Abstract
Genetic engineering technique offers myriads of applications in improvement of horticultural crops for biotic and abiotic stress tolerance, and produce quality enhancement. During last two decades, a large number of transgenic horticultural crops has been developed and more are underway. A number of genes including natural and synthetic Cry genes, protease inhibitors, trypsin inhibitors and cystatin genes have been used to incorporate insect and nematode resistance. For providing protection against fungal and bacterial diseases, various genes like chitinase, glucanase, osmotin, defensin and pathogenesis-related genes are being transferred to many horticultural crops world over. RNAi technique has been found quite successful in inducing virus resistance in horticultural crops in addition to coat protein genes. Abiotic stresses such as drought, heat and salinity adversely affect production and productivity of horticultural crops and a number of genes encoding for biosynthesis of stress protecting compounds including mannitol, glycine betaine and heat shock proteins have been employed for abiotic stress tolerance besides various transcription factors like DREB1, MAPK, WRKY, etc. Antisense gene and RNAi technologies have revolutionized the pace of improvement of horticultural crops, particularly ornamentals for color modification, increasing shelf-life and reducing post-harvest losses. Precise genome editing tools, particularly CRISPR/Cas9, have been efficiently applied in tomato, petunia, citrus, grape, potato and apple for gene mutation, repression, activation and epigenome editing. This review provides comprehensive overview to draw the attention of researchers for better understanding of genetic engineering advancements in imparting biotic and abiotic stress tolerance as well as on improving various traits related to quality, texture, plant architecture modification, increasing shelf-life, etc. in different horticultural crops.
Keywords: Genetic engineering, Horticultural crops, Abiotic and biotic stresses, Quality improvement, Genome editing
Introduction
Biotechnology has offered tremendous scope and potential to conventional methods of crop improvement, crop protection, crop quality management and improving other horticultural traits. It extends remarkable opportunities in fruit production enhancement by providing new genotypes for breeding purpose, supply of healthy and disease-free planting material, improvement in fruit quality, enhancing shelf-life, availability of biopesticides, biofertilizers, etc. Integration of specially desired traits through genetic engineering has been made possible in some horticultural crops. Genetic engineering consists of isolation of a gene of interest, ligating that gene with a desirable vector to form the recombinant-DNA molecule and then transferring that gene into the plant genome to create a new function. Transgenic technology has been rated as the fastest growing technology in agriculture (ISAAA 2017). It refers to a set of techniques used for transferring desirable gene(s) from any source (plants, animals, microorganisms or even artificially synthesized genes) across taxonomic boundaries into a certain plant by non-conventional methods. In contrast to conventional breeding which involves the random mixing of tens of thousands of genes present both in the resistant and susceptible plants, recombinant DNA technology allows the transfer of only the desirable genes to the susceptible plants and the preservation of valuable economic traits. Moreover, the genetic sources for resistance are not limited only to closely related plant species (Lurquin 2002). Combating various types of biotic and abiotic stresses is the foundation and crux of sustainable agriculture. Although conventional breeding and marker-assisted breeding nowadays are being used to develop more promising cultivars, however, in case of biennials or perennial horticultural crops, particularly fruit trees, such techniques are not feasible due to long sexual generation periods. The major advantages of transgenic technology lie in that the genes governing for various agronomically important traits can be sourced from any organism—plants or microorganisms, etc. and can be employed for plant transformation. Thus, novel traits from any background can be incorporated into the target plant with ease. However, for single gene transfer into elite backgrounds, the development and standardization of a high frequency, efficient plant regeneration and genetic transformation protocol is the utmost pre-requisite. A number of studies had been carried out in the past to develop suitable regeneration and genetic transformation protocol in many horticultural species including apple (Rustaee et al. 2007), pomegranate (Parmar et al. 2012, 2013, 2015), chilli (Sharma et al. 2006; Khan et al. 2011a), cucumber (Vasudevan et al. 2007), lily (Kathryn and Han 2008), sweet orange (Singh and Rajam 2010), broccoli (Kumar and Srivastava 2015), datepalm (Aslam et al. 2015), chrysanthemum (Naing et al. 2016), etc.
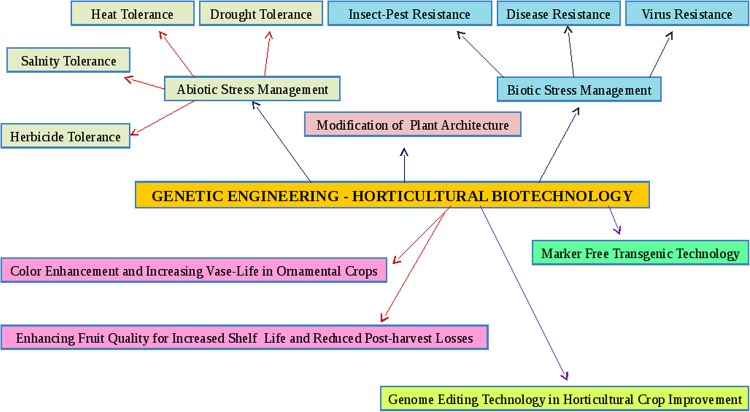
Horticultural biotechnology has been a leading example in many areas for more than two decades, right from the commercialization of the first ever transgenic crop in the form of ‘Flavr-Savr’ transgenic tomato with enhanced shelf-life trait. The first field trials of transgenic horticultural plants had been carried out in France and USA in 1986 (James and Krattiger 1996). Transgenic Flavr Savr tomato is the first successful example of genetically modified food crop and was approved for commercialization in USA in 1994. The main resistant traits introduced into horticultural plants and already commercialized are insect-pest resistance (Bt. toxin gene) and herbicide tolerance while other important studies concern virus resistance, male sterility, etc. Among various genetically modified (GM) horticultural crops, GM papaya showing resistance to Papaya ring spot virus contributes to approx. 53% of the total share of GM horticultural crops cultivated globally. Herbicide tolerance trait is dominating the GM horticultural crop acreage followed by insect resistance and virus resistance traits (ISAAA 2017). Also, the RNA interference (RNAi) technology has gained popularity in plant genetics and system biology during these days due to its stable transgene expression. The applications of this technology cover wide range from insect resistance, viral and disease resisance, drought, heat, salinity tolerance; developing designer flower colors by knocking down the expression of certain endogenous genes, increasing shelf-life, plant architecture modification etc. It is being used as a potential tool in tweaking the regulation of various metabolic pathways in plants and assigning functions to the genes involved, thereof. The most studied crop so far is tomato, but research activities had already been carried out in various horticultural crops such as fruits, vegetables and flowers. With the advancement of regeneration and genetic transformation protocols, extensive research efforts have been made to incorporate genes for various biotic and abiotic stress tolerance/resistance, enhancing shelf-life, modification of plant architecture and color and texture modification traits in a number of horticultural crops, which have been summarized and discussed in this review paper. An overview of various transgenic strategies targeting horticultural crop improvement is depicted in Fig. 1.
Fig. 1.
Overview of various transgenic strategies targeting horticultural crop improvement
Biotic stress management through transgenic approach
Insect-pest resistance
At present, insect-pest resistance is lacking generally in many crop plants. The use of chemical control measures is proving hazardous to the consumers and also not environmentally sustainable. From a grower’s perspective, any genetic improvement that could reduce the cost of chemical application to combat pests would be of significant benefit. Bt (Cry) gene isolated from a soil bacteria Bacillus thuringiensis has proven highly effective in controlling various lepidopteran insects in a number of crops successfully. Insect resistance was firstly reported in tomato using Bt. gene in 1987. Transgenic Bt. tomato plants exhibited resistance against Spodoptera litura and Heliothis virescens (Fischhoff et al. 1987). Fruit trees like transgenic persimmon carrying cryI gene were found resistant to Plodia interpunctata and Monema flavescens (Tao et al. 1997). Brinjal is among the highly consumed vegetables in Asia and specifically in Indian subcontinent. However, it is extremely damaged by a lepidopteran insect, i.e., Leucinodes orbonalis. Kumar et al. (1998) transformed a synthetic cry1Ab gene coding for an insecticidal crystal protein (ICP) to brinjal (Solanum melongena cv. Pusa Purple Long) by co-cultivating cotyledons with A. tumefaciens. Gene expression was evaluated by double-antibody sandwich ELISA analysis. The transgenic lines displayed significant differences in the insect mortality in fruit bioassays. It was suggested to express a very high level of insecticidal crystal protein to confer complete protection against Leucinodes orbonalis by employing some fruit-specific promoter for better inducible expression. Potato varieties engineered for resistance to Colorado potato beetle were in commercial production for several years and were technically and agronomically successful, allowing significant reductions in insecticide use (Shelton et al. 2002). Chakrabarty et al. (2002) transformed cauliflower var. Pusa Snowball K-1 with a synthetic cryIA(b) gene and the transgenic plants indicated the effectiveness of the transgene against infestation by diamondback moth (Plutella xylostella) larvae during insect bioassays. Paul et al. (2005) developed transgenic cabbage (Brassica oleracea var. capitata) line DTC 507 with a synthetic fusion gene of B. thuringiensis encoding a translational fusion product of cry1B and cry1Ab δ-endotoxins to confer resistant against diamondback moth (Plutella xylostella), the most destructive pest of cruciferous plants across the globe. Bt cabbage plants expressing the fusion protein in mature leaves caused 100% mortality to all the four larval stages of diamondback moth. Complete mortality of the neonate larvae had been observed within 24 h and within a period of 48 h in case of other three stages of larvae. Bt gene (Cry1Ac) has been successfully transformed and expressed in okra (Abelmoschus esculentus) for incorporating resistance against fruit and short borer (Earias vittella), which is the most serious insect-pest of this crop in Asia (Narendran et al. 2013). Okra is severely affected by Earias vittella and its larvae bore into pods and shoots of the plant and eat the internal tissues leading to withering of the plant and reduction in the market value of the pods. In insect bioassays, fruits from transgenic lines showed 100% larval mortality. Natural as well as synthetic insect resistance genes had been transferred into a number of horticultural crops for imparting resistance against various insect-pests. Zhang et al. (2015) transformed kiwifruit plant (Actinidia chinensis) with a synthetic chimeric gene SbtCry1Ac encoding for protein btCry1Ac, When the transgenic plants were screened for insect resistance in insect bioassays, an average of 75.2% Oraesia excavate inhibition rate was reported at 10 days of post-infection. This technology could be highly useful to protect yield losses of kiwifruit due to insect attack, which is an economically as well as nutritionally important fruit crop, offering a remarkably high vitamin C content.
Other genes such as protease inhibitors, trypsin inhibitors, lectins, etc. have also been employed for incorporation of insect-pest resistance in many crop species. Ding et al. (1998) developed insect-resistant transgenic Taiwan cauliflower Brassica oleracea var. botrytis cvs. Known You Early no. 2, Snow Lady and Beauty Lady expressing the trypsin inhibitor gene, isolated from local sweet potato. The transgenic plants expressed resistance to Spodoptera litura and Plutella xylostella in in-planta bioassays. Transgenic strawberry expressing cowpea (Vigna unguiculata) protease trypsin inhibitor (CpTi) gene under a constitutive promoter developed resistance against vine weevil (Otiorhynchus salcatus). The CpTi transgenic lines reduced the frequency of survival of weevil larvae and pupae during insect bioassays (Graham et al. 2002). Gessler and Patocchi (2007) developed transgenic apple lines with trypsin inhibitor encoding CpTI gene from cowpea and cry1A(c) gene of B. thuringiensis for incorporation of resistance against codling moth pest. Almost all the genotypes of chrysanthemum are infested by two aphids, namely Myzus persicae and Aphis gossypii, lowering the flower quality and also transmitting viruses. Valizadeh et al. (2013) transformed chrysanthemum genotype 1581 by Agrobacterium tumefaciens-mediated technique with SAE gene, under the control of chrysanthemum RbcS promoter to incorporate aphid resistance. The protease inhibitor sea anemone equistatin (SAE) has three domains for inhibition of both cysteine and aspartic proteases. In another study, Chrysanthemum morifolium WRKY48 (CmWRKY48) transcription factor over-expressing transgenic chrysanthemum plants were found to inhibit the population growth of aphids (Li et al. 2015).
Another category of plant pests, root-knot nematode (Meloidogyne incognita) causes severe yield losses in many horticultural crops. Genetic transformation of various proteinase inhibitor genes from plants is considered as the most potential strategy to prevent such yield losses. Cysteine proteinases are involved in the digestion process of root-knot nematodes and binding of various cystatins to the active sites of proteinases inhibits their activity by proteolytic digestion (Shingles et al. 2007). Roderick et al. (2012) developed transgenic plantain (Musa sp.) cv. Gonja manjaya plants expressing a maize cystatin gene that inhibits the digestive cysteine proteinases and a synthetic peptide that disrupts nematode chemoreception. The best level of resistance exhibited by the transgenic plants against the major pest species Radopholus similis was 84% for the cystatin, 66% for the peptide and 70% for the dual defense. In another study, Papolu et al. (2016) developed transgenic brinjal plants expressing a modified rice cystatin (OC-I∆D86) gene under a root-specific promoter, TUB-1 for inducing resistance against root-knot nematode (RKN). Transgenic plants were confirmed for gene integration and expression using PCR, Southern and Western blotting, ELISA and qPCR assays. When one transgenic line (single copy event) was challenged with root-knot nematode, 78.3% inhibition rate in reproduction of root-knot nematode had been reported. In an earlier study, transgenic banana plants expressing the same cystatin gene (OC-I∆D86) exhibited 70% resistance to the migratory endoparasite, R. similis (Atkinson et al. 2004). Lilley et al. (2004) had also reported a partial resistance (67%) against M. incognita in transgenic potato roots expressing same gene. Root lesion nematode (RLN), Pratylenchus penetrans, is one of the main pests of lily producers, particularly in USA, where lily (Lilium longiflorum) cv. ‘Nellie white’ assumes a great economic importance as cut flowers and constitutes one of the most valuable species. Vieira et al. (2015) developed transgenic lilies over-expressing the OC-I∆D86 gene which displayed an enhanced resistance to root lesion nematode infection by means of nematode reduction up to 75%. The transgenic lily plants also exhibited an increased biomass and better growth performance additionally as compared to non-transformed plants.
Disease resistance
The next major constraint limiting the production of horticultural crops is different diseases caused by pathogenic fungi, bacteria and viruses. Conventional breeding seems to have limited application due to non-availability of resistant gene(s) in gene pool of a particular crop. One of the main targets of genetic transformation is to improve tolerance or to incorporate resistance in plants against different pathogens. Genetic engineering of disease resistance in crops has become popular and valuable in terms of cost and efficacy. For imparting resistance against bacterial and fungal diseases, various genes like chitinase, glucanase, osmotin, defensin, etc. are being transferred into various horticultural crops world over. Various glycolytic enzymes encoded by genes like chitinase, glucanase, PR proteins, etc. inside the plant cells have cell wall degrading capabilities, which attract their use for developing transgenic plants for incorporation of resistance against fungal pathogens (Ceasar and Ignacimuthu 2012).
Among different strategies used for genetic engineering for disease resistance, the employment of systemic acquired resistance (SAR)-related genes is of paramount importance. SAR is long lasting and often associated with local and systemic accumulation of salicylic acid (SA) and induced expression of many genes including pathogenesis-related (PR) genes (Ryals et al. 1996). A gene for a PR protein from tomato (PR-5) had been expressed in transgenic sweet orange and regenerants showed increased tolerance to Phytophthora citrophthora (Fagoaga et al. 2001). Lin et al. (2004) introduced Arabidopsis thaliana-derived NPR-I gene into tomato. Transgenic tomato plants developed enhanced heat tolerance and resistance against tomato mosaic virus (ToMV). The transgenic lines also conferred significant level of resistance to bacterial wilt (BW) and Fusarium wilt (FW) along with moderate degree of enhanced resistance to gray leaf spot (GLS) and bacterial spot (BS). Malnoy and Aldwinckle (2007) developed transgenic apple lines over-expressing MpNPR1-1 (ortholog of AtNPR1), which exhibited broad spectrum resistance against V. inaequalis, Gymnosporangium juniper-virginianae, a causative agent of cedar apple rust and Erwinia amylovora, which causes fire blight. The Fuji apple, the most popular and most widely cultivated variety of apple in China, is highly susceptible to powdery mildew disease. Apple powdery mildew, which is caused by Podosphaera leucotricha, damages leaves and young fruits, thus leading to huge yield losses (Qu et al. 2009). Chen et al. (2012) introduced Malus hupehensis derived NPR (MhNPR1) gene into ‘Fuji’ cultivar of apple for development of resistance against powdery mildew disease. NPR1 gene plays a key role in regulating salicylic acid (SA)-mediated SAR in plants. MhNPR1 gene induced the expression of MdPRs and MdMLO genes which interact with powdery mildew as revealed by RT-PCR and the transgenic apple plants expressed enhanced resistance to powdery mildew disease. Over-expression of AtNPR1 gene in tomato and carrot plants also exhibited resistance to bacterial and fungal pathogens (Lin et al. 2004; Wally et al. 2009). Commercial sweet orange cultivars are suffering from this deadly disease. Similar findings were reported by over-expression of a Vitis vinifera NPR1,1 (Vv NPR1,1) gene which conferred to enhance resistance against powdery mildew in grapevine (Le et al. 2011). In the United States, Huanglongbing (HLB) is a very serious disease of citrus, which is associated with a phloem-limited bacterium Candidatus liberibacter asiaticus (CLas) (Duan et al. 2009). Dutt et al. (2015) over-expressed an Arabidopsis thaliana NPR1 gene under a constitutive promoter CaMV35S and also under a phloem-specific Arabidopsis SUC2 (AtSUC2) promoter in sweet orange cultivar ‘Hamlin’ and ‘Valencia’. NPR1 gene is involved in the induction of expression of several native genes involved in the plant defense signaling pathways. The transgenic plants exhibited reduced disease severity and a few lines remained disease-free even after three years of planting in a high-disease pressure field site.
Another category of genes imparting disease resistance is Chitinases, which are glycosyl hydrolases that catalyze the degradation of chitin, an insoluble, linear β-1,4-linked polymer of N-acetyl glucosamine, a cell wall component of various bacteria and fungi, and thus code for pathogen resistance. Chitinase gene has been transferred to a number of crops for harboring fungal resistance. In carrot, the tobacco class I ChiC gene has shown resistance against Botrytis cinerea (Punja and Raharjo, 1996), RCC2 gene, a rice chitinase displayed enhanced resistance to Sphaerotheca humuli in transgenic strawberry plants (Asao et al. 1997). In another study, Yamamoto et al. (2000) transformed a rice chitinase gene (RCC2) into the somatic embryos of grapevine cv. Neo Muscat and reported an increased resistance level against powdery mildew fungus, V. necator. Schestibratov and Dolgov (2005) also developed transgenic strawberry plants expressing thaumatin II gene from Thaumatococcus danielli and reported some level of resistance to B. cinerea during in vitro assays. Vellice et al. (2006) expressed a chitinase gene from Phaseolus vulgaris, a glucanase or a thaumatin-like protein, both from Nicotiana tabacum and a combination of both in strawberry cv. ‘Pajaro’. Two transgenic lines expressing chitinase gene showed enhanced tolerance to Botrytis cinerea. Khan et al. (2008) attempted to confer resistance to early blight of potato, caused by Alternaria solani, transformed a chitinase gene, ChiC, isolated from Streptomyces griseus strain HUT 6037, along with a bialaphos resistance (bar) gene into potato. The herbicide-resistant transgenic potato plants demonstrated enhanced resistance against Alternaria solani in in vitro bioassays. However, in another study, Moravcikova et al. (2004) reported that the high level of expression of cucumber class III ChiC gene in potato could not enhance resistance against the phytopathogenic fungus, Rhizoctonia solani (causing black scurf disease in potato) to any considerable level. Das and Rahman (2010) had also expressed bacterial chitinase (chi B) gene in litchi cv. Bedana, which, however, showed low level of chitinase activity and only partial resistance against Phomopsis sp. pathogen had been reported in transgenic plants. Various endo-chitinases genes such as CHIT42 and CHIT33 from Trichoderma harzianum had also been successfully transformed and expressed to impart increased fungal tolerance in potato (Lorito et al. 1998), apple (Bolar et al. 2001), broccoli (Mora and Earle 2001), carrot (Baranski et al. 2008) and lemon (Distefano et al. 2008). Girhepuje and Shinde (2011) developed transgenic tomato plants over-expressing a wheat chitinase gene, chi194, under the control of maize ubiquitin 1 promoter. The transgenic tomato lines showing higher expression of chitinase activity were found to be highly resistant to Fusarium wilt disease of tomato caused by Fusarium oxysporum f. sp. Lycopersici. In another study, transgenic litchi (Litchi chinensis) plants containing a rice chitinase gene were developed to increase the antifungal response. The transgenic lines exhibited higher chitinase activity and disease resistance than the non-transformed plants (Das et al. 2012). Guava wilt disease caused by a soil borne fungus Fusarium oxysporum f. sp. psidii is emanating as a serious threat to guava growers throughout the entire globe. To control this disease, Mishra et al. (2016) transferred a Trichoderma-endochitinase gene into guava (Psidium guajava). In vitro pathogen inhibition assay and spore germination assay revealed that the crude extract of the transformed plants inhibited the germination of fungal conidia and were resistance to wilt disease.
