Abstract
Vestibular compensation is a recovery process from vestibular symptoms over time after unilateral loss of peripheral vestibular end organs. The aim of the present study was to observe time-dependent changes in long-term potentiation (LTP) at Schaffer collateral-CA1 synapses in the CA1 area of the hippocampus during vestibular compensation. The input-output (I/O) relationships of fEPSP amplitudes and LTP induced by theta burst stimulation to Schaffer's collateral commissural fibers were evaluated from the CA1 area of hippocampal slices at 1 day, 1 week, and 1 month after unilateral labyrinthectomy (UL). The I/O relationships of fEPSPs in the CA1 area was significantly reduced within 1 week post-op and then showed a non-significant reduction at 1 month after UL. Compared with sham-operated animals, there was a significant reduction of LTP induction in the hippocampus at 1 day and 1 week after UL. However, LTP induction levels in the CA1 area of the hippocampus also returned to those of sham-operated animals 1 month following UL. These data suggest that unilateral injury of the peripheral vestibular end organs results in a transient deficit in synaptic plasticity in the CA1 hippocampal area at acute stages of vestibular compensation.
Keywords: Hippocampus, Long-term potentiation, Unilateral labyrinthectomy, Vestibular compensation
INTRODUCTION
Peripheral vestibular afferent signals arising from the vestibular hair cells in the labyrinth of the inner ear can control body posture or eye movement via reflexes. Peripheral vestibular information is also essential for spatial navigation or spatial memory formation mediated by higher brain structures such as the hippocampus and neocortex [1,2,3]. Sudden loss of these vestibular afferent signals by unilateral labyrinthectomy (UL) results in ocular or postural deficits as well as spatial disorientation. However, most of these clinical symptoms almost disappear within a few weeks after UL, in a process known as vestibular compensation (VC). Considering that the injured vestibular hair cells are never regenerated, VC is considered as a type of lesion-induced neural plasticity in the brain [4,5].
The hippocampal CA1 area is believed to be a critical structure for spatial navigation and memory, as place cells for coding for spatial location as well as the direction and speed of body movement are located in this area [6]. Long-term potentiation (LTP), a long-lasting enhancement of field excitatory postsynaptic potentials (fEPSPs), is easily induced by high-frequency electrical stimulation of Schaffer collaterals/commissural fibers in the CA1 area [7]. LTP has been shown to be the closest cellular mechanism for learning and memory storage in the hippocampus. Some papers have documented that LTP induction in the CA1 area is linked to maintenance of spatial memory [8,9].
Many experimental studies have documented the existence of functional connections between the vestibular system and hippocampus [3,10,11]. In humans, a radiological imaging study showed that excitation of the peripheral vestibular system by galvanic stimulation activates the hippocampus [12]. Vestibular afferent inputs can modulate the activity of place cells in the CA1 area, probably to contribute to spatial navigation. In contrast, loss of vestibular inputs produces deficits in hippocampal-dependent spatial learning and memory tasks such as the radial arm maze, Morris water maze, and a food foraging task [3,11,13]. In humans, bilateral vestibular loss is accompanied by a reduction of hippocampal volume as well as significant spatial memory and navigation deficits [14].
A few studies have demonstrated a role of peripheral vestibular sensory inputs in the induction of LTP in the hippocampus. Bilateral labyrinthectomy did not affect the induction and maintenance of LTP in the hippocampal CA1 area in rats 6 weeks after surgery [15]. The induction rate of LTP in the CA1 area by passive whole-body rotation was higher than by immobility in the CA1 area of behaving rats [16]. However, it has not been demonstrated how UL affects induction of LTP in the CA1. Therefore, the purpose of this study was to understand the temporal changes in LTP in the hippocampal CA1 region of rats using an in vitro multi-channel recording system during VC.
METHODS
Animals and surgical unilateral labyrinthectomy
All experimental procedures were approved by the guidelines of the Committee on Animal Research at Wonkwang University (approval No. WKU15-153). Nineteen male Sprague–Dawley rats (250~300 g) were housed in a controlled animal husbandry unit at 21±1℃ with ad libitum access to water. The rats were divided into 4 experimental groups: (1) sham operation with intact labyrinth (n=5, 15 slices each ipsilateral and contralateral) 1 day before recording; (2) UL 1 day before recording (n=5, 15 slices each ipsilateral and contralateral); (3) UL 1 week before recording (n=5, 15 slices each ipsilateral and contralateral); and (4) UL 1 month before recording (n=5, 15 slices each ipsilateral and contralateral).
