Abstract
The efficient utilization of lignocellulosic biomass for ethanol production depends on the fermentability of the biomass hydrolysate obtained after pretreatment. In this work we evaluated the kinetics of ethanol production from xylose using Pichia stipitis in acid-treated corn cob hydrolysate. Acetic acid is one of the main inhibitors in corn cob hydrolysate that negatively impacts kinetics of xylose fermentation by P. stipitis. Unstructured kinetic model has been formulated that describes cell mass growth and ethanol production as a function of xylose, oxygen, ethanol, and acetic acid concentration. Kinetic parameters were estimated under different operating conditions affecting xylose fermentation. This is the first report on kinetics of xylose fermentation by P. stipitis which includes inhibition of acetic acid on growth and product formation. In the presence of acetic acid in the hydrolysate, the model accurately predicted reduction in maximum specific growth rate (from 0.23 to 0.15 h−1) and increase in ethanol yield per unit biomass (from 3 to 6.2 gg−1), which was also observed during experimental trials. Presence of acetic acid in the fermentation led to significant reduction in the cell growth rate, reduction in xylose consumption and ethanol production rate. The developed model accurately described physiological state of P. stipitis during corn cob hydrolysate fermentation. Proposed model can be used to predict the influence of xylose, ethanol, oxygen, and acetic acid concentration on cell growth and ethanol productivity in industrial fermentation.
Keywords: Acetic acid, Corn cob, Kinetic parameter estimation, Pichia Stipitis
Introduction
Bio-ethanol from the fermentation process is considered as a potential alternative fuel for gasoline. Being a renewable energy source, bio-ethanol has important advantages when compared to gasoline. The emission of green house gases and particulate materials from ethanol combustion is lower as it is an oxygen-rich fuel (Sensoz et al. 2000). Technology of bio-ethanol production from lignocellulosic biomass such as corn cob, sugarcane bagasse, and grasses offers great potential. Most of these agricultural feedstock contains both hexose and pentose sugars. Though hexose sugars are being utilized today for ethanol production, it is also possible to utilize xylose (pentose) for the ethanol fermentation (Wright 2006). Successful utilization of xylose would help in improving the process economics for lignocellulosic biomass to ethanol production. In several reports, yeast Pichia stipitis has been identified for efficient conversion of xylose to ethanol under micro-aerobic conditions (Agbogbo et al. 2006).
Lignocellulosic hydrolysate generated from the agricultural residues contains high concentration of inhibitors that negatively affect microbial growth. Inhibitors in lignocellulosic hydrolysate generated from pretreatment include weak organic acids, sugar-derived compounds such as furfural and hydroxymethyl furfural, and lignin degradation products. Formation of inhibitors in the pretreatment step of xylan hydrolysis is the main hurdle in the fermentation of xylose (Heer and Sauer 2008). The presence of inhibitors in the pretreatment varies with the feedstock and type of pretreatment applied. If the pretreatment is performed at high severity, it leads to generation of variety of strong inhibitors. Acetyl groups from the biomass are converted into acetic acid and hemicellulosic fraction can get converted into furfural as inhibitor. Another inhibitor is 5-hydroxymethyl furfural (HMF), which is generated from the glucose degradation. These inhibitors have been shown to negatively impact on the fermentation performance of S. cerevisiae, which was used for fermentation of mixed sugar of glucose and xylose (Pampulha and Loureiro 1989; Palmqvist and Hahn-Hagerdal 2000).
Acetic acid is a weak acid generated from the deacetylation of hemicelluloses during pretreatment. At low pH, acetic acid is in the undissociated form, which has a major impact on cell growth. Generation of toxins such as HMF and furfural can be controlled and these exhibit lesser inhibition on fermentation as compared to acetic acid. In biomass hydrolysate, acetic acid concentration varies in the range of 3–10 g L−1, depending on the feedstock and type of pretreatment. Therefore, effect of these inhibitors on ethanol yield and productivity must be studied for commercialization of lignocellulosic ethanol process. (Nichols et al. 2008; Pampulha and Loureiro-Das 2000; Abbott and Ingledew 2004; Graves et al. 2000; Wang and Liu 2014).
Acetic acid present in undissociated form in the fermentation broth diffuses through the cellular membrane and penetrates into the cytoplasm leading to acidic conditions inside the cell which ultimately leads to lower cell mass yield. Pumping of H+ protons for pH balance also leads to decrease in volumetric productivity of ethanol (Taherzadeh et al. 1997; Thomas et al. 2002; Maiorella et al. 1983). The inhibitory effect of acetic acid on the kinetics of the ethanol production by xylose isomerase-based S. cerevisiae has been presented by Bellissimi et al. (2009) and Casey et al. (2010).