A number of defense mechanisms were evolved in plants over thousands of years to overcome pathogen attack/infection and the role of many genes or various pathways has been investigated and identified (Islam 2006). The disease resistance conferred by glucanase gene may be attributed to solubilizing elicitors from the fungal cell walls which induce production of antifungal phytoalexins (Keen and Yoshikawa 1993). Yoshikawa et al. (1993) also proposed the role of glucanase in the induction of the transcription of a plant defense gene, phenylalanine ammonia lyase in response to fungal attack. Further, glucanase is a hydrolytic enzyme, which breaks down the cell wall component, glucan of many necrotrophic fungal pathogens. An increased level of resistance in transgenic potato plants expressing soybean glucanase gene against Phytopthora infestans has been reported due to the increased glucanase activity (Borkowoska et al. 1998). Transgenic kiwifruit over-expressing soybean β-1,3 glucanase gene exhibited a six fold increased enzyme activity leading to a decrease in the disease lesion area caused by the gray mold fungus, Botrytis cinerea (Nakamura et al. 1999). Almost all the cultivated varieties of brinjal are susceptible to wilt disease caused by Verticillum dahliae and Fusarium oxysporum, which cause considerable yield losses annually (Najar et al. 2011). To generate wilt disease resistance in brinjal, Singh et al. (2014) transformed alfalfa glucanase gene coding for an acidic glucanase into brinjal cv. Pusa Purple Long. The selected transgenic lines, confirmed with DNA and protein blotting techniques, showed enhanced level of resistance against these wilt causing fungi with a delay of 5–7 days in disease development as compared to the non-transgenic plants. Sometimes, it has been found that the transgenes were capable of inducing disease resistance trait, but have altered the plant growth processes due to the use of a constitutive promoter. In a study, Mercado et al. (2015) expressed β-1,3-glucanase gene bgn13,1, isolated from Trichoderma harzianum in strawberry under the control of CaMV 35S promoter. The transgenic lines showed reduced anthracnose symptoms (from 61.0 to 16.5%) in leaf and crown than control plants after inoculation with Colletotrichum acutatum. However, most of the transgenic lines displayed stunted phenotype and reduced yield due to the reduction in number of fruits per plant and a reduced fruit size.
The use of various antimicrobial proteins coding genes like defensins had been advocated for combating a large class of fungal and bacterial pathogens (Collinge et al. 2010). Defensins represent a class of antimicrobial peptides which play an important defensive role against fungi, bacteria and protozoa, but are non-toxic to mammalian cells and plants. Defensin genes encoded proteins react by creating certain pores in the fungal hyphal membrane and, thus, disturb the ion-influx–outflux and kill the fungal pathogens. Zainal et al. (2009) reported an enhanced level of resistance in transgenic tomato expressing Capsicum annum defensin gene against various fungal pathogens. A bell pepper J1 defensin gene was also reported to confer resistance against anthracnose disease of mango, which is caused by Colletotrichum gloeosporioides (Rivera-Dominguez et al. 2011). Protein extract from processed embryos of transgenic mango cv. ‘Ataulfo’ inhibited the growth of C. gloeosporioides, Aspergillus niger and Fusarium spp. Transgenic banana plants over-expressing two defensin genes—PhDef1 and PhDef2 had been found resistant against Fusarium oxysporum f. sp. cubense (Ghag et al. 2012). In addition to that, genetic transformation of many non-plant antimicrobial compounds like cercopin, attacin, phytoalexins had been reported to enhance resistance level in plants expressing them (Mondal et al. 2012; Ahuja et al. 2012). Transgenic apple expressing maize Leaf color (Lc) gene exhibited resistance to fire blight and scab diseases (Flachowsky et al. 2010). In another study, transformation of a biotin binding protein (Markwick et al. 2003) and a proteinase inhibitor gene from Nicotiana alata exhibited resistance against light brown apple moth disease. Constitutive expression of a fungus-inducible carboxy esterase gene (PepEST) under CaMV35S promoter was reported to increase the anthracnose disease resistance in transgenic pepper (Capsicum annum) (Ko et al. 2016). PepEST gene expression in fruits leads to disease resistance development by generation of H2O2 and expression of pathogenesis-related (PR) genes, which encode for a number of small proteins having antimicrobial activity. On infection of the anthracnose fungus, Colletotrichum gloeosporioides on the transgenic fruits of pepper cv. Nokkang, a higher level of expression of PR genes, namely PR3, PR5, PR10 and PepThi was reported than the non-transgenic plants. Further, a lower rate of disease occurrence (30%) was reported in the transgenic fruits than in the wild-type plants.
Various types of polyamines including putrescine, spermidine and spermine play a key role in imparting tolerance/resistance to both biotic and abiotic stresses. Hazarika and Rajam (2011) transformed tomato cv. Pusa Ruby with a human S-adenosyl methionine decarboxylase (samdc) gene, which is involved in the biosynthesis of polyamines viz. spermidine and spermine. The transgenic tomato plants synthesized higher level of polyamines and also expressed enhanced level of resistance against Fusarium oxysporum causing wilt disease and Alternaria solani, the early blight causing fungus. In addition to that, the transgenic lines also expressed better tolerance to a variety of abiotic stresses including high temperature, drought, salinity and chilling stress. Shin et al. (2002) developed transgenic chilli pepper plants (Capsicum annum cv. Nockwang) with Tsi1 (tobacco stress-induced 1) gene via Agrobacterium tumefaciens-mediated gene transfer technique using cotyledon and hypocotyl explants. The protein product of Tsi1 gene has got some role in regulating stress-responsive genes and pathogenesis-related (PR) genes. The transgenic chilli plants expressed enhanced resistance to pepper mild mottle virus, cucumber mosaic virus, a bacterial pathogen—Xanthomonas campestris pv. Vesicatoria and a fungal pathogen—Phytophthora capsici. Genetic transformation of Vf gene, imparting scab disease resistance caused by Venturia inaequalis under CaMV 35S promoter in apple, had been found to impart scab resistance in susceptible cultivars of apple in a number of studies (Malnoy et al. 2008; Szankowski et al. 2009; Joshi et al. 2009). Banana (Musa sps.) is one of the most important fruit crops being cultivated in about 120 countries across the globe. India is the largest global producer of banana. Banana Xanthomonas wilt (BXW), which is caused by Xanthomonas campestris pv. musacearum, is considered as one of the most destructive diseases of this fruit crop, particularly in East and Central Africa (Tripathi et al. 2009). In a study, Namukwaya et al. (2012) expressed plant ferredoxin like protein (Pf1p) gene under the control of CaMV35S promoter in transgenic banana cv. ‘Sukali Ndiizi’ and ‘Nakinyika’ to develop resistance against BXW disease. In bioassay studies, 67% of the transgenic lines were found resistant to BXW and did not show any disease symptoms, while the wild-type plants expressed severe symptoms of wilting. In another study, grapevine rootstock of V. berlandieri × V. rupestris cv. Richter 110 had been transformed with an Agrobacterium oncogene-silencing gene to develop crown gall-resistant lines (Galambos et al. 2013). Lindow et al. (2014) reported a reduced severity of Pierce’s disease and pathogen mobility in transgenic grape cv. Freedom by the over-expression of an rpfF gene (from Xylella fastidiosa), which codes for the synthase for diffusible signal factors (DSFs). Cheng et al. (2016) transformed Vitis vinifera Thompson Seedless grape with a stilbene synthase gene, VqSTS6, isolated from Chinese wild-type V. quinquangularis accession Danfeng-2 under a fruit-specific promoter to develop resistance against powdery mildew disease, caused by Uncinula necator. The transgenic plants synthesized enhanced quantity of trans-resveratrol and other stilbene compounds as compared to the control plants and expressed enhanced resistance to powdery mildew fungus. It has been found that VqSTS6 gene is involved in resveratrol biosynthetic pathway in grapes and, thus, plays a key role in imparting protection against invading pathogens. Jiwan et al. (2012) reported antisense expression of the peach MLO gene in strawberry (Fragaria × ananasa) conferred cross-species resistance to Fragaria-specific powdery mildew. RNA-interference (RNAi) technology being used recently is quite successful in controlling various bacterial and fungal diseases in plants by switching off the expression of certain endogenous genes. In one such study, transgenic tomato plants expressing hpRNA constructs against Agrobacterium iaaM and ipt oncogenes were found to be resistant to crown gall disease (Escobar et al. 2001). Recently, Pessina et al. (2016) reported an increased level of resistance in grapevine to powdery mildew by RNAi-mediated silencing of the susceptible (S-gene) MLO-7.
Virus resistance
In fruit crops, the coat protein-mediated approach to engineer virus resistance has been in application to introduce resistance against various viral diseases. Papaya is grown in many tropical countries, but its cultivation is being threatened by Papaya Ring Spot Virus (PRSV), a disease that is considerably lowering its yield. Using biotechnological interventions, the coat protein gene of the virus has been transferred to papaya to confer PRSV resistance. Since 1998, GM papayas have been cultivated in Hawaii, USA, which had shown considerable resistance to PRSV. PRSV-resistant transgenic papaya varieties ‘SunUp’ and ‘Rainbow’ have now occupied >80% shelf-space in the US market. Strawberry is susceptible to various devastating fungi, bacteria and viruses. Finstad and Martin (1995) developed transgenic strawberry plants expressing a coat protein (cp) gene from strawberry mild yellow edge potexvirus (SMYELV-CP) and these lines conferred resistance to the virus. In another study, Lee et al. (2009) developed transgenic chilli pepper plants with a coat protein gene (CMVP0-CP). Three independent transgenic events, which were earlier highly tolerant to CMVP1 pathogen, were also found to be tolerant to CMVP0 pathogen. The production and productivity of watermelon (Citrullus lanatus) have been affected considerably by two viruses, namely Zucchini yellow mosaic virus (ZYMV) and papaya ring spot virus type W (PRSV W) worldwide. In an attempt to get rid of these two viruses altogether, Yu et al. (2011) transformed three watermelon cultivars, namely ‘Feeling’, ‘China rose’, and ‘Quality’ with chimeric construct containing truncated ZYMV coat protein (CP) and PRSV W CP genes via Agrobacterium tumefaciens-mediated gene transfer technique. Two completely immune transgenic lines of ‘Feeling’ cultivar had been obtained during greenhouse bioassays where these two lines showed complete resistance to ZYMV and PRSV W and no virus accumulation was detected by Western blotting from these transgenic lines.
Also, transgenic papaya plants with the mutated replicase (RP) gene from PRSV showed high resistance or immunity against PRSV in the field (Xiangdong et al. 2007). Borth et al. (2011) developed transgenic banana (cv. Dwarf Brazilian) plants resistant to banana bunchy top virus (BBTV) by transforming four gene construct derived from the replicase associated protein (Rep) gene of the Hawaiian isolate of BBTV. The transgenic plants showed no bunchy top symptoms, while the non-transgenic plants expressed bunchy top symptoms. Azadi et al. (2011) transformed lily cv. ‘Acapulco’ plants with a defective cucumber mosaic virus replicase gene and four transgenic lines were found to show enhanced level of virus resistance.
RNAi technology has been found successful to impart resistance to various viral diseases in plants. The expression of a self-complementary hairpin RNA under the control of rolC promoter controlled the systemic disease spread caused by plum pox virus without preventing local infection (Pandolfini et al. 2003). Using a hairpin RNA gene silencing strategy, transgenic poinsettia plants resistant to Poinsettia Mosaic Virus have been developed (Clarke et al. 2008). Praveen et al. (2010) developed transgenic plants of tomato with AC4 gene-RNAi construct and the transgenic plants were found to show the suppression of tomato leaf curl virus activity. Transgenic banana plants expressing siRNA targeted against viral replication initiation (Rep) gene were developed by Shekhawat et al. (2012), which showed high level of resistance to BBTV infection. Transgenic development work carried out in various horticultural crops for imparting biotic stress resistance has been summarized in Table 1.
Table 1.
Transgenic horticultural crops for biotic stress resistance/management
| S. no. | Crop species/cultivar | Gene and genetic transformation method used | Mechanism of action | Target trait | Trait improvement (transgenic vs normal plants) | References | |
|---|---|---|---|---|---|---|---|
| (A) Insect-pest resistance | |||||||
| 1. | Brinjal (egg plant) cv. Pusa Purple Long | Synthetic cry 1Ab gene; Agrobacterium tumefaciens-mediated gene transfer | Insect mid-gut dissolution | Resistance against fruit and shoot borer, Leucinodes orbonalis | The transgenic lines displayed significant differences in the insect mortality in fruit bioassays | Kumar et al. (1998) | |
| Brinjal | Modified rice cystatin gene (OC-I∆D86); Agrobacterium tumefaciens-mediated gene transfer | Binding of cystatin to the active sites of proteinases inhibit their activity, thus affecting their proteolytic digestion | Resistance against root-knot nematode (Meloidogyne incognita) | 78.3% inhibition rate in reproduction of root-knot nematode in transgenic plants | Papolu et al. (2016) | ||
| 2. | Banana cv. Gonja manjaya | Maize cystatin gene (CC-II); Agrobacterium tumefaciens-mediated gene transfer | Binding of cystatins to the active sites of proteinases inhibit their activity, thus affecting their proteolytic digestion | Resistance against reniform nematode (Potylenchulus reniformis) and Helicotylenchus multicinctus | Reduced infection of P. reniformis and H. multicinctus in transgenic plants | Roderick et al. (2012) | |
| Banana | Cystatin gene (OC-I∆D86); Agrobacterium tumefaciens-mediated gene transfer | Binding of cystatins to the active sites of proteinases inhibit their activity, thus affecting their proteolytic digestion | Resistance against Radopholus similis | Transgenic banana plants exhibited 70% resistance to the migratory endoparasite, R. similis | Atkinson et al. (2004) | ||
| 3. | Strawberry (Fragaria × ananasa) | CpTi (Cowpea protease trypsin inhibitor) gene; Agrobacterium tumefaciens-mediated gene transfer | CpTi protein inhibits the internal proteases within the plant cell on pest attack and thus protect the integral protein functions | Resistance against vine weevil (Otiorhynchus sulcatus) | CpTi transgenic lines reduced the frequency of survival of weevil larvae and pupae during insect bioassays | Graham et al. (2002) | |
| 4. | Cabbage (Brassica oleracea var. capitata) | Cry1B-cry1Ab fusion gene; Agrobacterium tumefaciens-mediated gene transfer | Insect mid-gut dissolution | Resistance against Diamond back moth (Plutella xylostella) | Reduced infestation of P. xylostella in transgenic plants | Paul et al. (2005) | |
| 5. | Okra (Abelmoschus esculentus), an inbred line of Maharashtra Hybrid Seeds Company Ltd., Jalna, India | Cry1Ac gene; Agrobacterium tumefaciens-mediated gene transfer | Insect mid-gut dissolution | Insect resistance (fruit and shoot borer) | In insect bioassays, fruits from transgenic lines caused 100% larval mortality | Narendran et al. (2013) | |
| 6. | Kiwifruit (Actinidia chinensis) | Synthetic chimeric gene sbtCryIAc; Agrobacterium tumefaciens-mediated gene transfer | Insect mid-gut dissolution | Insect resistance against Oraesia excavate | An average of 75.2% O. excavate inhibition rate at 10 days of post-infection during in vitro insect bioassay | Zhang et al. (2015) | |
| 7. | Potato | Cystatin gene (OC-I∆D86); Agrobacterium tumefaciens-mediated gene transfer | Binding of cystatins to the active sites of proteinases inhibit their activity, thus affecting their proteolytic digestion | Resistance against M. incognita | A partial resistance (67%) against M. incognita in transgenic potato roots | Lilley et al. (2004) | |
| 8. | Lily (Lilium longiflorum) cv. ‘Nellie white’ | Rice cystatin gene, OC-I∆D86; Agrobacterium rhizogenes-mediated gene transfer | Binding of cystatins to the active sites of proteinases inhibit their activity, thus affecting their proteolytic digestion | Root lesion nematode (Patrylenchus penetrans) resistance | Enhanced resistance to root lesion nematode infection by means of nematode reduction up to 75% and an increased biomass and better growth performance as compared to non-transformed plants | Vieira et al. (2015) | |
| 9. | Chrysanthemum morifolium | CmWRKY48 transcription factor; Agrobacterium tumefaciens-mediated gene transfer | WRKY48 TF works as positive regulator in various biotic stress responses in plants | Aphid resistance | Inhibition in the population growth of aphids in transgenic chrysanthemum plants | Li et al. (2015) | |
| C. morifolium genotype 1581 | Protease inhibitor sea anemone equistatin (SAE) gene; Agrobacterium tumefaciens-mediated gene transfer | SAE has three domains for inhibition of both cysteine and aspartic proteases of aphids | Aphid resistance | Enhanced resistance against Myzus persicae and Aphis gossypi | Valizadeh et al. (2013) | ||
| 10. | Taiwan cauliflower (Brassica oleracea var. botrytis) cvs. Known You Early no. 2, Snow Lady and Beauty Lady | Trypsin inhibitor gene; Agrobacterium tumefaciens-mediated gene transfer | Inhibits trypsin | Insect resistance | Enhanced resistance to Spodoptera litura and Plutella xylostella in transgenic plants in in-planta bioassays | Ding et al. (1998) | |
| (B) Fungal resistance | |||||||
| 1. | Strawberry (Fragaria × ananassa) | A rice chitinase gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3–60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Resistance against Sphaerotheca humuli | Enhanced resistance to Sphaerotheca humuli in transgenic strawberry plants | Asao et al. (1997) | |
| Strawberry | Osmotin; Agrobacterium tumefaciens-mediated gene transfer | Increase the level of proline accumulation | Resistance against gray mold disease caused by Botrytis cinerea | The transgenic plants showed higher levels of antifungal activity and significantly increased resistance to wilt and gray mold diseases | Martinelli et al. (1997) | ||
| Strawberry | Thaumatin II gene (from Thaumatococcus danielli; Agrobacterium tumefaciens-mediated gene transfer | Some role in fungal resistance | Resistance to Botrytis cinerea | Enhanced resistance to B. cinerea | Schestibratov and Dolgov (2005) | ||
| Strawberry cv. Pajaro | Chitinase and a glucanse gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinase and glucanase degrades chitin and glucan, cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Resistance to Botrytis cinerea | Two transgenic lines expressing chitinase gene showed enhanced tolerance to Botrytis cinerea | Vellice et al. (2006) | ||
| Strawberry | β-1,3-glucanase; Agrobacterium tumefaciens-mediated gene transfer | Dissolves glucan component of cell wall of fungi, thus imparting fungal resistance | Resistance to anthracnose crown rot caused by Colletotrichum acutatum | Reduced crown rot lesions in transgenic events as compared to the non-transgenic lines | Mercado et al. (2007) | ||
| Strawberry cv. Camarosa | β-1,3-glucanase gene (bgn13,1 from Trichoderma harzianum); Agrobacterium tumefaciens-mediated gene transfer | Dissolves glucan component of cell wall of fungi, thus imparting fungal resistance | Resistance to crown gall disease | The transgenic lines showed reduced anthracnose symptoms (from 61 to 16.5%) in leaf and crown than control plants | Mercado et al. (2015) | ||
| 2. | Carrot (Daucus carota) | Tobacco class I ChiC gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3-60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Resistance against Botrytis cinerea | Enhanced resistance against Botrytis cinerea in transgenic carrot plants | Punja and Raharjo (1996) | |
| Carrot | AtNPR1 gene (derived from Arabidopsis thaliana); Agrobacterium tumefaciens-mediated gene transfer | NPR1 gene plays a key role in regulating salicylic acid (SA)-mediated systemic acquired resistance (SAR) in plants | Resistance against bacterial and fungal pathogen | Enhanced resistance to bacterial and fungal pathogens | Wally et al. (2009) | ||
| 3. | Potato | Soybean glucanase gene; Agrobacterium tumefaciens-mediated gene transfer | Dissolves glucan component of cell wall of fungi, thus imparting fungal resistance | Resistance against Phytopthora infestans | An increased level of resistance in transgenic potato against Phytopthora infestans | Borkowoska et al. (1998) | |
| Potato | A cucumber class III ChiC gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3-60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Resistance against Rhizoctonia solani causing black scurf disease in potato | No resistance against the phytopathogenic fungus, Rhizoctonia solani | Moravcikova et al. (2004) | ||
| Potato cvs. Waseshiro, Benimaru and May Queen | A chitinase gene, ChiC from Streptomyces griseus and bar gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinases are glycosyl hydrolases which catalyze the degradation of chitin, an insoluble, linear β-1,4-linked polymer of N-acetyl glucosamine, a cell wall component of various bacteria and fungi, and thus codes for pathogen resistance | Resistance against early blight of potato caused by Alternaria solani | The herbicide resistant transgenic potato plants demonstrated enhanced resistance against A. solani in in vitro bioassays | Khan et al. (2008) | ||
| 4. | Kiwifruit | Soybean β-1,3-glucanase gene; Agrobacterium tumefaciens-mediated gene transfer | Dissolves glucan component of cell wall of fungi, thus imparting fungal resistance | Resistance against gray mold fungus Botrytis cinerea | Transgenic kiwifruit exhibited a six fold increased enzyme activity leading to a decrease in the disease lesion area caused by the gray mold fungus, Botrytis cinerea | Nakamura et al. (1999) | |
| 5. | Grapevine (Vitis vinifera) | Rice chitinase gene (RCC2); Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3–60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Powdery mildew resistance | Increased resistance level against powdery mildew fungus, V. necator | Yamamoto et al. (2000) | |
