Abstract
Growing cells on microcarriers may have overcome the limitation of conventional cell culture system. However, the surface functionality of certain polymeric microcarriers for effective cell attachment and growth remains a challenge. Polycaprolactone (PCL), a biodegradable polymer has received considerable attention due to its good mechanical properties and degradation rate. The drawback is the non-polar hydrocarbon moiety which makes it not readily suitable for cell attachment. This report concerns the modification of PCL microcarrier surface (introduction of functional oxygen groups) using ultraviolet irradiation and ozone (UV/O3) system and investigation of the effects of ozone concentration, the amount of PCL and exposure time; where the optimum conditions were found to be at 60,110.52 ppm, 5.5 g PCL and 60 min, respectively. The optimum concentration of carboxyl group (COOH) absorbed on the surface was 1495.92 nmol/g and the amount of gelatin immobilized was 320 ± 0.9 µg/g on UV/O3 treated microcarriers as compared to the untreated (26.83 ± 3 µg/g) microcarriers. The absorption of functional oxygen groups on the surface and the immobilized gelatin was confirmed with the attenuated total reflectance Fourier transformed infrared spectroscopy (ATR-FTIR) and the enhancement of hydrophilicity of the surface was confirmed using water contact angle measurement which decreased (86.93°–49.34°) after UV/O3 treatment and subsequently after immobilization of gelatin. The attachment and growth kinetics for HaCaT skin keratinocyte cells showed that adhesion occurred much more rapidly for oxidized surfaces and gelatin immobilized surface as compared to untreated PCL.
Keywords: Microcarrier, Polycaprolactone (PCL), Ultra violet ozone (UV/O3), Gelatin immobilization, Surface modification
Introduction
Microcarrier cell culture system served two significant purposes. First, it is to mass produce large amounts of certain bioproducts such as recombinant proteins, hormones, and vaccines, where animal cells are routinely cultured in a bioreactor to meet industrial demand (van der Velden-de Groot 1995) and clinical trial stage (Goh et al. 2013). The second purpose is to serve as delivery of cultured cells (Seland et al. 2011). Therefore, materials with appropriate degradation rate are favorable in microcarrier cell culture system to reduce the effect of toxic degradation products. Polycaprolactone (PCL), a semi-crystalline polymer, undergoes a two-stage degradation process, the non-enzymatic hydrolytic cleavage of ester group (amorphous area) followed by the second stage of intracellular degradation (Chen et al. 2000). In a work on smooth muscle cells cultured on PCL microcarrier, the initial degradation products and chemical leaching from the polymer were found to be non-toxic to cells (Steynberg et al. 2012). This suggests that the slow degradation rate of PCL causes only relatively small local pH changes thus does not exert unfavorable effects on the cells.
Bulk chemistry, surface charge, wettability and hydrophilicity of the microcarrier surface have been reported to be the primary factors in determining cell behavior for in vitro cultures (Mano et al. 2007). Cells will grow on the outer surface of a microcarrier until they form a confluent monolayer. Each microcarrier can accommodate 100–200 cells. The density of a microcarrier is approximately 1.02–1.04 g/cm3, which is slightly higher than that of the culture medium (GE Healthcare handbook 2005). The ideal size for a microcarrier is in the 100–500 µm range and narrow size distribution is important to ensure size uniformity and easy maintenance in suspension at low agitation speeds (Bock et al. 2009; Zhou et al. 2013).
Several surface modification techniques have been developed to improve wetting, adhesion, and compatibility of polymer surfaces to cells (Ratner et al. Ratner and Bryant 2004). Surface modification methods, such as plasma treatment, gamma irradiation, ozone- or photo-induced grafting, ultraviolet/ozone (UV/O3) and chemical surface oxidation, have been employed to introduce functional groups on surfaces with minimal alteration of bulk properties, resulting in improved cell attachment, spreading, and proliferation (Yang et al. 2003; Ma et al. 2002; Shen et al. 2007; Yusilawati et al. 2010). Among these techniques, UV/O3 and plasma treatment are widely used.
UV/O3 has been shown to be a highly successful method for controlled modification of polymer and or production of surfaces for enhanced cell attachment (Teare et al. 2000, 2001; Mitchell et al. 2004). UV/O3 treatment has many advantages over other methods for surface modification of polymer because it is cost effective and involves only simple apparatus with no requirement of vacuum systems. UV/O3 treatment can also give precise control over the modification process even with the absence of any wet chemistry. Ozonation treatment with UV light can easily be carried out in various gasses, solvents and solutions at room temperature and this method is suitable for heat-unstable materials such as organic substrates (Clark et al. 2000; Davidson et al. 2004).
Recently, it became of interest to immobilize proteins or oligopeptides onto polymer surface as it significantly improves the biocompatibility of the polymer (Ma et al. 2002). Immobilization of biologically active molecules can generate a specific, predictable and controlled response from the cells seeded on the materials (Yuan et al. 2012). Several mechanisms can be employed in protein immobilization, such as physical or chemical adsorption, physical encapsulation and chemical combination (Kwon et al. 2006; Khan et al. 2007). Carbodiimide coupling reagents are the most popular type of zero-length crosslinker in the immobilization process. Water-soluble carbodiimides are the most common choice for biochemical conjugations because most macromolecules of biological origins are soluble in aqueous buffer solutions. Not only the carbodiimide itself is able to dissolve in the reaction medium, but the by-product of the reaction, an isourea, is also water-soluble, facilitating easy purification. Among the carbodiimide; EDAC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride) and EDAC/NHS (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride/N-hydroxysuccinimide) are frequently used to immobilize biomolecules such as gelatin on functionalized carrier (Zhu et al. 2002; Khan et al. 2007). EDAC/NHS-coupled reactions are highly efficient and can usually, increase the yield of conjugation than EDAC alone. Khan et al. (2007) claimed that EDAC/NHS strategy provides a good platform to improve the biocompatibility for various biomedical purposes.
