Abstract
Background
Mesenchymal stem cells (MSCs) have emerged as an attractive alternative to modulating immune response after transplantation. Recent studies have shown that systemically administered MSCs enter the inflamed intestine. In the present study, we propose a strategy to improve the efficacy of MSC-based cellular therapy for inflammation using Astragaloside and Baicalein to enhance cell survival, inhibit apoptosis, and modulate inflammatory response in vitro.
Material/Methods
MSCs were induced with lipopolysaccharide (LPS) as an inflammatory model before being treated for 48 h with Astragaloside, Baicalein, and the combination of both. MSCs proliferation was determined using the MTT method. The cell cycle situation was monitored using flow cytometry, and the apoptosis ability of MSCs was detected with Annexin-V flow cytometry. The levels of cytokine IL-1β, IL-8, and TNF-α, and their relations with the ERK pathway were measured using ELISA, RT-PCR, and Western blot.
Results
Compared to the control groups (containing no drug), each drug-treated group showed the ability to promote epithelial differentiation and cell growth and to inhibit apoptosis. The combination group had reduced levels of IL-1β, IL-8, and TNF-α in LPS-induced MSCs, much more than in the other 2 groups. Compared with the other groups, the combination of Astragaloside and Baicalin more efficiently reduced IL-1β, IL-8, and TNF-α levels in the LPS-induced MSCs model, and ERK inhibitor was capable of recovering the inflammatory effect.
Conclusions
The results demonstrated that Astragaloside and Baicalin can promote epithelial differentiation and proliferation, inhibit apoptosis, and reduce inflammatory effects.
MeSH Keywords: Anti-Inflammatory Agents, Cell Differentiation, Mesenchymal Stromal Cells
Background
Mesenchymal stem cells (MSCs) have emerged as an attractive model for cell therapy for inflammatory and autoimmune diseases. MSCs are multipotent stem cells that can differentiate into different cell types [1,2]. To ensure that MSCs reach the damaged mucosa lesions, sufficient MSCs in the peripheral blood circulation are needed for MSCs transplantation for autoimmune diseases. Recent studies have shown that some Traditional Chinese Medicines and their extracts can stimulate MSCs proliferation and differentiation [3–6], such as ozone, which can promote MSCs in intestinal tissue [7,8].
Our former studies showed that both Astragaloside and Baicalin can promote MSCs to move to damaged intestine tissues, in which case more MSCs home to the site of damage to support repair of the gut mucosal barrier (data not shown). However, MSCs are relatively unable to effectively promote the rebuilding of intermucosal barrier, as intestinal bacteria have negative effects on MSCs in the injured intestine [9]. Therefore, maintaining the differentiation capacity of MSCs and improving their proliferation are of significance to the treatment of ulcerative colitis. In the present study, we propose a hypothesis that Astragaloside and Baicalin enhance cell survival, inhibit apoptosis, and regulate inflammation of LPS-induced MSCs in vitro.
Material and Methods
Material
Male Sprague-Dawley rats aged 6 weeks were purchased from the animal facility of Nanjing Medical University Experimental Animal Center.
Astragaloside (20 mg, FY13081102) and Baicalin (20 mg, FY12960708) were purchased from Nanjing Feiyu Biological Technology Company.
Methods
MSCs isolation and cultivation
MSCs were isolated and cultured as described below. Briefly, 6-week-old adult mice (n=4) were mildly anesthetized using sodium pentobarbital (100 mg/kg, i.p.). Their femoral bones were obtained aseptically, then soaked in 75% ethanol for 10 min. Bone marrow cell suspension was obtained by flushing the marrow cavity with phosphate-buffered saline (PBS). The bone marrow cell suspension was aspirated and then centrifuged at 1500 rpm for 5 min. The marrow supernatant was discarded. The obtained marrow cells were then suspended in M199 medium containing 10% FBS and 1% double antibody. The marrow suspension was seeded into culture flasks. Next, the cells were incubated for 24 h at 37°C in an atmosphere of 5% humidified CO2. The medium was changed every 3–4 days until MSCs reached 80% confluence. MSCs were used for experiments within passages 3–6.