| Grapevine | Vv NPR1,1 gene (derived from Vitis vinifera); Agrobacterium tumefaciens-mediated gene transfer | NPR1 gene plays a key role in regulating salicylic acid (SA)-mediated systemic acquired resistance (SAR) in plants | Resistance against powdery mildew | Enhanced resistance to powdery mildew disease | Le et al. (2011) | ||
| Grapevine rootstock of V. berlandieri × V. rupestris cv. Richter 110 | Agrobacterium oncogene-silencing gene; Agrobacterium tumefaciens-mediated gene transfer | Oncogenes when silenced lead to no crown gall formation | Resistance against crown gall disease | Enhanced resistance to crown gall-disease | Galambos et al. (2013) | ||
| Grapevine cv. Freedom | rpfF gene (from Xylella fastidiosa); Agrobacterium tumefaciens-mediated gene transfer | rpfF gene codes for the synthase for diffusible signal factors (DSFs) imparting resistance against Pierce’s disease | Resistance against Pierce’s disease | A reduced severity of Pierce’s disease and pathogen mobility in transgenic grape | Lindow et al. (2014) | ||
| Grapevine cv. Thompson Seedless grape | A stilbene synthase gene, VqSTS6 from wild grapevine, V. quinquangularis; Agrobacterium tumefaciens-mediated gene transfer | VqSTS6 gene is involved in resveratrol biosynthetic pathway and plays a key role in imparting protection against invading pathogens | Resistance against powdery mildew disease caused by Uncinula necator | The transgenic plants synthesized enhanced quantity of trans-resveratrol and other stilbene compounds and expressed enhanced resistance to powdery mildew fungus | Cheng et al. (2016) | ||
| Grapevine | RNAi-mediated silencing of MLO-7 gene; Agrobacterium tumefaciens-mediated gene transfer | MLO-7 is a powdery mildew susceptible gene, its silencing by RNAi imparts resistance trait | Resistance against powdery mildew disease | Enhanced resistance against powdery mildew disease in transgenic plants | Pessina et al. (2016) | ||
| Grape cv. Chardonnay | MLO-7 gene; Agrobacterium tumefaciens-mediated gene transfer | Genome editing to bring targeted mutagenesis | Resistance against powdery mildew | Enhanced resistance against powdery mildew in transgenic plants | Malnoy et al. (2016) | ||
| 6. | Apple (Malus domestica) | Malus pumila NPR1 (MpNPR1) gene; Agrobacterium tumefaciens-mediated gene transfer | NPR1 gene plays a key role in regulating salicylic acid (SA)-mediated systemic acquired resistance (SAR) in plants | Fungal and bacterial resistance | Resistance to two fungal pathogens, Venturia inaequalis and Gymnosporangium juniperi-verginianae, and a bacteria, Erwinia amylovora | Malnoy et al. (2007) | |
| Fuji Apple (Fuji Naga-fu no. 6) | Mh-NPR1 gene (derived from Malus hupehensis); Agrobacterium tumefaciens-mediated gene transfer | NPR1 gene plays a key role in regulating salicylic acid (SA)-mediated systemic acquired resistance (SAR) in plants | Resistance against apple powdery mildew, caused by Podosphaera leucotricha | MhNPR1 gene induced the expression of MdPRs and MdMLO genes which interact with powdery mildew and the transgenic apple plants expressed enhanced resistance to powdery mildew disease | Chen et al. (2012) | ||
| Apple cv. Golden delicious | DIPM-1, DIPM-2 and DIPM-4 gene; Agrobacterium tumefaciens-mediated gene transfer | Genome editing to bring targeted mutagenesis | Fire blight disease resistance | Enhanced resistance to fire blight disease in transgenic plants | Malnoy et al. (2016) | ||
| 7. | Litchi (Litchi chinensis) cv. Bedana | Bacterial chitinase gene (ChiB); Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3–60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Resistance against Phomopsis sp. | Transgenic plants showed low level of chitinase activity and only partial resistance against Phomopsis sp. pathogen | Das and Rahman (2010) | |
| Litchi cv. Bedana | Chitinase gene (rice-derived); Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3–60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Fungal resistance | The transgenic lines exhibited higher chitinase activity and disease resistance than the non-transformed plants | Das et al. (2012) | ||
| 8. | Mango (Mangifera indica) cv. Ataulfo | Defensin J1 gene from bell pepper; Biolistic-mediated DNA delivery method | defensin gene acts as proteinase inhibitor, also creates pores in cell membrane of fungal hyphae | Resistance against anthracnose disease caused by Colletotrichum gloeosporioides | Protein extract from processed embryos of transgenic mango cv. ‘Ataulfo’ inhibited the growth of C. gloeosporioides, Aspergillus niger and Fusarium sps. | Rivera- Dominguez et al. (2011) | |
| 9. | Tomato | Defensin gene form chilli; Agrobacterium tumefaciens-mediated gene transfer | Defensin gene acts as proteinase inhibitor, also creates pores in cell membrane of fungal hyphae | Various fungal pathogens | Enhanced level of resistance in transgenic tomato plants | Zainal et al. (2009) | |
| Tomato cv. Pusa Ruby | Samdc (a human derived S-adenosyl methionine carboxylase gene); Agrobacterium tumefaciens-mediated gene transfer | Samdc is a key gene involved in polyamine biosynthesis, imparting tolerance to a variety of biotic and abiotic stresses | Biotic and abiotic stress resistance/tolerance | The transgenic tomato plants expressed enhanced level of resistance against Fusarium oxysporum and Alternaria solani and also expressed better tolerance high temperature, drought, salinity and chilling stress | Hazarika and Rajam (2011) | ||
| Tomato cv. Pusa Ruby | chi194, a wheat endochitinase gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3–60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Resistance against tomato wilt caused by Fusarium oxysporum f. sp. lycopersici | The transgenic tomato lines found to be highly resistant to Fusarium wilt | Girhepuje and Shinde (2011) | ||
| Tomato | At NPR1 gene (derived from Arabidopsis thaliana); Agrobacterium tumefaciens-mediated gene transfer | NPR1 gene plays a key role in regulating salicylic acid (SA)-mediated systemic acquired resistance (SAR) in plants | Resistance against bacterial and fungal pathogen | Enhanced resistance to bacterial and fungal pathogens | Lin et al. (2004) | ||
| Tomato | Wasabi defensin (WD) gene from Wasabia japonica; Agrobacterium tumefaciens-mediated gene transfer | Defensins are antimicrobial proteins, inhibit proteases and create pores in fungal hyphal membrane | Fungal resistance | The transgenic tomato plants exhibited enhanced resistance against a number of fungi including Alternaria solani, Botrytis cinerea, Fusarium oxysporum and Erysiphe lycopersic. | Khan et al. (2011b) | ||
| 10. | Brinjal (Solanum melongena) cv. Pusa Purple Long | Glucanase gene from alfalfa; Agrobacterium tumefaciens-mediated gene transfer | Dissolves glucan component of cell wall of fungi, thus imparting fungal resistance | Resistance against Verticillium dahliae and Fusarium oxysporum | The transgenic lines showed enhanced level of resistance against these wilt causing fungi with a delay of 5–7 days in disease development as compared to the non-transgenic plants | Singh et al. (2014) | |
| 11. | Guava (Psidium guajava) | Trichoderma-endochitinase gene; Agrobacterium tumefaciens-mediated gene transfer | Chitinase degrades chitin, which constitutes 3–60% of cell wall composition of fungi, by hydrolysis and thus codes for disease resistance | Wilt disease resistance | Crude extract of the transformed plants inhibited the germination of fungal conidia | Mishra et al. (2016) | |
| 12. | Pepper cv. Nokkang | A pepper esterase gene (PepEST); Agrobacterium tumefaciens-mediated gene transfer | PepEST gene expression leads to generation of H2O2 and expression of pathogenesis-related (PR) genes, which encode for a number of small proteins having antimicrobial activity | Anthracnose disease resistance (caused by Colletotrichum gloeosporioides) | A lower rate of disease occurrence in the transgenic fruits, approximately 30% of than in the wild-type plants | Ko et al. (2016) | |
| 13. | Citrus (sweet orange) cv. ‘Hamlin’ and ‘Valencia’ | AtNPR1 gene (from Arabidopsis thaliana); Agrobacterium tumefaciens-mediated gene transfer | NPR1 gene is involved in the induction of expression of several native genes involved in the plant defense signaling pathways | Resistance against Huanglongbing (HLB) disease, caused by a bacterium Candidatus Liberibacter asiaticus (CLas) | The transgenic plants exhibited reduced disease severity and a few lines remained disease-free even after 3 years of planting in a high-disease pressure field site | Duan et al. (2009) | |
| (C) Virus resistance | |||||||
| 1. | Potato cv. ‘spuncta’ | ds RNA of coat protein gene of PVY; Agrobacterium tumefaciens-mediated gene transfer | Antisense gene inhibition | Resistance against Potato virus Y (PVY) | Transgenic plants were found highly resistant to three strains of PVY, each belonging to three different subtypes of the virus (PVY N, PVY O and PVY NTN) | Missiou et al. (2004) | |
| 2. | Poinsettia | RNAi; Agrobacterium tumefaciens-mediated gene transfer | siRNA-mediated gene silencing | Poinsettia mosaic virus resistance | Enhanced resistance to Poinsettia mosaic virus | Clarke et al. (2008) | |
| 3. | Papaya | PRSV Coat protein (CP) gene; Agrobacterium tumefaciens-mediated gene transfer | Coat protein-mediated virus resistance | Papaya ring spot virus | Enhanced resistance against PRSV infection | Suzuki et al. (2008) | |
| 4. | Watermelon (Citrullus lanatus), three cvs. namely ‘Feeling’, ‘China rose’ and ‘Quality’ | A chimeric gene construct containing truncated ZYMV-cp and PRSV W cp genes; Agrobacterium tumefaciens-mediated gene transfer | RNA mediated post-transcriptional gene silencing (PTGS) | Resistance against two viruses namely, ZYMV and PRSV W | Two completely immune transgenic line of ‘Feeling’ cultivar showed complete resistance to ZYMV and PRSV W | Yu et al. (2011) | |
| 5. | Banana cv. Rasthali | ihp-RNA-Rep and ihp-RNA-ProRep; Agrobacterium tumefaciens-mediated gene transfer | siRNA, derived from the transgene sequence coded for resistance against BBTV by establishing RNAi mechanism to silence Rep gene transcript | Resistance against banana bunchy top virus (BBTV) | Transgenic plants were found resistant to BBTV | Shekhawat et al. (2012) | |
| 6. | Strawberry | CP gene from strawberry mild yellow edge potexvirus; Agrobacterium tumefaciens-mediated gene transfer | Coat protein-mediated virus resistance | Resistance against strawberry mild yellow edge potex virus | Transgenic lines were found resistant to SMYEPV | Finstad and Martin (1995) | |
| 7. | Lily cv. ‘Acapulco’ | A defective cucumber mosaic virus replicase gene; Agrobacterium tumefaciens-mediated gene transfer | Defective-viral genome-mediated resistance | CMV virus resistance | Four transgenic lines showed enhanced level of virus resistance | Azadi et al. (2011) | |
Abiotic stress management through transgenic approach
Abiotic stresses such as heat, drought and salinity are the major environmental constraints affecting production and productivity of almost all horticultural crops. Conventional plant breeding has not been proved that much successful in addressing abiotic stress mitigation so far. The reason might be that the traits are controlled by a number of genes present at a quantitative trait locus (QTL). To combat the negative effects of various abiotic stresses, it is pre-requisite to identify potential candidate genes or QTLs (gene networks) associated with broad-spectrum multiple abiotic stress tolerance. Various abiotic stresses including drought, high temperature, salinity, frost and flood, etc. adversely affect overall crop growth and productivity by affecting the vegetative and reproductive stages of growth and development. These stresses generally trigger a series of physiological, biochemical and molecular changes in the plants which often result in damage to the cellular machinery (Rai et al. 2011). These changes include the disruption of cellular osmotic balance leading to dysfunctional homeostasis, ion distribution and oxidative stresses which cause denaturation of integral proteins of plants. Plants respond to such stresses in a variety of mechanisms which trigger the cell signaling process, transcriptional controls and production of a number of stress conditions related tolerant proteins, antioxidants and osmotic solutes to maintain homeostasis and to protect and repair the damaged integral proteins. Generally, plants which are stress sensitive are unable to synthesize such compounds under stress conditions and, thus, are rendered liable to various stresses which hamper their overall growth. A number of genes have been identified in a number of plants/organisms, closely or distantly related, which code for the synthesis of these stress protecting compounds and thus can be targeted for genetic transformation into sensitive genotypes. Such genes have been classified into three categories as: (a) genes which code for the synthesis of various osmolytes such as mannitol, glycine betain, proline, and heat shock proteins, (b) genes responsible for ion and water uptake and transport like aquaporins and ion transporter, etc. and (c) genes regulating transcriptional controls and signal transduction mechanism, examples MAPK, DREBI, etc. Research on genetic modification of various horticultural crops for improved abiotic stress tolerance has been explored.
Drought tolerance
Various genes controlling signaling and gene regulatory pathways offer certain key targets for genetic engineering for abiotic stress tolerance. Transcription factors (TFs) that regulate or switch on the expression of a number of genes involved in imparting abiotic stress tolerance in plants have been proposed as the most efficient targets for genetic transformation (Bhatnagar-Mathur et al. 2008). These transcription factors include DREB1 gene family, Myb gene family, etc. Tsai-Hung et al. (2002) transformed tomato plants with a DNA cassette containing an Arabidopsis C repeat/dehydration-responsive element binding factor 1 (CBF1) cDNA and a nos terminator, driven by a cauliflower mosaic virus 35S promoter. These transgenic tomato plants were more resistant to water deficit stress than the wild-type plants. Pasquali et al. (2008) reported improved tolerance to cold and drought stress in transgenic apple by the over-expression of a cold-inducible Osmyb 4 gene from rice, which codes for a TF belonging to Myb family. The over-expression of DREB1b TF gene had also been reported to induce cold tolerance and drought tolerance in transgenic grapevine (Jin et al. 2009). Chrysanthemum is one of the leading ornamental cut flowers across the globe and its production is severely hampered by various environmental conditions (Gao et al. 2012). Drought stress harms this crop to the maximum extent by retarding its growth. WRKY transcription factors (TFs) work as positive or sometimes negative regulators in various abiotic stress responses in plants. Fan et al. (2016) transformed a CmWRKY1 TF derived from Chrysanthemum morifolium and over-expressed it in chrysanthemum cultivar ‘Jinba’. It was found that CmWRKY1 regulates an ABA-mediated pathway by suppressing the expression levels of various genes including PP2C, ABI1 and ABI2, and activating the expression levels of genes like PYL2, SnRK2-2, ABF4, MYB2, RAB18 and DREB1A in a positive regulation. The transgenic plants displayed increased drought tolerance in polyethylene glycol (PEG) stress as compared to control plants. Also, multiple abiotic stress tolerance in banana had been reported by the over-expression of MusaWRKY71 gene, which is a very potential abiotic stress-responsive WRKY TF gene, cloned from Musa species cv. Karibale Monthan (Shekhawat and Ganapathi 2013).
A bacterial mannitol-1-phosphate dehydrogenase (mtlD) gene driven by the constitutive cauliflower mosaic virus (CaMV) 35S promoter was transferred into tomato plants which provides improved abiotic stress tolerance in the transformed plants (Khare et al. 2010). Drought (polyethylene glycol in medium) and salinity (sodium chloride in medium) tolerance tests revealed that transgenic lines exhibited a higher tolerance for abiotic stresses than non-transformed plants. To impart tolerance to various abiotic stresses in potato, Gangadhar et al. (2016) transformed a potato-derived gene StnsLTP1 into potato (Solanum tuberosum cv. Desiree) using Agrobacterium tumefaciens-mediated genetic transformation method. Under stress conditions, transgenic potato lines displayed enhanced cell membrane integrity by reduced membrane lipid peroxidation activity and H2O2 content, comparatively. Also an increased level of antioxidant enzyme activity with enhanced accumulation of ascorbates and upregulation of various stress-related genes including StAPX, StCAT, StSOD, etc. was reported in transgenic potato plants. In an attempt to improve abiotic stress tolerance in mulberry, a very important plant of silk industry, Hva1 gene encoding for late embryogenesis abundant protein from barley, was transformed by Agrobacterium tumefaciens-mediated method (Checker et al. 2012). LEA proteins comprise a group of hydrophillins, which are induced as a response to dessication in seeds and are also stimulated under various abiotic stress conditions like dehydration, salinity, chilling or high temperature stress in vegetative tissues of plants (Khurana et al. 2008). The transgenic lines displayed an enhanced level of tolerance to drought, salinity and cold conditions than normal plants as quantified by free proline, membrane stability index (MSI) and PS II activity. Glycine betaine plays an important role in drought stress tolerance by scavenging oxidative stress-inducing molecule (free radicals) and it also protects the photosynthetic system in plants. Cheng et al. (2013) transformed choline oxidase gene (CodA) isolated from Arthrobacter globiformis, which is involved in the biosynthesis of glycine betaine, into potato cv. ‘Superior’ under an oxidative stress-inducible SWAP2 promoter for inducing drought stress tolerance trait. Under water-stress conditions, transgenic potato plants showed expression of codA gene and an accumulation of glycine betaine with a higher leaf water potential as compared to the non-transformed plants. In the stress-recovery treatment, transgenic potato plants displayed a stronger antioxidant activity, higher chlorophyll content, more efficient photosynthesis and better recovery, comparatively. Plant micro RNA (miRNA) regulates several developmental and physiological phenomena inside the plants including drought responses. Zhang et al. (2011a, b) transformed tomato with an miR 169 family member, Sly-miR169c, which can effectively down-regulate the transcripts of the target genes—three nuclear factor Y subunit genes (SINF-YA1/2/3) and one multidrug resistance-associated protein gene (SIMRP1), which are down-regulated under drought stress. The transgenic tomato plants over-expressing Sly-miR169c displayed reduced stomatal opening, reduced leaf water loss and transcription rate with enhanced drought tolerance traits.
Heat tolerance
Under heat stress, many reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide are produced inside the plant cells, leading to various kinds of physiological disorders in plants which affect crop growth and productivity. These ROS denature enzymes and damage various cellular components inside the plant cells. Tolerance to heat stress is straightway correlated with the increased capacity of plants to scavenge ROS (Chaitanya et al. 2002). Thus, it is very important to scavenge ROS to maintain normal growth and metabolism of plants. Plants have developed a variety of mechanisms to combat ROS by the production of various enzymatic systems like superoxide dismutase (SOD) to remove superoxide ions, glutathione reductase (GR) and peroxidase to scavenge peroxide ions (H2O2), etc. (Noctor and Foyer 1998). Thus, over-expression of ROS scavenging enzymes in plants via genetic transformation offers a much potential strategy to overcome heat stress. Wisniewski et al. (2002) reported over-expression of cytosolic ascorbate peroxidase (cAPX) gene improved tolerance to heat stress in transgenic apple. Wang et al. (2006) developed transgenic tomato plants which over-expressed cAPX gene with enhanced tolerance to heat (40 °C), In field tests, detached fruits from field grown transgenic tomato plants showed enhanced resistance with the exposure to direct sunlight as compared to the fruits from wild-type (non-transgenic) plants. Over-expression of Cu/Zn superoxide dismutase (Cu/Zn SOD) gene (derived from Manihot esculenta) under an oxidative stress inducible promoter SWPA2 in potato led to enhanced heat stress tolerance (Tang et al. 2006). Cu/Zn SOD is an ROS scavenging enzyme and, thus, helps in quenching of free radicals released under heat stress in plants. Transgenic plants expressed enhanced tolerance to 250 μM methyl viologen and the visible damage due to heat stress was around 25% in the transgenic plants as compared to the non-transgenic/wild-types, which were destroyed completely under heat stress.
The non-enzymatic methods involve the production of a variety of chemical compounds including polyamines, carotenoids, ascorbic acid, tocopherol, etc., which directly react with ROS, scavenge them and thus provide protection to the plants against heat stress. Polyamines play an important role in imparting thermal stress tolerance in plants. S-adenosyl-I-methionine decarboxylase (SAMDC) is one of the key regulatory target enzymes in polyamines biosynthesis. Cheng et al. (2009) over-expressed SAMDC cDNA, isolated from Saccharomyces cerevisae, in tomato plants for enhanced polyamines production. Transgenic lines produced 1.7–2.4-fold higher levels of spermidine and spermine with enhanced antioxidant enzyme activity and better protection of membrane lipid peroxidation as compared to wild-type plants, leading to enhanced tolerance to high temperature stress (38 °C). Over-expression of heat shock proteins in plants has been proposed as one of the potential strategies to combat heat stress. HSPs function as molecular chaperons, who are involved in correct protein folding, assembly, translocation, degradation and they also provide stability to integral proteins and cell membranes under heat stress (Boston et al. 1996). Song et al. (2014) over-expressed CgHSP70 gene conferring for heat tolerance in chrysanthemum. The transgenic lines exhibited an increased peroxidase (POD) activity, higher proline content and reduced malondialdehyde (MDA) content. Proline is an important osmoprotectant that protects cells from damage under heat stress and transgenic plants were better able to tolerate heat stress than wild-type plants.