In the present study, the surface polarity of PCL was modified using ultraviolet ozone (UV/O3) system with the goal to afford microcarriers that readily provide appropriate surface for gelatin immobilization with the introduction of surface hydroxyl, carbonyl, and carboxylic acid groups. The UV/O3 system involved a simple apparatus with no vacuum systems or sophisticated gas handling. The surface modification process was statistically optimized in order to achieve optimum gelatin immobilization, thus improved cell attachment on the microcarrier. Topographical and chemical changes induced upon optimization of PCL microcarrier surfaces were characterized using scanning electron microscopy (SEM), attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) and contact angle. Gelatin was then immobilized on the optimized surface-modified PCL microcarrier. Human keratinocytes (HaCaT) cell growth and expansion on the untreated, UV/O3 treated and UV/O3-gelatin coated PCL microcarriers were observed and compared.
Materials and methods
Materials, chemicals and reagents
Pellet polycaprolactone (PCL) (Mn = 45,000), dichloromethane (DCM), polyvinyl alcohol (PVA), sodium hydroxide and hydrochloric acid were supplied by Sigma-Aldrich (St. Louis, MO, USA). N-hydroxysuccinimide (NHS), sodium bicarbonate and sodium dodecyl sulfate (SDS) were purchased from Merck Millipore (Darmstadt, Germany). Toluidine Blue O (TBO) was supplied by Bendosen Laboratory Chemical (Selangor, Malaysia). 2-(N-morpholino) ethane sulfonic acid (MES), Ca2+ and Mg2+-phosphate buffer (PBS) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC) were purchased from EMD Chemical Inc. (Cincinnati, OH, USA). Pure oxygen gas (99%) was supplied by Linde Malaysia Sdn. Bhd (Selangor, Malaysia) and Total Collagen Assay Kit was purchased from QuickZyme Bioscience (Leiden, Netherlands). Dulbecco’s modification of Eagle’s medium (DMEM) in powder form, fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin, 0.1 g/l streptomycin) were supplied by Gibco (Grand Island, NY, USA). Accutase was obtained from Innovative Cell Technologies (San Diego, CA, USA) whereas bovine gelatin was purchased from Halagel (Kedah, Malaysia).
Cell line
A spontaneous immortalized continuous HaCaT (skin keratinocytes of adult human) was used throughout the study. The cell line was purchased from Cell Lines Service (CLS, Eppelheim, Germany) Cat. No. 300493. Cells were kept cryopreserved in liquid nitrogen until further use.
Preparation of PCL microcarriers
Polycaprolactone (PCL) microcarriers were prepared according to emulsion solvent evaporation process as described by Maia and Santana (2004) with several modifications. PCL (10 g) were dissolved in dichloromethane (90 ml) and the mixture was stirred on a hot plate magnetic stirrer (Ika C mag HS7, Staufen, Germany) to obtain a homogeneous solution. The dissolved mixture was then added dropwise into a polyvinyl alcohol (PVA) solution which acts as a surfactant. The resulting emulsion was stirred using an overhead stirrer (Ika RW 20 digital) at 250 rpm for 6 h at 25 °C. The microcarriers were collected by vacuum filtration (Sartorius Stedim, Göttingen, Germany) and were washed with distilled water and dried in 30 °C oven for 12 h. The particles were then stored in vacuum at 5 °C until ready to use.
Preparation of PCL film
Polycaprolactone (PCL) film was prepared by a solution casting method by dissolving PCL in dichloromethane (9 wt%). The dissolved PCL was placed in a glass Petri dish. The solvent was allowed to evaporate at 25 °C. The film was then removed from the Petri dish after overnight drying at 25 °C.
UV/O3 treatment process
The prototype system used for oxidization of microcarriers’ surface was set following Murakami et al. (2005). The system was equipped with an ozone generator (Absolute ozone Nano, Edmonton, Alberta, Canada) that can supply 5–10% (w/w) ozone with oxygen (O2) flow rate of 0.1–4 lpm. Pure supply of oxygen gas was controlled by a flow rate meter with maximum flow rate of 5 lpm at a constant standard working pressure of 20 psig. The O2 gas was channeled to an O2 inlet port lined with polytetrafluoroethylene (PTFE) gas tubing. Ozone was generated as the O2 gas flows through an electrical discharge and channeled out through the outlet port to the Dresher bottle placed in a self-fabricated UV box. UV-C germicidal lamps (Sanyo Denki, Tokyo, Japan) were mounted in the UV box. The lamps emit radiant energy of 253.7 nm wavelength and a controlled amount of 184.9 nm radiation. The Dresher bottle containing the microcarriers was mounted on an orbital shaker (Lab Companion SK 300, Seoul, Korea) to ensure even radiation on PCL microcarrier surface. Microcarriers were treated under direct exposure to ozone and irradiation with UV (air). They were treated by ozone aerations {direct exposure}, UV irradiation {air}, and the combination of ozone aeration with UV irradiation (UV/O3) in accordance to the experimental design.
