Summary
Background
In a recent study, it was reported that transcatheter arterial embolization with spherical embolic material for life-threatening hemorrhages in various cancer patients was safe and effective. Calibrated microspheres are able to access distal regions of the target arteries, which results in the disappearance of tumor staining. However, there are few reports on the pathological behavior of EmboSpheres in gastric cancer specimens. In this case, we succeeded in salvage embolization for advanced gastric cancer with hemorrhagic shock using spherical embolic material. To our knowledge, this is the first report of a pathological evaluation of spherical embolic microspheres in a gastric cancer specimen.
Case Report
A 70-year-old man with scirrhous gastric cancer was admitted to our hospital for staging laparoscopy. Unfortunately, he had a sudden onset of hematemesis and melena leading to hemorrhagic shock due to bleeding from the gastric cancer. While undergoing a rapid blood transfusion, he underwent emergent embolization to achieve hemostasis. The left gastric and right gastroepiploic arteries were embolized with spherical embolic material, and the patient survived. Two days later, the patient was able to undergo gastrectomy. A large number of microspheres were observed in areas of hemorrhage. The range and median diameter of the minor axis were 177–1048 μm and 281 μm, respectively.
Conclusions
Transcatheter arterial embolization using spherical embolic material could become one of safe and effective options, especially when there is no extravasation or pseudoaneurysm but only tumor staining from the clinical and pathological point of view.
MeSH Keywords: Catheterization, Peripheral; Embolization, Therapeutic; Hemostasis; Microspheres; Stomach Neoplasms
Background
Advanced gastric cancer patients often experience sudden hematemesis, melena and syncope owing to severe anemia [1]. Patients can suddenly and readily experience a collapse in their hemodynamic status, and rapid rescue measures must be taken to stop hemorrhage. For non-variceal, acute upper gastrointestinal bleeding, there are three major treatments; namely, emergency gastrectomy (EG), endoscopic therapy (ET), and transcatheter arterial embolization (TAE). EG is most invasive and has a high rate of complications such as re-bleeding and death after surgery [2], and hence this treatment should be avoided if possible. ET is usually the first choice, because its rate of successful hemostasis has been reported to be 67% to 100% [3]. In cases of massive bleeding, however, the failure rate of ET may increase and then TAE should be chosen as an alternative [1,4–6].
As regards the selection of embolic material, in cases in which digital subtraction angiography (DSA) displays apparent extravasation or pseudoaneurysm, a coil or gelatin sponge particles are usually selected [7]. When there is a decreased coagulation ability, N-butyl cyanoacrylate is effective [8,9]. However, when DSA does not clearly display extravasation or pseudoaneurysm, particularly when only tumor staining is observed, there is no established consensus regarding the type of embolic material that should be utilized. In a recent study, it was reported that TAE with EmboSpheres for life-threatening hemorrhages in patients with gastric cancer [10,11], breast cancer [12] and neurofibroma [13] was safe and effective. Calibrated microspheres are able to access distal regions of the target arteries resulting in disappearance of tumor staining. However, there are no reports on the pathological behavior of EmboSpheres in a gastric cancer specimen. In this study, we performed TAE with EmboSpheres and succeeded in rescuing a patient who had advanced gastric cancer and was undergoing massive hemorrhage. This enabled the patient to survive until surgery. In the present study, we evaluated the actual distribution of EmboSpheres and the diameter of the embolized vessels for the first time in a gastric cancer specimen.