Xylose to ethanol fermentation process could be further optimized by the development of realistic growth and fermentation models. In aerobic fermentation process, oxygen should be continuously supplied to achieve higher productivities. Ethanol yield maximization becomes challenging as higher oxygen supply increases the ethanol productivity only up to a threshold level. Beyond threshold level of oxygen supply, reduction in ethanol yield is observed due to respiratory action by cell mass (Hahn-Hagerdal et al. 1994). Farias et al. (2014) have proposed a model for P. stipitis consisting of a three-equation system including xylose, ethanol, and cell mass but it did not include the impact of dissolved oxygen and acetic acid on xylose fermentation. Slininger et al. (2014) have proposed a kinetic model for P. stipitis with additional equation for oxygen consumption rate with xylose, ethanol, and biomass concentration. All the above models have been developed based on experimental data in a synthetic xylose medium and did not account for the effects of inhibitors that are generated during acid hydrolysis of hemicelluloses.
This is the first report for development of a mathematical model which considers acetic acid inhibition for xylose to ethanol fermentation by P. stipitis. The focus of this work was to estimate the effect of xylose, ethanol, oxygen, and acetic acid concentration on rates of cell growth and ethanol production. Kinetic model consisting of linear differential equation has been developed that describes cell mass, ethanol production, xylose consumption, and oxygen concentration with time.
Material and analytical methods
Microorganism
Pichia stipitis ATCC 58784 was adapted by serial propagation in corn cob xylose hydrolysate. The adapted strain was designated as P. stipitis PSA30. It was maintained at −80 °C in the form of glycerol stock in xylose-rich hydrolysate.
Media and bioreactor assembly
Aerobic batch fermentations were carried out in 1-L New Brunswick BioFlow fermenters equipped with automatic pH and DO control. Oxygen supply in the fermenter was maintained by sparging air into the fermentation broth. All trials were conducted at constant aeration of 0.2 vvm. The inoculum for the fermenter was prepared by growing P. stipitis strain in 250 mL of YP media (1% yeast extract, 2% peptone) containing 3% xylose in a 500-mL flask. The inoculated flasks were incubated at 32 °C in a rotary shaker at 250 rpm for 24 h. Two-stage inoculations were done to prepare inoculum for main fermentation. After incubation, samples were analyzed for cell mass concentration and cell viability. Shake flask culture was used as inoculum (10%) for main fermentation of 1-L working volume in YP media with corn cob hydrolysate.
Lignocellulosic feedstock
Corn cob was used as a feedstock for studying xylose fermentation using P. stipitis. Corn plants were grown in Abhyudaya Biotech, Nashik (18.33°N 73.16°E), Maharashtra, India. Corn cobs (moisture content of 10%) were harvested in October 2014. Corncobs were stored in a dry place at room temperature. The dry matter content (DM) was estimated to be approximately 92% of the feedstock.
Pretreatment for corn cob
Corn cobs having total solids of about 92% by weight, containing cellulose (34.10%), hemicelluloses (29.12%), and lignin (12.90%) were used as feedstock. It was subjected to mechanical shearing (Hammer mill, Bhide & Sons, India) for size reduction to about 20–50-mm particle size. The size-reduced material was soaked in water for about 30 min at room temperature. The pretreatment was performed with 10–20% dry matter (DM) as per requirement in the experiment. The pretreatment was done in a continuous high-pressure digester with constant supplies of acid and steam to maintain desired temperature of 140–200 °C. The slurry was prepared with size-reduced corn cob at a total solid concentration of 30%. The slurry was mixed with sulphuric acid at a concentration of 2% w/w of corn cob dry solid (i.e., 0.6% w/w on slurry basis) and oxalic acid at a concentration of 1% w/w of corn cob dry solid (i.e., 0.3% w/w on slurry basis). The resultant reaction mixture was then subjected to hydrolysis in a digester at 160 °C and pressure of about 6 bar for 15 min. Due to the addition of steam and acid, the total solids of pretreated slurry were diluted to about 15–22% w/w. The acid hydrolysate was then transferred to filter press (Andritz USA) for separation of xylose-rich stream. Filter press operation was carried out under pressure of 12 bar absolute. Xylose stream obtained from the filtration contained between 3 and 5% w/w xylose depending on the initial solids content in pretreatment. The pH of the hydrolysate was adjusted to 5.5 for xylose fermentation by P. stipitis.
Fermentation
Corn cob hydrolysate fermentations were performed in bioreactors with 1-L working volume with two rushton impellers of six blades. Culture developed in shake flask was inoculated to fermentation media after adjusting pH to 5.5. The agitation was set to 150 rpm and temperature was controlled at 32 °C. Aeration rate was maintained at 0.2 vvm. Dissolved oxygen was monitored using O2 electrode (InPro 6800 Polarographic oxygen sensor, Mettler Toledo). The pH of the medium was controlled at 5.5 ± 0.2 using automatic addition of hydrochloric acid (3 N) and sodium hydroxide (3 N). Samples were drawn after every 6 h for analysis of biomass, sugars, and byproducts.
Analytical methods
Feedstock composition was determined by laboratory analytical procedures (LAPs) developed by NREL starting from sample preparation to summative mass closure (Sluiter and Sluiter 2011). Sugars (cellobiose, glucose, xylose), fermentation products (ethanol, xylitol, glycerol), and inhibitors (formic acid, acetic acid) were analyzed by high-performance liquid chromatography (HPLC) Agilent 1100 system equipped with UV and RI (Refractive index) detector. Bio-Rad Aminex HPX-87H column (300 × 7.8 mm I.D.) was used for separation of organic compounds at 55 °C. Sulfuric acid (0.005 M) was used as the mobile phase at a flow rate of 0.6 mL min−1. Culture samples were filtered using 0.2-μm filter and filtrate was loaded on the column.