Identification of MSCs
MSCs were digested with 0.25% trypsin and then centrifuged at 1000 rpm for 5 min. The supernatant was decanted. The centrifuge tubes were washed 2 times with PBS to produce the cell suspension (1×106/L). The cell suspension was added into 2 tubes. Each tube, containing 0.1 ml cell suspension, was treated with either 20 ul PE-labeled CD45 or CD90 antibody. Both were incubated for 30 min at room temperature in the dark, and analyzed using flow cytometry after centrifugation.
LPS-induced MSCs preparation and experimental design
For LPS-induced MSCs preparation, after the bone marrow cell suspension was aspirated, the obtained cells were rinsed with PBS for 3 times and then shifted into serum-free medium containing 500 ng/ml LPS. Next, the cells were incubated for 24 h before supernatant collection.
MSCs were randomly assigned to the following 4 groups: the Baicalin group, the Astragaloside group, the combination group, and the control group. LPS-induced MSCs were incubated in cultures containing Astragaloside and Baicalin at concentrations of 0 ng/ml, 5 ng/ml, 10 ng/ml, 50 ng/ml, 100 ng/ml, and 500 ng/ml. The experimental effects were examined at 24 h and 48 h after treatment.
Assessment of MSCs proliferation
After incubation for 24 h and 48 h, MSCs were digested with 0.25% trypsin (containing no EDTS) and then washed with PBS by centrifugation at 2000 rpm for 5 min. MSCs (5×105) were collected. The single-cell suspension was fixed with 70% ethanol for 2 h (or overnight) at 4°C. After washing, 100 μL of RNase was added and incubated at 37°C for 30 min. The cells were stained with 400 μL of PI in the dark for 30 min at 37°C. The cells were analyzed using flow cytometry.
The single-cell suspension was seeded into 6-well cell culture plates and then incubated with MTT solution, with DMSO as a blank control. The sample was detected using microplate spectrophotometer absorbance.
Assessment of MSCs apoptosis
LPS-stimulated MSCs were seeded into cell culture plates. The Astragaloside, Baicalin, and the combination of both were added into the culture wells. After gently shaking, the cells were assessed using annexin-V fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay and analyzed by flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
The amounts of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β in the culture medium supernatants were measured with a commercial ELISA kit purchased from R&D Systems (R&D Systems, USA) according to the instructions.
Assessment of inflammatory cytokines with real-time fluorescence quantitative polymerase chain reaction (qPCR)
RNA extraction was carried out using the Trizol extraction kit according to the manufacturer’s instructions, and the purified RNA samples were stored at −80°C until being used. cDNAs were produced by retrograde transcription using Accupower, RocketScript, and RT PreMix commercial kits. Specific DNA fragments were amplified using RealMasterMix (Probe) and miRcute miRNA with a Stratagene cycler. The following forward (F) and reverse (R) primers were used for qPCR amplifications (synthesized by Bioneer China):
IL-8-F: CCGGGGCCCGCAAGGTCATC,
IL-8-R: CCACTTGTCCCGGGCATAG;
IL-1β-F: CAACTCCATGTTTCATAAC,
IL-1β-R: CGTGGTCTAACTTGTTGGC;
TNF-α-F: CGTGAAGAACGACCTAAC;
TNF-α-R: CATGTATCTTGCAGTTCC.
Assessment of inflammatory cytokines with Western blot analysis
Total proteins were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% milk and incubated with primary antibodies against IL-8, L-1β, and TNF-α, as well as GAPDH, at 4°C overnight and then incubated with secondary antibody at room temperature for 1 h. The bands were visualized by enhanced chemiluminescence (Millipore, Billerica, MA, USA) and GAPDH was used as a loading control. All data analyses were repeated 3 times independently.
Statistical analysis
Data analysis was performed using SPSS version 17.0 for Windows. The Pearson’s chi-squared test was used to compare qualitative variables, and comparisons among different groups were performed using one-way analysis of variance. A two-tailed P value test was used in all analyses, and a p value less than 0.05 was considered statistically significant. All experiments presented here were performed independently at least 3 times.