Salinity tolerance
Salinity or salt stress is one of the most prevalent abiotic stresses that severely affect the quality and quantity of different horticultural produce. Around 20% of the world irrigated agricultural land is affected with salinity problem (Rengasamy 2006). Salinity tolerance is a complex mechanism governed by many genes (Bojorquez-Quintal et al. 2014). Plants which are exposed to abiotic stress conditions produce several pathogenesis-related proteins to compensate the adverse effect of stress conditions. Osmotin is one of the important pathogenesis-related proteins, which is produced by the plants to combat various biotic and abiotic stresses. Husaini and Abdin (2008) over-expressed tobacco osmotin gene in strawberry (Fragaria × ananasa Duch.) and found that the transgenic strawberry plants exhibited tolerance to salt stress. Chilli plants are not easily amenable to tissue culture and genetic transformation, thus limiting the scope of genetic improvement for various biotic and abiotic stresses (Kothari et al. 2010). Subramanyam et al. (2011) could successfully improve the tolerance of chilli pepper (Capsicum annum L. cv. Aiswarya 2103) plants by the ectopic expression of tobacco osmotin gene via Agrobacterium tumefaciens-mediated gene transfer technique. T2 generation of transgenic pepper plants revealed enhanced levels of chlorophyll, proline, glycine betaine, ascorbate peroxidase (APX), superoxide dismutase (SOD), glutathione reductase (GR) and relative water content (RWC) in biochemical analysis and survived in salinity level up to 300 mM NaCl concentration. In comparison to other horticultural crops, citrus species are the most sensitive to soil salinity, which greatly limit growth and productivity of citrus crops across the globe. Cervera et al. (2000) transformed Carrizo citrange, an excellent rootstock of citrus with a yeast-derived halotolerance gene, HAL 2, which is involved in salt tolerance mechanism. HAL2 gene is involved in the methionine biosynthetic pathway and confers tolerance to lithium and sodium ions. It encodes for a salt-sensitive biphosphate nucleotidase, which is required for sulfate accumulation. The transgenic lines expressing HAL2 protein showed improved tolerance to salinity than the wild-type plants. Tomato is considered as one of the most important vegetable crops worldwide for the commercial value it offers. Wang et al. (2005) developed transgenic tomato plants expressing tolerance to chilling and salt stress by incorporation of cytosolic ascorbate peroxidase (cAPX) gene, derived from pea (Pisum sativum L.). Ascorbate peroxidase plays a key role in quenching hydrogen peroxide (H2O2) in plant cells, thus providing protection against oxidative injury induced by chilling and salt stress. The transgenic plants showed better seed germination rate (26–37%) than the wild type (3%) when the seeds were placed at 9 °C for 5 weeks. APX activity was found 10–25 folds higher in transgenic plants under salinity stress (200–250 mM) conditions, thus ensuring minimum damage to the leaves comparatively.
Various abiotic stresses including salinity, chilling and oxidative stresses are the critical factors limiting the cultivation and productivity of sweet potato (Ipomoea batatas), a root vegetable crop. It has been observed that the increased production of glycine betaine in plant cells improves their tolerance level towards these stresses. Fan et al. (2012) transformed sweet potato cv. Sushu-2 with a chloroplastic betaine aldehyde dehydrogenase (SoBADH) gene from Spinacia oleracea, which is involved in the biosynthesis of glycine betaine. The over-expression of SoBADH gene in transgenic sweet potato improved tolerance towards salinity, oxidative stress and low temperature by providing protection against cell damage by maintaining cell membrane integrity, stronger photosynthetic activity, reduced ROS production and activation of ROS scavenging mechanism. To enhance the tolerance of tomato plants to salinity stress, Lim et al. (2016) transformed a strawberry D-galacturonic acid reductase (GalUR) gene into cherry tomato (Solanum lycopersicum) lines to increase the ascorbic acid content. Transgenic tomato plants enriched with high fruit ascorbic acid contents had been found more tolerant to abiotic stress induced by viologen, NaCl and mannitol as compared to the wild-type plants. The transgenic events could survive at a salt stress up to 200 mM and also showed higher expression levels of antioxidant genes including APX and CAT, responsible for imparting additional capabilities to the transgenic plants for salt tolerance. Under high salinity stress conditions, ion-homeostasis within the plant cells get disturbed altering the overall metabolism. Bulle et al. (2016) developed transgenic chilli pepper (Capsicum annuum) plants expressing wheat Na+/H+ antiporter gene (TaNHX2) to develop tolerance towards salinity stress. Transgene integration and expression were confirmed by PCR, Southern hybridization and RT-PCR in T1 generation. In biochemical assays, transgenic lines gave enhanced levels of proline, chlorophyll, superoxide dismutase, ascorbate peroxidase, relative water content and reduced level of H2O2 and malondialdehyde as compared to the non-transformed plants under salt stress conditions. Over-expression of TaNHX2 gene has already been evaluated in tomato to combat salinity stress (Yarra et al. 2012). To improve salt tolerance in bottle gourd, Han et al. (2015) transformed a bottle gourd line ‘G5’ with Arabidopsis thaliana-derived H+-pyrophosphatase AVP gene. The AVP1-expressing transgenic lines exhibited an improved salt tolerance and maintained higher relative water content under salt stress regime in glasshouse. When watermelon plants were grafted onto the transgenic bottle gourd root stock, they also exhibited greater salt tolerance generating higher biomass and photosystem II quantum yields.
Herbicide tolerance
Herbicide tolerance in bedding plants can be expected to significantly reduce the cost of weeding in a landscape environment. The herbicide glyphosate is a potent inhibitor of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSP) in higher plants. A complementary DNA (cDNA) clone encoding EPSP synthase was isolated from a complementary DNA library of a glyphosate-tolerant Petunia hybrida cell line (MP4-G) that overproduces the enzyme. This cell line was shown to overproduce EPSP synthase messenger RNA as a result of a 20-fold amplification of the gene. A chimeric EPSP synthase gene was constructed with the use of the cauliflower mosaic virus 35S promoter to attain high-level expression of EPSP synthase and introduced into petunia cells. Transformed petunia cells as well as regenerated transgenic plants were found tolerant to glyphosate (Shah et al. 1986). Transgenic pineapple plants transformed with the bar gene for bialaphos resistance were developed (Sripaoraya et al. 2006) and evaluated for tolerance to herbicide Basta. Seven months after transfer to the field, plants were found tolerant to 1600 ml/rai of the herbicide Basta® X (stock concentration 15% w/v glufosinate ammonium), this being twice the dose recommended for field application of the herbicide. Transgenic plants tolerant to Glufosinate ammonium should facilitate more effective weed control in pineapple plantations without damage to the crop. Transgenic development work in various horticultural crops for imparting abiotic stress tolerance/resistance has been summarized in Table 2.
Table 2.
Transgenic horticultural crops for abiotic stress tolerance/management
| S. no. | Crop species/cultivar | Gene and genetic transformation method used | Mechanism of action | Target trait | Trait improvement | References |
|---|---|---|---|---|---|---|
| (A) Drought tolerance | ||||||
| 1. | Apple | Osmyb4; Agrobacterium tumefaciens-mediated gene transfer | A Myb family transcription factor, leads to accumulation of various solutes compatible to abiotic stress tolerance | Drought and cold tolerance | Enhanced tolerance to drought and low temperature stress in transgenic plants | Pasquali et al. (2008) |
| MdC1PK61; Agrobacterium tumefaciens-mediated gene transfer | Synthesizes a CBL-interacting protein kinase (C1PK) | Salt, drought and chilling tolerance | Enhanced tolerance to drought and low temperature and salt stress in transgenic plants | Wang et al. (2012) | ||
| 2. | Banana | MusaWRKY71; Agrobacterium tumefaciens-mediated gene transfer | Encodes a WRKY transcription factor | Multiple abiotic stress tolerance | Enhanced tolerance to drought, salinity and high temperature | Shekhawat and Ganapathi (2013) |
| MusaSAP1; Agrobacterium tumefaciens-mediated gene transfer | Encodes for stress associated proteins (SAPs) | Multiple abiotic stress tolerance | Enhanced tolerance to drought, salinity and high temperature | Sreedharan et al. (2012) | ||
| 3. | Citrus | P5CS gene; Agrobacterium tumefaciens-mediated gene transfer | Encodes for the biosynthesis of proline | Drought tolerance | Enhanced tolerance to drought and low temperature stress in transgenic plants | De Carvalho et al. (2013) |
| P5CSF129A; Agrobacterium tumefaciens-mediated gene transfer | Provides protection against reactive oxygen species(ROS) by altering enzymes activity | Drought tolerance | Enhanced tolerance to drought and low temperature stress in transgenic plants | De Campos et al. (2011) | ||
| 4. | Strawberry | Osmotin; Agrobacterium tumefaciens-mediated gene transfer | Increase the level of proline accumulation | Salinity tolerance | Enhanced tolerance to salinity stress in transgenic plants | Husaini and Abdin (2008) |
| 5. | Mulberry cv. K2 | Osmotin; Agrobacterium tumefaciens-mediated gene transfer | Increase the level of proline accumulation | Salinity and drought tolerance | Enhanced tolerance to drought and salinity stress in transgenic plants | Das et al. (2011) |
| 6. | Chrysanthemum (Chrysanthemum morifolium) cv. ‘Jinba’ | CmWRKY1 transcription factor (derived from C. morifolium); Agrobacterium tumefaciens-mediated gene transfer | CmWRKY1 works as positive regulator in drought stress | Drought stress tolerance | The transgenic plants displayed increased drought tolerance in PEG stress as compared to control plants | Fan et al. (2016) |
| 7. | Tomato (Solanum lycopersicum cv. Aika Craig) | Sly-miR169c, an miR169 family member; Agrobacterium tumefaciens-mediated gene transfer | Down regulates the transcripts of target genes namely; three nuclear factor Y subunit genes (S1NF-YA1/2/3) and one multidrug resistance-associated protein (slMRP1) gene | Drought tolerance | Transgenic plants exhibited reduced stomatal opening, decreased transpiration rate, lowered leaf water loss and enhanced drought tolerance | Zhang et al. (2011a, 2011b) |
| 8. | Potato cv. Superior | Cod A gene (from Arthrobacter globiformis); Agrobacterium tumefaciens-mediated gene transfer | Cod A gene codes for glycine betaine, which scavenges oxidative stress-inducing molecule (free radicals) and it also protects the photosynthetic system in plants | Drought tolerance | The transgenic potato plants displayed a stronger antioxidant activity, higher chlorophyll content, more efficient photosynthesis and better recovery, comparatively | Cheng et al. (2013) |
| 9. | China rose (Rosa chinensis) | RcXET and MtDREBIC genes; Agrobacterium tumefaciens-mediated gene transfer | XET and DREBIC genes are up-regulated in response to various abiotic stresses and imparts tolerance to the plant cells | Freezing and drought tolerance | Increased EC%, proline content, soluble sugar, photosynthesis rate, negative water potential and turgor loss point in transgenic plants leading to a better tolerance towards drought and freezing | Chen et al. (2016) |
| (B) Heat tolerance | ||||||
| 1. | Tomato (Solanum lycopersicum) | Yeast SAMDC gene; Agrobacterium tumefaciens-mediated gene transfer | SAMDC (S-adenosyl-methionine decarboxylase) is one of the key enzymes involved in the biosynthesis of polyamines, which protect the plants against heat stress | Heat stress | Transgenic plants produced 1.7–2.4 times, higher level of spermidine and spermine than the wild-type plants and expressed tolerance to heat stress with enhanced antioxidant enzyme activity and protection of membrane lipid peroxidation | Cheng et al. (2009) |
| Tomato cv. Zhongshu No. 5 | cAPX gene; Agrobacterium tumefaciens-mediated gene transfer | APX encodes for antioxidant enzyme which removes H2O2, a reactive oxygen species (ROS) produced under heat stress | UV-B and heat stress tolerance | Transgenic plants and fruits expressed enhanced tolerance to heat (40 °C) and UV-B stress as compared to wild-type plants | Wang et al. (2006) | |
| 2. | Chrysanthemum morifolium cv. ‘Zhongshanzigui’ | CgHSP70 gene (from C. morifolium); Agrobacterium tumefaciens-mediated gene transfer | HSPs function as molecular chaperons, which are involved in correct protein folding, assembly, translocation, degradation and they also provide stability to integral proteins and cell membranes under heat stress | Heat tolerance | The transgenic lines exhibited an increased peroxidase (POD) activity, higher proline content and reduced malondialdehyde (MDA) content and were better able to tolerate heat stress than wild-type plants | Song et al. (2014) |
| 3. | Potato (Solanum tuberosum) cv. Desiree | StnsLTP1 gene (from potato); Agrobacterium tumefaciens-mediated gene transfer | StnsLTPI gene imparts tolerance to various abiotic stresses as a result of enhanced activation of antioxidative defense mechanisms via cyclic scavenging of reactive oxygen species (ROS) and co-coordinating the expression of stress related genes | Heat, drought and salinity tolerance | Transgenic potato lines displayed enhanced cell membrane integrity and an increased level of antioxidant enzyme activity with enhanced accumulation of ascorbates and upregulation of various stress related genes including StAPX, StCAT, StSOD etc. under stress conditions | Gangadhar et al. (2016) |
| Potato | Cu/Zn SOD (from Manihot esculenta); Agrobacterium tumefaciens-mediated gene transfer | ROS scavenging enzyme and thus helps in quenching of free radicals released under heat stress in plants | Heat stress | Transgenic plant expressed enhanced tolerance to 250 μM methyl viologen and reduced visible damage due to heat stress | Tang et al. (2006) | |
| (C) Salinity tolerance | ||||||
| 1. | Apple | MdNHX1 gene; Agrobacterium tumefaciens-mediated gene transfer | Acts as Na+/H+ antiporter | Salinity tolerance | Enhanced tolerance to salinity stress in transgenic plants | Li et al. (2010) |
| 2. | Banana | MusaDHN-I gene; Agrobacterium tumefaciens-mediated gene transfer | Over-expression of dehydrin gene and a LEA protein | Drought and salinity | Enhanced tolerance to drought and salinity stress | Shekhawat et al. (2011) |
| 3. | Kiwi | AtNHX1 gene; Agrobacterium tumefaciens-mediated gene transfer | Keeps K+/Na+ ratio high during salinity stress conditions | Salinity | Enhanced tolerance to salinity stress in transgenic plants | Tian et al. (2011) |
| 4. | Pear | SAMDC2 gene; Agrobacterium tumefaciens-mediated gene transfer | Encodes for the biosynthesis of polyamines | Salinity | Enhanced tolerance to salinity stress in transgenic plants | He et al. (2013) |
| Pear | SPDS gene; Agrobacterium tumefaciens-mediated gene transfer | Encodes for the biosynthesis of polyamines | Salinity | Enhanced tolerance to salinity stress in transgenic plants | Wen et al. (2010) | |
| 5. | Tomato (Solanum lycopersicum) cv. Zhongshu No. 5 | cAPX gene; Agrobacterium tumefaciens-mediated gene transfer | Ascorbate peroxidase plays a key role in quenching hydrogen peroxide (H2O2) in plant cells, thus providing protection against oxidative injury induced by chilling and salt stress | Chilling and salinity | The transgenic plants showed better seed germination rate (26–37%) than the wild type (3%) when the seeds were placed at 9 °C for 5 weeks and higher APX activity under salinity stress (200–250 mM) conditions | Wang et al. (2005) |
| Cherry tomato—C, H and F lines | GalUR gene (D-galacturonic acid reductase gene), derived from strawberry; Agrobacterium tumefaciens-mediated gene transfer | GalUR gene codes for higher level of ascorbic acid biosynthesis, which imparts tolerance to salinity stress | Salinity stress | Transgenic tomato plants were found more tolerant to abiotic stress-induced viologen, Nacl and mannitol and with higher expression levels of APX and CAT, responsible for imparting additional capabilities for salt tolerance | Lim et al. (2016) | |
| Tomato | Na+/H+ antiporter gene (TaNHX2) from wheat; Agrobacterium tumefaciens-mediated gene transfer | TaNHX2 gene leads to the production of osmolytes to maintain the cell membrane stability | Salinity tolerance | Better salinity tolerance in transgenic plants comparatively | Yarra et al. (2012) | |
| 6. | Citrus (Carrizo citrange root stock) | Yeast HAL2 gene; Agrobacterium tumefaciens-mediated gene transfer | HAL-2 gene is involved in the pathway of methionine biosynthesis and confers tolerance to Lithium and Sodium. | Salinity tolerance | Transgenic plants showed better tolerance to salt stress than non-transgenic plants | Cervera et al. (2000) |
| 7. | Sweet potato (Ipomoea batatas) cv. Sushu-2 | SoBADH (Spinacia oleracea derived betaine aldehyde dehydrogenase gene); Agrobacterium tumefaciens-mediated gene transfer | Glycine betaine protects the plant cells from abiotic stress by providing protection against cell damage by maintaining cell membrane integrity, stronger photosynthetic activity, reduced ROS production and activation of ROS scavenging mechanism | Salinity, oxidative stress and chilling/cold stress | The transgenic plants improved tolerance towards salinity, oxidative stress and low temperature | Fan et al. (2012) |
| 8. | Chilli pepper (Capsicum annum L.) cv. Aiswarya 2103 | Osmotin gene; Agrobacterium tumefaciens-mediated gene transfer | Over-expression of osmotin gene induces biosynthesis of proline and confers tolerance to osmotic stress | Salinity tolerance | Transgenic pepper plants could survive salinity level up to 300 mM Nacl concentration | Subramanyam et al. (2011) |
| Chilli pepper cv. Q4 | Na+/H+ antiporter gene (TaNHX2) from wheat; Agrobacterium tumefaciens-mediated gene transfer | TaNHX2 gene leads to the production of osmolytes to maintain the cell membrane stability | Salinity tolerance | Transgenic lines revealed enhanced levels of proline, chlorophyll, superoxide dismutase, ascorbate peroxidase, relative water content and reduced level of H2O2 and malondialdehyde as compared to the non-transformed plants under salt stress conditions | Bulle et al. (2016) | |
| 9. | China rose (Rosa chinensis) | AtDREB2A-CA gene; Agrobacterium tumefaciens-mediated gene transfer | Over-expression of AtDREB2A-CA gene enhances salinity stress tolerance in Chinese rose by altering the leaf ultra structure in response to salt stress | Salinity stress | Enhanced salinity tolerance in transgenic plants | Josine et al. (2015) |
| 10. | Chrysanthemum morifolium | CmWRKY17 transcription factor; Agrobacterium tumefaciens-mediated gene transfer | WRKY transcription factors work sometime as positive and sometime as negative regulators in a variety of abiotic stress responses in plants | Salinity stress | Over-expression of CmWRKY17 TF in chrysanthemum increased plants sensitivity to salinity stress | Li et al. (2015) |
| 11. | Mulberry (Morus indica) cv. K-2 | HvaI gene (from barley); Agrobacterium tumefaciens-mediated gene transfer | LEA proteins (encoded by HvaI gene) protect cells against abiotic stresses by sequestering ions, stabilization of macro molecules and membranes, and act as antioxidants | Salinity, drought and cold tolerance | The transgenic lines displayed an enhanced level of tolerance to drought, salinity and cold conditions than normal plants as quantified by free proline, membrane stability index (MSI) and PSII activity | Checker et al. (2012) |
| 12. | Bottle gourd line ‘G5′ | AVP1 gene derived from Arabidopsis thaliana; Agrobacterium tumefaciens-mediated gene transfer | AVP1 gene is involved in imparting salt tolerance | Salinity tolerance | The transgenic lines exhibited an improved salt tolerance and maintained higher relative water content under salt stress regime in glasshouse | Han et al. (2015) |
| (D) Herbicide tolerance | ||||||
| 1. | Petunia hybrida | EPSP synthase gene; Agrobacterium tumefaciens-mediated gene transfer | Over-production of the enzyme renders inhibition by glyphosate ineffective | Glyphosate tolerance | Transformed petunia cells as well as regenerated transgenic plants were found tolerant to glyphosate | Shah et al. (1986) |
| 2. | Pineapple | Bar gene; Particle bombardment -mediated gene transfer | Bar gene codes for enzyme PAT, which acetylates bialaphos rendering it inactive | Bialaphos resistance/tolerance | Transgenic plants were found tolerant to 1600 ml/rai of the herbicide Basta® X (stock concentration 15% w/v glufosinate ammonium), this being twice the dose recommended for field application of the herbicide | Sripaoraya et al. (2006) |
| 3. | Potato | Bar gene; Agrobacterium tumefaciens-mediated gene transfer | Bar gene codes for enzyme PAT, which acetylates bialaphos rendering it inactive | Bialaphos resistance/tolerance | Transgenic potato expresses tolerance to bialaphos | Khan et al. (2008) |
Enhancing fruit quality for increased shelf-life and reduced post-harvest losses
Excessive softening is the main factor limiting fruit shelf-life and storage. Transgenic plants modified for the expression of cell wall modifying enzymes have been used to investigate the role of particular activities in fruit softening during ripening. Fruit ripening has been modified by altering the activity of cell wall enzymes such as polygalacturonases that are involved in tissue softening and deterioration. The biosynthesis of ethylene, the fruit ripening hormone, has also been blocked in several ways to delay fruit ripening. Calgene Inc., USA (1994) developed the first commercialized transgenic plant, a long shelf-life tomato by the suppression of polygalacturonase (PG) gene by antisense strategy (Smith et al. 1988). PG gene encodes for polygalacturonase enzyme which degrades pectin, the major component of fruit cell wall. Calgene Inc. has given the brand name ‘Mac Gregor’ to its transgenic tomato and the fruits of this plant had enhanced shelf-life for approximately 2 weeks longer without softening. The Flavr Savr tomatoes have improved flavor and total soluble solids (TSS), in addition to the enhanced shelf-life. However, this Flavr Savr variety was withdrawn from the market three years later because of its disease susceptibility and lack of productivity. The plant hormone ethylene is involved in senescence in many flowers and fruits and their vase life can be extended by either blocking ethylene biosynthesis or ethylene reception (Bovy et al. 1999). Later on, other tomato varieties with increased shelf-life were developed through antisense RNA inhibition of ACC synthase or ACC oxidase, two ethylene precursors. Delayed leaf senescence has been achieved in tobacco and petunia by manipulation of cytokinin biosynthesis (Clark et al. 2003). Researchers at the Horticultural Research International, the United Kingdom, have identified the genes which control the taste, smell and color of strawberries. As a result, it would now be possible to create super strawberries that taste sweeter using transgenic approaches. Blackheart is a fruit defect caused by exposure of pineapples to higher temperatures which stimulates polyphenol oxidase (PPO) activity. Stewart et al. (2001) cloned a PPO gene from pineapple fruits under conditions that produce blackheart. The PPO gene has been silenced in transformed plants and transgenic plants are under field evaluation. Also, Park et al. (2005) demonstrated that fruit from tomato plants expressing Arabidopsis thaliana H+/cation exchanger (CAX) gene has more calcium (Ca2+) and prolonged shelf-life when compared to controls. Nambeesan et al. (2010) expressed a yeast spermidine synthase (ySpdSyn) gene under constitutive (CaMV35S) promoter and fruit-ripening specific (E8) promoter in Solanum lycopersicum (tomato). The ySpdSyn transgenic fruits had a longer shelf-life, reduced shriveling and delayed decay symptom development in comparison with the wild-type (WT) fruits. Crop maturity indicated by the percentage of ripening fruits on the vine was delayed in a CaMV35S-ySpdSyn genotype, with fruits accumulating higher levels of the antioxidant lycopene. Notably, whole plant senescence in the transgenic plants was also delayed compared with wild-type plants. Sajeevan et al. (2017) over-expressed AtSHN1, a transcription factor associated with epicuticular wax biosynthesis to increase leaf surface wax load in mulberry. It has been proposed that leaf surface waxes contribute for cuticular resistance, protect mesophyll cells from dessication and, thus, reduce post-harvest water losses. Transgenic mulberry plants expressing AtSHN1 gene displayed dark green shiny appearance with increased leaf surface wax content and showed significant improvement in leaf moisture retention capacity even 5 h after the harvest as compared to wild-type plants.