Case Report
Our report was exempted by our institutional review board and informed consent was obtained from the patient. A 70-year-old man presented to our hospital with anorexia (4-kg weight loss over previous 6 months) and a positive fecal occult blood test. Upper gastrointestinal (GI) endoscopy and upper GI series were performed and he was diagnosed with scirrhous gastric cancer with an ulcer in the lesser curvature. Contrast-enhanced computed tomography (CECT) displayed thickening of the entire circumference of the gastric body wall and an enlarged lymph node at the lesser curvature (Figure 1). Neither liver nor lung metastasis was observed. Two weeks later, at the time of an outpatient visit, the patient’s hemoglobin level was reduced from 10.0 to 6.0 g/dL, and he was immediately hospitalized. The patient’s severe anemia was treated with a transfusion of 10 units of red cell concentrate (reduced leukocytes) which improved his hemoglobin level from 6.0 to 9.4 g/dL, and this level was maintained. No coagulopathy was observed. Five days later, however, the patient experienced syncope following an episode of hematemesis and melena. Endoscopic hemostasis was difficult because of a massive hemorrhage. Emergent endovascular embolization was performed under transfusion to stop the hemorrhage from the gastric cancer.
Figure 1.
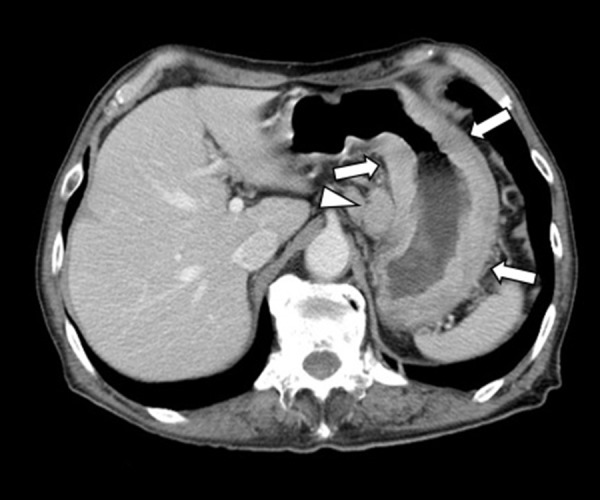
Initial contrast-enhanced computed tomography images. Thickening of the entire circumference of the gastric body wall (arrows) was displayed. A swollen lymph node was displayed in the lesser curvature (arrowhead).
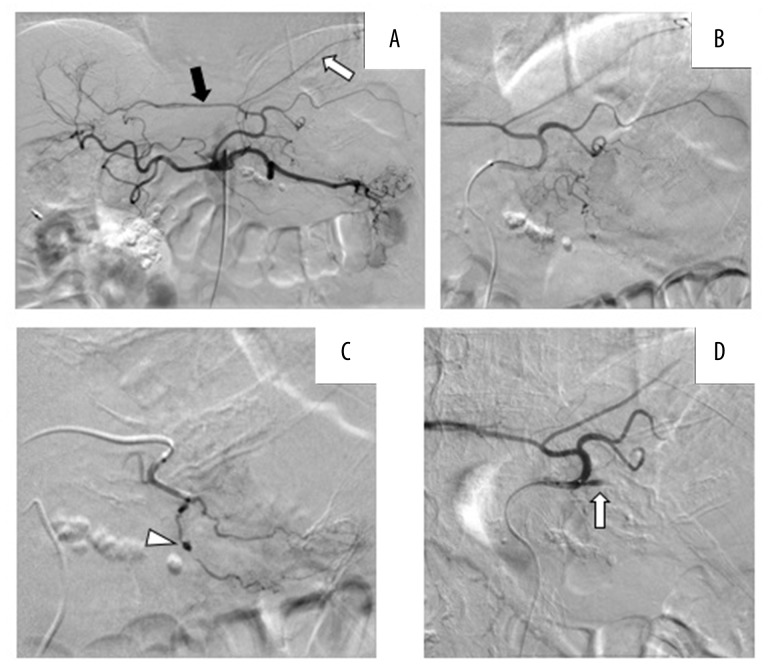
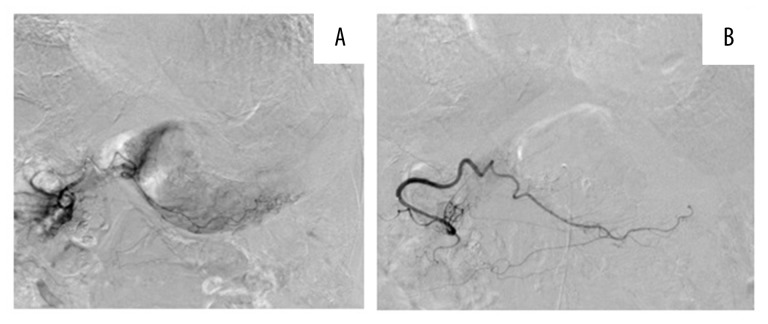
We punctured the right femoral artery under local anesthesia. A 4-Fr guiding catheter (MM1, Gadelius Medical K.K., Japan) was inserted and advanced to the celiac artery. Digital subtraction angiography (DSA) displayed the left subphrenic and left hepatic arteries branching from the left gastric artery (Figure 2A). In addition, a 2.8-Fr outer microcatheter (Renegade HI-FLO, Boston Scientific, Japan) and a 1.8-Fr inner microcatheter (Carnelian MARVEL, Tokai Medical Products, Japan) were selected as a triple co-axial system. A pseudoaneurysm was observed in a branch of the left gastric artery (Figure 2B, 2C). The inner microcatheter was advanced to this branch of the left gastric artery and gelatin sponge particles were infused until the proximal side of the branch was completely embolized (Figure 2D). However, the patient’s blood pressure remained low, and