ULs were performed as previously described in detail [17]. In brief, after induction of anesthesia with 4% isoflurane, the left middle ear was opened and the ossicular bones were removed to expose the oval window through the ventral approach under a surgical microscope. The inner ear was destroyed surgically by opening the oval window with a needle and aspiration with a suction pump. UL was confirmed by the appearance of spontaneous nystagmus just after recovery from anesthesia.
Hippocampal slice preparation
All procedures for preparation of hippocampal slices and LTP recording were performed according to the protocol recommended by Alpha MED Scientific Inc. (Japan). Briefly, animals were deeply anesthetized with urethane and decapitated by a guillotine. The whole brain was rapidly removed from the cranium and immersed in ice-cold artificial cerebrospinal fluid (aCSF in mM; NaCl 124, KCl 3, NaH2PO4 1.25, MgCl2 1.3, NaHCO3 26, glucose 10, CaCl2 2.5) for 2 min and bubbled with 95% O2/5% CO2. The brain was trimmed and mounted on the stage of a vibrating microtome (Model VT1000S; Leica, Wetzlar, Germany) with super glue in such a way that the blade would cut through the hemispheres at an angle of 20°~30° relative to their horizontal planes. Five brain slices containing medial segments of the hippocampus from one hemisphere were cut at a thickness of 350 mm using stainless steel blades in an ice-cold sucrose cutting solution (in mM) (sucrose 110, NaCl 60, NaHCO3 28, NaH2PO4 1.25, KCl 3, MgSO4 7, CaCl2 0.5, glucose 5, sodium ascorbate 0.6), and then transferred to a chamber (100 ml) containing aCSF at 25℃. In each slice, the hippocampus was selectively isolated from surrounding brain tissue using a surgical knife under a surgical microscope. Hippocampal slices were again transferred to a slice holding chamber (500 ml) containing aCSF and recovered at 32℃ for at least 1 hour. After the recovery, the slice holding chamber containing hippocampal slices was kept in a 25℃ water bath until the recording.
Hippocampal slice electrophysiology
Individual hippocampal slices were positioned on an MED64 microelectrode array (8×8, 150 µm inter-electrode spacing, Alpha MED Scientific Inc., Osaka, Japan) and then immobilized using self-made sliver slice anchors under an inverted microscope (Olympus, Tokyo, Japan). Slices were perfused with oxygenated 30℃ aCSF at a rate of 2 ml/min using an automatic temperature controller and peristaltic pump (Harvard Apparatus, Cambridge, USA). fEPSPs was evoked by electrical stimulation to Schaffer collateral/commissural fibers at the stratum radiatum (SR) layer of the CA1 area through one electrode (Fig. 1B). Biphasic pulses (0.1 ms, 10~90 µA, 0.05 Hz) were delivered using a pulse generator and Performer® software in the MED64 recording system. The fEPSPs were sampled at 20 kHz using a MED 64 multi-channel amplifier, and were digitized and graphically displayed using the LTP module of Performer® software.
Fig. 1. Typical recording of field excitatory post-synaptic potentials (fEPSPs) evoked by application of single pulse electrical shock to Schaffer collaterals-commissural fibers in the CA1 area of the hippocampus.