Recombinant-DNA technology also finds its multifaceted applications in improvement of the nutritional quality of fruits. To improve the vitamin C accumulation in fruits and make them more health supportive to the consumers, Zhang et al. (2011a, b) used RNAi technology to silence the expression of a mitochondrial APX gene in tomato fruit. Ascorbate peroxidase (APX) oxidizes the reduced l-ascorbic acid in ascorbate metabolic pathway and, thus, reduces vitamin C content. The mitochondrial APX enzyme activity in transgenic lines was reduced by 0.4–0.8 times than the non-transformed plants and this led to 1.4–2.2 times increase in ascorbic acid content in tomato fruits. Altering the fruit texture leading to softening of fruits is a desirable trait from the processing point of view. Kaur et al. (2010) transformed tomato cv. Pusa Uphar with a fruit-specific expansion gene, LeEXP1 under the control of a fruit-specific promoter LeACS4. The over-expression of LeEXP1 gene resulted in enhanced fruit softening, as determined by force required to rupture the fruit pericarp than the non-transgenic plants with increased red coloration of fruits at different stages of ripening. This trait may find usefulness in tomato processing industry. Behboodian et al. (2012) silenced the expression of ACC oxidase (ACO1) gene in tomato using RNAi approach to lower down ethylene production, which is involved in regulating fruit ripening and flower senescence. The transgenic tomato lines harboring the hpRNA-ACO1 gene exhibited lower ethylene production and a longer fruit shelf-life of 32 days as compared to 10 days for wild-type fruits. Further, fruits of transgenic tomato demonstrated reduced level of firmness loss as a result of decrease in pectin methylesterase (PME) and polygalacturonase (PG) activities.
Color enhancement and increasing vase life in various ornamental crops
Genetic engineering techniques had so far limited impact in ornamental horticulture field. However, ornamental horticulture and particularly floriculture are very well suited to the approach of genetic engineering technology. The primary focus on color modification is important in cut flowers because flower color is an important driver of new variety development. Ornamental plants generally have eye-catching attraction and aesthetic importance. Altering flower color or plant architecture has been an important area of work for all the floriculturists. Several ornamental plants, including carnation, rose and gerbera, have been engineered for modified flower color. The primary color imparting pigments present in flowers are a wide class of flavonoids, carotenoids and betains, which are the targets of genetic engineering for color alterations/modifications. Flower color modification venture includes modifying the flavonoid biosynthetic pathway through the introduction of new genes, over-expression of certain key regulatory genes or by silencing the expression of those target genes by co-suppression strategy, antisense gene technology or by RNAi technique. Research has been focused on the manipulation of either anthocyanins (red and blue colors) or carotenoids (yellow and orange colors), with the intent of creating a wider range of flower colors than occurs naturally, as well as to produce natural dyes for industrial purposes (Lu et al. 2003). The first application of genetic engineering to modify flower color led to the production of an orange pelargonidin-producing Petunia variety, which produced flowers with pale brick color. This was achieved by the expression of dihydroflavonol-4 reductase (dfr) gene from maize in a petunia line (Meyer et al. 1987). Chalcone synthase (Chs) is another gene which had been used for production of pink, white and variegated flowers (sense and antisense genes were used) in petunias, chrysanthemum, gerbera and roses (van der Krol et al. 1988). Transgenic violet carnations have been successfully produced with the introduction of F3′5′H gene from Petunia hybrida which encodes a flavonoid required for the biosynthesis of delphinidin (Holton et al. 1993). The vase life of flowers can be altered by manipulating the biosynthesis of ethylene. The enzymes ACC synthase and ACC oxidase are encoded by genes acs and aco, respectively, and both have been cloned from many species including carnation. Transgenic carnation having antisense aco gene had been produced and exhibited longer vase life. Flowers of the transgenic carnation plants exhibited low climacteric ethylene production and a markedly delayed petal senescence (Savin et al. 1995).
Till date, the only genetically modified product commercialized on a significant scale are the color modified carnations developed in a joint venture by Suntory Ltd. and Florigene Ltd. Florigene is selling transgenic Moon series carnations engineered for dark violet–purple color around the world. The varieties are developed in Australia and flowers are produced primarily in South America for marketing in the United States and Japan. In another study, fruit-specific RNAi-mediated suppression of a photomorphogenesis regulatory gene (DETI) was reported to enhance the carotenoid and flavonoid content in tomatoes (Davuluri et al. 2005). Recently, in 2009, transgenic blue roses had been developed by two companies, namely Florigen Ltd. and Suntory Ltd. in Australia. A package of three genes was transferred to red rose plants as; a synthetic RNAi gene that switches off the red rose dihydroflavonol reductase (DFR) gene, a delphinidin gene from blue pansy and a DFR gene from iris that had an affinity for producing delphinidin (Katsumoto et al. 2007). The resultant roses exclusively accumulated delphinidin in the petals, and the flowers had blue hues, not so far achieved by hybridization breeding. Worldwide, chrysanthemum is considered as one of the most economically valuable flowers. Research efforts have been focused on improving various traits in chrysanthemum including flower color and architectural variants. Chrysanthemums are typically used as cut flowers or potted plants. Obtaining bright red and blue colored flowers has always been the charm of chrysanthemum growers. In an attempt to develop red colored flowers, He et al. (2013) down-regulated CmF3′H gene using RNAi approach and over-expressed the Senecio cruentus F3′5′H (PCFH) gene in chrysanthemum. The transgenic chrysanthemum plants developed bright red flowers and exhibited a significantly increased cyanidin content.
Modification of plant architecture
Genetic engineering has succeeded in relevance to modifying plant architecture to satisfy the instinct of beauty of mankind. Ornamental plants are being grown for the purpose of beautifying, embellishing or improving human environments. Differential expression of a number of diverse genes in different backgrounds has led to diversification in plant forms. Reduction in height is a major consideration during commercial production of chrysanthemum. In an attempt to reduce plant height, Zheng et al. (2001) ectopically expressed a tobacco phytochrome B1 (PHY-B1) gene in ‘Iridon’ chrysanthemum under the control of CaMV35S promoter. The transgenic plants developed a short stature as compared to the wild-type plants looking similar to that caused by the application of a commercial growth retardant. The leaves of transgenic plants developed more intense color inferring the biosynthesis of higher levels of chlorophyll pigment. Later in 2003, Petty et al. reported decrease in chrysanthemum plant height by introducing Arabidopsis GA insensitive (gai) gene. In another study, Aida et al. (2008) transferred CAG gene in antisense orientation into Chrysanthemum morifolium and observed alteration in gynoecium and androecium to corolla-like tissues. Khodakovskaya et al. (2009) developed transgenic chrysanthemum by transforming ipt (isopentyl transferase) gene, a cytokinin biosynthetic gene, in an attempt to change the plant architecture. The transgenic plants were found shorter in height with more number of flowers, but with smaller size and late flowering period than the wild-type plants. In another study, Jiang et al. (2010) reported lessened branches formation in transgenic chrysanthemum transformed with DgLsL gene in antisense orientation. Dwarfed transgenic plants of petunia had also been developed by transforming gai (gibberellic acid insensitive) gene from Arabidopsis thaliana by Tanaka et al. (2005). Further, Han et al. (2007) reported that Ls-like sense gene expression in transgenic carnation resulted in lack of axillary branches formation. Cultivars of many crops having shorter stems generally exhibit higher harvest index, offering additional commercial advantage to the growers. In another study, the ectopic expression of a PHYA gene in potato plants significantly inhibited stem elongation and increased the harvest index through hypersensitivity to far red (FR) light (Robson et al. 1996). The potential applications of transgenic technology for improving quality, color, texture, shelf-life and plant architecture of horticultural crops have been compiled in Table 3.
Table 3.
Transgenic horticultural crops for improvement of other desirable traits
| S. No. | Crop species/cultivar | Gene and genetic transformation method used | Mechanism of action | Target trait | Trait improvement | References |
|---|---|---|---|---|---|---|
| (A) Modification of fruit quality, increasing shelf-life and reducing post-harvest losses | ||||||
| 1. | Tomato | Polygalacturonase gene in antisense orientation; Agrobacterium tumefaciens-mediated gene transfer | PG gene encodes for polygalacturonase enzyme which degrades pectin, the major component of fruit cell wall, its antisense expression inhibits that activity | To increase shelf-life of tomato fruit | The fruits of transgenic tomato got shelf for approx. 2 weeks longer without softening and developed improved flavor and total soluble solids (TSS) | Smith et al. (1988) |
| Tomato | Arabidopsis thaliana H+/cation exchanger (CAX) gene; Agrobacterium tumefaciens-mediated gene transfer | Increased expression of CAX gene in plants causes dramatic increase in Ca2+ ions leading to increased shelf-life | Prolonged shelf-life | Fruit from transgenic tomato plants had more calcium (Ca2+) and prolonged shelf-life when compared to controls | Park et al. (2005) | |
| Tomato | Yeast spermidine synthase (ySpdSyn) gene; Agrobacterium tumefaciens-mediated gene transfer | Polyamines, particularly Spd aids in increasing fruit shelf-life probably by reducing post-harvest senescence and decay | Increased shelf-life | The ySpdSyn transgenic fruits had a longer shelf-life, reduced shriveling and delayed decay symptom development in comparison to the wild-type | Nambeesan et al. (2010) | |
| Tomato | RNAi based silencing of mitochondrial APX gene; Agrobacterium tumefaciens-mediated gene transfer | Ascorbate peroxidase (APX) oxidizes the reduced l-ascorbic acid in ascorbate metabolic pathway and thus reduces vitamin C content | Increased vitamin content | The mitochondrial APX enzyme activity in transgenic lines was reduced by 0.4–0.8 times than the non-transformed plants and this led to 1.4–2.2 times increase in ascorbic acid content in tomato fruits | Zhang et al. (2011a, b) | |
| Tomato cv. Pusa Uphar | LeEXP1 gene; Agrobacterium tumefaciens-mediated gene transfer | Over-expression of LeEXP1 gene influence fruit texture and juice viscosity in tomato and thus assists in fruit softening | Enhanced fruit softening | The over-expression of LeEXP1 gene resulted in enhanced fruit softening. This trait may find usefulness in tomato processing industry | Kaur et al. (2010) | |
| Tomato | RNAi-mediated suppression of DET1 gene; Agrobacterium tumefaciens-mediated gene transfer | DETI is a phytomorphogenesis regulatory gene | To improve carotenoid and flavonoid content in fruits | Enhanced carotenoid and flavonoid content in transgenic tomato fruits | Davuluri et al. (2005) | |
| Tomato cv. MI1 | RNAi gene construct of ACO1 gene; Agrobacterium tumefaciens-mediated gene transfer | To inhibit ethylene production | To increase shelf-life | The transgenic tomato lines exhibited lower ethylene production and a longer fruit shelf-life of 32 days as compared to 10 days for wild-type fruits | Behboodian et al. (2012) | |
| Tomato cv. Micro-Tom and Ailsa Craig | SlIAA-9 gene; Agrobacterium tumefaciens-mediated gene transfer | Genome editing to bring targeted mutagenesis | To introduce parthenocarpy | To mutants showed morphological changes in leaf shape and seedless fruit, which is a characteristic of parthenocarpic tomato | Ueta et al. (2017) | |
| 2. | Watermelon (Citrullus lanatus) | CIPDS gene; Agrobacterium tumefaciens-mediated gene transfer | Genome editing to bring targeted mutagenesis in CIPDS gene by CRISPR/Cas9 | To develop CIPDS gene knockouts for albino phenotype | All the transgenic watermelon plants harbored CIPDS mutations and developed clear or mosaic albino phenotype | Tian et al. (2017) |
| (B) Color enhancement and increasing vase life in various ornamental crops | ||||||
| 1. | Petunia | acc and aco genes in antisense orientation; Agrobacterium tumefaciens-mediated gene transfer | Inhibition of ethylene | Delayed leaf senescence | Delayed leaf senescence in transgenic petunia than wild-type | Clark et al. (2003) |
| Petunia | dfr gene from maize; Agrobacterium tumefaciens-mediated gene transfer | dfr gene from maize is capable of synthesizing pelargonidin pigment, thus giving brick red colored flowers | Color modification | Transgenic orange pelargonidin-producing Petunia variety, which produced flowers with pale brick color | Meyer et al. (1987) | |
| 2. | Petunia, Carnation, Gerbera and Rose | chs gene in sense and antisense orientation; Agrobacterium tumefaciens-mediated gene transfer | Alteration in flavonoid biosynthetic pathway | Color modification | Development of pink, white and variegated flowers in transgenic petunia, chrysanthemum, gerbera and rose | van der Krol et al. (1988) |
| 3. | Carnation | F3′5′H gene from Petunia hybrida; Agrobacterium tumefaciens-mediated gene transfer | F3′5′H gene encodes a flavonoid required for the biosynthesis of delphinidin | Color modification | Transgenic violet carnations | Holton et al. (1993) |
| Carnation | Antisense aco gene; Agrobacterium tumefaciens-mediated gene transfer | aco gene is involved in ethylene biosynthesis | Increased vase life | Flowers of the transgenic plants exhibited low climacteric ethylene production and a markedly delayed petal senescence | Savin et al. (1995) | |
| 4. | Red rose | A package of 3 gene viz, DFR gene in RNAi vector, delphinidin gene from blue pansy and a DFR gene from iris; Agrobacterium tumefaciens-mediated gene transfer | Synthetic RNAi gene switches off the red rose dihydroflavonol reductase (DFR) gene, delphinidin gene and DFR gene have an affinity for producing delphinidin | Modified flower color | Transgenic roses exclusively accumulated delphinidin in the petals, and the flowers had blue hues | Katsumoto et al. (2007) |
| 5. | Chrysanthemum morifolium | RNAi suppression of CmF3′H gene and over-expression of Senecio cruentus F3′5′H gene; Agrobacterium tumefaciens-mediated gene transfer | Delphinidin biosynthetic pathway modification leading to increased synthesis of cyanidin and thus flower color modification | Flower color modification | The transgenic chrysanthemum plants developed bright red flowers and exhibited a significantly increased cyanidin content | He et al. (2013) |
| (C) Modification of plant architecture | ||||||
| 1. | Chrysanthemum | ipt gene; Agrobacterium tumefaciens-mediated gene transfer | ipt (isopentyl transferase) gene is a cytokinin biosynthetic gene | Modified plant architecture | The transgenic plants were found shorter in height with more number of flowers, but with smaller size and late flowering period than the wild-type plants | Khodakovskaya et al. (2009) |
| Chrysanthemum | gai gene from Arabidopsis thaliana; Agrobacterium tumefaciens-mediated gene transfer | gai gene inhibits the effect of gibberellic acid in plants and thus results in dwarfism | Modified plant architecture | Decrease in transgenic chrysanthemum plant height | Petty et al. (2003) | |
| Chrysanthemum cv. Iridon | Tobacco phytochrome B1 (PHYB1) gene; Agrobacterium tumefaciens-mediated gene transfer | The ectopic expression of the tobacco PHYB1 gene increases the sensitivity of plants to red wavelengths of light, resulting in the inhibition of stem elongation | Reduction in plant height | The transgenic plants developed a short stature as compared to the wild-type plants | Zheng et al. (2001) | |
| 2. | Petunia | gai gene from Arabidopsis thaliana; Agrobacterium tumefaciens-mediated gene transfer | gai gene inhibits the effect of gibberellic acid in plants and thus results in dwarfism | Modified plant architecture | Decrease in transgenic petunia plant height | Tanaka et al. (2005) |
| 3. | Potato | PHYA gene; Agrobacterium tumefaciens-mediated gene transfer | The ectopic expression of PHYA gene increases the sensitivity of plants to far-red wavelengths of light, resulting in the inhibition of stem elongation | Reduction in plant height | Dwarfism in transgenic potato plants with increased harvest index through hypersensitivity to far red (FR) light | Robson et al. (1996) |
Though at present, transgenic crops are being cultivated over an acreage of 179.7 m ha by 70–80 million farmers in 28 countries across the globe (ISAAA, 2017), still people have many more apprehensions in their minds regarding biosafety, health and environmental risks posed by the consumption and commercialization of genetically modified crops. This has led to the development of new technologies to address such concerns, referred to as marker-free (Clean-gene) transgenic technology and genome editing technology.
Marker-free transgenic technology
Generally, the methods of genetic transformation employ selection markers such as antibiotic resistance genes or herbicide tolerance genes for the selection of desirable transgene expression in transformed cells (Bevan et al. 1983; Akama et al. 1995). However, except for the role as a selectable marker, these genes do not have any relevant function inside the plant cell and, thus, they exert an extra burden on the plant genome. Also, the constitutive expression of these genes encoded proteins affects the plant metabolism in a negative way. Further, use of marker genes, particularly those coding for antibiotic resistance, has been facing a strong criticism and opposition, particularly in edible crops including fruits and vegetables. Developing marker-free plants or finding out suitable alternatives of antibiotic or herbicide tolerance genes has been proposed with the hope of increasing consumers’ acceptance for genetically modified crops. A set of new technologies has been developed which involve the elimination of marker genes during transgenic plants development, which come under the recent molecular advent as ‘Marker-free transgenic technology’ or ‘Clean-gene technology’. Such technologies would be helpful to minimize biosafety concerns during biosafety research trials and the transgenic products would fetch wider consumer acceptance. Site-specific recombination has been suggested by many workers as a potential strategy (Dale and David 1991; Gleave et al. 1999; Puchta 2000). Hare and Chua (2002) proposed chemically inducible site-specific recombination systems as valuable tools for excision of transgenes when their expressions are not required. De Vetten et al. (2003) suggested the use of marker-free gene construct for genetic transformation of potato followed by polymerase chain reaction (PCR)-based selection of transformed cells for identification of transformants. Co-transformation with two gene constructs followed by segregation of marker gene and gene of interest in segregating generation has been explored. Sun et al. (2009) devised a strategy to eliminate public concerns regarding proliferation of antibiotic and herbicide resistance genes into the environment by constructing a super binary vector having two T-DNA systems to generate marker-free transgenic chrysanthemum plants. The vector system was designed having two T-DNA regions—one having hygromycin phosphotransferase (hpt) selectable marker gene and the other T-DNA containing β-glucuronidase (uidA) gene, placed adjacent to each other with no intervening region. A total of 17 hpt-resistant/gus positive To chrysanthemum plants were evaluated for segregation in T1 generation and among those, approximately 15.7% carried the transgene. Suitability of a twin T-DNA system for generating marker-free transgenic plants has been established.
For the development of selectable marker-free transgenic plants, Multi-Auto-Transformation (MAT) vector system (Ebinuma et al. 1997), which combines positive selection using isopentenyl transferase (ipt) gene, the first enzyme of cyokinin biosynthesis, with a site-specific recombination (Barry et al. 1984) and removal system offers a very potential tool (Sugita et al. 1999). Marker gene is removed from the transformed cells by the mechanism of homologous recombination. The MAT vector backbone is composed of yeast site-specific recombination R/RS system to excise the DNA fragment and the ipt gene cloned between two directly oriented recombination sites (Araki et al. 1987). The MAT vector system has been employed in a number of crops to develop marker-free transgenic plants including Antirrhinum majus (Minlong et al. 2000), citrus (Ballester et al. 2007), Kalanchoe blossfeldiana (Thirukkumaran et al. 2009) and Petunia hybrida (Khan et al. 2010). Khan et al. (2011b) used MAT vector in which ipt gene was used as a selection marker and Wasabi defensin (WD) gene, isolated from Wasabia japonica as a target gene, to transform tomato plants. The marker-free transgenic tomato plants exhibited enhanced resistance against a number of fungi including Alternaria solani, Botrytis cinerea, Fusarium oxysporum and Erysiphe lycopersici. Also, phosphomannose isomerase (PMI) gene derived from E. coli had been developed as an efficient positive selection marker for apple transformation, which induced the capability to grow on mannose supplemented medium in the transformed cells (Degenhardt and Szankowski 2006; Degenhardt et al. 2007). Further, plastid engineering has also been advocated as one of the most viable techniques to avoid transgene spread to other related crop (non-target species).
Cisgenic crops represent a step towards a new generation of genetically modified crops. Development of genetically modified crops, which do not possess any selectable marker (e.g., antibiotic resistance or herbicide tolerance) gene in the end product and also, if the inserted gene is derived from the same organism/plant, would be a welcome step to increase consumers’ acceptance for that product and to minimize the environmental risks associated with genetically modified crops. In this direction, Vanblaere et al. (2011) developed cisgenic apple plants by inserting the endogenous scab resistance gene HcrVf2 under the control of its own regulatory sequences into the scab susceptible apple cultivar ‘Gala’ using R/RS vector system to develop marker-free transgenic plants. Dhekney et al. (2011) also used cisgenic approach to develop disease-resistant apple.