Results
MSCs identification
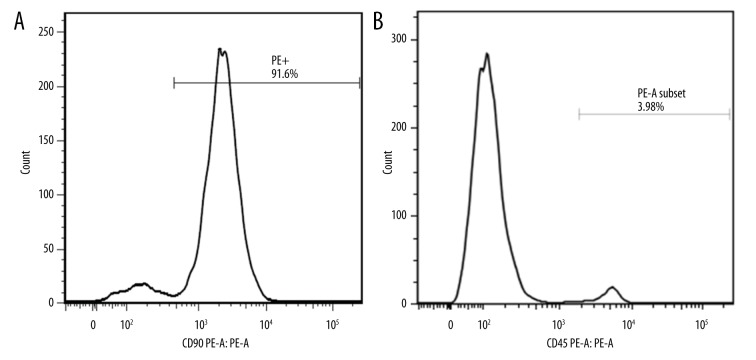
The MSCs were positive for CD90 and negative for CD45 (Figure 1).
Figure 1.
After 3 passages, MSCs isolated from rat bone marrow were already stabilized, and the surface marker (CD90+/CD45−) can be identified by flow cytometry. (A) 91.6% of the third-generation cells showed CD90 positivity. (B) 3.98% of the third-generation cells showed CD90 positivity.
MSCs proliferation and differentiation
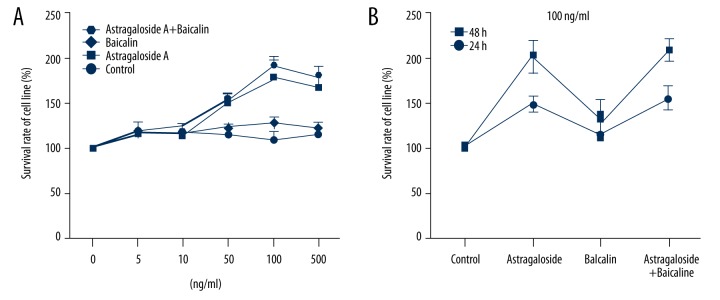
MTT assay was used to observe the MSCs proliferation; when drug concentration reached 100 ng/ml, the proliferation ability was obvious. The combination group had the best cell proliferation ability, followed by the Astragaloside group. At the drug concentration of 100 ng/ml, the cells grew more when incubated for 48 h than for 24 h (Figure 2).
Figure 2.
Effects of appropriate concentrations and time of Astragaloside and Baicalin treatment on the differentiation and proliferation of cells. (A) Effects of different drug concentrations on LPS-induced MSCs proliferation; when drug concentration was 100 ng/ml, the proliferation ability was obvious, and the combination group was the best, followed by the Astragaloside group. (B) Effects of different time points on LPS-induced MSCs proliferation; at drug concentration of 100 ng/ml, the cells incubated for 48 h were expanded more than those for incubated for 24 h.
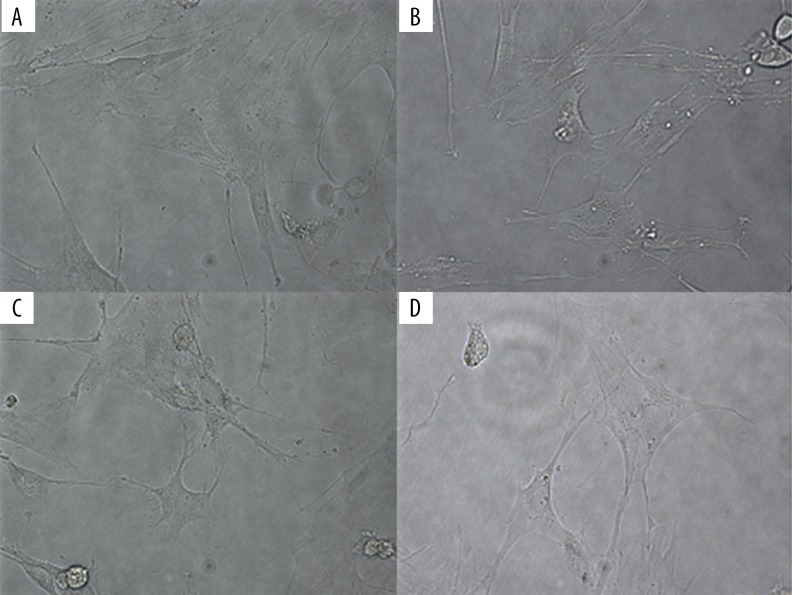
Cell morphology was observed with a microscope. MSCs in the visible model group were spindle-shaped cells. Compared to spindle-shaped MSCs in the visible model group, MSCs treated with drugs were oval in shape, with microvilli-like protrusions on the surface. The micrographs demonstrated that all the drugs tested can stimulate the differentiation of epithelial cells (Figure 3).