Experimental design
The experimental run was design by statistical software, Design Expert® 7.0. The influence of ozone concentration (Factor A), PCL sample amount (Factor B) and exposure time (Factor C) on the concentration of carboxyl (COOH) groups (Y) deposited on the surface of microcarriers were evaluated using three levels faced centered composite design (FCCD) that require 20 formulations which include six replicates at the center point to represent process variations. This design was used to explore the quadratic response based on the second order polynomial model. The independent variables and their levels are presented in Table 1. A significant difference between the mean of three independent conditions was based on statistical analysis of variance (ANOVA).
Table 1.
Factors and levels used in 23 faced centered composite design (FCCD) for UV/O3 treatment on PCL microcarriers surface
| Variable | Unit | Symbol | Uncoded level | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Ozone concentration | ppm | A | 42,492.8 | 60,110.5 | 77,728.2 |
| Sample amount | g | B | 1 | 5.5 | 10 |
| Exposure time | min | C | 30 | 60 | 90 |
Quantification of carboxylic (COOH) functional group on microcarriers’ surface
Carboxylic (COOH) functional groups introduced on the surface of PCL microcarriers were determined by Toluidine Blue O (TBO) assay. TBO solution (0.1%) was prepared by dissolving the TBO powder in 1 mM NaOH. PCL microcarrier (1 g) was placed in the 0.1% TBO solution and incubated at 40 °C in an incubator shaker (Infors HT, Bottmingen, Switzerland) at 400 rpm for 30 min. Using vacuum filtration, the PCL microcarriers were then washed with 1 mM NaOH until the filtrate becomes colorless. This step ensures complete removal of any unbound dye that did not adsorb onto the surface. Collected microcarriers were placed in a tube containing 10 ml of 20% SDS. The microcarriers were then incubated at 40 °C and shaken at 1300 rpm for 30 min to desorb dyes from the microcarriers’ surface. Once the dyes were desorbed from the surface, microcarriers were pelleted by centrifugation at 25 °C, 12,000 rpm for 15 min. The dye intensity in the supernatant was then measured at 625 nm using a Multiskan™ GO plate reader (Thermo Scientific, Waltham, MA, USA). A standard curve was generated using TBO concentrations of 5, 10, 20, 50, 75, 100, 150, and 200 µM. Calculation of TBO concentration was performed using the following equation, fitting the curve to a power plot:
| 1 |
where A is the absorbance, k and n are constants, and c is the concentration of TBO in the solution.
Gelatin immobilization
The UV/O3-treated PCL microcarriers were covalently immobilized with gelatin using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDAC) and N-hydroxysuccinimide (NHS) (EDAC/NHS). The microcarriers were dispersed in 10 mg/ml EDAC and 5 mM NHS in MES buffer (50 mM, pH 6). The mixture was stirred and incubated at 4 °C for 24 h. The microcarriers were collected by vacuum filtration and washed with 50 mM MES buffer. The microcarriers were then dispersed in 100 ml MES buffer and incubated for another 10 min at 4 °C to ensure the unbound cross-linkers were totally washed out. Next, the microcarriers were collected by vacuum filtration and washed with distilled water. After washing, the microcarriers were directly immersed in 10 mg/ml gelatin solution. The mixture was incubated at 37 °C in an incubator shaker (Infors HT) at 100 rpm for 48 h. After incubation, the microcarriers were collected by vacuum filtration and dispersed (washed) in PBS solution for 30 min in an incubator shaker. The washing steps were repeated in distilled water prior to final retrieval using vacuum filtration and drying at 30 °C overnight. The dry gelatin immobilized PCL microcarriers were stored in a desiccator until ready to use.
Quantitative measurement of immobilized gelatin on microcarrier
Immobilized gelatin was analyzed using the method suggested by Prockop and Udenfriend (1960) employing hydroxyproline collagen assay. First, 300 mg of microcarriers were placed in 1.5 ml screw cap tube containing 6 M hydrochloric acid to hydrolyze the gelatin. The mixtures were then incubated at 95 °C for 22 h. After incubation, the tubes were cooled to room temperature prior to centrifugation at 1300 rpm for 10 min. The supernatant (35 µl) was pipetted into the appropriate well in a 96 well microplate and 75 µl of buffer assay was added to each well. The microplate was covered with adhesive seal lid and incubated at 25 °C for 20 min with shaking (IKA). Detection reagent was prepared fresh according to the ratio of reagent A and B (2:3). The detection reagent (75 µl) was then added to each well and the microplate was again covered with an adhesive seal lid. The microplate was momentarily shaken to mix the solution before incubating at 60 °C for 60 min. After the incubation, the microplate was cooled to room temperature prior to quantification of the amount of gelatin (hydroxyproline) at 570 nm.
Characterization
Characterization of the physically optimized surface of PCL microcarriers was carried out to elucidate the improvement of wettability and biocompatibility of the microcarriers. The water contact angle was measured using Phoenix 300 Contact Angle analyzer (S.E.O. Co., Inc, Gyeonggi-do, Korea) by means of sessile drop method. The drops image was captured by a video camera and Surfaceware 8 software was used to calculate the contact angle from the shape of the water drop on the raw (untreated), UV/O3 treated and gelatin coated PCL film. Next, the surface structure and morphology of PCL microcarriers were compared using scanning electron microscopy (Hitachi S3400 N, Tokyo, Japan). Surface chemistry of UV/O3 treated PCL was analyzed by attenuated total reflectance (ATR) mode using Nicolet™iS™ 50 FT-IR spectrometer (Thermo Scientific) to observe the addition of functional oxygen groups after UV/O3-treatment and the presence of amide bond on the gelation coated PCL microcarrier.