Genome editing technology in horticultural crop improvement
Recent technology relies on certain engineered endonucleases (EEN) that cleave DNA in sequence-specific manner due to the presence of a sequence-specific DNA-binding domain. These endonucleases recognize specific DNA sequence and, thus, efficiently and precisely cleave the target genes. The double-strands breaks (DSBs) of DNA result in cellular DNA repair mechanisms, including homology-directed repair (HDR) and non-homologous end joining breaks (NHEJ), leading to gene modification at the target sites in the genome of plants. Generally, this technology employs three types of engineered endonucleases viz. Zinc finger nucleases (ZFNs), TALENs and CRISPR/CaS for site-specific cleavage. The CRISPR/Cas9 system has originated from bacteria and archae (Wiedenheft et al. 2012). CRIPSR/Cas9 is comparatively easy to prepare, affordable and can be better upscaled than ZFNs and TALENs. Cas9-induced double strand breaks in the plant genome are repaired by non-homologous end joining (NHEJ) method (Li et al. 2013). During this repair, small insertions or deletions may occur disturbing the open reading frame of a protein or introduces a stop codon (Belhaj et al. 2013). Zinc finger nucleases (ZFNs) and transcription activator-like effector (TALENs) technologies of genome editing affected from the disadvantages of high technical complexity and low efficiency. On the other hand, the clustered regularly interspersed short palindromic repeats (CRISPR)-associated protein 9 (CRISPR/Cas9) technology has revolutionized genome editing by overcoming the disadvantages of ZFNs and TALENs due to a high efficiency, low cost involvement, simplicity and versatility (Cardi and Stewart 2016). CRIPSR/Cas9 genome editing technology will probably avoid the current GM regulations mechanisms as the Cas9 protein-guide RNA complexes get rapidly decomposed in the regenerating cell cultures and thus will broaden the utility of this technology with greater global acceptance levels in comparison to the transgenic technology. A gene-edited crop does not necessarily contain any transgene and, thus, does not require very stringent regulation and thus such crops may find quick acceptance among consumers (Jones 2015). Precise genome editing offers a wonderful technology to decipher plant gene functions and in improvement of crop plants. CRISPR/Cas9 editing tools have been efficiently applied in a number of horticultural crops including tomato, petunia, citrus, grape, potato and apple for gene mutation, repression, activation and epigenome editing (Nishitani et al. 2016; Ren et al. 2016; Song et al. 2016).
Genome editing in plants has been revolutionized with the development of CRISPR/Cas9 technology. Recently, this technology has found application in developing resistance against many viruses (Ali et al. 2015; Baltes et al. 2015). Chandrasekaran et al. (2016) could successfully develop virus resistance in cucumber using Cas9/subgenomic RNA (sgRNA technology) to disrupt the function of recessive eIF4E (eukaryotic translation initiation factor 4E) gene. Homozygous T3 transgenic cucumber plant which was targeted to both eIF4E sites developed immunity to cucumber vein yellowing virus (Ipomovirus) infection and resistance to the potyviruses—Zucchini yellow mosaic virus and Papaya ring spot mosaic virus-w. In a recent study, Malnoy et al. (2016) reported the direct delivery of purified CRISPR/Cas9 ribonucleoproteins (RNPs) targeting to bring mutagenesis in MLO-7, a susceptible gene in order to increase resistance to powdery mildew in grape cultivar Chardonnay and DIPM-1, DIPM-2 and DIPM-4 genes in apple cultivar Golden delicious to increase resistance to fire blight disease. Tian et al. (2017) demonstrated the usefulness of genome editing technology, CRIPSR/Cas9—as a powerful tool to effectively create knockout mutations in watermelon. They targeted CIPDS (phytoene desaturase) gene for mutagenesis for developing albino phenotype. All the transgenic watermelon plants harbored CIPDS mutations and developed clear or mosaic albino phenotype, indicating that CRIPSR/Cas9 system has 100% genome editing efficiency in transgenic watermelon lines to introduce new functions. Induction of parthenocarpy has always been desired in horticultural crop plants for various industrial purposes and for eating quality. Ueta et al. (2017) demonstrated a CRISPR/Cas9 system-based breeding strategy to generate parthenocarpic tomato plants. Using CRISPR/Cas9 system, they could effectively introduce 100% somatic mutations into SlIAA9—a key gene controlling parthenocarpy in To tomato plants. Regenerated To mutants showed morphological changes in leaf shape and seedless fruit, which is a characteristic of parthenocarpic tomato. In a very recent study, Kishi-Kaboshi et al. (2017) reported for the first time gene editing in chrysanthemum using CRISPR/Cas9 system and developed transgenic chrysanthemum plants expressing the yellowish-green fluorescent protein (CpYGFP) gene from Chiridius poppei. Two sgRNAs were selected to target different positions in the CpYGFP gene and they obtained transgenic calli containing mutated CpYGFP genes (CRISPR-CpYGFP-chrysanthemum). Finally, the CRISPR-CpYGFP-chrysanthemum shoot containing a mutation in the CpYGFP gene was obtained.
Conclusion
The applications of recombinant-DNA technology or genetic engineering in crop improvement are immense to solve the problem of global hunger as population is increasing day by day with depriving sustainable intensification. However, horticultural crops have got less attention in this area so far. In contrast to the increasing global adoption of biotech field crops, biotechnology has limited commercial success to date in horticultural crops including fruits, vegetables, flowers and landscape plants. At this juncture of time, we cannot ignore the potential of this technology for the genetic enhancement of our horticultural crops to combat various production constraints like biotic or abiotic stresses and fruit quality improvement. Transgenic technology provides a potential technique for genetic enhancement using desirable trait of interest in plants. There is a need to address various regulatory obstacles for commercial release of various transgenic crops so that the real benefit of this wonderful technology may reach the consumers, the end users. After the advent of Next Generation Sequencing (NGS) technologies, many horticultural crops including strawberry, papaya, grapevine, sweet orange, mango, etc. have been sequenced, which has now solved the problem of lack of genomic information and thus facilitated the target gene/site to be modified using genome editing technology. This has also improved the breeding efficiency as various genes/QTLs coding for various horticulturally important traits have been identified. In addition to that, transcriptome sequences of a number of horticultural crops are now available in public databases. This vast information will assist in identifying various genes governing various important traits and will help in identifying the target sites for genome editing and genetic transformation.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Aida R, Komano M, Saito M, Nakase K, Murai K. Chrysanthemum flower shape modification by suppression of chrysanthemum-AGAMOUS gene. Plant Biotechnol. 2008;25:55–59. doi: 10.5511/plantbiotechnology.25.55. [DOI] [Google Scholar]
- Akama K, Puchta H, Hohn B. Efficient Agrobacterium-mediated transformation of Arabidopsis thaliana using the bar gene as selectable marker. Plant Cell Rep. 1995;14:450–454. doi: 10.1007/BF00234053. [DOI] [PubMed] [Google Scholar]
- Ali Z, Abulfaraj A, Idris A, Ali S, Tashkandi M, Mahfouz MM. CRISPR/Cas9-ediated viral interference in plants. Genome Biol. 2015;16:238. doi: 10.1186/s13059-015-0799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki H, Jearnpipatkula A, Tatsumi H, Sakurai T, Ushino K, Muta T. Molecular and functional organization of yeast plasmid pSR1. J Mol Biol. 1987;182:191–203. doi: 10.1016/0022-2836(85)90338-9. [DOI] [PubMed] [Google Scholar]
- Asao H, Nishizawa Y, Arai S, Sato T, Hirai M, Yoshida K, Shinmyo A, Hibi T. Enhanced resistance against a fungal pathogen Sphaerotheca humuli in transgenic strawberry expressing a rice chitinase gene. Plant Biotechnol. 1997;14:145–149. doi: 10.5511/plantbiotechnology.14.145. [DOI] [Google Scholar]
- Aslam J, Khan SA, Azad MAK. Agrobacterium-mediated genetic transformation of datepalm (Phoenix dactylifera L.) cultivar “Khalasah” via somatic embryogenesis. Plant Sci Today. 2015;2:93–101. doi: 10.14719/pst.2015.2.3.119. [DOI] [Google Scholar]
- Atkinson HJ, Grimwood S, Johnston K, Green J. Prototype demonstration of transgenic resistance to the nematode Radopholus similis conferred on banana by a cystatin. Transgenic Res. 2004;13:135–142. doi: 10.1023/B:TRAG.0000026070.15253.88. [DOI] [PubMed] [Google Scholar]
- Azadi P, Otang NV, Supaporn H, Khan RS, Chin DP, Nakamura I, Mii M. Increased resistance to cucumber mosaic virus (CMV) in Lilium transformed with a defective CMV replicase gene. Biotechnol Lett. 2011;33:1249–1255. doi: 10.1007/s10529-011-0550-7. [DOI] [PubMed] [Google Scholar]
- Ballester R, Cervera M, Pena L. Efficient production of transgenic citrus plants using isopentenyl transferase positive selection and removal of the marker gene by site-specific recombination. Plant Cell Rep. 2007;26:39–45. doi: 10.1007/s00299-006-0197-3. [DOI] [PubMed] [Google Scholar]
- Baltes NJ, Hummel AW, Konecna E, Cegan R, Bruns AN, Bisaro DM, Voytas DF. Conferring resistance to geminiviruses with the CRISPR-Cas prokaryotic immune system. Nat Plants. 2015 doi: 10.1038/nplants.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranski R, Klocke E, Nothnagel T. Chitinase CHIT36 from Trichoderma harzianum enhances resistance of transgenic carrot to fungal pathogens. J Phytopathol. 2008;156:13–521. doi: 10.1111/j.1439-0434.2008.01417.x. [DOI] [Google Scholar]
- Barry GF, Rogers SG, Fraley RT, Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci USA. 1984;81:4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboodian B, Ali ZM, Ismail I, Zainal Z. Postharvest analysis of lowland transgenic tomato fruits harboring hpRNAi-ACO1 construct. Sci World J. 2012;1:1–17. doi: 10.1100/2012/439870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhaj K, Chaparro-Garcia A, Kamoun S, Nekrasov V. Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods. 2013;9:39. doi: 10.1186/1746-4811-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MW, Flavell RB, Chilton MD. A chimeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature. 1983;304:184–187. doi: 10.1038/304184a0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar-Mathur P, Vadez V, Sharma KK. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep. 2008;27:411–424. doi: 10.1007/s00299-007-0474-9. [DOI] [PubMed] [Google Scholar]
- Bojorquez-Quintal E, Velarde-Buendıa A, Ku-Gonzalez A, Carillo-Pech M, Ortega-Camacho D, Echevarrıa-Machado I, Pottosin I, Martínez-Estévez M. Mechanisms of salt tolerance in habanero pepper plants (Capsicum chinense Jacq.): proline accumulation, ions dynamics and sodium root-shoot partition and compartmentation. Front Plant Sci. 2014;5:10–3389. doi: 10.3389/fpls.2014.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolar JP, Norelli JL, Harman GE, Brown SK, Aldwinkle HS. Synergistic activity of endochitinase and exochitinase from Trichoderma atroviride (T. harzianum) against the pathogenic fungus (Venturia inaequalis) in transgenic apple plants. Transgenic Res. 2001;10:533–543. doi: 10.1023/A:1013036732691. [DOI] [PubMed] [Google Scholar]
- Borkowoska M, Krzymowska M, Talarczyk A, Awan MF, Yakovleva L, Kleczkowski K, Wielgat V. Transgenic potato plants expressing soybean beta-1,3-endoglucanase gene exhibit an increased resistance to Phytophthora infestans. Z Naturforsch. 1998;53:1012–1016. doi: 10.1515/znc-1998-11-1212. [DOI] [PubMed] [Google Scholar]
- Borth W, Perez E, Cheah K, Chen Y, Xie WS, Gaskill D, Khalil S, Sether D, Melzer M, Wang M, Manshardt R, Gonsalves D, Hu JS. Transgenic banana plants resistant to banana bunchy top virus infection. Acta Hortic. 2011 [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Post-transcriptional control of gene expression in plants. Dordrecht: Springer; 1996. pp. 191–222. [DOI] [PubMed] [Google Scholar]
- Bovy AG, Angenent GC, Dons HJM, Van-Atvorst AG. Heterologous expression of the Arabidopsis etr1-1 allele inhibits the senescence of carnation flowers. Mol Breed. 1999;5:301–308. doi: 10.1023/A:1009617804359. [DOI] [Google Scholar]
- Bulle M, Yarra R, Abbagani S. Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol Breed. 2016;36:36. doi: 10.1007/s11032-016-0451-5. [DOI] [Google Scholar]
- Cardi T, Stewart CN., Jr Progress of targeted genome modification approaches in higher plants. Plant Cell Rep. 2016;35:1401–1416. doi: 10.1007/s00299-016-1975-1. [DOI] [PubMed] [Google Scholar]
- Ceasar SA, Ignacimuthu S. Genetic engineering of crop plants for fungal resistance: role of antifungal genes. Biotechnol Lett. 2012;34:995–1002. doi: 10.1007/s10529-012-0871-1. [DOI] [PubMed] [Google Scholar]
- Cervera M, Ortega C, Navarro A, Navarro L, Pena L. Generation of transgenic citrus plants with the tolerance-to-salinity gene HAL2 from yeast. J Hortic Sci Biotechnol. 2000;75:26–30. doi: 10.1080/14620316.2000.11511195. [DOI] [Google Scholar]
- Chaitanya KV, Sundar D, Masilamani S, Reddy AR. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002;36:175–180. doi: 10.1023/A:1015092628374. [DOI] [Google Scholar]
- Chakrabarty R, Viswakarma N, Bhat SR, Kirti PB, Singh BD, Chopra VL. Agrobacterium-mediated transformation of cauliflower, optimization of protocol and development of Bt-transgenic cauliflower. J Biosci. 2002;27:495–502. doi: 10.1007/BF02705046. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal-on A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol. 2016;17(7):1140–1153. doi: 10.1111/mpp.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checker VG, Chhibbar AK, Khurana P. Stress-inducible expression of barley Hva1 gene in transgenic mulberry displays enhanced tolerance against drought, salinity and cold stress. Transgenic Res. 2012;21:939–957. doi: 10.1007/s11248-011-9577-8. [DOI] [PubMed] [Google Scholar]
- Chen XK, Zhang JY, Zhang Z, Du XL, Du BB, Qu SC. Overexpressing MhNPR1 in transgenic Fuji apples enhances resistance to apple powdery mildew. Mol Biol Rep. 2012;39:8083–8089. doi: 10.1007/s11033-012-1655-3. [DOI] [PubMed] [Google Scholar]
- Chen JR, Chen YB, Ziemiańska M, Liu R, Deng ZN, Niedźwiecka-Filipiak I, Li YL, Jio JX, Xiong XY. Co-expression of MtDREB1C and RcXET enhances stress tolerance of transgenic China rose (Rosa chinensis Jacq.) Plant Growth Regul. 2016;35:586–599. doi: 10.1007/s00344-015-9564-z. [DOI] [Google Scholar]
- Cheng L, Zou Y, Ding S, Zhang J, Yu X, Cao J, Lu G. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integ Plant Biol. 2009;51:489–499. doi: 10.1111/j.1744-7909.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Deng XP, Kwak SS, Chen W, Eneji AE. Enhanced tolerance of transgenic potato plants expressing choline oxidase in chloroplasts against water stress. Bot Stud. 2013;54:30. doi: 10.1186/1999-3110-54-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Xie X, Xu Y, Zhang C, Wang X, Zhang J, Wang U. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from Chinese wild Vitis quinquangularis. Planta. 2016;243:1041–1053. doi: 10.1007/s00425-015-2459-1. [DOI] [PubMed] [Google Scholar]
- Clark DG, Loucas H, Shibuya K, Underwood B, Barry K, Jandrew J. Biotechnology of floricultural crops-scientific questions and real world answers. In: Vasil IK, editor. Plant biotechnology 2002 and beyond. Dordrecht: Kluwer Academic; 2003. pp. 337–342. [Google Scholar]
- Clarke JL, Spetz C, Haugslien S, Xing S, Dees MW, Moe R, Blystad DR. Agrobacterium tumefaciens-mediated transformation of poinsettia, Euphorbia pulcherrima, with virus-derived hairpin RNA constructs confers resistance to Poinsettia mosaic virus. Plant Cell Rep. 2008;27:1027–1038. doi: 10.1007/s00299-008-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge DB, Jorgensen HJ, Lund OS, Lyngkjaer MF. Engineering pathogen resistance in crop plants: current trends and future prospects. Annu Rev Phytopathol. 2010;48:269–291. doi: 10.1146/annurev-phyto-073009-114430. [DOI] [PubMed] [Google Scholar]
- Dale EC, David OW. Gene transfer with subsequent removal of the selection gene from the host genome. Proc Natl Acad Sci USA. 1991;88:558–562. doi: 10.1073/pnas.88.23.10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das DK, Rahman A. Expression of a bacterial chitinase (ChiB) gene enhances antifungal potential in transgenic Litchi chinensis Sonn. (cv. Bedana) Curr Trends Biotechnol Pharm. 2010;41:820–833. [Google Scholar]
- Das M, Chauhan H, Chhibbar A, Mohd Q, Haq R, Khurana P. High-efficiency transformation and selective tolerance against biotic and abiotic stress in mulberry, Morus indica cv. K2, by constitutive and inducible expression of tobacco osmotin. Transgenic Res. 2011;20:231–246. doi: 10.1007/s11248-010-9405-6. [DOI] [PubMed] [Google Scholar]
- Das MP, Rebecca LJ, Sharmila S, Banerjee A, Kumar D. Identification and optimization of cultural conditions for chitinase production of Bacillus amyloliqufaciens SM3. J Chem Pharma Res. 2012;4:969–4974. [Google Scholar]
- Davuluri GR, Tuinen A, Fraser PD, Manfredonia A, Newman R, Burgess D. Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat Biotechnol. 2005;23:890–895. doi: 10.1038/nbt1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Campos MKF, Carvalho K, Souza FS, Marur CJ, Pereira LFP, Bespalhok FJC, Vieira LGE. Drought tolerance and antioxidant enzymatic activity in transgenic Swingle citrumelo plants over-accumulating proline. Environ Exp Bot. 2011;72:242–250. doi: 10.1016/j.envexpbot.2011.03.009. [DOI] [Google Scholar]
- De Carvalho K, de Campos MKF, Domingues DS, Pereira LFP, Vieira LGE. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol Biol Rep. 2013 doi: 10.1007/s11033-012-2402-5. [DOI] [PubMed] [Google Scholar]
- De Vetten N, Wolters AM, Raemakers K, Van Der Meer I, Stege R, Heeres EA. Transformation method for obtaining marker-free plants of a cross-pollinating and vegetatively propagated crop. Nat Biotechnol. 2003;21:439–442. doi: 10.1038/nbt801. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Szankowski I. Transformation of apple (Malus domestica Borkh.) using the phosphomannose isomerase gene as a selectable marker. Acta Hortic. 2006;725:811–814. doi: 10.17660/ActaHortic.2006.725.113. [DOI] [Google Scholar]
- Degenhardt J, Poppe A, Rosner L. Alternative selection systems in apple transformation. Acta Hortic. 2007;738:287–292. doi: 10.17660/ActaHortic.2007.738.31. [DOI] [Google Scholar]
- Dhekney SA, Li ZT, Gray DJ. Grapevines engineered to express cisgenic Vitis vinifera thaumatin-like protein exhibit fungal disease resistance. In Vitro Cell Dev Biol Plant. 2011;47:458–466. doi: 10.1007/s11627-011-9358-3. [DOI] [Google Scholar]
- Ding LC, Hu CY, Yeh KW, Wang PJ. Development of insect-resistant transgenic cauliflower plants expressing the trypsin inhibitor gene isolated from local sweet potato. Plant Cell Rep. 1998;17:854–860. doi: 10.1007/s002990050497. [DOI] [PubMed] [Google Scholar]