Figure 3.
MSCs differentiation in vitro. The inflammatory cell model was induced by 500 ng/ml LPS, after which the LPS-induced MSCs were treated with Astragaloside, Baicalin, or the combination of both. In the control group, LPS-induced MSCs were spindle-shaped, like fibroblasts. In the drug-treated groups, the cells became oval in shape, with microvilli-like protrusions on the surface. (A) Control group (LPS-MSCs). (B) Astragaloside-MSCs. (C) Baicalin-MSCs. (D) Astragaloside+Baicalin-MSCs.
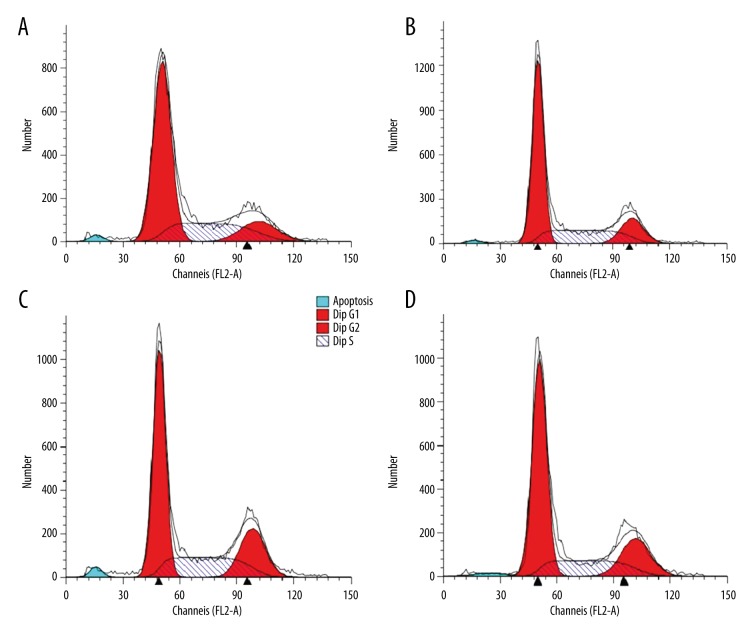
After incubation of LPS-stimulated MSCs with drugs, flow cytometry was used to detect the drug-relevant effects on MSCs cell cycle. The results demonstrated that LPS can inhibit the growth of MSCs at G1 phase, and the drugs can counteract the inhibition effect (Figure 4).
Figure 4.
Growth condition of MSCs. LPS-induced MSCs were treated with Astragaloside, Baicalin, or the combination of both, and cell cycle was detected by flow cytometry. The figure shows that LPS induced MSCs cell cycle arrest at G1 phase. However, after the addition of the drugs, their proliferation ability was recovered. (A) LPS. (B) Astragaloside. (C) Baicalin. (D) Astragaloside+Baicalin.
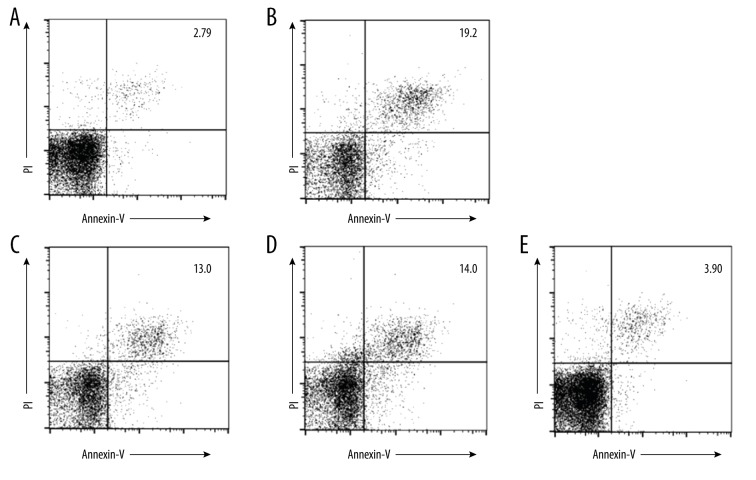
MSCs Apoptosis
The annexin-V-positive LPS-stimulated MSCs were detected by flow cytometry. The apoptotic cells were increased compared with the normal MSCs group. Compared to the LPS group, those in the drug groups were significantly reduced (P<0.01). Furthermore, although the number of apoptotic cells in the Baicalin group or Astragaloside group was higher compared with the LPS group, there was no significant difference between the 2 groups. The combination group had fewer apoptotic cells compared to the LPS group (P<0.01) (Figure 5).L2 MSCs inflammation and the possible mechanism
Figure 5.