Microcarrier culture of HaCaT cells
Monolayer cultivation
Cryopreserved stocks of HaCaT cells were thawed and 1 × 105 cells/ml were seeded into 25 cm2 tissue culture flasks containing complete DMEM medium supplemented with 10% (v/v) FBS and 1% (v/v) of 100 U/ml penicillin, 0.1 g/l streptomycin. The flasks were incubated at 37 °C in a humidified 5% CO2 atmosphere. Upon reaching 80% confluence, cells were detached using accutase and subcultured at the same cell density in 175 cm2 culture flask. Cells were fed every other day by replacing 100% of the spent medium with fresh medium. Subsequently, cells were detached using accutase and then resuspended in the culture medium as described above, for use in microcarrier culture.
Microcarrier preparation
Polycaprolactone (PCL) microcarrier was subjected to sterilization using 70% ethanol. The microcarriers were dispersed in PBS, and once the microcarriers have settled at the bottom, the supernatant was decanted and the microcarriers were washed twice in 70% ethanol solution prior to overnight incubation in 70% (v/v) ethanol (50–100 ml per gram of microcarrier). The ethanol solution was removed and the microcarriers were rinsed three times in sterile PBS (50 ml/g microcarrier) and equilibrated in culture medium (50 ml/g microcarrier) before use.
Microcarrier culture cultivation
Inoculum for spinner flasks was obtained from static monolayer growth in 175 cm2 tissue culture flasks. Spent medium was discarded and the flask was washed twice with PBS and then incubated for 15 min in accutase until complete cell removal from the flask’s surface. The accutase was inactivated by the addition of fresh culture medium to the cell suspension. The suspension was then centrifuged at 100×g for 5 min. The supernatant was discarded and the remaining pellet was resuspended in 30 ml fresh complete medium. Cell concentration was determined using Neubauer hemocytometer under an inverted phase microscope (Olympus CK40, Tokyo, Japan). Five hundred milliliter spinner vessel (BellCo, Vineland, NJ, USA) with 200 ml working volume was coated with 5% silicon oil in ethyl acetate (to prevent microcarrier from attaching to the inner surface). PCL microcarriers (3 g/ml) suspended in 50 ml culture medium and cells at a concentration of 1.5 × 105 cell/ml were inoculated in the spinner flask and culture medium was added to a final volume of 200 ml. Samples (2 ml) of cell suspension were taken every 12 h and analyzed for cell viability and morphology.
Sampling and cell counting
One millilitre of microcarriers culture was aseptically pipetted out from the spinner flask culture and placed into a 15 ml tube. The microcarriers were allowed to settle. Supernatant was discarded. Microcarriers were washed twice with PBS before treatment with Accutase and the tube was incubated in CO2 incubator for 15 min at 37 oC. After 15 min the mixture was gently flushed to detach the immobilised cells. The concentration of cells in the suspension was determined using a haemacytometer with the aid of trypan blue. Twenty microlitres of cell suspension were mixed with an equal volume of trypan blue dye. Ten microlitres of the mixture were placed on the haemacytometer and allowed to spread by capillary action. Cells were counted under an inverted microscope and concentration of cells (cells/ml) was calculated using Eq. 2.
| 2 |
c = cell concentration (cells/ml), n = number of cells, v = volume counted (ml)
Standard heamacytometers used have the depth of chamber of 1 mm and the area of the central grid is 1 mm2. Therefore v = 0.1 mm3. The formula then becomes
| 3 |
or
| 4 |
If the cells were too concentrated, the cell suspension was diluted and the dilution factor was added in the calculation as follows:
| 5 |
Results and discussion
Effect of surface modification by ultraviolet (UV), ozone, and combination of UV/O3
The amount of carboxylic acid (COOH) group introduced on the surface of microcarriers treated with either UV, ozone, and combination of UV and ozone (UV/O3) treatment was measured (Fig. 1). The amount of COOH in the PCL sample was highest in the combination treatment of UV/O3 (836.32 nmol/g) as compared to untreated PCL (283.42 nmol/g), ozone treatment (348.65 nmol/g) and UV treatment (635.94 nmol/g). The high amount of surface COOH is expected to improve the wettability of the PCL microcarriers.
Fig. 1.
Amount of carboxylic acid (COOH) functional group introduced on the surface of untreated microcarrier and microcarrier treated with either UV/O3treatment, ozone aeration (only) treatment, UV irradiation (only) treatment, respectively. Oxygen flow rate (2 lpm), sample amount (1 g), exposure time (10 min) and UV intensity (22 mW/cm2) were held constant for the respective treatment
UV/O3 treatment is a practical technique to modify the surface of PCL microcarrier to provide preferable surface charge density for adsorption of proteins and anchoring dependent cells (Zhou et al. 2013). Ozone did not measurably change the polymer surface unless in the presence of energy (such as heat or light) that can degrade the ozone into molecular oxygen and oxygen atom which then reacts with the polymer surface (Callen et al. 1995). Meanwhile, UV irradiation resulted in scission of the ester linkage resulting in the increase of surface oxygen content on the polymer surface; thus increased COOH group on the PCL surface in the presence of air (Callen et al. 1995; Murakami, Fukushima and Hirano 2003). However, due to limitation of oxygen in atmospheric condition, the reaction is minimal. In the UV/O3 combination treatment, the effect is more pronounced where UV radiation activated and degraded the ozone into molecular oxygen and an oxygen atom, thus allowing ozone to react with the polymer surface.