- Distefano G, LaMalfa S, Vitale A, Lorito M, Deng Z, Gentile A. Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Transgenic Res. 2008;17:873–879. doi: 10.1007/s11248-008-9172-9. [DOI] [PubMed] [Google Scholar]
- Duan Y, Zhou L, Hall DG, Li W, Doddapaneni H, Lin H. Complete genome sequence of citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol Plant Microbe Interact. 2009;22:1011–1020. doi: 10.1094/MPMI-22-8-1011. [DOI] [PubMed] [Google Scholar]
- Dutt M, Barthe G, Irey M, Grosser J. Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; Citrus greening) PLoS One. 2015;10:e0137134. doi: 10.1371/journal.pone.0137134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinuma H, Sugita K, Matsunaga E, Yamakado M. Selection of marker-free transgenic plants using the isopentenyl transferase gene as a selectable marker. Proc Natl Acad Sci USA. 1997;94:2117–2121. doi: 10.1073/pnas.94.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar MA, Civerolo EL, Summerfelt KR, Dandekar AM. RNAi-mediated oncogene silencing confers resistance to crown gall tumorigenesis. Proc Natl Acad Sci USA. 2001;98:13437–13442. doi: 10.1073/pnas.241276898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagoaga C, Rodrigo I, Conejero V, Hinarejos C, Tuset JJ, Arnau J, Pina JA, Navarro L, Pena L. Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Mol Breed. 2001;7:175–185. doi: 10.1023/A:1011358005054. [DOI] [Google Scholar]
- Fan W, Zhang M, Zhang H, Zhang P. Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One. 2012;7:e37344. doi: 10.1371/journal.pone.0037344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Song A, Jiang Zhang T, Sun H, Wang Y. CmWRKY1 enhances the dehydration tolerance of chrysanthemum through the regulation of ABA-associated genes. PLoS One. 2016;11:e0150572. doi: 10.1371/journal.pone.0150572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstad K, Martin RR. Transformation of strawberry for virus resistance. Acta Hortic. 1995;385:6–90. [Google Scholar]
- Fischhoff DA, Bowdish KS, Perlak FJ, Marrone PG, McCormick SM, Niedermeyer JG, Dean DA, Kusano-Kretzmer K, Mayer EJ, Rochester DE, Rogers SG, Fraley RT. Insect tolerant tomato plants. BioTechnol. 1987;5:807–813. [Google Scholar]
- Flachowsky H, Szankowski I, Fischer TC. Transgenic apple plants overexpressing the Lc gene of maize show an altered growth habit and increased resistance to apple scab and fire blight. Planta. 2010;231:623–635. doi: 10.1007/s00425-009-1074-4. [DOI] [PubMed] [Google Scholar]
- Galambos A, Zok A, Kuczmog A, Olah R, Putnoky P, Ream W, Szegedi E. Silencing Agrobacterium oncogenes in transgenic grapevine results in strain-specific crown gall resistance. Plant Cell Rep. 2013;32:1751–1757. doi: 10.1007/s00299-013-1488-0. [DOI] [PubMed] [Google Scholar]
- Gangadhar BH, Sajeesh K, Venkatesh J, Baskar V, Abhinandan K, Yu JW, Prasad R, Mishra RK. Enhanced tolerance of transgenic potato plants over-expressing non-specific lipid transfer protein-1 (StnsLTP1) against multiple abiotic stresses. Front Plant Sci. 2016;7:1228. doi: 10.3389/fpls.2016.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Song A, Zhu X, Chen F, Jiang J. The heterologous expression in Arabidopsis of a highly tolerant to a new CMV pathotype. Plant Cell Rep. 2012;28:223–232. [Google Scholar]
- Gessler C, Patocchi A. Recombinant DNA technology in apple. Adv Biochem Engin/Biotechnol. 2007;107:13–132. doi: 10.1007/10_2007_049. [DOI] [PubMed] [Google Scholar]
- Ghag SB, Shekhawat UKS, Ganapathi TR. Petunia floral defensins with unique prodomains as novel candidates for development of Fusarium wilt resistance in transgenic banana plants. PLoS One. 2012;7:39557. doi: 10.1371/journal.pone.0039557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girhepuje PV, Shinde GV. Transgenic tomato plants expressing a wheat endochitinase gene demonstrate enhanced resistance to Fusarium oxysporum f. sp. Lycopersici. Plant Cell Tiss Organ Cult. 2011;105:243–251. doi: 10.1007/s11240-010-9859-5. [DOI] [Google Scholar]
- Gleave AP, Mitra DS, Mudge SR, Morris BA. Selectable marker-free transgenic plants without sexual crossing: transient expression of cre recombinase and use of a conditional lethal dominant gene. Plant Mol Biol. 1999;40:223–235. doi: 10.1023/A:1006184221051. [DOI] [PubMed] [Google Scholar]
- Graham J, Gordon SC, Smith K, McNcol RJ, McNcol JW. The effect of the cowpea trypsin inhibitor in strawberry on damage by vine weevil under field conditions. J Hortic Sci Biotechnol. 2002;77:33–40. doi: 10.1080/14620316.2002.11511453. [DOI] [Google Scholar]
- Han BH, Suh EJ, Lee SY, Shin HK, Lim YP. Selection of nonbranching lines induced by introducing Ls-like cDNA into Chrysanthemum (Dendranthema × grandiflorum (Ramat.) Kitamura) Shuho-no-chikara. Sci Hortic. 2007;115:70–75. doi: 10.1016/j.scienta.2007.07.012. [DOI] [Google Scholar]
- Han JS, Park LI, Jeon SM, Park S, Naing AH, Kim CK. Assessments of salt tolerance in a bottle gourd line expressing the Arabidopsis H+-pyrophosphatase AVP1 gene and in a watermelon plant grafted onto a transgenic bottle gourd rootstock. Plant Breed. 2015;134:233–238. doi: 10.1111/pbr.12253. [DOI] [Google Scholar]
- Hare PD, Chua NH. Excision of selectable marker genes from transgenic plants. Nat Biotechnol. 2002;20:575–580. doi: 10.1038/nbt0802-843a. [DOI] [PubMed] [Google Scholar]
- Hazarika P, Rajam MV. Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol Mol Biol Plants. 2011;17:115–128. doi: 10.1007/s12298-011-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Ke H, Keting H, Qiaoyan X, Silan D. Flower colour modification of chrysanthemum by suppression of F3′H and overexpression of the exogenous Senecio cruentus F3′5′H gene. PLoS One. 2013;8(11):e74395. doi: 10.1371/journal.pone.0074395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton TA, Brugliera F, Tanaka Y. Cloning and expression of flavonol synthase from Petunia hybrida. Plant J. 1993;4:1003–1010. doi: 10.1046/j.1365-313X.1993.04061003.x. [DOI] [PubMed] [Google Scholar]
- Husaini AM, Abdin MZ. Overexpression of tobacco osmotin gene leads to salt stress tolerance in strawberry (Fragaria × ananassa Duch.) plants. Indian J Biotech. 2008;7:465–471. [Google Scholar]
- ISAAA (2017). http://www.isaaa.org. Accessed 25 May 2017
- Islam A. Fungus resistant transgenic plants: strategies, progress and lessons learnt. Plant Tiss Cult Biotechnol. 2006;16:117–138. [Google Scholar]
- James C, Krattiger AF. Global review of the field testing and commercialization of transgenic plants: 1986 to 1995. Briefs: ISAAA; 1996. p. 1. [Google Scholar]
- Jiang B, Miao H, Chen S, Zhang S, Chen F, Fang W. The lateral suppressor-like gene, DgLsL, alternated the axillary branching in transgenic chrysanthemum (Chrysanthemum × morifolium) by modulating IAA and GA content. Plant Mol Biol Rep. 2010;28:144–151. doi: 10.1007/s11105-009-0130-3. [DOI] [Google Scholar]
- Jin WM, Dong J, Hu YL, Lin ZP, Xu XF, Han ZH. Improved cold-resistant performance in transgenic grape (Vitis vinifera L.) overexpressing cold-inducible transcription factors AtDREB1b. Hortic Sci. 2009;44:35–39. [Google Scholar]
- Jiwan D, Roalson EH, Main D, Dhingra A. Antisense expression of peach mildew resistance locus O (PpMlo1) gene confers cross-species resistance to powdery mildew in Fragaria × ananassa. Transgenic Res. 2012;22:1119–1131. doi: 10.1007/s11248-013-9715-6. [DOI] [PubMed] [Google Scholar]
- Jones HD. Regulatory uncertainty over genome editing. Nat. Plants. 2015 doi: 10.1038/nplants.2014.11. [DOI] [PubMed] [Google Scholar]
- Joshi SG, Soriano JM, Kortstee A. Development of cisgenic apples with durable resistance to apple scab. Acta Hortic. 2009;839:403–406. doi: 10.17660/ActaHortic.2009.839.53. [DOI] [Google Scholar]
- Josine T, Ji J, Wang G, Zhao Q, Yang HL, Wang YR, Wu WD. AtDREB2A-CA gene over-expression in Rosa Chinensis Jacq. affect leaf ultrastructure response to salt stress. Int J Agri Crop Sci. 2015;8:463–476. [Google Scholar]
- Kathryn KK, Han BH. Biolistic-mediated transformation of Lilium longiflorum cv. Nellie White. Hortic Sci. 2008;43(6):1864–1869. [Google Scholar]
- Katsumoto Y, Fukuchi-Mizutani M, Fukui Y, Brugliera F, Holton TA, Karan M. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007;48:1589–1600. doi: 10.1093/pcp/pcm131. [DOI] [PubMed] [Google Scholar]
- Kaur P, Samuel DVK, Bansal KC. Fruit-specific over-expression of LeEXP1 gene in tomato alters fruit texture. J Plant Biochem Biotechnol. 2010;19(2):177–183. doi: 10.1007/BF03263338. [DOI] [Google Scholar]
- Keen NT, Yoshikawa M. β-1,3-endoglucanase from soybean releases elicitor-active carbohydrates from fungus cell walls. Plant Physiol. 1993;71:460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RS, Sjahril R, Nakamura I, Mii M. Production of transgenic potato exhibiting enhanced resistance to fungal infections and herbicide applications. Plant Biotechnol Rep. 2008;2:13–20. doi: 10.1007/s11816-008-0043-x. [DOI] [Google Scholar]
- Khan RS, Nakamura I, Mii M. Production and selection of marker-free transgenic plants of Petunia hybrida using site specific recombination. Biol Plant. 2010;54:265–271. doi: 10.1007/s10535-010-0046-7. [DOI] [Google Scholar]
- Khan H, Siddique I, Anis M, Khan PR. In vitro organogenesis from internode derived callus cultures of Capsicum annuum L. J Plant Biochem Biotechnol. 2011;20:84–89. doi: 10.1007/s13562-010-0029-y. [DOI] [Google Scholar]
- Khan RS, Nakamura I, Mii M. Development of disease-resistant marker-free tomato by R/RS site-specific recombination. Plant Cell Rep. 2011;30:1041–1053. doi: 10.1007/s00299-011-1011-4. [DOI] [PubMed] [Google Scholar]
- Khare N, Goyary D, Singh NK, Shah P, Rathore M, Anandhan S. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tiss Organ Cult. 2010;2:267–277. doi: 10.1007/s11240-010-9776-7. [DOI] [Google Scholar]
- Khodakovskaya M, Vankova R, Malbeck J, Li A, Li Y, McAvoy R. Enhancement of flowering and branching phenotype in chrysanthemum by expression of ipt under the control of a 0.821 kb fragment of the LEACO1 gene promoter. Plant Cell Rep. 2009;28:1351–1362. doi: 10.1007/s00299-009-0735-x. [DOI] [PubMed] [Google Scholar]
- Khurana P, Vishnudasan D, Chhibbar AK. Genetic approaches towards overcoming water deficit in plants special emphasis on LEAs. Physiol Mol Biol Plants. 2008;14:277–298. doi: 10.1007/s12298-008-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi-Kaboshi M, Aida R, Sasaki K. Generation of gene-edited Chrysanthemum morifolium using multicopy transgenes as targets and markers. Plant Cell Physiol. 2017;58(2):216–226. doi: 10.1093/pcp/pcw222. [DOI] [PubMed] [Google Scholar]
- Ko M, Cho JH, Seo HH, Lee HH, Kang HY, Nguyen TS. Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants. Planta. 2016;244:379–392. doi: 10.1007/s00425-016-2514-6. [DOI] [PubMed] [Google Scholar]
- Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N. Chilli peppers—a review on tissue culture and transgenesis. Biotechnol Adv. 2010;28:35–48. doi: 10.1016/j.biotechadv.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kumar P, Srivastava DK. High frequency organogenesis in hypocotyl, cotyledon, leaf and petiole explants of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Physiol Mol Biol Plants. 2015;21(2):279–285. doi: 10.1007/s12298-015-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Mandaokar A, Sreenivasu K, Chakrabarti SK, Bisaria S, Sharma SR. Insect-resistant transgenic brinjal plants. Mol Breed. 1998;4:3–37. doi: 10.1023/A:1009694016179. [DOI] [Google Scholar]
- Le HG, Farine S, Kieffer-Mazet F, Miclot AS, Heitz T, Mestre P, Bertsch C, Chong J. Vitis vinifera VvNPR1,1 is the functional ortholog of AtNPR1 and its overexpression in grapevine triggers constitutive activation of PR genes and enhanced resistance to powdery mildew. Planta. 2011;234(2):405–417. doi: 10.1007/s00425-011-1412-1. [DOI] [PubMed] [Google Scholar]
- Lee YH, Jung M, Shin SH, Lee JH, Choi SH, Her NH. Transgenic peppers that are highly tolerant to a new CMV pathotype. Plant Cell Rep. 2009;28:223–232. doi: 10.1007/s00299-008-0637-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Feng F, Liang D, Cheng L, Ma F, Shi S. Overexpression of a Malus vacuolar Na+/H+ antiporter gene (MdNHX1) in apple rootstock M26 and its influence on salt tolerance. Plant Cell Tiss Organ Cult. 2010;102:337–345. doi: 10.1007/s11240-010-9738-0. [DOI] [Google Scholar]
- Li JF, Norville JE, Aach J, McCormack M, Zhang D, Bush J, Church GM, Sheen J. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol. 2013;31:688–691. doi: 10.1038/nbt.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Song A, Gao C, Jiang J, Chen S, Fang W. The over-expression of a chrysanthemum WRKY transcription factor enhances aphid resistance. Plant Physiol Biochem. 2015;95:26–34. doi: 10.1016/j.plaphy.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Lilley CJ, Urwin PE, Johnston KA, Atkinson HJ. Preferential expression of a plant cystatin at nematode feeding sites confers resistance to Meloidogyne and Globodera spp. Plant Biotechnol J. 2004;2:3–12. doi: 10.1046/j.1467-7652.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Lim MY, Jeong BR, Jung M, Harn CH. Transgenic tomato plants expressing strawberry D-galacturonic acid reductase gene display enhanced tolerance to abiotic stresses. Plant Biotechnol Rep. 2016;10:105–116. doi: 10.1007/s11816-016-0392-9. [DOI] [Google Scholar]
- Lin WC, Lu CF, Wu JW, Cheng ML, Lin YM, Yang NS. Transgenic tomato plants expressing the Arabidopsis NPR1 gene display enhanced resistance to a spectrum of fungal and bacterial diseases. Transgenic Res. 2004;13:67–581. doi: 10.1007/s11248-004-2375-9. [DOI] [PubMed] [Google Scholar]
- Lindow S, Newman K, Chatterjee S, Baccari C, Lavarone AT, Ionescu M. Production of Xylella fastidiosa diffusible signal factor in transgenic grape causes pathogen confusion and reduction in severity of pierce’s disease. Mol Plant Microbe Interact. 2014;27:244–254. doi: 10.1094/MPMI-07-13-0197-FI. [DOI] [PubMed] [Google Scholar]
- Lorito M, Woo SL, Fernandez IG, Colucci G, Harman GE, Pintor-Toro JA, Filipone E, Muccifora S, Lawrence CB, Zoina A, Tuzun S, Scala F. Genes from mycoparasitic fungi as source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA. 1998;95:7860–7865. doi: 10.1073/pnas.95.14.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Chandler SF, Mason JG, Brugliera F. Florigene flowers: from laboratory to market. In: Vasil IK, editor. Plant biotechnology 2002 and beyond. Dordrecht: Kluwer Academic; 2003. pp. 333–336. [Google Scholar]
- Lurquin P. High tech harvest: understanding genetically modified food plants. Cambridge: Westview; 2002. [Google Scholar]
- Malnoy M, Aldwinckle HS. Development of fire blight resistance by recombinant DNA technology. Plant Breed Rev. 2007;29:315–358. doi: 10.1002/9780470168035.ch6. [DOI] [Google Scholar]
- Malnoy M, Jin Q, Borejsza-Wysocka EE, He SY, Aldwinckle HS. Overexpression of the apple MpNPR1 gene confers increased disease resistance in Malus × domestica. Mol Plant Microbe Interact. 2007;20:1568–1580. doi: 10.1094/MPMI-20-12-1568. [DOI] [PubMed] [Google Scholar]
- Malnoy M, Xu M, Borejsza-Wysocka E, Korban SS, Aldwinckle HS. Two receptor like genes, Vf1 and Vf2, confer resistance to the fungal pathogen Venturia inaequalis inciting apple scab disease. Mol Plant Microbe Interact. 2008;21:448–458. doi: 10.1094/MPMI-21-4-0448. [DOI] [PubMed] [Google Scholar]
- Malnoy M, Viola R, Jung MH, Koo OJ, Kim S, Kim JS, Velasco R, Nagamangala KC. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci. 2016;7:1904. doi: 10.3389/fpls.2016.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwick NP, Docherty LC, Phung MM. Transgenic tobacco and apple plants expressing biotin-binding proteins are resistant to two cosmopolitan insect pests, potato tuber moth and light brown apple moth, respectively. Transgenic Res. 2003;12:671–681. doi: 10.1023/B:TRAG.0000005103.83019.51. [DOI] [PubMed] [Google Scholar]
- Martinelli A, Gaiani A, Cella R. Agrobacterium-mediated transformation of strawberry cultivar Marmolada onebar. Acta Hortic. 1997;439:169–173. doi: 10.17660/ActaHortic.1997.439.22. [DOI] [Google Scholar]
- Mercado JA, Martín-Pizarro C, Pascual L, Quesada MA, Pliego-Alfaro F, de los Santos B, Romero F, Gálvez J, Rey M, de la Vin˜a G, Llobell A, Yubero-Serrano E-M, Mun˜oz-Blanco J, Caballero JL. Evaluation of tolerance to Colletotrichum acutatum in strawberry plants transformed with Trichoderma-derived genes. Acta Hortic. 2007;738:383–388. doi: 10.17660/ActaHortic.2007.738.46. [DOI] [Google Scholar]
- Mercado JA, Barcelo M, Pliego C, Rey M, Caballero JL, Munoz-Blanco J, Ruano-Rosa D, Lopez-Herrera C, Santos B, Romero-Munoz F, Pliego-Alfaro F. Expression of the β-1,3-glucanase gene bgn13,1 from Trichoderma harzianum in strawberry increases tolerance to crown rot diseases but interferes with plant growth. Transgenic Res. 2015;24:979–989. doi: 10.1007/s11248-015-9895-3. [DOI] [PubMed] [Google Scholar]
- Meyer P, Heidmann I, Forkmann G, Saedler H. A new petunia flower colour generated by transformation of a mutant with a maize gene. Nature. 1987;330:677–678. doi: 10.1038/330677a0. [DOI] [PubMed] [Google Scholar]
- Minlong C, Takayanagi K, Kamada H, Nishimura S, Handa T. Transformation of Antirrhinum majus L. by a rol-type multi-auto-transformation (MAT) vector system. Plant Sci. 2000;159:273–280. doi: 10.1016/S0168-9452(00)00351-4. [DOI] [PubMed] [Google Scholar]
- Mishra M, Jalil SU, Mishra RK, Kumari S, Pandey BK. In vitro screening of guava plantlets transformed with endochitinase gene against Fusarium oxysporum f. sp. psidii. Czech J Genet Plant Breed. 2016;52:6–13. doi: 10.17221/74/2015-CJGPB. [DOI] [Google Scholar]
- Missiou A, Kalantidis K, Boutla A, Tzortzakaki S, Tabler M, Tsagris M. Generation of transgenic potato plants highly resistant to potato virus Y (PVY) through RNA silencing. Mol Breeding. 2004;14:185–197. doi: 10.1023/B:MOLB.0000038006.32812.52. [DOI] [Google Scholar]
- Mondal SN, Dutt M, Grosser JW, Dewdney MM. Transgenic citrus expressing the antimicrobial gene Attacin E (attE) reduces the susceptibility of ‘Duncan’ grapefruit to the citrus scab caused by Elsinoe fawcettii. Eur J Plant Pathol. 2012;133:391–404. doi: 10.1007/s10658-011-9912-1. [DOI] [Google Scholar]
- Mora AA, Earle ED. Resistance to Alternaria brassicicola in transgenic broccoli expressing Trichoderma harzianum endochitinase gene. Mol Breed. 2001;8:1–9. doi: 10.1023/A:1011913100783. [DOI] [Google Scholar]
- Moravcikova J, Matusikova I, Libantova J, Bauer M, Mlynarova L. Expression of a cucumber class III chitinase and Nicotiana plumbaginifolia class I glucanase genes in transgenic potato plants. Plant Cell Tiss Organ Cult. 2004;79:161–168. doi: 10.1007/s11240-004-0656-x. [DOI] [Google Scholar]
- Naing AH, Ai TN, Jeon SM, Lim SH, Kim CK. An efficient protocol for Agrobacterium-mediated genetic transformation of recalcitrant chrysanthemum cultivar Shinma. Acta Physiol Plant. 2016;38:38. doi: 10.1007/s11738-015-2059-5. [DOI] [Google Scholar]
- Najar AG, Anwar A, Masoodi L, Khar MS. Evaluation of native biocontrol agents against Fusarium solani f. sp. melongenae causing wilt disease of brinjal in Kashmir. J Phytol. 2011;3:31–34. [Google Scholar]
- Nakamura Y, Sawada H, Kobayashi S, Nakajima I, Yoshikawa M. Expression of soybean β-1,3-glucanase cDNA and effect on disease tolerance in kiwifruit plants. Plant Cell Rep. 1999;18:527–532. doi: 10.1007/s002990050616. [DOI] [Google Scholar]