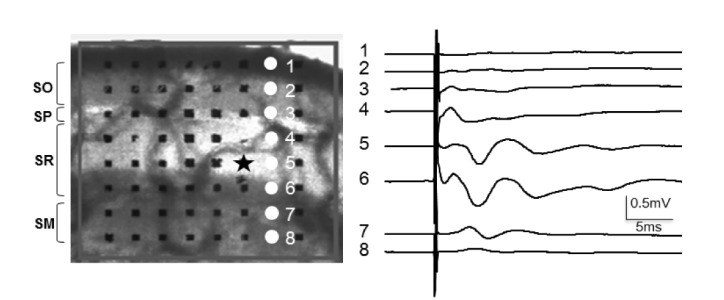
Basal synaptic excitability and transmission was assayed by plotting the stimulus amplitude (µA) against amplitudes of fEPSPs to generate input-output (I/O) relationships. Once the threshold stimulus amplitude to initially elicit fEPSPs was determined, sequential changes in fEPSPs were recorded as the next stimulus was given with double the initial stimulus amplitude. The Performer® software analyzed the amplitude and initial slope of the negative fEPSPs waves and then plotted I/O relationships in one recording electrode that showed the most prominent fEPSPs waves. As a baseline recording, fEPSPs with half maximal amplitude were recorded at 20-second intervals and the slope of each fEPSPs was automatically calculated by the Performer® software. After 30 min of stable baseline recording, LTP was induced by applying patterned theta-burst stimuli (TBS) via the stimulation electrode. TBS comprised three pulse trains administered at 20-s intervals with 10 bursts administered at 5 Hz per train and 4 pulses administered at 100 Hz per burst. fEPSPs recording was continued for 60 min after the theta burst stimulation. During the LTP recording session, the software automatically calculated the amplitude and initial slope of fEPSPs. After the end of LTP recording, the software automatically displayed a plot that expressed the percentage of the fEPSP values relative to the average values of the first five baseline points in each recording channel.
Data analysis
Data recorded during these experiments were analyzed with the Performer® software and Excel (Microsoft Office 2013; Microsoft Corporation, Redmond, WA, USA). To analyze I/O responses, the amplitudes of fEPSPs at each stimulus intensity were calculated from one recording channel of each slice and then averaged from 3 slices in each experimental animal. To compare LTP induction rates among experimental groups, LTP induction rates expressed in % in the Performer software were collected and averaged from 4 recording electrodes in each slice. This analysis of LTP induction rates was performed from 3 slices from each experimental animal. Data for LTP induction rates and fEPSPs amplitudes of I/O responses are expressed as mean±SD from 5 rats in each group. Differences between groups were detected using an ANOVA followed by Dunn's post hoc tests. A P-value less than 0.05 denotes statistical significance.
RESULTS
When we applied a single pulse of electrical stimulation to Schaffer collaterals in the medial SR layer, negative deflections of fEPSPs were noted in the whole area of the SR layer, representing synaptic activation of apical dendrites of pyramidal cells. Large amplitude fEPSPs were observed on recording electrodes located medially or distally in the SR layer (electrodes 5 and 6 in Fig. 1). The stratum lacunosum-moleculare (SM) layer showed smaller negative waves of fEPSPs (electrodes 7 and 8 in Fig. 1).
To determine the effects of UL on baseline excitability and transmission, we measured changes in fEPSPs amplitude by comparing I/O responses between experimental groups. The threshold amplitude of electrical stimulation to elicit fEPSPs was 10.5±3.2 µA (mean±SD) with small variations among slices. Both bilateral slices of UL animals at 1 day and 1 week after UL exhibited statistically significant reductions in the fEPSP amplitudes between 30 µA and 60 µA of stimulus intensities compared with those of sham-operated animals, indicating a rightward shift on the I/O plot (Fig. 2). At 1 day after UL, reductions in the fEPSPs amplitude were more prominent on the ipsilateral side than the contralateral side. The fEPSPs amplitudes in slices from UL at 1 month post-op were likely to be lower than those of sham-operated animals, though without statistical significance across the range of stimulation intensities.
Fig. 2. Stimulus intensity-dependent changes in the amplitudes of fEPSPs in the stratum radiatum layer of the CA1 area at 1 day, 1 week, and 1 month after UL.