For biomass analysis, samples were centrifuged at 8000 r.p.m. for 5 min and filtrate was discarded. Dry cell weights were measured by keeping centrifuged samples overnight in vacuum oven at 60 °C. Cell growth was also measured using spectrophotometer in terms of optical density at A 600nm. Cell viability was measured by dilution plating of samples at different time intervals.
Data processing-model fit
The accuracy of the kinetic model was evaluated using the residual standard deviation (RSD), where RSD is defined as:
| 1 |
where
where dp and Xp are values of selected variable predicted by model and those obtained from experimental trials at any given time. Model is more accurate when the value of RSD is lower.
Mathematical modeling
Mathematical equations describing rates of cell growth, substrate consumption, ethanol production, and oxygen consumption are given by:
| 2 |
| 3 |
| 4 |
| 5 |
The kinetics of cell death was considered to be negligible based on cell viability observed during experimental trials.
Monod model was used for the cell growth under oxygen-limited conditions (Eq. 6). Cell growth and maintenance were also found to be uniquely influenced by substrate and product inhibitions. To account for the effect of acetic acid inhibition and oxygen concentration on cell growth, Andrews and Levenspiel equations were modified (Eq. 7).
| 6 |
where μ is defined by
| 7 |
The new terms C A and C Amax describe the inhibition by acetic acid on yeast growth. It was observed that using mild conditions of pH and temperature in the pretreatment, furfural and HMF formation is reduced. Furfural and HMF inhibition terms were not considered in the model due to low concentration of these inhibitors in the xylose-rich stream.
The rate of substrate uptake is given by
where
| 8 |
The ethanol production by P. stipitis was defined as in Eq. (4)
where
| 9 |
The oxygen concentration in the fermentation broth is a function of oxygen uptake rate and the oxygen transfer rate.
| 10 |
Oxygen uptake rate is proportional to the cell mass concentration and the specific growth rate Y x/ox is defined as yield of biomass per unit oxygen consumed.
Results and discussion
Fermentation with pretreated corn cob hydrolysate
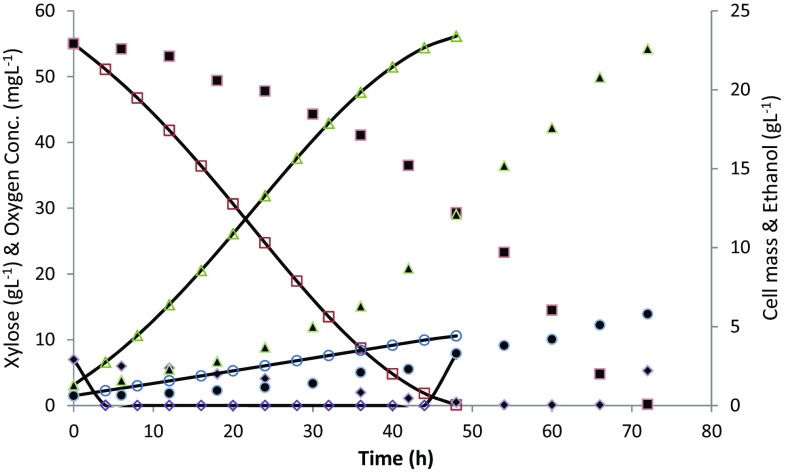
Pretreated corn cob hydrolysate was selected as a source of xylose for fermentation by P. stipitis. The acid hydrolysate stream was concentrated by evaporation to achieve the desired concentration of xylose in the fermentation broth. Experimental data of batch fermentation trials are mentioned in Table 1. It was observed that the batches with high initial xylose concentrations produced higher ethanol concentrations. The ethanol yield coefficient (Y p/s) was found to be dependent on initial substrate concentration as shown in Table 1. Earlier studies of kinetic modeling of xylose fermentation by P. stipitis were carried out in pure synthetic xylose (Slininger et al. 2014; Farias et al. 2014); however, in the present study, acid-treated corn cob hydrolysate stream was used for experimental data generation which mimicked actual process conditions expected in the industrial environment. The experimental observations during corn cob hydrolysate fermentation showed a significant reduction in the xylose consumption rate, which led to an increase in fermentation time and lower productivity as compared to synthetic xylose fermentation. The experimental data generated from corn cob hydrolysate fermentation was compared with the predicted values from the model proposed by Slininger et al. (2014) which includes terms of substrate inhibition, product inhibition, and effect of oxygen concentration on xylose fermentation. As expected, the model did not predict the fermentation profiles for pretreated corn cob hydrolysate as shown in Fig. 1. The major reason for poor fitting of Slininger model could be the absence of terms describing inhibition effects of major inhibitors such as acetic acid.
Table 1.
