Abstract
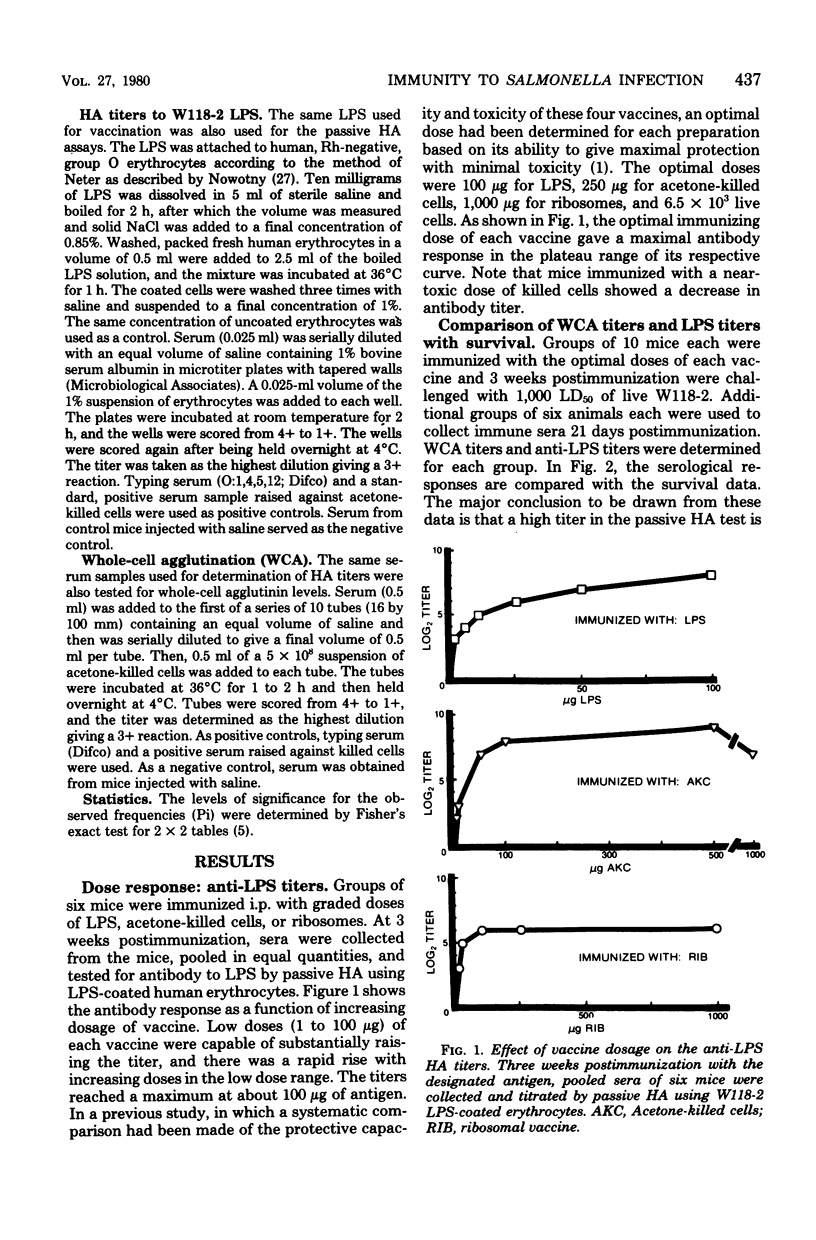
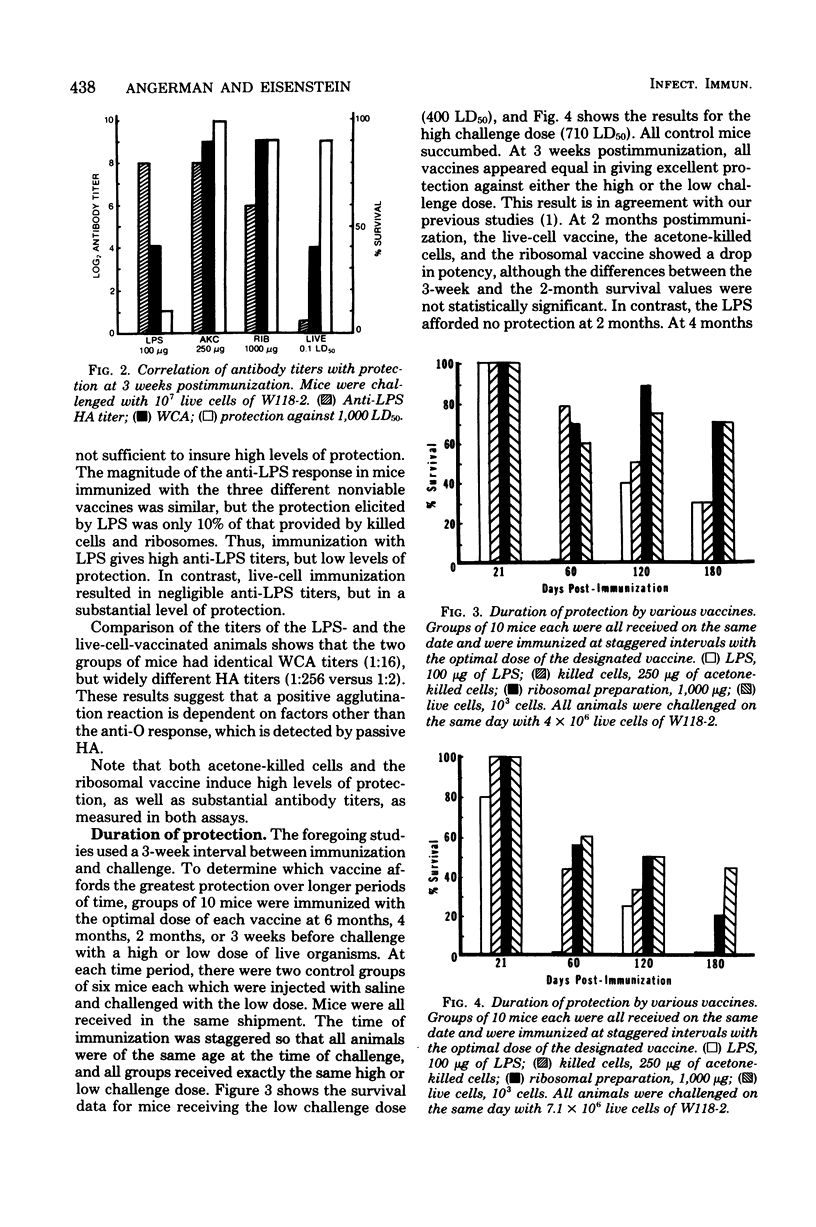
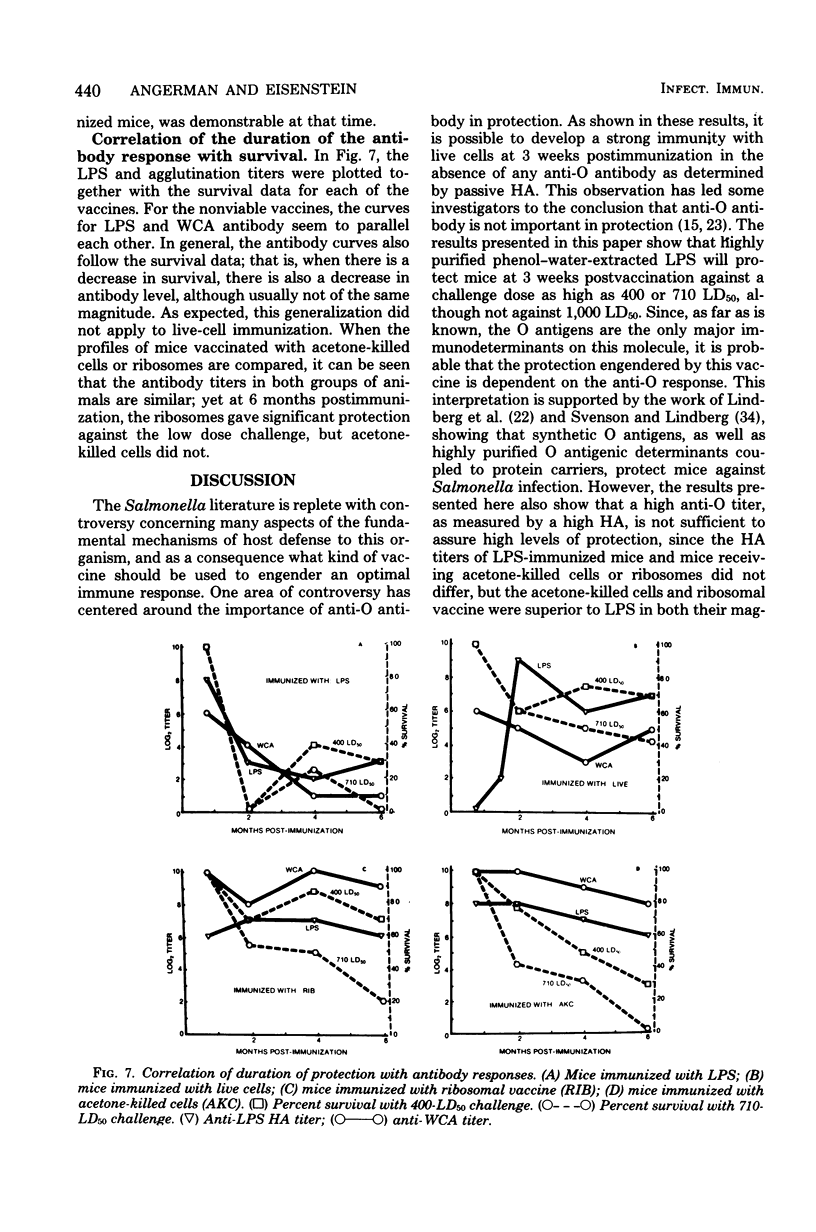
Groups of mice were immunized with optimal doses of the following vaccines of Salmonella typhimurium W118-2: acetone-killed cells, lipopolysaccharide, ribosomes, and live cells. At 3 weeks, 1 month, 2 months, 4 months, or 6 months postimmunization, sera were collected from control and vaccinated animals, and the anti-lipopolysaccharide and whole-cell agglutination titers of the sera were determined. Other groups of similarly vaccinated mice were tested for resistance to infection by challenging with live W118-2 and scoring the number of survivors 30 days postinfection. It was found that only ribosomes and live cells afforded significant protection 6 months after immunization. Thus, in duration of protection ribosomes were superior to the other nonviable vaccines tested. At all time intervals tested, purified lipopolysaccharide was the least effective vaccine. Protection afforded by the acetone-killed cell and ribosomal vaccines correlated better with the whole-cell agglutination titers than with the anti-lipopolysaccharide titers. However, the longer duration of protection afforded by the ribosomal vaccine, as compared with the acetone-killed vaccine, could not be accounted for by differences in whole-cell agglutination titers. These studies show that ribosomal vaccines are equal in all parameters to acetone-killed cells and have the advantage of providing longer-lasting immunity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft M. T., Singh B., Nicholson C. C., Ritchie J. M., Sorryan E., Williams F. A seven-year field trial of two typhoid vaccines in Guyana. Lancet. 1967 Nov 18;2(7525):1056–1059. doi: 10.1016/s0140-6736(67)90335-2. [DOI] [PubMed] [Google Scholar]