the pulse of the femoral artery remained non-palpable in spite of continuous pumping transfusions. DSA was performed from the splenic artery showing that the posterior and short gastric arteries were not the feeding arteries. No other obvious extravasation or pseudoaneurysm were observed in any feeding artery. We suspected undetectable bleeding from the tumor. We decided to use one vial of EmboSpheres (100–300 μm, NIPPON KAYAKU, Co., Ltd., Tokyo), which was mixed with 11 mL of contrast agent to block the more distal and smaller vessels of the other branches of the left gastric artery. Similarly, we performed DSA from the right gastroepiploic artery, which displayed tumor blush in the greater curvature (Figure 3A). We then infused another vial of EmboSpheres (100–300 μm). Finally, tumor staining disappeared. After the embolization, the main trunk of the right gastroepiploic artery remained visible on DSA (Figure 3B). At this point, the patient’s blood pressure increased and became stable.
Figure 2.
Digital subtraction angiography (DSA) images of the celiac artery and left gastric artery. (A) Selective celiac artery angiography. The left hepatic artery (black arrow) and left subphrenic artery (white arrow) branched from the left gastric artery. (B) Selective left gastric artery angiography. (C) The pseudoaneurysm was located on a branch from the left gastric artery (arrowhead). (D) After the embolization using gelatin sponge and EmboSpheres the target branch was occluded completely.
Figure 3.
DSA images of the right gastroepiploic artery. (A) Although extravasation and pseudoaneurysm were not displayed, the tumor staining was seen along the gastric wall in the greater curvature. (B) After embolization using EmboSpheres only, final angiography showed disappearance of tumor staining and the remaining main trunk of the right gastroepiploic artery (arrow).
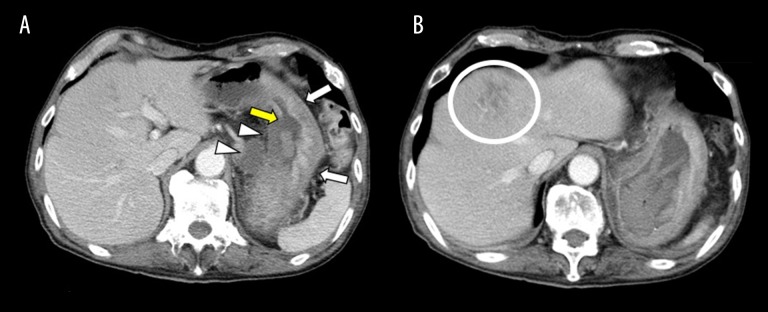
Two days after the TAE, CECT showed no re-bleeding and extravasation, and the gastric wall of the greater curvature had preserved perfusion (Figure 4A). On the other hand, the gastric wall in the lesser curvature and lymph nodes of the lesser curvature were completely unenhanced, which indicated necrosis. Necrosis was observed specifically in segment 4 of the liver (Figure 4B). On the same day, total gastrectomy was performed for the prevention of re-bleeding. Local liver infarction improved after three weeks with conservative treatment. Six months after discharge, the patient died owing to progression of gastric cancer.