Experimental data for corn cob hydrolysate fermentation at various concentrations of pretreated xylose stream
| Initial xylose concentration (g L−1) | Acetic acid concentration @ 0 h (g L−1) | Fermentation time (h) | Residual xylose concentrationa (g L−1) | Maximum cell mass concentrationa (g L−1) | Maximum ethanol concentrationa (g L−1) | Ethanol yield (gg−1) |
|---|---|---|---|---|---|---|
| 10 | 0.8 | 16 | 0.3 (0.05) | 1.5 (0.14) | 5 (0.21) | 0.38 |
| 30 | 2.5 | 36 | 0.7 (0.1) | 3.4 (0.10) | 12.4 (0.7) | 0.38 |
| 40 | 3.2 | 48 | 0.5 (0.07) | 4.1 (0.14) | 16.3 (0.28) | 0.38 |
| 55 | 4 | 72 | 0.2 (0.07) | 5.8 (0.17) | 22.6 (0.35) | 0.39 |
| 100 | 6 | 176 | 2.3 (0.2) | 9.3 (0.21) | 41.6 (0.63) | 0.41 |
| 125 | 7 | 240 | 38 (0.8) | 7.9 (0.2) | 40.3 (0.4) | 0.45 |
aStandard deviations are mentioned in bracket
Fig. 1.
Experimental and simulated data by Slininger et al. model for acid-treated corn cob hydrolysate at 55 and 4 g L−1 initial xylose and acetic acid, respectively. Experimental data were shown by xylose (solid square); oxygen conc. (solid diamond); cell mass (solid circle); ethanol (solid triangle). Model-predicted data were shown by xylose (empty square); oxygen conc. (empty diamond); cell mass (empty circle); ethanol (empty triangle)
For developing a kinetic model for xylose fermentation using P. stipitis, it is necessary to consider different aspect of xylose utilization and substrate inhibition; ethanol inhibition, and inhibition due to byproducts present in biomass hydrolysate. Proposed kinetic model has been developed from basic equation suggested by Andrews and Levenspiel which describes substrate and product inhibition in the fermentation process. The modified model in the present study incorporates leanings of Andrews and Levenspiel, as well as Slininger to accurately predict xylose fermentation kinetics using P. stipitis in a process close to industrial environment. The proposed model was fitted with experimental data generated from corn cob hydrolysate fermentations to estimate the kinetic parameters.
Kinetic parameter estimation
Kinetic parameters such as μ max, K s, K La, Y p/x, and P max were evaluated based on experimental data of corn cob hydrolysate fermentation. Other parameters in Eqs. (2)–(10) such as n, m s, K ox, m, C Amax, and K I were estimated by unconstrained nonlinear optimization. The parameter estimation technique was based on minimization of an objective function, formed by experimental data and predicted values by the model simulation. For simultaneous estimation of kinetic parameters, a quasi-Newton algorithm was used.