- Nambeesan S, Datsenka T, Ferruzzi MG, Malladi A, Mattoo AK, Handa AK. Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J. 2010;63:836–847. doi: 10.1111/j.1365-313X.2010.04286.x. [DOI] [PubMed] [Google Scholar]
- Namukwaya B, Tripathi L, Tripathi JN, Arinaitwe G, Mukasa SB, Tushemereirwe WK. Transgenic banana expressing Pflp gene confers enhanced resistance to Xanthomonas wilt disease. Transgenic Res. 2012;21:855–865. doi: 10.1007/s11248-011-9574-y. [DOI] [PubMed] [Google Scholar]
- Narendran M, Deole SG, Harkude S, Shirale D, Nanote A, Bihani P, Parimi S, Char BR, Zehr UB. Efficient genetic transformation of okra (Abelmoschus esculentus (L.) Moench) and generation of insect-resistant transgenic plants expressing the cry1Ac gene. Plant Cell Rep. 2013;32:1191–1198. doi: 10.1007/s00299-013-1415-4. [DOI] [PubMed] [Google Scholar]
- Nishitani C, Hirai N, Komori S, Wada M, Okada K, Osakabe K, et al. Efficient genome editing in apple using a CRISPR/Cas9 system. Sci Rep. 2016;6:31481. doi: 10.1038/srep31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pandolfini T, Molesini B, Avesani L, Spena A, Polverari A. Expression of self-complementary hairpin RNA under the control of the rolC promoter confers systemic disease resistance to plum pox virus without preventing local infection. BMC Biotech. 2003;3:7. doi: 10.1186/1472-6750-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papolu PK, Dutta TK, Tyagi N, Urwin PE, Lilley CJ, Rao U. Expression of a cystatin transgene in eggplant provides resistance to root-knot nematode, Meloidogyne incognita. Front Plant Sci. 2016;7:1122. doi: 10.3389/fpls.2016.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Cheng NH, Pittman JK, Yoo KS, Park J, Smith RH. Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ Plant Physiol. 2005;139:1194–1206. doi: 10.1104/pp.105.066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar N, Kamlesh K, Thakur AK. In vitro organogenesis from cotyledon derived callus cultures of Punica granatum L. cv. Kandhari Kabuli. Natl Acad Sci Lett. 2012;35:215–220. doi: 10.1007/s40009-012-0041-y. [DOI] [Google Scholar]
- Parmar N, Kanwar K, Thakur AK. High efficiency plant regeneration via direct organogenesis in Punica granatum L. cv. Kandhari Kabuli from hypocotyl explants. Proc Natl Acad Sci India Sect-B Biol Sci. 2013;83:569–574. doi: 10.1007/s40011-013-0178-6. [DOI] [Google Scholar]
- Parmar N, Kanwar K, Thakur AK. High efficiency plant regeneration from cotyledon explants of pomegranate (Punica granatum L.) cv. Kandhari Kabuli. Vegetos. 2015;28:160–165. [Google Scholar]
- Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M. Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep. 2008;27:1677–1686. doi: 10.1007/s00299-008-0587-9. [DOI] [PubMed] [Google Scholar]
- Paul A, Sharma SR, Sresty TVS, Devi S, Bala S, Kumar PS, Saradhi PP, Frutos R, Altosaar I, Kumar PA. Transgenic cabbage (Brassica oleracea var. capitata) resistant to Diamondback moth (Plutella xylostella) Indian J Biotech. 2005;4:72–77. [Google Scholar]
- Pessina S, Lenzi L, Perazzolli M, Campa M, DallaCosta L, Urso S, Vale G, Salamini F, Velasco R, Malnoy M. Knockdown of MLO genes reduces susceptibility to powdery mildew in grapevine. Hortic Res. 2016;3:16016. doi: 10.1038/hortres.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty LM, Harberd NP, Carre IA, Thomas B, Jackson SD. Expression of the Arabidopsis gai gene under its own promoter causes a reduction in plant height in chrysanthemum by attenuation of the gibberellin response. Plant Sci. 2003;164:75–182. doi: 10.1016/S0168-9452(02)00380-1. [DOI] [Google Scholar]
- Praveen S, Ramesh SV, Mishra AK, Koundal V, Palukaitis P. Silencing potential of viral derived RNAi constructs in tomato leaf curl virus-AC4 gene suppression in tomato. Transgenic Res. 2010;19:45–55. doi: 10.1007/s11248-009-9291-y. [DOI] [PubMed] [Google Scholar]
- Puchta H. Removing selectable marker genes: taking the shortcut. Trends Plant Sci. 2000;5:273–274. doi: 10.1016/S1360-1385(00)01684-8. [DOI] [PubMed] [Google Scholar]
- Punja ZK, Raharjo V. Response of transgenic cucumber and carrot plants expressing different chitinase enzymes to inoculation with pathogens. Plant Dis. 1996;80:999–1005. doi: 10.1094/PD-80-0999. [DOI] [Google Scholar]
- Qu SC, Dong L, Zhang Z. Research advances of resistant genes in apple. J Agric Sci Technol. 2009;11:36–41. [Google Scholar]
- Rai MK, Kalia RK, Singh R, Gangola MP, Dhawan AK. Developing stress tolerant plants through in vitro selection—an overview of the recent progress. Environ Exp Bot. 2011;71:89–98. doi: 10.1016/j.envexpbot.2010.10.021. [DOI] [Google Scholar]
- Ren C, Liu X, Zhang Z, Wang Y, Duan W, Li S, Liang Z. CRISPR/Cas9—mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.) Sci Rep. 2016;6:32289. doi: 10.1038/srep32289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy P. World salinization with emphasis on Australia. J Exp Bot. 2006;57:1017–1023. doi: 10.1093/jxb/erj108. [DOI] [PubMed] [Google Scholar]
- Rivera-Dominguez M, Astorga-Cienfuegos KR, Vallejo-Cohen S, Vargas-Arispuro I, Sanchez- Sanchez E. Transgenic mango embryos (Mangifera indica) cv. ‘Ataulfo’ with the defensin J1 gene. Revista Mexicana de Fitopathol. 2011;29:78–80. [Google Scholar]
- Robson PRH, McCormac AC, Irvine AS, Smith H. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nat Biotechnol. 1996;14:995–998. doi: 10.1038/nbt0896-995. [DOI] [PubMed] [Google Scholar]
- Roderick H, Tripathi L, Babirye A, Wang D, Tripathi J, Urwin PE. Generation of transgenic plantain (Musa spp.) with resistance to plant pathogenic nematodes. Mol Plant Pathol. 2012;13:842–851. doi: 10.1111/j.1364-3703.2012.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustaee M, Nazeri S, Ghadimzadeh M, Malboobi MA. Optimizing in vitro regeneration from Iranian native dwarf rootstock of apple (Malus domestica Borkh) Int J Agric Biol. 2007;9:775–778. [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajeevan RS, Nataraja KN, Shivashankara KS, Pallavi N, Gurumurthy DS, Shivanna MB. Expression of Arabidopsis SHN1 in Indian mulberry (Morus indica L.) increases leaf surface wax content and reduces post-harvest water loss. Front Plant Sci. 2017;8:418. doi: 10.3389/fpls.2017.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin KW, Baudinette SC, Graham MW, Michael MZ, Nugent GD, Lu C, Chandler SF, Cornish EC. Antisense ACC oxidase RNA delays carnation petal senescence. Hortic Sci. 1995;30:970–972. [Google Scholar]
- Schestibratov KA, Dolgov SV. Transgenic strawberry plants expressing a thaumatin II gene demonstrate enhanced resistance to Botrytis cinerea. Sci Hortic. 2005;106:177–189. doi: 10.1016/j.scienta.2005.03.016. [DOI] [Google Scholar]
- Shah DM, Horsch RB, Klee HJ, Kishore G, Winter JA, Tumer NE, Hironaka CM, Sanders PR, Gasser CS, Aykent S, Siegel NR, Rogers SG, Fraley RT. Engineering herbicide tolerance in transgenic plants. Science. 1986;233:478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Sharma S, Thakur AK, Srivastava DK. Plant regeneration and Agrobacterium-mediated gene transfer in Bell pepper (Capsicum annuum L.) Crop Improv. 2006;33:1–8. [Google Scholar]
- Shekhawat UKS, Ganapathi TR. MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One. 2013;8:e75506. doi: 10.1371/journal.pone.0075506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat UKS, Ganapathi TR, Srinivas L. MusaDHN-1, a novel multiple stress-inducible SK3-type dehydrin gene, contributes affirmatively to drought- and salt-stress tolerance in banana. Planta. 2011;234:915–932. doi: 10.1007/s00425-011-1455-3. [DOI] [PubMed] [Google Scholar]
- Shekhawat UKS, Ganapathi TR, Hadapad AB. Transgenic banana plants expressing small interfering RNAs targeted against viral replication initiation gene display high-level resistance to banana bunchy top virus infection. J Gen Virol. 2012;93:1804–1813. doi: 10.1099/vir.0.041871-0. [DOI] [PubMed] [Google Scholar]
- Shelton AM, Zhao JZ, Roush RT. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu Rev Entomol. 2002;47:845–881. doi: 10.1146/annurev.ento.47.091201.145309. [DOI] [PubMed] [Google Scholar]
- Shin R, Park JM, An JM, Paek KH. Ectopic expression of Tsi1 in transgenic hot pepper plants enhances host resistance to viral, bacterial and oomycete pathogens. Mol Plant Microb Interact. 2002;15:983–989. doi: 10.1094/MPMI.2002.15.10.983. [DOI] [PubMed] [Google Scholar]
- Shingles J, Lilley CJ, Atkinson HJ, Urwin PE. Meloidogyne incognita: molecular and biochemical characterization of acathepsin L cysteine proteinase and the effect on parasitism following RNAi. Exp Parasitol. 2007;115:114–120. doi: 10.1016/j.exppara.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Singh S, Rajam MV. Highly efficient and rapid plant regeneration in Citrus sinensis. J Plant Biochem Biotechnol. 2010;19:195–202. doi: 10.1007/BF03263340. [DOI] [Google Scholar]
- Singh D, Ambroise A, Haicour R, Sihachakr D, Rajam MV. Increased resistance to fungal wilts in transgenic eggplant expressing alfalfa glucanase gene. Physiol Mol Biol Plants. 2014;20:143–150. doi: 10.1007/s12298-014-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJS, Watson CF, Ray J, Bird CR, Morris PC, Schuch W, Grierson D. Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature. 1988;334:724–726. doi: 10.1038/334724a0. [DOI] [Google Scholar]
- Song A, Zhu X, Chen F, Gao H, Jiang J, Chen S. A chrysanthemum heat shock protein confers tolerance to abiotic stress. Int J Mol Sci. 2014;15:5063–5078. doi: 10.3390/ijms15035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Jia M, Chen K, Kong X, Khattak B, Xie C, Li A, Mao L. CRISPR/Cas9: a powerful tool for crop genome editing. Crop J. 2016;4:75–82. doi: 10.1016/j.cj.2015.12.002. [DOI] [Google Scholar]
- Sreedharan S, Shekhawat UKS, Ganapathi TR. MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol Biol. 2012;80:503–517. doi: 10.1007/s11103-012-9964-4. [DOI] [PubMed] [Google Scholar]
- Sripaoraya S, Keawsompong S, Insupa P, Power JB, Davey MR, Srinives P. Genetically manipulated pineapple: transgene stability, gene expression and herbicide tolerance under field conditions. Plant Breed. 2006;125:411–413. doi: 10.1111/j.1439-0523.2006.01229.x. [DOI] [Google Scholar]
- Stewart RJ, Sawyer BJB, Bucheli CS, Robinson SP. Polyphenol oxidase is induced by chilling and wounding in pineapple. Aus J Plant Physiol. 2001;28:181–191. [Google Scholar]
- Subramanyam K, Sailaja KV, Subramanyam K, Rao DM, Lakshmidevi K. Ectopic expression of an osmotin gene leads to enhanced salt tolerance in transgenic chilli pepper (Capsicum annum L.) Plant Cell Tiss Organ Cult. 2011;105:181–192. doi: 10.1007/s11240-010-9850-1. [DOI] [Google Scholar]
- Sugita K, Matsunaga E, Ebinuma H. Effective selection system for generating marker-free transgenic plants independent of sexual crossing. Plant Cell Rep. 1999;18:941–947. doi: 10.1007/s002990050688. [DOI] [Google Scholar]
- Sun S, Zhou L, Lu M, Cai M, Jiang XW, Zhang QX. Marker-free transgenic chrysanthemum obtained by Agrobacterium-mediated transformation with twin T-DNA binary vectors. Plant Mol Biol Rep. 2009;27:102–108. doi: 10.1007/s11105-008-0062-3. [DOI] [Google Scholar]
- Suzuki JY, Tripathi S, Fermin G, Hou S, Saw J, Ackerman CM, Yu Q, Schatz MC, Pitz KY, Yepes M, Fitch MMM, Manshardt RM, Slightom JL, Ferreira SA, Salzberg S, Alam M, Ming R, Moore PH, Gonsalves D (2008) Efforts to deregulate Rainbow papaya in Japan: molecular characterization of transgene and vector inserts. In: Second international symposium on papaya, 9–12 December 2008, Madurai, India, p 56
- Szankowski I, Waidmann S, Degenhardt J, Patocchi A, Paris R, Silfverberg-Dilworth E, Broggini G, Gessler C. Highly scab-resistant transgenic apple lines achieved by introgression of HcrVf2 controlled by different native promoter lengths. Tree Genet Genomes. 2009;5:349–358. doi: 10.1007/s11295-008-0191-8. [DOI] [Google Scholar]
- Tanaka Y, Katsumoto Y, Brugliera F, Mason J. Genetic engineering in floriculture. Plant Cell Tiss Org Cult. 2005;80:1–24. doi: 10.1007/s11240-004-0739-8. [DOI] [Google Scholar]
- Tang L, Kwon SY, Kim SH, Kim JS, Choi JS, Cho KY, Sung CK, Kwak SS, Lee HS. Enhanced tolerance of transgenic potato plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against oxidative stress and high temperature. Plant Cell Rep. 2006;25:1380–1386. doi: 10.1007/s00299-006-0199-1. [DOI] [PubMed] [Google Scholar]
- Tao R, Dandekar AM, Uratsu SL, Vail PV, Tebbets JL. Engineering genetic resistance against insects in Japanese Persimmon using the cryI(A)c gene of Bacillus thuringiensis. J Am Soc Hortic Sci. 1997;122:764–771. [Google Scholar]
- Thirukkumaran G, Khan RS, Chin DP, Nakamura I, Mii M. Isopentenyl transferase gene expression offers the positive selection of marker-free transgenic plant of Kalanchoe blossfeldiana. Plant Cell Tiss Organ Cult. 2009;97:237–242. doi: 10.1007/s11240-009-9519-9. [DOI] [Google Scholar]
- Tian N, Wang J, Xu ZQ. Overexpression of Na+/H+ antiporter gene AtNHX1 from Arabidopsis thaliana improves the salt tolerance of kiwifruit (Actinidia deliciosa) South Afr J Bot. 2011;77:160–169. doi: 10.1016/j.sajb.2010.07.010. [DOI] [Google Scholar]
- Tian S, Jiang L, Gao Q, Zhang J, Zong M, Zhang H, Ren Y, Guo S, Gong G, Liu F, Xu Y. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 2017;36:399–406. doi: 10.1007/s00299-016-2089-5. [DOI] [PubMed] [Google Scholar]
- Tripathi L, Mwangi M, Abele S, Aritua V, Tushemereirwe WK, Bandyopadhyay R. Xanthomonas wilt: a threat to banana production in east and central Africa. Plant Dis. 2009;93:440–451. doi: 10.1094/PDIS-93-5-0440. [DOI] [PubMed] [Google Scholar]
- Tsai-Hung H, Jent-turn L, Yee-yung C, Ming-Tsair C. Heterology expression of the Arabidopsis C-Repeat/Dehydration Response Element Binding Factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2002;130:618–626. doi: 10.1104/pp.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta R, Abe C, Watanabe T, Sugano SS, Ishihara R, Ezura H, Osakabe Y, Osakabe K. Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci Rep. 2017;7:507. doi: 10.1038/s41598-017-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh M, Deraison C, Kazemitabar SK, Rahbe Y, Jongsma MA. Aphid resistance in florist’s chrysanthemum (Chrysanthemum morifolium Ramat.) induced by sea anemone equistatin overexpression. Afr J Biotechnol. 2013;12:6922–6930. [Google Scholar]
- van der Krol AR, Lenting PE, Veenstra J, van der Meer IM, Koes RE, Gerats AGM, Mol JNM, Stuitje AR. An antisense chalcone synthase gene in transgenic plants inhibits flower pigmentation. Nature. 1988;333:866–869. doi: 10.1038/333866a0. [DOI] [Google Scholar]
- Vanblaere T, Szankowski I, Schaart J, Schouten H, Flachowsky H, Broggini GAL, Gessler C. The development of a cisgenic apple plant. J Biotechnol. 2011;154:304–311. doi: 10.1016/j.jbiotec.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Vasudevan A, Selvaraj N, Ganapathi A, Choi CW. Agrobacterium-mediated genetic transformation in cucumber (Cucumis sativus L.) Am. J Biotechnol Biochem. 2007;3:24–32. doi: 10.3844/ajbbsp.2007.24.32. [DOI] [Google Scholar]
- Vellice GR, Ricci JCD, Hernandez L, Castagnaro AP. Enhanced resistance to Botrytis cinerea mediated by the transgenic expression of the chitinase gene ch5B in strawberry. Transgenic Res. 2006;15:57–68. doi: 10.1007/s11248-005-2543-6. [DOI] [PubMed] [Google Scholar]
- Vieira P, Wantoch S, Lilley JL, Chitwood DJ, Atkinson HJ, Kamo K. Expression of a cystatin transgene can confer resistance to root lesion nematodes in Lilium longiflorum cv. ‘Nellie White’. Transgenic Res. 2015;24:421–432. doi: 10.1007/s11248-014-9848-2. [DOI] [PubMed] [Google Scholar]
- Wally O, Jayaraj J, Punja ZK. Broad-spectrum disease resistance to necrotrophic and biotrophic pathogens in transgenic carrots (Daucus carota L.) expressing an Arabidopsis NPR1 gene. Planta. 2009;231:131–141. doi: 10.1007/s00425-009-1031-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wisniewsky M, Meilan R, Cui M, Webb R, Fuchigamy L. Over-expression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. J Am Soc Hortic Sci. 2005;130:167–173. [Google Scholar]
- Wang Y, Wisniewski M, Meilan R, Cu M, Fuchigami L. Transgenic tomato (Lycopersicon esculentum) overexpressing cAPX exhibits enhanced tolerance to UV-B and heat stress. J Appl Hortic. 2006;8:87–90. [Google Scholar]
- Wang RK, Li LL, Cao ZH, Zhao Q, Li M, Zhang LY, Hao YJ. Molecular cloning and functional characterization of a novel apple MdCIPK6L gene reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Plant Mol Biol. 2012 doi: 10.1007/s11103-012-9899-9. [DOI] [PubMed] [Google Scholar]
- Wen XP, Ban Y, Inoue H, Matsuda N, Moriguchi T. Spermidine levels are implicated in heavy metal tolerance in a spermidine synthase overexpressing transgenic European pear by exerting antioxidant activities. Transgenic Res. 2010;19:91–103. doi: 10.1007/s11248-009-9296-6. [DOI] [PubMed] [Google Scholar]
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- Wisniewski M, Fuchigami L, Wang Y, Srinivasan C, Norilli J (2002) Overexpression of a cytosolic ascorbate peroxidase gene in apple improves resistance to heat stress. In: XXXVI International Horticultural Congress and Exhibition, p 147 (Abstr.)
- Xiangdong WEI, Congyul LAN, Zhijing LU, Changming YE. Analysis on virus resistance and fruit quality for T4 generation of transgenic papaya. Front Biol China. 2007;2:284–290. [Google Scholar]
- Yamamoto T, Iketani H, Ieki H, Nishizawa Y, Notsuka K, Hibi T, Hayashi T, Matsuta N. Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep. 2000;19:639–646. doi: 10.1007/s002999900174. [DOI] [PubMed] [Google Scholar]
- Yarra R, He SJ, Abbagani S, Ma B, Bulle M, Zhang WK. Overexpression of a wheat Na+/H+ antiporter gene (TaNHX2) enhances tolerance to salt stress in transgenic tomato plants (Solanum lycopersicum L.) Plant Cell Tiss Organ Cult. 2012;111(1):49–57. doi: 10.1007/s11240-012-0169-y. [DOI] [Google Scholar]
- Yoshikawa M, Tsuda M, Takeuchi Y. Resistance to fungal disease in transgenic tobacco plants expressing the phytoalexin elicitor-releasing factor, β-1,3-glucanase from soybean. Naturwissenschaften. 1993;80:417–420. doi: 10.1007/BF01168337. [DOI] [Google Scholar]
- Yu TA, Chiang CH, Wu HW, Li CM, Yang CF, Chen JH, Chen YW, Yeh SD. Generation of transgenic watermelon resistant to Zucchini yellow mosaic virus and Papaya ringspot virus type W. Plant Cell Rep. 2011;30:359–371. doi: 10.1007/s00299-010-0951-4. [DOI] [PubMed] [Google Scholar]
- Zainal Z, Marouf E, Ismail I, Fei CK. Expression of the Capsicuum annum (Chilli) defensin gene in transgenic tomatoes confers enhanced resistance to fungal pathogens. Am J Plant Physiol. 2009;4:70–79. doi: 10.3923/ajpp.2009.70.79. [DOI] [Google Scholar]
- Zhang X, Zou Z, Gong P, Zhang J, Ziaf K, Li H, Xiao F, Ye Z. Over-expression of microRNA169 confers enhanced drought tolerance to tomato. Biotechnol Lett. 2011;33:403–409. doi: 10.1007/s10529-010-0436-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li H, Shu W, Zhang C, Ye Z. RNA interference of a mitochondrial APX gene improves vitamin C accumulation in tomato fruit. Sci Hortic. 2011;129:222–226. [Google Scholar]
- Zhang HY, Liu HM, Liu XZ. Production of transgenic kiwifruit plants harboring the SbtCry1Ac gene. Genet Mol Res. 2015;14:8483–8489. doi: 10.4238/2015.July.28.16. [DOI] [PubMed] [Google Scholar]
- Zheng ZL, Yang Z, Jang JC, Metzger JD. Modification of plant architecture in chrysanthemum by ectopic expression of the tobacco phytochrome B1 gene. J Am Soc Hortic Sci. 2001;126:19–26. [Google Scholar]