Interaction of carboxylic functional group (COOH) and amount of immobilized gelatin
Recent investigations have demonstrated that immobilization of certain biologically active molecules on polymer surface is an effective strategy to promote cell adhesion and proliferation (Darain et al. 2010; Yuan et al. 2012; Zhu et al. 2002). For instance, gelatin was covalently immobilized on the PCL surface through the formation of a peptide bond between carboxylic acid and amine (NH2) group from gelatin (Zhu et al. 2002). Figure 2 shows a direct proportional interaction between COOH and amount of gelatin immobilized on the surface of the microcarrier. The concentration of COOH increased with time, subsequently, the amount of gelatin increased as the concentration of COOH increased.
Fig. 2.
Interaction between carboxyl (COOH) concentration on microcarrier surface and amount of gelatin immobilized. Kim et al. (2009) found that carboxyl functional group increased proportionally with the exposure time of oxygen plasma treatment. Subsequently, the efficiency of the protein immobilization on the treated surface was increased thus generating functional amine (NH2) group for cell adhesion
Optimization of process conditions of UV/O3 treatment
Based on the results shown in the previous sections, the UV/O3 combination treatment was found to be superior to the UV or ozone treatment alone. Therefore, the optimization of UV/O3 process conditions using central composite design was carried out with the aim to maximize the concentration of functional carboxylic acid (COOH) group introduced on the PCL microcarrier surface to enhance immobilization of gelatin. Table 2 shows the combined effects of three variables namely ozone concentration (A), the amount of samples (B) and exposure time (C) on the concentration of COOH. Based on the regression analysis, the second order polynomial model for concentration of COOH (Y nmol/g) was obtained as a function of variables and can be represented as follows:
| 6 |
Table 2.
Measured and predicted carboxyl (COOH) concentration in the experiments obtained by faced centered composite design (FCCD)
| Run | Experimental factors | Y (COOH conc) (nmol/g) | |||
|---|---|---|---|---|---|
| A (ppm) | B (gram) | C (min) | Measured | Predicted | |
| 1 | 60110.52 | 5.5 | 30 | 1044.95 | 993.43 |
| 2 | 42,492.8 | 5.5 | 60 | 1398.22 | 1406.19 |
| 3 | 42,492.8 | 1 | 30 | 510.99 | 551.02 |
| 4 | 42,492.8 | 10 | 30 | 1046.13 | 1026.83 |
| 5 | 77,728.24 | 5.5 | 60 | 1464.57 | 1452.39 |
| 6 | 60,110.52 | 1 | 60 | 1117.19 | 1027.76 |
| 7 | 42,492.8 | 10 | 90 | 1213.98 | 1185.64 |
| 8 | 42,492.8 | 1 | 90 | 948.64 | 948.28 |
| 9 | 77,728.24 | 10 | 30 | 1046.60 | 1048.01 |
| 10 | 60,110.52 | 5.5 | 60 | 1452.50 | 1432.96 |
| 11 | 60,110.52 | 5.5 | 60 | 1495.92 | 1432.96 |
| 12 | 77,728.24 | 1 | 90 | 999.16 | 1019.51 |
| 13 | 77,728.24 | 1 | 30 | 469.46 | 498.85 |
| 14 | 60,110.52 | 5.5 | 60 | 1445.57 | 1432.96 |
| 15 | 60,110.52 | 5.5 | 60 | 1476.90 | 1432.96 |
| 16 | 77,728.24 | 10 | 90 | 1369.19 | 1330.21 |
| 17 | 60,110.52 | 5.5 | 60 | 1481.53 | 1432.96 |
| 18 | 60,110.52 | 10 | 60 | 1335.80 | 1421.01 |
| 19 | 60,110.52 | 5.5 | 90 | 1285.86 | 1333.17 |
| 20 | 60,110.52 | 5.5 | 60 | 1236.93 | 1432.96 |
A = Ozone concentration, B = Amount of sample, C = Exposure time
The optimum process conditions were as in Run 11 with ozone concentration of 60110.52 ppm, amount of samples of 5.5 g and exposure time at 60 min with carboxyl concentration of 1495.92 nmol/g
COOH concentrations were found to be in the range of 469.46 nmol/g (run 13) to 1495.92 nmol/g (run 11) (Table 2). The optimum process conditions were as in Run 11 with ozone concentration of 60110.52 ppm, amount of samples of 5.5 g and exposure time at 60 min with carboxyl concentration of 1495.92 nmol/g.
The analysis of variance (ANOVA) of the model is shown in Table 3. The quality of the model can be analyzed by several measures. The first measure is the Fisher ratio (F-value) which demonstrates the significance of the model. F-value of the model (25.03) indicates that the model was highly significant and effect of factors was real (Anderson and Whitcomb 2015). The probability (p value) is also a measure of the significance of a regression model and each parameter that contributes to the model equation (Hinkelmann and Kempthorne 2008). The very low probability value (p < 0.0001) as seen in Table 3 ensures the valid measures of variation in the data about its mean. The smaller the value indicates the higher the significance of the model and the higher the relevance of the corresponding parameters contribution to the model. Model coefficients of all linear terms and quadratic terms except for ozone concentration were found to be significant with p value of <0.0001 and <0.05, respectively. Linear terms for the amount of samples (B) and exposure time (C) show the highly significant effects to the model (p < 0.0001). However, no interaction terms were significant. The significance of the model was also evaluated by lack-of-fit test, which measures the failure of the model to represent the data in the experimental domain at points that were not included in the regression (Gomathi and Neogi 2009). Lack of fit of the model was found to be insignificant which is strongly desirable for the model.
Table 3.