| 11 |
where
is the vector of kinetic parameters.
Xen, Sen, Pen, and Coxen are experimental values of cell mass, substrate, ethanol, and oxygen concentration, respectively, at sampling times n.
X n, S n, and Coxn are simulated values by the model.
are the maximum values of the measured variables.
is the minimized error.
Set of ODE (ordinary differential equations) describing rates of cell growth, substrate consumption, product formation, and oxygen consumption was solved in MATLAB. Simultaneously, a least square objective function was minimized for estimation of kinetic parameters. The optimization toolbox FMINSEARCH in MATLAB was used for minimizing the objective function.
Model prediction
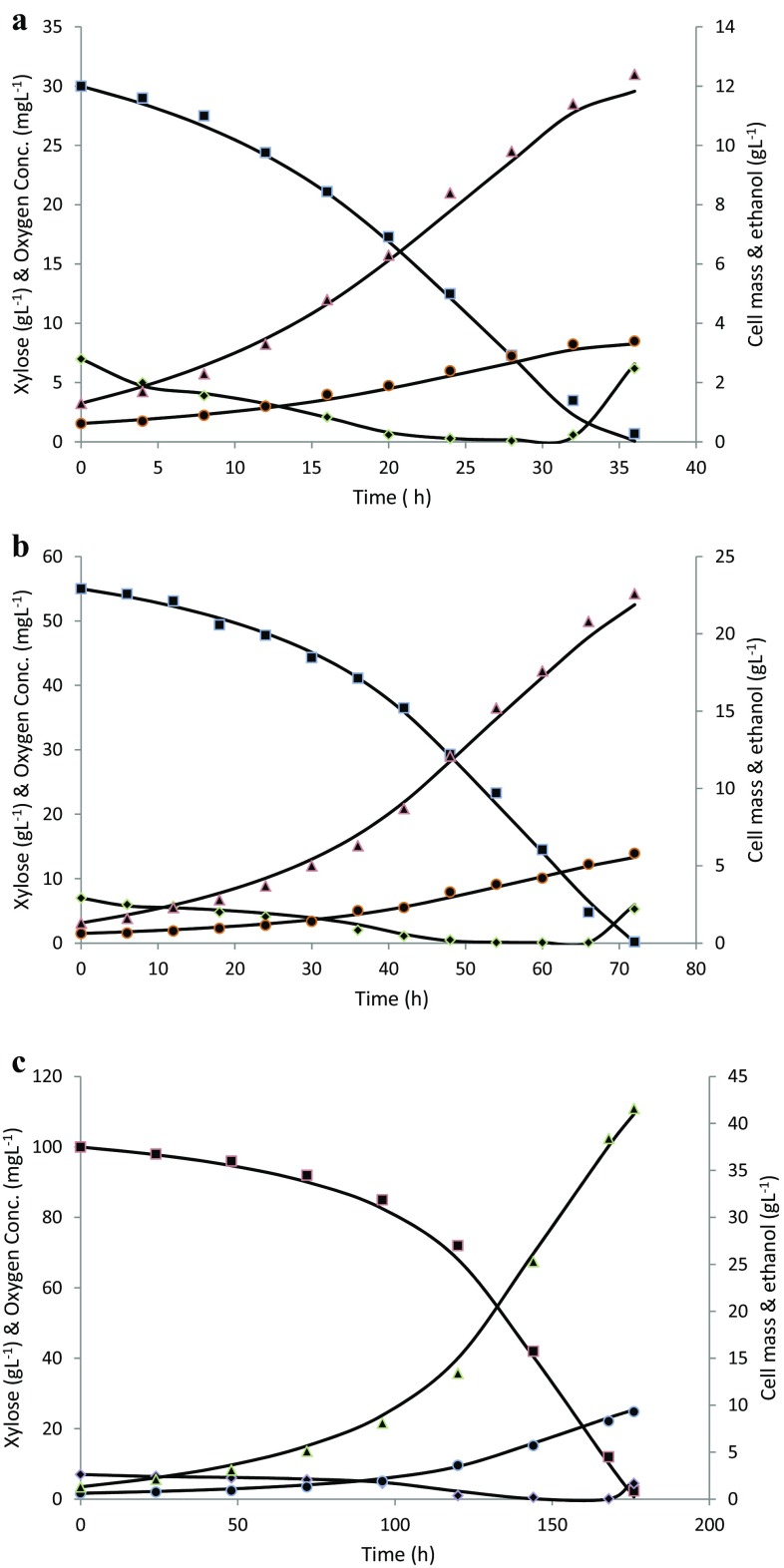
During fermentation of xylose by P. stipitis, initial substrate concentration adversely affected the specific growth rate and xylose consumption rate as shown in Fig. 4. To evaluate the influence of substrate concentration on cell growth, the K s and μ max values were calculated based on Monod equation and linearization method of Lineweaver–Burk plot. The constants of Monod equation were calculated in the range of lower xylose concentration where P. stipitis followed Monod kinetics. The values of K s = 2.9 g L−1 and μ max = 0.15 h−1 were calculated from the graph of 1/μ vs 1/S 0 based on experimental data at different initial xylose concentrations.
Fig. 4.
Experimental and simulated data by model (line) for various concentrations of xylose hydrolysate streams. a 30 g L−1 initial xylose; b 55 g L−1 initial xylose; c 100 g L−1 initial xylose; experimental data are shown for xylose (filled square); oxygen conc. (filled diagonal); cell mass (filled circle); ethanol (filled triangle)
Ethanol production by P. stipitis was defined by Luedeking–Piret equation.
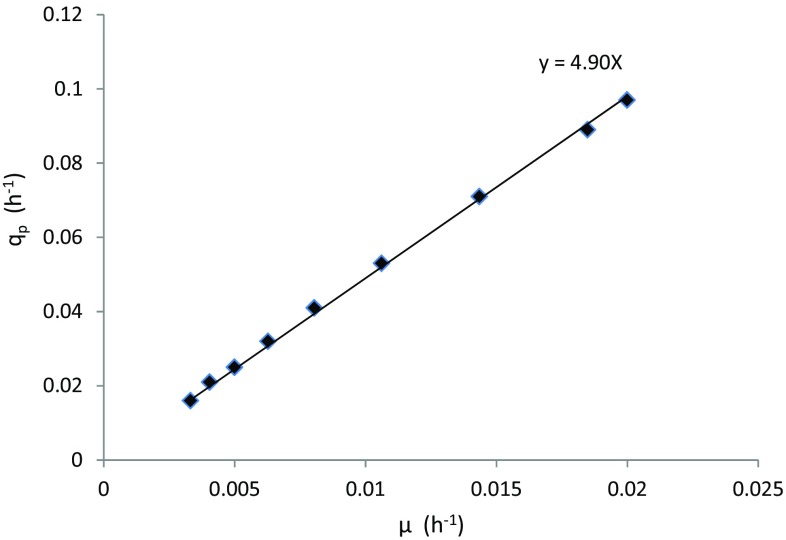
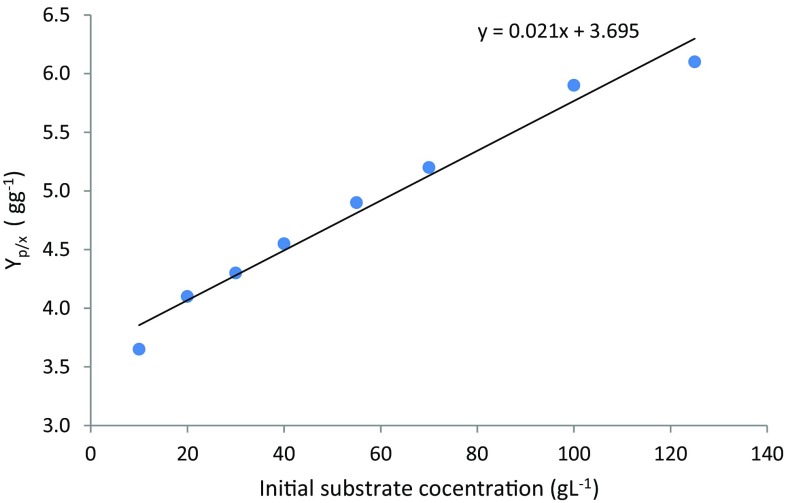
As the ethanol production by P. stipitis followed growth associated pattern, the value of was found to be negligible and was equivalent to Y p/x. Cell mass to product conversion factor (Y p/x) estimation was done by plotting values of μ and q p (from Eq. 9) as shown in Fig. 2. The slope of the line is equivalent to Y p/x as per Luedeking–Piret expression. It was observed from experimental data that the conversion factor Y p/x increased linearly with the increase in substrate concentration (as shown in Fig. 3), which showed that cells produced higher ethanol with increasing initial substrate concentration. Following co-relation describes the change in Y p/x with initial substrate concentration.