(A) Without any treatment, only a small amount of cell apoptosis in the control group. (B) After LPS treatment, the cell apoptosis was enhanced (19.2). (C) After Astragaloside treatment, the cell apoptosis was reduced (13.0). (D) After Baicalin treatment, cell apoptosis was reduced (14.0). (E) After treatment with the drug combination, cell apoptosis was further decreased.
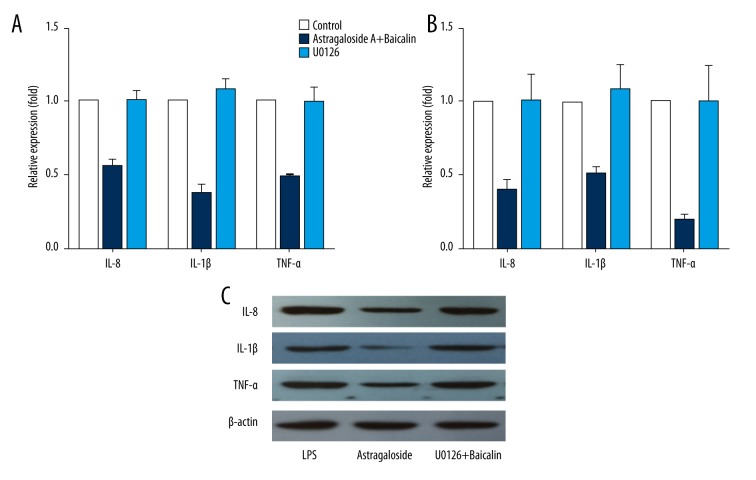
It has been established that the MAPK/ERK pathway is a major contributor to cell proliferation. To investigate how Astragaloside and Baicalin promote cell proliferation and differentiation, as well as how they inhibit MSCs apoptosis and influence the expression of the inflammatory cytokines, MAPK/ERK pathway inhibitor U0126 was used as a control. IL-8, IL-1β, and TNF-α expression were detected using ELISA, RT-PCR, and Western blot. The results showed that, compared with the combination group, the inhibitor group recovered inflammation (Figure 6).
Figure 6.
Effects of Astragaloside and Baicalin on IL-8, IL-1β, and TNF-α expression and the relationship with the MAPK/ERK pathway. After the addition of U0126 and MAPK/ERK pathway inhibitor, we used ELISA, RT-PCR, and Western blot to detect the levels of inflammatory cytokines to clarify whether the effects involved the MAPK/ERK pathway. (A) IL-8, IL-1β, and TNF-α expression of MSCs supernatant with ELISA. (B) IL-8, IL-1β, and TNF-α expression of MSCs supernatant with RT-PCR. (C) IL-8, IL-1β, and TNF-α expression of MSCs supernatant with Western blot.
Discussion
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes, and adipocytes [10,11]. MSCs can be easily isolated, cultured, and expanded in vitro, maintaining their biological properties, such as proliferative capacity and differentiation potential. Owing to their properties, MSCs transplantation has been used for the treatment of various diseases [12–14]. Although recent studies have shown that systemically delivered MSCs can locate in inflamed intestinal tissues, the results are far from satisfactory. Indeed, we have to isolate and expand MSCs in vitro to improve the efficacy of MSCs treatment because they are limited in number, only accounting for about 0.001~0.01% of the nucleated cells derived from bone marrow. Besides, only a few MSCs survive and settle to the site of damage. A study reported that only 0.13% of human MSCs homed to the intestine at 3 days after the injection of the cells into sublethally irradiated mice [15–17]. Therefore, suppressing apoptosis is important to effectively ameliorate the inflammatory response [18–20].