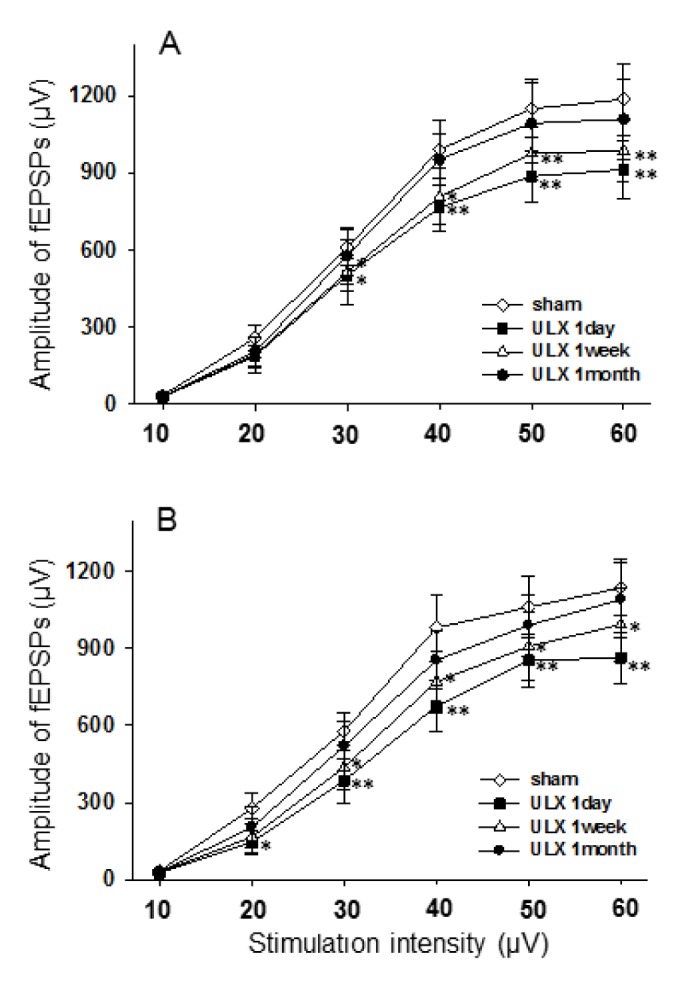
(A) Contralesional CA1 area, (B) ipsilesional CA1 area (n=15, *p<0.05, **p<0.01, sham vs. UL).
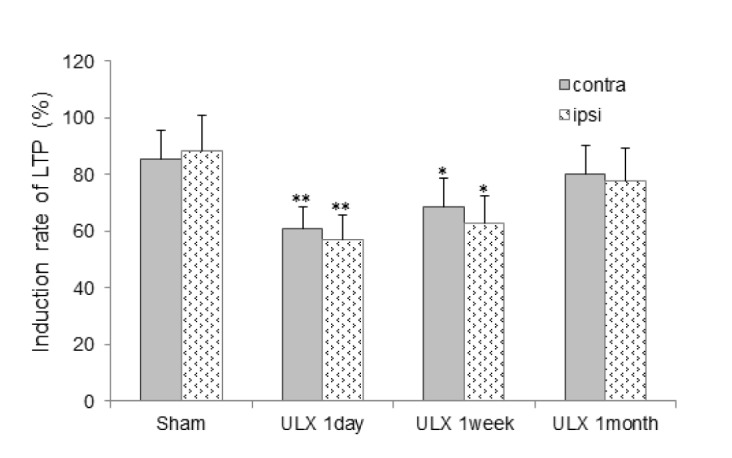
We used a TBS protocol TBS to induce LTP in the SR layer of the CA1 area following a 15-min period of measuring baseline responses. Following TBS, a persistent potentiation of fEPSPs was easily produced in slices of all groups by the end of observation period. In slices from the sham group, TBS caused robust potentiation of fEPSPs, and the induction rate of LTP was 85.3~88.8% (n=15) 60 min after TBS. In contrast, there was a significant decrease in induction rate of LTP in bilateral slices, especially ipsilateral slices (56.9±8.6 %, n=15), after TBS in bilateral slices of UL animals at 1 day post-op (p<0.01) (Fig. 3). This reduced induction rate of LTP in bilateral slices remained at 1 week post-UL (p<0.05) but disappeared at 1 month post-UL (Fig. 4).
Fig. 3. Typical recordings of long-term potentiation induction (LTP) of fEPSPs in the stratum radiatum layer of the CA1 area evoked by theta burst stimulation.