| 12 |
Fig. 2.
Determination of kinetic parameter Y p/x for batch experiment. Data are shown for acid-treated hydrolysate at 55 and 4 g L−1 initial xylose and acetic acid, respectively
Fig. 3.
Dependence of kinetic parameter Y p/x on initial xylose concentration
In the present work, the maximum ethanol concentration reached up to 41 g L−1 as compared to that reported in the literature in the range of 42–55 g L−1. The difference in the maximum ethanol concentration achieved was mainly due to the use of hydrolysate base media in the present study as compared to synthetic xylose fermentation as reported in the literature.
Based on experimental trials, values of key parameters μ max, K s, K La, and P max were found to be 0.15 h−1, 2.9 g L−1, 6.8 h−1, and 41.6 g L−1, respectively. Batch simulation was performed using Eqs. (2)–(12) and with parameters derived from experimental data of corn cob hydrolysate fermentation. To predict the fermentation profiles, kinetic parameters (Y x/s, n, m s, K ox, Y x/ox, m, K I, and C Amax) were estimated by unconstrained nonlinear optimization. Based on parameter estimation procedure, values of kinetic parameters such as Y x/s, n, m s, K ox, Y x/ox, m, K I, and C Amax were estimated to be 0.09 gg−1, 0.3, 0.001 gg−1 h−1, 0.12 mg L−1, 0.0024 g mg−1, 2.9, 0.12 g L−1, and 16 g L−1, respectively. The xylose fermentation profiles predicted by model simulation and those obtained from experimental trials are shown in Fig. 4.
The standard residual deviation described by Eq. (1) provides the average percentage deviation of the experimental and model-predicted values and was used to characterize the quality of model predictions. It was observed that the residual deviations were in the range of 2.4–14% for cell mass, xylose, ethanol, and oxygen concentration as shown in Table 2. Relatively low value of RSD (%) showed good quality of data fitting and accurate model prediction. In the case of oxygen, deviations were found to be higher as compared to other variables. This could be attributed to interference caused in oxygen measurement due to the presence of higher solids at increasing xylose concentration.
Table 2.
Residual standard deviations for experimental and model data after parameter estimation
| Initial xylose concentration (g L−1) | RSD (%) for variables | |||
|---|---|---|---|---|
| Cell mass | Xylose | Ethanol | Oxygen Conc. | |
| 10 | 6.38 | 5.68 | 7.15 | 7.84 |
| 30 | 7.01 | 3.30 | 5.36 | 6.49 |
| 40 | 6.37 | 2.39 | 5.31 | 10.81 |
| 55 | 6.55 | 2.40 | 5.80 | 12.86 |
| 100 | 6.07 | 3.01 | 5.13 | 14.08 |
| 125 | 5.26 | 2.43 | 4.80 | 12.14 |
Farias et al. (2014), Andrade et al. (2007), and Rivera et al. (2006) also used Levenspiel equation to evaluate product inhibition and reported that the model fitted satisfactorily with the experimental data. Main objective of the present work was to define the fermentation kinetics in the presence of inhibitors such as acetic acid. The proposed model was able to predict the fermentation profiles of xylose to ethanol fermentation with high accuracy. Xylose fermentation in the presence of acetic acid showed low value of maximum specific growth rate of 0.15 h−1 as compared to 0.23 h−1 in the absence of acetic acid. Similar observation was also reported by Narendranath et al. (2001) and Andrade et al. (2012) for glucose fermentation to ethanol in the presence of acetic acid using Saccharomyces cerevisiae.