Figure 4.
Contrast-enhanced computed tomography images. (A) Two days after embolization neither the gastric body wall nor the swollen lymph node in the lesser curvature were enhanced (arrowheads). On the other hand, the gastric body wall in the greater curvature remained enhanced (arrows). An area of the gastric wall mucosa membrane in the lesser curvature was collapsed (yellow arrows). (B) A low-density area was located in segment 4 of the liver, indicating infarction (circle).
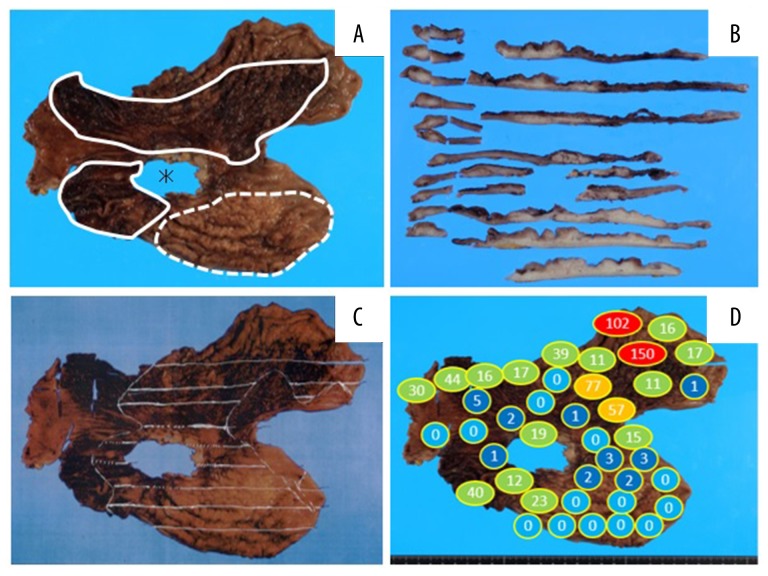
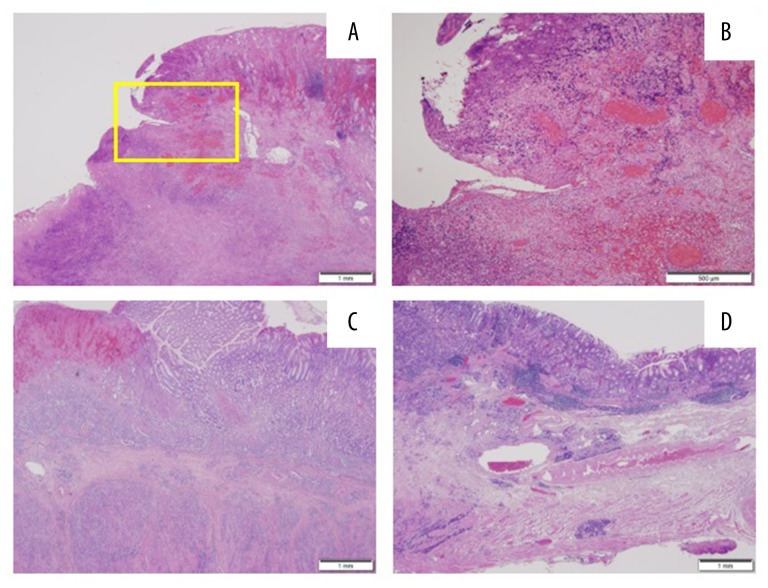
Surgical findings included infiltration of the tumor into the pancreas and strong adherence of the main region of the tumor with the ulcer in the lesser curvature to the pancreas as well as to the right and left gastric arteries, making total removal of the stomach impossible. The stomach was removed as extensively as possible. Many blood clots and a small amount of oozing were observed in the stomach. Macroscopic and microscopic pathological findings of the specimen are shown in Figures 5–7. The patient was diagnosed with advanced gastric cancer of poorly differentiated tubular adenocarcinoma, scirrhous type. Cancer cells were found mainly in the submucosa and muscularis propria in the posterior wall of the gastric body. The cancer cells spread horizontally over the anterior wall, cardiac area and antrum. Among the regional lymph nodes, 12 lymph nodes were identified to contain cancer cells. The gastric specimen lacked a region of the stomach around the lesser curvature, which could not be removed owing to its strong adhesion to the pancreas.