Astragaloside, a main active substance of Astragalus, has many pharmacological functions, such as anti-virus and anti-tumor effects. Studies have shown that Astragaloside can promote MSCs proliferation and differentiation, while decreasing apoptosis. Baicalin, extracted from the root of Scutellaria, has bactericidal, anti-inflammatory, antioxidant, antispasmodic, and anti-tumor effects. Recent studies have shown that Baicalin can also promote cell proliferative capacity and differentiation potential. However, the effects of Astragaloside and Baicalin on MSCs under inflammatory conditions have not been reported in detail thus far [21–24].
Patients with ulcerative colitis always have disordered intestinal flora. LPS, derived from the outer membrane of gram-negative bacteria, can induce MSCs to differentiate into inflammatory cells and secrete inflammatory cytokines [9]. Both Astragaloside and Baicalin can promote MSCs to locate in damaged intestine. However, how to increase the number of MSCs in the damaged intestine and how to suppress the differentiation of MSCs to inflammatory cells remained to be studied.
In this study, we treated MSCs with LPS to induce the inflammation model. Flow cytometry analysis showed that LPS-induced MSCs cell cycle was arrested at G1 phase, and cell apoptosis was enhanced. MTT results indicated that their proliferative potential was suppressed due to the cell cycle arrest and enhanced apoptosis. We also found that LPS-induced MSCs had some morphologic similarities to inflammation cells under the microscope. The data revealed that the expression of IL-1β, IL-8, and TNF-α were increased. In fact, the cytokines contribute to the accumulation and activation of inflammatory cells, as they can facilitate the release of inflammatory mediators, which exacerbate inflammation.
A previous study revealed that Astragaloside increased T lymphocyte and B lymphocyte proliferation, while inhibiting production of IL-1β and TNF-α from macrophages in vitro [25]. Also, Baicalin has an anti-fibrosis effect on bleomycin-induced pulmonary fibrosis [26]. Similar phenomena were also observed in our study. After the addition of the drugs, their proliferation ability was recovered, the cell apoptosis was suppressed, the expression levels of the inflammatory factors were inhibited, and LPS-induced MSCs were obviously differentiated into epithelial cells. All this further demonstrates that both Astragaloside and Baicalin can reduce the cells apoptosis while promoting cell proliferation. Therefore, they also help to differentiate LPS-treated MSCs into epithelial cells to repair injured tissue in patients with ulcerative colitis.
The MAPK/ERK pathway has been reported to be involved in many pharmacological functions induced by Baicalin and Astragaloside, including cell proliferation and apoptosis [24–28]. Therefore, we analyzed the function of the MAPK/ERK pathway in this study. When we treated the cells with U0126, which is a MAPK/ERK pathway inhibitor, we found that U0126 can recover the effects of Astragaloside and Baicalin on cytokines released by LPS-induced MSCs. This means that the effects of the 2 drugs are involved in the MAPK/ERK pathway. Further studies are needed to investigate the specific receptor and the potential pathway that may be involved.
Conclusions
We presented experimental evidence that Astragaloside and Baicalin can promote epithelial differentiation and cell growth, and reduce apoptosis and inflammatory effects.
Acknowledgement
There is no conflict of interest regarding the publication of this paper.