It was observed that volumetric ethanol productivity decreased due to the presence of acetic acid in corn cob hydrolysate. Maximum ethanol productivity was found to be 0.53 g ethanol L−1 h−1 in the absence of acetic acid. With increase in acetic acid concentration from 4 to 7 g L−1, the ethanol productivity was decreased to 0.31 and 0.17 g ethanol L−1 h−1, respectively. Although major inhibition effect of acetic acid was on cell growth and fermentation time, the ethanol yields on xylose were not decreased when compared with the yields observed in the absence of acetic acid. It was observed that higher ethanol yield was achieved with increasing concentration of xylose and acetic acid. This could be attributed due to increase in Y p/x and reduction in substrate consumed for cell mass production. Lower cell mass yield is due to the acetic acid which is present in undissociated form in the fermentation broth at pH 5.5. Undissociated form of acetic acid diffuses through the cell membrane and penetrates to the cytoplasm leading to acidic conditions inside the cell. To maintain the internal pH, ATP is utilized by the cell to pump H+ protons out of the membrane leading to reduction in ATP pool available for cell growth. This ultimately leads to lower cell mass yield. Pumping of H+ protons for pH balance also leads to decrease in volumetric productivity of ethanol.
Model-predicted fermentation profile was compared with the experimental values as shown in Fig. 4. It showed that proposed model accurately described the kinetics of xylose fermentation in the presence of acetic acid. To validate the model, batch experiment was performed in corn cob hydrolysate with initial xylose concentration of 70 g L−1 and acetic acid concentration 5 g L−1. Model-predicted fermentation profile was compared with the experimental values. The RSD (%) values for model prediction and experimental data were found to be lower (<8%) for the cell growth, xylose, ethanol, and oxygen concentrations. Relatively lower RSD values suggest a good fit of experimental data to the proposed model.
Conclusion
In an industrial-scale lignocellulosic ethanol process irrespective of the choice of pretreatment, inhibitors such as acetic acid will be generated as it is a part of hemicellulosic side chain. It is very important to develop a kinetic model which can accurately predict fermentation profiles in an industrial-scale process. This is the first study on kinetic model development of xylose fermentation process which includes inhibition in terms of acetic acid along with other important parameters of substrate inhibition, product inhibition, and oxygen concentration. Presence of acetic acid in corn cob hydrolysate strongly affected μ max and Y p/x parameters. The proposed model can contribute towards development of an optimal process for ethanol production from xylose by P. stipitis. Approach described in this work can be used in process optimization, design and control, simulation, and optimization of the process which may aid to reduce the development costs of lignocellulosic ethanol technology. Our model can help in optimizing aeration rate and mixing conditions, help in selection of appropriate process configuration like fed batch or continuous process depending on substrate or product inhibition kinetics. Overall, kinetic modeling study can lead to reduction in production cost of ethanol from lignocellulosic biomass.
Acknowledgements
The authors gratefully acknowledge Praj industries Ltd. Pune for financial support to conduct the research work. The authors are also thankful to analytical team of Praj Matrix—R&D Center for providing assistance with analytical measurements.
Abbreviations
- dp′
Average of experimental values
- dp
Experimental value
- Xp
Value predicted by mathematical model
- np
Number of experiment points
- μ
Specific growth rate (h−1)
- qs
Specific substrate uptake rate (h−1)
- qp
Specific ethanol production rate (h−1)
- Cox
Dissolved oxygen concentration (mg L−1)
- X
Cell mass concentration (g L−1)
- μmax
Maximum specific growth rate (h−1)
- S
Xylose concentration (g L−1)
- KS
Saturation constant governing xylose-limited growth (g L−1)
- KI
Substrate inhibition constant for growth (g L−1)
- P
Ethanol concentration (g L−1)
- n
Exponents governing ethanol inhibition of growth
- Pmax
Maximum ethanol concentration allowing growth (g L−1)
- Kox
Saturation constant for oxygen-limited growth (mg L−1)
- CA
Acetic acid concentration (g L−1)
- CAmax
Maximum concentration of acetic acid at which cell growth ceases (g L−1)
- m
Parameter used to describe acetic acid inhibition
- Yx/s
Biomass yield (g g−1)
- mS
Maintenance coefficient (g g−1 h−1)
- Yp/x
Yield of ethanol per biomass formed (g g−1)
- KLa
Volumetric mass transfer coefficient (s−1)
- Cox*
Critical dissolved oxygen concentration (mg L−1)
- Yx/ox
Yield of biomass per oxygen consumed (g mg−1)
- h
Time (h)
Compliance with ethical standards
Conflict of interest
The author(s) declare that they have no competing or conflict of interest.