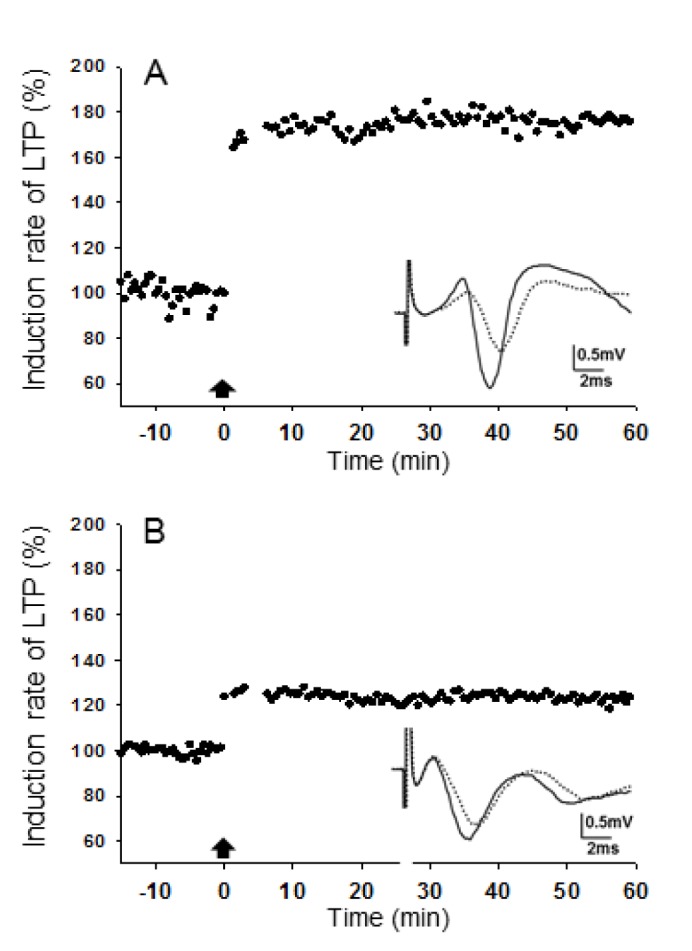
(A) Sham animal, (B) UL 1 day. Arrows indicate application of theta burst stimulation to induce LTP.
Fig. 4. Comparison of induction rates of LTP between the contralesional (contra) and ipsilesional (ipsi) CA1 areas of hippocampal slices following UL (n=15, *p<0.05, **p<0.01).
DISCUSSION
The present study evaluated basal synaptic excitability and transmission by observing the I/O relationships of fEPSPs amplitudes in the hippocampal CA1 area slices following UL. The I/O relationships of fEPSPs in the CA1 area was significantly reduced 1 week post-op and then showed a non-significant reduction at 1 month after UL. Similarly, Zheng et al.[18] reported that the fEPSPs slopes on I/O curves in the CA1 area of hippocampal slices was slightly reduced relative to controls at 4~6 weeks post-UL. However, the population spike amplitude and the fEPSPs slopes in CA1 and CA2 were significantly decreased at 5~6 months post-UL [18]. This experimental evidence suggests that permanent unilateral loss of peripheral vestibular end organs may induce profound changes in synaptic excitability in the hippocampal CA1 area at acute stages of VC (<1 week).
Several lines of experimental evidence have demonstrated that the vestibular system can affect electrical excitability of the hippocampal formation. For example, a transient inactivation of vestibular hair cells by injection of lidocaine in the middle ear causes severe reduction in the firing rates of place cells and an absence of theta rhythms (4~10 Hz) in the CA1 area over 3 days after the injection [19,20]. Otherwise, vestibular activation by passive whole-body rotation results in a continuous induction of the theta rhythm and alteration of fEPSPs amplitudes in the CA1 area [16]. Electrical stimulation of the medial vestibular nucleus in the brainstem increases firing rates of CA1 complex spike cells corresponding anatomically to pyramidal cells in rats [21].
One other major finding of the present study is the significant reduction of LTP induction in bilateral CA1 areas with ipsilateral predominance at 1 day post-UL that persisted at 1 week post-UL (p<0.05) but disappeared at 1 month post-UL. A previous experimental study observed no significant changes in LTP induction in the CA1 area and dentate gyrus of awake and anesthetized rats 1~7 months following bilateral labyrinthectomy [15]. Transient activation of the peripheral vestibular system by passive whole-body rotation enhances LTP via activation of cholinergic septohippocampal cells in the CA1 area in freely behaving rats [16]. Therefore, as far as we know, we report here for first time the observation of reduced LTP induction, indicating impaired synaptic plasticity in the CA1 area following the removal of peripheral vestibular end organs.