Figure 5.
Gastric cancer specimen. (A) The area of the ulcer that adhered to and remained on the patient’s pancreas (asterisk), areas of hemorrhage (surrounded by solid lines) and a thick gastric wall (region surrounded by the dotted line) were macroscopically observed. (B) Cross-section after sectioning showed thick and white regions, which indicated cancer cell infiltration. (C) The spread of cancer cells is indicated with white lines. (D) The number of EmboSpheres detected in each region of the specimen.
Figure 6.
Pathological findings on hematoxylin and eosin staining. (A) The lesion due to the ulcer near the mucosa formed a slope. (B) High-power field of the area within the square in (A). Hemorrhage, venous stasis and necrotic cancer cells were observed in the submucosal layer. (C) The posterior wall in the greater curvature – numerous and dense cancer cells were observed in the submucosa and between the muscles. (D) The anterior wall in the greater curvature - few cancer cells were observed compared with the posterior wall.
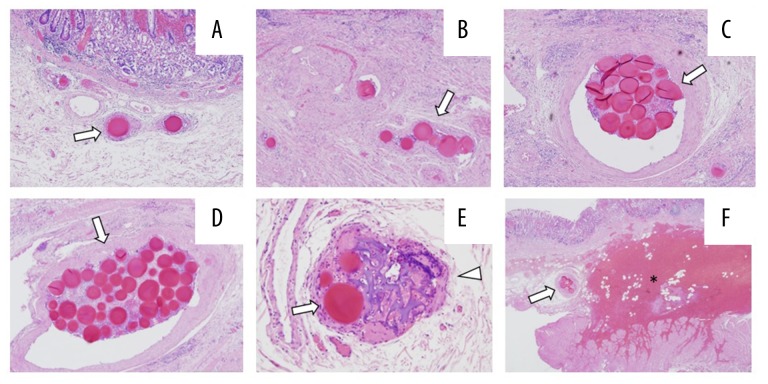
Figure 7.
Pathological images showing embolic agents embolizing all arteries in the submucosa of the gastric wall. (A) An EmboSpehre. (B) 6 EmboSpheres. (C) 19 EmboSpheres. (D) 42 EmboSpheres. (E) EmboSpheres (arrows) and gelatin sponge (arrow head) embolizing one artery. (F) EmboSpheres (arrow), embolization and hemorrhage (asterisk).
In the surrounding tissue near the ulcerative and missing region, a part of the ulcer had hemorrhage, congestion and tumor necrosis in the submucosa layer. This region became the most ischemic after TAE performed through the left gastric artery with gelatin sponge particles and EmboSpheres.
Furthermore, we investigated the distribution of the EmboSpheres and diameters of the embolized vessels. The actual number of EmboSpheres was counted in all of the gastric specimen slides (Figure 5D) A large number of EmboSpheres was observed in areas that were fed from the left gastric artery and right gastroepiploic artery. The minor axis of 12 randomly selected arteries that were embolized by EmboSpheres are listed in Table 1. The range, average and median diameter of the minor axis were 177–1048 μm, 451 μm, and 281 μm, respectively.
Table 1.