- Behling U. H., Nowotny A. Immune adjuvancy of lipopolysaccharide and a nontoxic hydrolytic product demonstrating oscillating effects with time. J Immunol. 1977 May;118(5):1905–1907. [PubMed] [Google Scholar]
- Behling U. H., Nowotny A. Long-term adjuvant effect of bacterial endotoxin in prevention and restoration of radiation-caused immunosuppression. Proc Soc Exp Biol Med. 1978 Mar;157(3):348–353. doi: 10.3181/00379727-157-40051. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Effect of adjuvant on immunogenicity of a heat-killed salmonella vaccine. J Infect Dis. 1972 Jul;126(1):69–76. doi: 10.1093/infdis/126.1.69. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey K. J., Cooper G. N. Oral immunization against experimental salmonellosis I. Development of temperature-sensitive mutant vaccines. Infect Immun. 1970 Mar;1(3):263–270. doi: 10.1128/iai.1.3.263-270.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Sypherd P. S. Extraction and isolation of individual ribosomal proteins from Escherichia coli. J Bacteriol. 1968 Aug;96(2):358–364. doi: 10.1128/jb.96.2.358-364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in experimental salmonellosis. 3. Comparative immunization with viable and heat-inactivated cells of Salmonella typhimurium. Infect Immun. 