Although the correlation between enhanced LTP induction in the CA1 area and hippocampal-dependent learning performance is not yet fully understood, many studies have documented that chemical or environmental manipulations that cause reduced LTP induction deteriorate hippocampal learning performance [22,23]. Additionally, a lowered threshold of LTP induction or the enhancement of synaptic efficacy in the CA1 area occurs in the initial phase of spatial memory formation [8,24].
Sudden unilateral loss of peripheral vestibular afferent activity produces severe deficits in the vestibulo-ocular reflex, such as ocular nystagmus, which produces apparent movement of the visual world, and “oscillopsia.” UL is also accompanied by severe vegetative symptoms such as nausea and vomiting, indicating abnormal activation of the autonomic nervous system. UL-induced oscillopsia and dysregulation of the autonomic nervous system at acute stages of UL may affect functions of higher brain structures including spatial memory and spatial perception [11,25,26]. In the acute stage after unilateral surgical vestibular lesion, patients showed marked reductions in performance on spatial navigation tasks that are predominantly mediated by the hippocampus. These impairments disappeared by 1 month after vestibular lesion [27,28]. Animals with UL are impaired in terms of performing another hippocampal-dependent task, spatial food-seeking behavior in the dark, at 3 months after the lesion, and this impairment disappeared at 6 months after UL [29]. This suggests that unilateral suppression of vestibular information would induce transitory spatial memory disorganization at a high level of information processing. Therefore, the reduced LTP induction in the CA1 area observed in the present study may be the electrophysiological substrate for the impairment of hippocampus-related performance at acute phases of VC.
There is also considerable evidence that UL results in neurochemical and biomolecular changes in the hippocampus over the course of VC. The hippocampus shows rapid neurochemical alterations in nitric oxide activity and the concentrations of amino acids and noradrenaline, even with regional specificity, within 24 hours after UL in the guinea pig [30]. Expression of glutamate ionotropic receptor NMDA type subunit 2A was altered in the ipsilateral CA1 at 10 hours post-UVD in guinea pigs [31]. An anatomical study has documented that vestibular axonal projections reach to major structures responsible for autonomic functions such as hypothalamus, locus coeruleus, and solitary nucleus [32]. Experimental evidence linking unilateral vestibular deafferentation to the long-lasting activation of the hypothalamic–pituitary–adrenal (HPA) axis has also been shown [33]. Activation of the HPA axis includes the observation of upregulation of cFos-positive neurons in the hypothalamus and a significant increase in cortisol concentrations at night [34,35]. At 2 weeks post-UL, there was a significant decrease in glucocorticoid receptor expression in the ipsilateral CA1 and a significant increase in glucocorticoid receptor expression in the contralateral dentate gyrus at 2 weeks post-UL [36]. LTP in the CA1 area was impaired at 2 but not 4 days after stressor cessation [37]. Collectively, stress-induced neuroendocrine plasticity and biomolecular changes induced by UL may contribute to changes in LTP induction at early stages of VC.
Recently, a study reported that induction of long-term depression (LTD) in the hippocampus may play an essential role not only in novelty acquisition but also in novelty-mediated memory enhancement [38]. A recent paper has shown that LTD induction in the CA1 area can be a possible cellular substrate for the consolidation of long-term spatial memory [39]. Considering these findings, further research is needed to observe changes of LTD induction at acute phases of VC.
In summary, we observed that unilateral loss of the peripheral vestibular end organs resulted in a transient deficit in LTP induction in the CA1 hippocampal area at acute stages of VC. This transient impairment of LTP can possibly be a neural substrate for disorders of spatial navigation or memory in patients with UL.