Diameters of vessels embolized with embospheres.
| Vessel number | Minor axis (μm) |
|---|---|
| 1 | 441 |
| 2 | 259 |
| 3 | 264 |
| 4 | 206 |
| 5 | 177 |
| 6 | 382 |
| 7 | 240 |
| 8 | 1.048 |
| 9 | 298 |
| 10 | 196 |
| 11 | 1.007 |
| 12 | 893 |
| Range | 177–1.048 |
| Median | 281 |
| Mean | 451 |
Discussion
The diameters of the blood vessels embolized with EmboSpheres have been investigated both in vivo (in the kidneys and uteri of sheep) and in human pathological specimens of brain tumors and arteriovenous malformations, particularly of the head and neck [14,15]. Verret et al. reported the median diameter of vessels occluded with EmboSpheres (500–700 μm) to be 414 μm [14]. In the present case, the median diameter of the vessels occluded using EmboSpheres (sized 100–300 μm) was 281 μm. Although these data cannot be directly compared with the previous report, as the original size of the EmboSpheres was different, this value was larger than we expected.
In cases of transcatheter arterial chemoembolization for hepatocellular carcinoma, adequate dilution of EmboSpheres is generally recommended to avoid aggregation. However, in our present case, rapid treatment without excessive dilution was crucial. As a result, clusters of microspheres were found in the specimens (Figure 7C, 7D).
The anterior wall of the gastric body largely retained its normal structure, and the normal mucosa, submucosa and muscularis propria structures contained a large number of arteries between 200 μm and 1 mm that were occluded by large amounts of EmboSpheres. On the other hand, in the posterior wall of the gastric body, few arteries were larger than 50 μm in caliber, because the space around the submucosa and muscularis propria was tightly filled with numerous cancer cells. Macroscopically, the posterior wall was markedly thickened and had a white color (Figure 5B).
In general, hypervascular tumors form new blood vessels and continue to enlarge by obtaining nutrition and oxygen. In this situation, spherical embolic agents are carried by free flow and embolize the distal arteries of the tumor and areas of hemorrhage. However, in more advanced cancers, such as in the present case, there are no enlarged arteries because of the densely packed and numerous cancer cells. This suggests that microspheres cannot reach distal small arteries in the vicinity of such densely packed cancer cells, if the diameter of the tumor-feeding arteries is less than that of the spherical embolic agent. The most interesting finding of the present case was the difference in the degree of necrosis between embolization using gelatin sponge particles and EmboSpheres versus EmboSpheres only (Table 2). A pseudoaneurysm on the branch of the left gastric artery was completely embolized with gelatin sponge particles and EmboSpheres, which resulted in disappearance of the proximal artery on DSA. CECT performed two days after the embolization displayed no enhancement of the gastric wall and lymph nodes. This indicated strong embolic and ischemic effects; however, as this area of tissue could not be removed, it was not evaluated pathologically. On the other hand, the trunks of the left gastric artery and right gastroepiploic artery were embolized with EmboSpheres only until the disappearance of tumor staining. CECT displayed continued enhancement on the gastric wall in the greater curvature with little necrosis and inflammation (foreign body reaction) on the same site. The reason for this was considered to be preservation of the proximal artery, specific characteristics of this type of tumor and the short time between embolization and evaluation.
Table 2.
Outcomes of transarterial embolization.
| Target artery | Feeding area | DSA imaging | Embolic agent | CECT imaging | Adverse events |
|---|---|---|---|---|---|
| Branch of the left gastric artery | Gastric wall and lymph nodes in the lesser curvature | Pseudoaneurysm | Gelatin sponge | No enhancement of the gastric wall and lymph nodes | None |
| Common trunk of the left gastric and left hepatic artery | Tumor stain | EmboSphere | Infarction of segment 4 of the liver | ||
| Right gastroepiploic artery | Gastric wall in the greater curvature | Tumor stain | EmboSphere | Enhancement of gastric wall remaining | None |
Conclusions
Transcatheter arterial embolization using spherical embolic material could become one of safe and effective options, especially when there is no extravasation or pseudoaneurysm but only tumor staining from the clinical and pathological point of view.