ANOVA for response surface quadratic model
| Source | Sum of squares | F-value | p value |
|---|---|---|---|
| Model | 1,648,458.77 | 25.03 | <0.0001 |
| A-ozone concentration | 5337.00 | 0.73 | 0.4130 |
| B-amount of sample | 38,6621.53 | 52.84 | <0.0001 |
| C-exposure time | 288,553.51 | 39.44 | <0.0001 |
| AB | 2689.67 | 0.37 | 0.5578 |
| AC | 7612.82 | 1.04 | 0.3317 |
| BC | 28,429.07 | 3.89 | 0.0770 |
| A2 | 37.16 | 0.005 | 0.9446 |
| B2 | 11,9636.98 | 16.35 | 0.0023 |
| C2 | 199,979.83 | 27.33 | 0.0004 |
R2 = 0.9575; Adj R2 = 0.9193; Pred R2 = 0.8433
In the present study, limited range of ozone concentration available from the ozone generator machine might contribute to the poor relationship between ozone concentration and concentration of carboxyl group deposited on the surface. This constraint was also encountered by Macmanus et al. (1999) where no clear relationship can be seen between ozone concentration and wettability of polymer surface.
The coefficient of determination, R2 = 0.9575 estimated for the above equation implies that 95.75% of the variability in the response (COOH concentration) could be explained by the model and that only 4% of total variation was not explained by the model. The adjusted R2 = 0.9193 was also found to be very high which is particularly useful when comparing models with a different number of terms, therefore, indicating a high degree of significance of the model (Anderson and Whitcomb 2015).
The interactive effects of process condition on gelatin immobilization
Three-dimensional response surface and two-dimensional contour plots of the parameter interactions which were plots to determine the optimal values of each parameters. The maximum response of the parameters and the optimum process conditions could be identified by obtaining an elliptical contour plot which means the perfect interaction between independence variable is achieved (Anderson and Whitcomb 2015). Figure 3 shows the effects of interaction between ozone concentration, exposure time and amount of samples on concentration of carboxylic acids introduced on the microcarrier surface.
Fig. 3.
3-D response surface and 2-D contour plot of interaction between a ozone concentrations and amount of samples; b exposure time and ozone concentration; c exposure time and amount of samples
These plots show that the COOH concentration increase with increase in factor level up to center points and then decreased for exposure time and amount of samples. The decrease in the COOH concentration as the amount of the sample increased after it reached maximum would be due to the less probability of the particle to be exposed to UV light and ozone caused by tight and limited space. The best interaction (elliptical contour plot) was between independent variables of exposure time and the amount of samples.
Amount of gelatin immobilized on UV/O3 treated microcarrier (gelatin coated PCL)
The introduction of oxygen functional group onto PCL microcarrier not only improved the hydrophilicity, indeed it provides a favorable site for biomacromolecules such as protein, peptide, polysaccharide or growth factor. The immobilization of gelatin onto the modified surface was achieved through covalent bonding using zero-length crosslinker as described in materials and methods section. The UV/O3 treated microcarriers obtained from the optimization work were used in this current phase of study and subsequent work.
Table 4 shows the amount of gelatin being immobilized onto the microcarrier before and after UV/O3 treatment. The measurement was based on the detection of hydroxyproline from the hydrolyzed gelatin that is immobilized on the microcarrier surface. There was an increase in concentration of gelatin immobilized on the microcarrier surface after the optimization of the process condition by 91.6% of the untreated PCL microcarrier.
Table 4.
Concentration of absorbed gelatin on untreated PCL microcarrier and UV/O3 treated PCL microcarrier
| Microcarrier | Concentration of immobilized gelatin (µg/g) | % increased |
|---|---|---|
| Untreated PCL | 26.83 ± 3.0 | NA |
| UV/O3treated | 320.00 ± 0.9 | 91.6 |
Characterization of UV/O3 treated PCL microcarrier and gelatin coated PCL microcarrier
To facilitate further discussion, the gelatin coated-UV/O3 treated microcarrier is referred to as ‘gelatin coated PCL’. In order to further clarify the effects of oxidization and introduction of functional groups on the surface of PCL microcarrier, the hydrophilicity and composition of the treated surface were investigated. Surface contact angle measures the hydrophilicity of the treated microcarrier and attenuated reflection–Fourier transform infra red (ATR-FTIR) confirmed the addition of functional groups on the microcarrier surface. The morphology of microspheres surface was studied by scanning electron microscope (SEM).
Contact angle measurement
The hydrophilic property of PCL microcarrier was evaluated by water contact angle that utilized de-ionized water as test liquid and by measuring the binding energy. The wettability of UV/O3 treated film was compared to the untreated and gelatin coated film. Table 5 shows the water contact angle values and surface energy of untreated PCL film, UV/O3 treated PCL film and gelatin coated PCL film. Treatment was made by relative measurement under similar condition as for microcarriers.
Table 5.
Contact angle values and surface energy of untreated, UV/O3 treated and UV/O3 treated and gelatin coated PCL microspheres
| Microcarrier | Contact angle (o) | Surface energy (mJ/m2) |
|---|---|---|
| Untreated PCL | 86.93 | 24.94 |
| UV/O3 treated PCL | 69.34 | 41.12 |
| Gelatin coated PCL | 49.34 | 61.28 |
From Table 5 the value of contact angle of untreated microcarrier (86.93°) imposed a relatively hydrophobic behavior. The angle was consequently decreased to 69.34° after UV/O3 treatment thus increased the surface energy from 24.94 to 41.12 mJ/m2. This could be due to the incorporation of functional oxygen-containing groups like O–C=O, C=O, C–O and OH (Darain et al. 2010) on the surface of the microcarrier. According to Gomathi and Neogi (2009) an increase in surface energy is due to the incorporation of the polar components on the surface by the presence of the polar groups, electric charges and free radicals. The introduction of functional and polar components on the PCL microcarrier surface not only improved its hydrophilicity but may also accommodate biomolecules components such as protein and cell growth factors to make the surface more biocompatible for cell growth and proliferation (Zhu et al. 2002). Drastic decrease in contact angle from 69.34° to 49.34° for gelatin coated PCL microcarrier was observed. This indicates further improvement in hydrophilicity as compared to UV/O3 treated PCL microcarrier which could be due to the presence of large amount of amino terminal and carboxyl groups (Yuan et al. 2012).