References
- Abbott DA, Ingledew WM. Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth of Saccharomyces cerevisiae and ethanol production. Biotech Lett. 2004;26:1313–1316. doi: 10.1023/B:BILE.0000044924.76429.71. [DOI] [PubMed] [Google Scholar]
- Agbogbo FK, Coward-Kelly G, Torry-Smith M, Wenger KS. Fermentation of glucose/xylose mixtures using Pichia stipitis. Process Biochem. 2006;41(11):2333–2336. doi: 10.1016/j.procbio.2006.05.004. [DOI] [Google Scholar]
- Andrade RR, Rivera EC, Costa A, Atala DIP, Maugeri F. Estimation of temperature dependent parameters of a batch alcoholic fermentation process. Appl Biochem Biotechnol. 2007;136(140):753–763. doi: 10.1007/s12010-007-9095-6. [DOI] [PubMed] [Google Scholar]
- Andrade RR, Rabelo SC, Filho RM, Costa A. Evaluation of the alcoholic fermentation kinetics of enzymatic hydrolysates from sugarcane bagasse (Saccharum officinarum L.) J Chem Technol Biotechnol. 2012 [Google Scholar]
- Bellissimi E, van Dijken JP, Pronk JT, van Maris AJA. Effect of acetic acid on the kinetics of xylose fermentation by an engineered, xylose-isomerase-based Saccharomyces cerevisiae strain. FEMS Yeast Res. 2009;9:358–364. doi: 10.1111/j.1567-1364.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Casey E, Sedlak M, Nancy WY, Mosier NS. Effect of acetic acid and pH on cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res. 2010;10:385–393. doi: 10.1111/j.1567-1364.2010.00623.x. [DOI] [PubMed] [Google Scholar]
- Farias D, Andrade RR, Maugeri F. Kinetic modeling of ethanol production by Scheffersomyces stipitis from xylose. Appl Biochem Biotechnol. 2014;172:361–379. doi: 10.1007/s12010-013-0546-y. [DOI] [PubMed] [Google Scholar]
- Graves T, Narendranath NV, Dawson K, Power R. Effect of pH and lactic or acetic acid on ethanol productivity by Saccharomyces cerevisiae in corn mash. J Ind Microbiol Biotechnol. 2000;33(6):469–474. doi: 10.1007/s10295-006-0091-6. [DOI] [PubMed] [Google Scholar]
- Hahn-Hagerdal B, Jeppsson H, Skoog K, Prior BA. Biochemistry and physiology of xylose fermentation by yests. Enzyme Microb Technol. 1994;16:933–943. doi: 10.1016/0141-0229(94)90002-7. [DOI] [Google Scholar]
- Heer D, Sauer U. Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb Biotechnol. 2008;1:497–506. doi: 10.1111/j.1751-7915.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorella B, Blanch HW, Wilke CR. By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng. 1983;25:103–121. doi: 10.1002/bit.260250109. [DOI] [PubMed] [Google Scholar]
- Narendranath NV, Thomas KC, Ingledew WM. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol. 2001;26:171–177. doi: 10.1038/sj.jim.7000090. [DOI] [PubMed] [Google Scholar]
- Nichols NN, Sharma LN, Mowery RA, Chambliss CK, van Walsum GP, Dien BS, Lten LB. Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzyme Microb Technol. 2008;42:624–630. doi: 10.1016/j.enzmictec.2008.02.008. [DOI] [Google Scholar]
- Palmqvist E, Hahn-Hagerdal B. Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol. 2000;74:17–24. doi: 10.1016/S0960-8524(99)00160-1. [DOI] [Google Scholar]
- Pampulha ME, Loureiro V. Interaction of the effects of acetic acid and ethanol on inhibition of fermentation in Saccharomyces cerevisiae. Biotechnol Lett. 1989;11:269–274. doi: 10.1007/BF01031576. [DOI] [Google Scholar]
- Pampulha ME, Loureiro-Das MC. Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol Lett. 2000;184(1):69–72. doi: 10.1111/j.1574-6968.2000.tb08992.x. [DOI] [PubMed] [Google Scholar]
- Rivera EC, Costa A, Atala DIP, Maugeri F, Maciel RW, Filho RM. Evaluation of optimization techniques for parameter estimation: application to ethanol fermentation considering the effect of temperature. Process Biochem. 2006;41:1682–1687. doi: 10.1016/j.procbio.2006.02.009. [DOI] [Google Scholar]
- Sensoz S, Angin D, Yorgun S. Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): fuel properties of bio-oil. Biomass Bioenergy. 2000;19:271–279. doi: 10.1016/S0961-9534(00)00041-6. [DOI] [Google Scholar]
- Slininger PJ, Dien BS, Lomont JM, Bothast RJ, Ladisch MR, Okos MR. Evaluation of kinetic model for computer simulation of growth and fermentation by Scheffersomyces (Pichia) stipitis fed d-xylose. Biotechnol Bioeng. 2014;111(8):1532–1540. doi: 10.1002/bit.25215. [DOI] [PubMed] [Google Scholar]
- Sluiter J, Sluiter A (2011) Laboratory analytical procedure (LAP) review and integration. https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html. Accessed 15 Oct 2015
- Taherzadeh MJ, Niklasson C, Liden G. Acetic acid—friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae. Chem Eng Sci. 1997;52:2653–2659. doi: 10.1016/S0009-2509(97)00080-8. [DOI] [Google Scholar]
- Thomas KC, Hynes SH, Ingledew WM. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic acid and lactic acids. Appl Environ Microb. 2002;68:1616–1623. doi: 10.1128/AEM.68.4.1616-1623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu S. Kinetic modeling of ethanol batch fermentation by Escherichia coli FBWHR using hot-water sugar maple wood extract hydrolyzate as substrate. Energies. 2014;7:8411–8426. doi: 10.3390/en7128411. [DOI] [Google Scholar]
- Wright L. Worldwide commercial development of bioenergy with a focus on energy crop-based projects. Biomass Bioenergy. 2006;30:706–714. doi: 10.1016/j.biombioe.2005.08.008. [DOI] [Google Scholar]