Footnotes
Source of support: Supported by the Natural Science Foundation of China (No. 81373606), Special Scientific Research for Traditional Chinese Medicine of State Administration of Traditional Chinese Medicine of China (No.201407001); and the Jiangsu Provincial Special Program of Medical Science (No. BL2014100)
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47. [PubMed] [Google Scholar]
- 2.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Maxson S, Lopez EA, Yoo D, et al. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1(2):142–49. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Gang EJ, Hong SH, Jeong JA, et al. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2004;321(1):102–8. doi: 10.1016/j.bbrc.2004.06.111. [DOI] [PubMed] [Google Scholar]
- 6.Anokhina EB, Buravkova LB. Heterogeneity of stromal precursor cells isolated from rat bone marrow. Tsitologiia. 2007;49(1):40–47. [PubMed] [Google Scholar]
- 7.Isik A, Peker K, Gursul C, et al. The effect of ozone and naringin on intestinal ischemia/reperfusion injury in an experimental model. Int J Surg. 2015;21:38–44. doi: 10.1016/j.ijsu.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Isik A, Peker K, Firat D. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedia S, Rampal R, Paul J, Ahuja V. Gut microbiome diversity in acute infective and chronic inflammatory gastrointestinal diseases in North India. J Gastroenterol. 2016;51(7):660–71. doi: 10.1007/s00535-016-1193-1. [DOI] [PubMed] [Google Scholar]
- 10.Park JS, Yi TG, Park JM, et al. Therapeutic effects of mouse bone marrow-derived clonal mesenchymal stem cells in a mouse model of inflammatory bowel disease. J Clin Biochem Nutr. 2015;57(3):192–203. doi: 10.3164/jcbn.15-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamou C, Callaghan MJ, Thangarajah H, et al. Mesenchymal stem cells can participate in ischemic neovascularization. Plast Reconstr Surg. 2009;123(2 Suppl):45s–50s. doi: 10.1097/PRS.0b013e318191be4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 13.Levy YS, Bahat-Stroomza M, Barzilay R, et al. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy. 2008;10(4):340–52. doi: 10.1080/14653240802021330. [DOI] [PubMed] [Google Scholar]
- 14.Komori M, Tsuji S, Tsujii M, et al. Efficiency of bone marrow-derived cells in regeneration of the stomach after induction of ethanol-induced ulcers in rats. J Gastroenterol. 2005;40(6):591–99. doi: 10.1007/s00535-005-1593-0. [DOI] [PubMed] [Google Scholar]
- 15.Semont A, Mouiseddine M, Francois A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17(6):952–61. doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–39. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 17.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6(2):82–93. doi: 10.4252/wjsc.v6.i2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in thedetails. Cell Stem Cell. 2009;4(3):206–16. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Yu J, Li Y, et al. CXCR4 positive bone mesenchymal stem cells migrate to human endothelial cell stimulated by ox-LDL via SDF-1alpha/CXCR4 signaling axis. Exp Mol Pathol. 2010;88(2):250–55. doi: 10.1016/j.yexmp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7(1):19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZC, Li SJ, Yang YZ, et al. Effect of astragaloside on cardiomyocyte apoptosis in murine coxsackievirus B3 myocarditis. J Asian Nat Prod Res. 2007;9(2):145–51. doi: 10.1080/10286020412331286506. [DOI] [PubMed] [Google Scholar]
- 22.Xing Y, Lv A, Wang L, Yan X. The combination of angiotensin II and 5-azacytidine promotes cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells. Mol Cell Biochem. 2012;360(1–2):279–87. doi: 10.1007/s11010-011-1067-z. [DOI] [PubMed] [Google Scholar]
- 23.Li QB, You Y, Chen ZC, et al. Role of Baicalein in the regulation of proliferation and apoptosis in human myeloma RPMI8226 cells. Chin Med J (Engl) 2006;119(11):948–52. [PubMed] [Google Scholar]
- 24.Kumagai T, Muller CI, Desmond JC, et al. Scutellaria baicalensis, a herbal medicine: Anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res. 2007;31(4):523–30. doi: 10.1016/j.leukres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Wang YP, Li XY, Song CQ, Hu ZB. Effect of astragaloside IV on T, B lymphocyte proliferation and peritoneal macrophage function in mice. Acta Pharmacol Sin. 2002;23(3):263–66. [PubMed] [Google Scholar]
- 26.Huang X, He Y, Chen Y, et al. Baicalin attenuates bleomycin-induced pulmonary fibrosis via adenosine A2a receptor related TGF-beta1-induced ERK1/2 signaling pathway. BMC Pulm Med. 2016;16(1):132. doi: 10.1186/s12890-016-0294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Gu J, Fan Y, et al. Baicalin attenuates acute myocardial infarction of rats via mediating the mitogen-activated protein kinase pathway. Biol Pharm Bull. 2013;36(6):988–94. doi: 10.1248/bpb.b13-00021. [DOI] [PubMed] [Google Scholar]
- 28.Zhou QM, Wang S, Zhang H, et al. The combination of Baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009;30(12):1648–58. doi: 10.1038/aps.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]