ATR-FTIR analysis
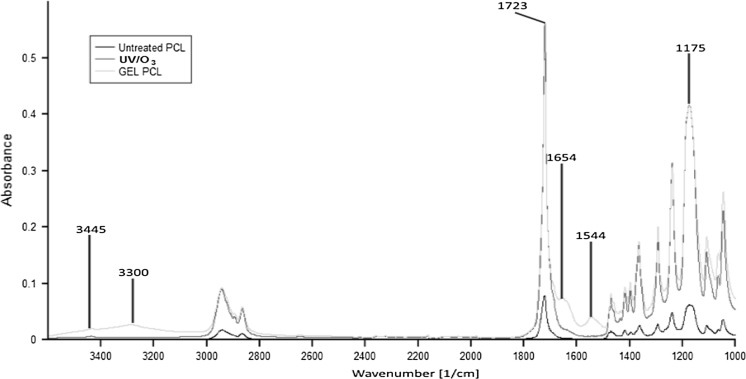
Attenuated total reflectance Fourier transformed infrared spectroscopy (ATR-FTIR) analysis was performed to determine the surface elements of PCL microcarriers. The peaks at 1723, 1175 and 1230 cm−1 (Fig. 4) are the signature peaks of polyesters which correspond to C=O, C–O–C and C–C, respectively, in the IR spectra (Kemala et al. 2012).
Fig. 4.
ATR-FTIR spectra of untreated PCL, UV/O3 treated PCL (UV/O3 PCL) and gelatin coated PCL (GEL PCL)
Interestingly, Fig. 4 shows the presence of a new peak in UV/O3 PCL spectra at wave number 3445 cm−1. This suggests the change in O–H group absorption resulted from oxidation process by UV/O3 could introduce O–H group in the main chain of PCL (Sabino 2007). Meanwhile, marked broadening in the region of 1600–1750 cm−1 could be attributed to the free acids, modification of chemical environment around the carbonyl group or the formation of vinyl groups (Wu 2002). Subsequently, the successful immobilization of gelatin onto the UV/O3 treated PCL surface could be deduced by the presence of a broadband at 3300 cm−1, possibly due to the overlapping of stretching vibrations of a hydroxyl group (O–H) and of an amine group (N–H). Increase in the relative intensity of amide I band (at 1654 cm−1) and amide II at (1544 cm−1)(Yuan et al. 2012) also attributed to the successful gelatin immobilization.
SEM analysis
The morphology of the PCL microcarrier was examined by SEM, as displayed in Fig. 5. The microcarriers were observed to be spherical with a uniform particle size in the range of 100–150 µm. The surface of untreated PCL (Fig. 5a) appears to be smoother than the surface of UV/O3 treated PCL microcarrier (Fig. 5b). Upon UV/O3 treatment the surface appears to crease and have plenty of holes which can be clearly seen in Fig. 5d.
Fig. 5.
SEM images show the untreated PCL microcarrier (a), UV/O3 treated PCL microcarrier (b), and gelatin coated PCL microcarrier (c). d, e and f are higher magnification of (a–c), respectively
Further, microcarriers coated with gelatin exhibited rather smooth surface as compared to UV/O3 treated PCL (Fig. 5c, f). The creases were covered by gelatins that were immobilized onto the surface through covalent bonding with intermediate crosslink. The immobilization was uniform over the microcarrier surface and this may help improve the biocompatibility of the microcarrier (Hong et al. 2005).
HaCaT growth on microcarrier culture
The importance of surface functionalization and wettability in microcarrier cell culture has been studied by many researchers due to its importance for cell growth and proliferation (Goh et al. 2013; Teare et al. 2000; Yanagisawa et al. 2006). The different types of PCL-based microcarriers studied in this present research (untreated PCL microspheres, UV/O3 treated PCL and gelatin coated PCL microcarrier) were further tested for their performance to support the attachment and growth of HaCatT cells. Figure 6 shows the growth kinetics of HaCaT cells on untreated PCL microcarrier, UV/O3 treated PCL microcarrier and gelatin coated PCl microcarrier.
Fig. 6.
Growth kinetics of human keratinocytes cells (HaCaT) on different microcarriers cultured in stirred spinner vessels (x) UV/O3 PCL, (filled circle) gelatin immobilized, (filled diamond) untreated PCL. Result were based on three independent experiment (n = 3, mean ± SD)
Microcarriers (3 g/l) were used to support cell growth in 500 ml spinner vessels with 200 ml medium (working volume) at a seeding concentration of 1.5 × 105 cells/ml for each type of microcarrier, respectively. Low agitation (30 rpm) was applied for the first 2 h to allow cells to attach and distribute efficiently. Subsequently, the agitation speed was increased to 60 rpm to maintain homogeneity of suspension culture, ensure good gas exchange and prevent aggregation of the microcarrier.