References
- 1.Lee HH, Park JM, Chun HJ, et al. Transcatheter arterial embolization for endoscopically unmanageable non-variceal upper gastrointestinal bleeding. Scand J Gastroenterol. 2015;50(7):809–15. doi: 10.3109/00365521.2014.990503. [DOI] [PubMed] [Google Scholar]
- 2.So JBY, Yam A, Cheah WK, et al. Risk factors related to operative mortality and morbidity in patients undergoing emergency gastrectomy. Br J Surg. 2000;87(12):1702–7. doi: 10.1046/j.1365-2168.2000.01572.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim YI, Choi IJ. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin Endosc. 2015;48(2):121–27. doi: 10.5946/ce.2015.48.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loffroy R, Rao P, Ota S, et al. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: Results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33(6):1088–100. doi: 10.1007/s00270-010-9829-7. [DOI] [PubMed] [Google Scholar]
- 5.Koh KH, Kim K, Kwon DH, et al. The successful endoscopic hemostasis factors in bleeding from advanced gastric cancer. Gastric Cancer. 2013;16(3):397–403. doi: 10.1007/s10120-012-0200-3. [DOI] [PubMed] [Google Scholar]
- 6.Loffroy R, Guiu B, D’Athis P, et al. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: Predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7(5):515–23. doi: 10.1016/j.cgh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Lee HJ, Shin JH, Yoon HK, et al. Transcatheter arterial embolization in gastric cancer patients with acute bleeding. Eur Radiol. 2009;19(4):960–65. doi: 10.1007/s00330-008-1216-2. [DOI] [PubMed] [Google Scholar]
- 8.Hongsakul K, Pakdeejit S, Tanutit P. Outcome and predictive factors of successful transarterial embolization for the treatment of acute gastrointestinal hemorrhage. Acta Radiol. 2013;55(2):186–94. doi: 10.1177/0284185113494985. [DOI] [PubMed] [Google Scholar]
- 9.Shin JH. Recent update of embolization of upper gastrointestinal tract bleeding. Korean J Radiol. 2012;13(Suppl 1):S31–39. doi: 10.3348/kjr.2012.13.S1.S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meehan T, Stecker MS, Kalva SP, et al. Outcomes of transcatheter arterial embolization for acute hemorrhage originating from gastric adenocarcinoma. J Vasc Interv Radiol. 2014;25(6):847–51. doi: 10.1016/j.jvir.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Keeling AN, McGrath FP, Thornton J, et al. Emergency percutaneous transcatheter embolisation of acute arterial haemorrhage. Ir J Med Sci. 2010;179(3):385–91. doi: 10.1007/s11845-009-0400-y. [DOI] [PubMed] [Google Scholar]
- 12.Moriarty JM, Xing M, Loh CT. Particle embolization to control life-threatening hemorrhage from a fungating locally advanced breast carcinoma: A case report. J Med Case Rep. 2012;6:186. doi: 10.1186/1752-1947-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno T, Takagi Y, Yamada H, et al. Life-threatening intratumoral hemorrhage in plexiform neurofibroma: A case report. JPRAS Open. 2015;5:24–28. [Google Scholar]
- 14.Verret V, Ghegediban SH, Wassef M, et al. The arterial distribution of embozene and embosphere microspheres in sheep kidney and uterus embolization models. J Vasc Interv Radiol. 2011;22(2):220–28. doi: 10.1016/j.jvir.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Laurent A, Wassef M, Chapot R, et al. Location of vessel occlusion of calibrated tris-acryl gelatin microspheres for tumor and arteriovenous malformation embolization. J Vasc Interv Radiol. 2004;15(5):491–96. doi: 10.1097/01.rvi.0000124952.24134.8b. [DOI] [PubMed] [Google Scholar]