Footnotes
Author contributions: G.W.L. and J.H.K. equally contributed to this paper as co-first author. G.W.L.: Acquisition of data, drafting of manuscript. J.H.K.: Analysis and interpretation of data, drafting of manuscript. M.S.K.: Study conception and design, drafting of manuscript, critical revision.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Hüfner K, Strupp M, Smith P, Brandt T, Jahn K. Spatial separation of visual and vestibular processing in the human hippocampal formation. Ann N Y Acad Sci. 2011;1233:177–186. doi: 10.1111/j.1749-6632.2011.06115.x. [DOI] [PubMed] [Google Scholar]
- 2.Besnard S, Machado ML, Vignaux G, Boulouard M, Coquerel A, Bouet V, Freret T, Denise P, Lelong-Boulouard V. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus. 2012;22:814–826. doi: 10.1002/hipo.20942. [DOI] [PubMed] [Google Scholar]
- 3.Smith PF. Vestibular-hippocampal interactions. Hippocampus. 1997;7:465–471. doi: 10.1002/(SICI)1098-1063(1997)7:5<465::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol. 2000;62:313–325. doi: 10.1016/s0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- 6.Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S. Hippocampal representational organization and spatial context. Hippocampus. 1999;9:444–451. doi: 10.1002/(SICI)1098-1063(1999)9:4<444::AID-HIPO10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Hölscher C. Long-term potentiation: a good model for learning and memory? Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:47–68. doi: 10.1016/s0278-5846(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 8.Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S. Long-term potentiation in the hippocampal CA1 area and dentate gyrus plays different roles in spatial learning. Eur J Neurosci. 2003;17:341–349. doi: 10.1046/j.1460-9568.2003.02458.x. [DOI] [PubMed] [Google Scholar]
- 9.Qi X, Zhang K, Xu T, Yamaki VN, Wei Z, Huang M, Rose GM, Cai X. Sex differences in long-term potentiation at temporoammonic-ca1 synapses: potential implications for memory consolidation. PLoS One. 2016;11:e0165891. doi: 10.1371/journal.pone.0165891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitier M, Besnard S, Smith PF. Vestibular pathways involved in cognition. Front Integr Neurosci. 2014;8:59. doi: 10.3389/fnint.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PF, Horii A, Russell N, Bilkey DK, Zheng Y, Liu P, Kerr DS, Darlington CL. The effects of vestibular lesions on hippocampal function in rats. Prog Neurobiol. 2005;75:391–405. doi: 10.1016/j.pneurobio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Vitte E, Derosier C, Caritu Y, Berthoz A, Hasboun D, Soulié D. Activation of the hippocampal formation by vestibular stimulation: a functional magnetic resonance imaging study. Exp Brain Res. 1996;112:523–526. doi: 10.1007/BF00227958. [DOI] [PubMed] [Google Scholar]
- 13.Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J Neurosci. 2003;23:6490–6498. doi: 10.1523/JNEUROSCI.23-16-06490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005;128:2732–2741. doi: 10.1093/brain/awh617. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Mason-Parker SE, Logan B, Darlington CL, Smith PF, Abraham WC. Hippocampal synaptic transmission and LTP in vivo are intact following bilateral vestibular deafferentation in the rat. Hippocampus. 2010;20:461–468. doi: 10.1002/hipo.20645. [DOI] [PubMed] [Google Scholar]
- 16.Tai SK, Leung LS. Vestibular stimulation enhances hippocampal long-term potentiation via activation of cholinergic septohippocampal cells. Behav Brain Res. 2012;232:174–182. doi: 10.1016/j.bbr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Jin BK, Chun SW, Lee MY, Lee SH, Kim JH, Park BR. Role of vestibulocerebellar N-methyl-D-aspartate receptors for behavioral recovery following unilateral labyrinthectomy in rats. Neurosci Lett. 1997;222:171–174. doi: 10.1016/s0304-3940(97)13371-7. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Kerr DS, Darlington CL, Smith PF. Unilateral inner ear damage results in lasting changes in hippocampal CA1 field potentials in vitro. Hippocampus. 2003;13:873–878. doi: 10.1002/hipo.10174. [DOI] [PubMed] [Google Scholar]
- 19.Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Lesions of the vestibular system disrupt hippocampal theta rhythm in the rat. J Neurophysiol. 2006;96:4–14. doi: 10.1152/jn.00953.2005. [DOI] [PubMed] [Google Scholar]