1972 May;5(5):792–797. doi: 10.1128/iai.5.5.792-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON D. Resistance to reinfection in experimental mouse typhoid. J Hyg (Lond) 1957 Sep;55(3):334–343. doi: 10.1017/s0022172400037244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Early antibody response in mice to either infection or immunization with Salmonella typhimurium. J Bacteriol. 1967 Mar;93(3):773–778. doi: 10.1128/jb.93.3.773-778.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashige S., Osawa N., Kawakami M., Mitsuhashi S. Experimental salmonellosis. X. Cellular immunity and its antibody in mouse mononuclear phagocytes. J Bacteriol. 1967 Oct;94(4):902–906. doi: 10.1128/jb.94.4.902-906.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDY M. Enhancement of the immunogenicity of typhoid vaccine by retention of the V1 antigen. Am J Hyg. 1953 Sep;58(2):148–164. doi: 10.1093/oxfordjournals.aje.a119596. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Rosenberg L. T., Ljunggren A., Garegg P. J., Svensson S., Wallin N. H. Effect of synthetic disaccharide-protein conjugate as an immunogen in Salmonella infection in mice. Infect Immun. 1974 Sep;10(3):541–545. doi: 10.1128/iai.10.3.541-545.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., KAWAKAMI M., YAMAGUCHI Y., NAGAI M. Studies on the experimental typhoid. 1. A comparative study of living and killed vaccines against the infection of mice with S. enteritidis. Jpn J Exp Med. 1958 Aug;28(4):249–258. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- Margolis J. M., Bigley N. J. Cytophilic macroglobulin reactive with bacterial protein in mice immunized with ribonucleic acid-protein fractions of virulent Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):390–397. doi: 10.1128/iai.6.3.390-397.