):985–995. doi: 10.1084/jem.144.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN K. E. Diagnosis of influenza by serologic methods. Am Rev Respir Dis. 1961 Feb;83(2):120–124. doi: 10.1164/arrd.1961.83.2P2.120. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Downie J. C., Webster R. G. Diversity of the antibody response to the different antigenic determinants on the hemagglutinin subunits of influenza viruses. J Immunol. 1976 Feb;116(2):336–341. [PubMed] [Google Scholar]
- Laver W. G., Downie J. C., Webster R. G. Studies on antigenic variation in influenza virus. Evidence for multiple antigenic determinants on the hemagglutinin subunits of A-Hong Kong-68 (H3 N2) virus and the A-England-72 strains. Virology. 1974 May;59(1):230–244. doi: 10.1016/0042-6822(74)90218-9. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Gerhard W., Webster R. G., Frankel M. E., Air G. M. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1425–1429. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. The structure of influenza viruses. IV. Chemical studies of the host antigen. Virology. 1966 Sep;30(1):104–115. doi: 10.1016/s0042-6822(66)81014-0. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- SHARP D. G., BEARD J. W. Counts of virus particles by sedimentation on agar and electron micrography. Proc Soc Exp Biol Med. 1952 Oct;81(1):75–79. doi: 10.3181/00379727-81-19782. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Henry-Aymard M., Pereira H. G. A quantitative, single-radial-diffusion test for immunological studies with influenza virus. J Gen Virol. 1972 Aug;16(2):231–236. doi: 10.1099/0022-1317-16-2-231. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., Dowdle W. R., Coleman M., Pereira M. S., Chakraverty P. Antigenic variation in current influenza A viruses: evidence for a high frequency of antigenic 'drift' for the Hong Kong virus. Bull World Health Organ. 1974;51(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Schild G. C. Studies with antibody to the purified haemagglutinin of an influenza Ao virus. J Gen Virol. 1970 Dec;9(3):191–200. doi: 10.1099/0022-1317-9-3-191. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Kilbourne E. D. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus: distinctiveness of hemagglutinin antigen of Hong Kong-68 virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):326–333. doi: 10.1073/pnas.63.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Estimation of the number of surface projections on myxo- and paramyxoviruses. Virology. 1970 Jun;41(2):392–394. doi: 10.1016/0042-6822(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Postlethwaite R., Schild G. C., Allison A. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. I. Thymus dependence of antibody formation and thymus independence of immunological memory. J Exp Med. 1974 Dec 1;140(6):1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J., Waterfield M. Evidence from studies with a cross-linking reagent that the haemagglutinin of influenza virus is a trimer. Virology. 1977 Jun 15;79(2):446–448. doi: 10.1016/0042-6822(77)90371-3. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Laver W. G., Downie J. C. Binding of antibodies to isolated haemagglutinin and neuraminidase molecules of influenza virus observed in the electron microscope. J Mol Biol. 1977 Jan 25;109(3):405–421. doi: 10.1016/s0022-2836(77)80020-x. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Webster R. G., Gerhard W. U. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature. 1979 May 17;279(5710):246–248. doi: 10.1038/279246a0. [DOI] [PubMed] [Google Scholar]