- 21.Horii A, Russell NA, Smith PF, Darlington CL, Bilkey DK. Vestibular influences on CA1 neurons in the rat hippocampus: an electrophysiological study in vivo. Exp Brain Res. 2004;155:245–250. doi: 10.1007/s00221-003-1725-9. [DOI] [PubMed] [Google Scholar]
- 22.Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann N Y Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- 23.Diamond DM, Park CR, Campbell AM, Woodson JC. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus. 2005;15:1006–1025. doi: 10.1002/hipo.20107. [DOI] [PubMed] [Google Scholar]
- 24.Pavlowsky A, Wallace E, Fenton AA, Alarcon JM. Persistent modifications of hippocampal synaptic function during remote spatial memory. Neurobiol Learn Mem. 2017;138:182–197. doi: 10.1016/j.nlm.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist's best friend. J Neurol. 2016;263(Suppl 1):S54–S64. doi: 10.1007/s00415-015-7903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hüfner K, Strupp M, Smith P, Brandt T, Jahn K. Spatial separation of visual and vestibular processing in the human hippocampal formation. Ann N Y Acad Sci. 2011;1233:177–186. doi: 10.1111/j.1749-6632.2011.06115.x. [DOI] [PubMed] [Google Scholar]
- 27.Péruch P, Borel L, Gaunet F, Thinus-Blanc G, Magnan J, Lacour M. Spatial performance of unilateral vestibular defective patients in nonvisual versus visual navigation. J Vestib Res. 1999;9:37–47. [PubMed] [Google Scholar]
- 28.Péruch P, Borel L, Magnan J, Lacour M. Direction and distance deficits in path integration after unilateral vestibular loss depend on task complexity. Brain Res Cogn Brain Res. 2005;25:862–872. doi: 10.1016/j.cogbrainres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Darlington CL, Smith PF. Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rats. Hippocampus. 2006;16:368–378. doi: 10.1002/hipo.20149. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Smith PF, Darlington CL. Subregional analysis of amino acid levels in the guinea pig hippocampus following unilateral vestibular deafferentation. J Vestib Res. 1999;9:335–345. [PubMed] [Google Scholar]
- 31.Liu P, Zheng Y, King J, Darlington CL, Smith PF. Long-term changes in hippocampal n-methyl-D-aspartate receptor subunits following unilateral vestibular damage in rat. Neuroscience. 2003;117:965–970. doi: 10.1016/s0306-4522(02)00878-3. [DOI] [PubMed] [Google Scholar]
- 32.Markia B, Kovács ZI, Palkovits M. Projections from the vestibular nuclei to the hypothalamic paraventricular nucleus: morphological evidence for the existence of a vestibular stress pathway in the rat brain. Brain Struct Funct. 2008;213:239–245. doi: 10.1007/s00429-008-0172-6. [DOI] [PubMed] [Google Scholar]
- 33.Saman Y, Bamiou DE, Gleeson M, Dutia MB. Interactions between stress and vestibular compensation: a review. Front Neurol. 2012;3:116. doi: 10.3389/fneur.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron SA, Dutia MB. Lesion-induced plasticity in rat vestibular nucleus neurones dependent on glucocorticoid receptor activation. J Physiol. 1999;518:151–158. doi: 10.1111/j.1469-7793.1999.0151r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustave Dit Duflo S, Gestreau C, Tighilet B, Lacour M. Fos expression in the cat brainstem after unilateral vestibular neurectomy. Brain Res. 1999;824:1–17. doi: 10.1016/s0006-8993(99)01172-5. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay L, Liu P, Gliddon C, Zheng Y, Smith PF, Darlington CL. Cytosolic glucocorticoid receptor expression in the rat vestibular nucleus and hippocampus following unilateral vestibular deafferentation. Exp Brain Res. 2005;162:309–314. doi: 10.1007/s00221-004-2168-7. [DOI] [PubMed] [Google Scholar]
- 37.Shors TJ, Gallegos RA, Breindl A. Transient and persistent consequences of acute stress on long-term potentiation (LTP), synaptic efficacy, theta rhythms and bursts in area CA1 of the hippocampus. Synapse. 1997;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 38.Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J Neurosci. 2012;32:11980–11990. doi: 10.1523/JNEUROSCI.0984-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge Y, Dong Z, Bagot RC, Howland JG, Phillips AG, Wong TP, Wang YT. Hippocampal long-term depression is required for the consolidation of spatial memory. Proc Natl Acad Sci U S A. 2010;107:16697–16702. doi: 10.1073/pnas.1008200107. [DOI] [PMC free article] [PubMed] [Google Scholar]