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A., Wilson B. M. Protective effects of a supernatant factor from Salmonella typhimurium on Salmonella typhimurium infection of inbred mice. Infect Immun. 1978 Oct;22(1):125–131. doi: 10.1128/iai.22.1.125-131.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- Rowley D., Auzins I., Jenkin C. R. Further studies regarding the question of cellular immunity in mouse typhoid. Aust J Exp Biol Med Sci. 1968 Aug;46(4):447–463. doi: 10.1038/icb.1968.38. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Immunochemistry of Salmonella O-antigens: preparation of an octasaccharide-bovine serum albumin immunogen representative of Salmonella serogroup B O-antigen and characterization of the antibody response. J Immunol. 1978 May;120(5):1750–1757. [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USHIBA D., SAITO K., AKIYAMA T., NAKANO M., SUGIYAMA T., SHIRONO S. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn J Microbiol. 1959 Apr;3:231–242. doi: 10.1111/j.1348-0421.1959.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Ushiba D. Two types of immunity in experimental typhoid; "cellular immunity" and "humoral immunity". Keio J Med. 1965 Jun;14(2):45–61. doi: 10.2302/kjm.14.45. [DOI] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. The effect of metabolic inhibitors and hydroxylamine on the immune response in mice to mycobacterial ribonucleic acid vaccines. J Immunol. 1974 Jan;112(1):271–284. [PubMed] [Google Scholar]