Cell attachment and growth are influenced by the types of the surface of a microcarrier (Goh et al. 2013; Murakami et al. 2005; Yusilawati et al. 2010). Figure 6 shows that the culture of untreated (raw) PCL was observed to have slight cell loss within 12 h of inoculation. The loss might be due to the low efficiency of cell attachment and spreading on the hydrophobic, uncharged microcarrier surface (Goh et al. 2013). It was also observed that there was no growth in cultures on untreated PCL (Table 6).
Table 6.
Growth kinetics of HaCaT cells on different types of microcarrier
| Microcarrier | Maximum cell concentration (×105 cells/ml) | Growth rate, µ (h−1) | Doubling time, td (h) |
|---|---|---|---|
| Untreated PCL | 1.1 ± 2.8 | - | - |
| UV/O3 PCL | 18.5 ± 9.8 | 0.0299 | 23.18 |
| Gelatin coated PCL | 17.6 ± 14.1 | 0.0257 | 27.02 |
Based on growth kinetics data (Table 6), UV/O3 treated microcarriers provide better surface for HaCaT cell attachment. The maximum cell concentration on UV/O3 PCL was 18.5 ± 9.8 cells/ml with a growth rate of 0.0299 h−1, which is observed to be slightly higher as compared to gelatin coated microcarrier with 17.6 ± 14.1 cell/ml maximum cell concentration and a cell growth rate of 0.0257 h−1. Moreover, the cells grown on UV/O3 PCL reached a doubling time of 23.18 h as compared to those grown on gelatin coated PCL (doubling time at 27.02 h).
The difference in HaCaT cell growth rate between the three types of microcarrier could be due to the absorption of different serum proteins (Jacobson and Ryan 1982). There are three different microcarriers with different surface properties (charged, uncharged, gelatin coated), which absorb different serum proteins that lead to differences in cell adhesion for each microcarrier. Yet there is very limited information on the interaction between serum proteins and the culture surface (Teare et al. 2001). There are two major classes of proteins in serum, fibrous and globular types (GE Healthcare handbook 2005). Globular proteins (albumin and γ-globulin (IgG)), types of non-adhesive protein, prevent cell attachment by blocking expression of fibrous protein or become competitively absorbed to the polymer surface. Meanwhile, fibrous proteins (fibronectin, vitronectin, type I collagen, hormone, growth factor and spreading factor) play a significant role in binding of a cell to the surface since they contain common sequences to all adhesive proteins, the ARG-GLY-ASP (RGD) sequence.
In the presence of 10% serum in the culture medium, as discussed by Teare et al. (2001), the hydrophobic surface is likely to absorb the non-adhesive proteins or the proteins that possess unfavorable conformation for the cells to attach. In contrast, as for the surface containing oxygen functional groups (UV/O3), the surfaces are made favorable to absorb proteins that promote cell attachment (Teare et al. 2001). An investigation conducted by Jacobson and Ryan (1982) discovered that due to the negative charges on the oxygen containing functional group surface, the amount of serum protein absorbed was about two or three times of that the positively charged surface and protein-coated surface. Experiments on oxidizing the culture dish using UV/O3 treatment conducted by Teare et al. (2001), show that fibronectin from serum preferentially absorbed on the surface. The combination effects of electrostatic interaction as well as favorable attachment site by serum protein enhance the HaCaT cell attachment rate onto the UV/O3 treated microcarrier, therefore, improved the cell concentration.
According to Zhou et al. (2013), each and every cell will have different attachment ligands and adherence forces to adhere to a surface. In the case of gelatin coating, it was reported that serum proteins were also absorbed on the surface but the amount was comparatively low as compared to negatively charged microcarrier (Jacobson and Ryan 1982). Gelatin may inherently contain specific biologically active cell attachment proteins which are favorable for HaCaT cell attachment and growth. In this present study, gelatin coated PCL may not show superior performance as compared to UV/O3 PCL for HaCaT cells but other types of cells, particularly stem cells may show specificity for attachment towards particular form of protein (gelatin).
Figure 7 shows the morphology of HaCaT cells on untreated PCL, UV/O3 PCL and gelatin coated PCL observed at 96 h of cultivation using inverted light microscope and SEM. At this time point the cells were in confluent state. Cells appeared to be flattened and spread over the surface of the microcarriers with comparatively large aggregates formed for UV/O3 PCL microcarrier.
Fig. 7.

Micrograph of HaCaT at 96 h on a untreated PCL, b UV/O3 PCL, c Gelatin coated PCL visualized using an inverted phase contrast microscope. SEM image of d HaCaT cells on UV/O3 PCL and e HaCaT cells on gelatin-coated PCL
Conclusion
Based on the statistical optimization, the optimum conditions for maximizing the carboxyl group concentration (1495.92 nmol/g) on the surface of PCL were as follows: ozone concentration of 60,110.52 ppm, sample amount of 5.5 g, and exposure time of 60 min. The effect of exposure time and sample amount was found to be significant as an interaction effect. Higher gelatin immobilization was achieved on UV/O3 treated (320 ± 0.9 µg/g) microcarriers as compared to the untreated (26.83 ± 3 µg/g) microcarriers indicating that the introduction of oxygen functional group on the surface of microcarriers was successful. Lastly, the growth kinetics of HaCaT cells were compared between untreated, UV/O3 treated and gelatin coated microcarrier. In the light of the selection of dermal related cells (keratinocytes) as model in this study, the aim was to demonstrate the usability of the biodegradable PCL microcarrier in tissue applications. Microcarriers offer the advantage of mass production of cells, large scale production of bioproduct, as well as more specific application such as transplantation tools to carry cells in wound healing therapies. Keratinocytes have been reported to have great potential for treating skin diseases such as genetic abnormalities, infections and skin cancer (Deyrieux and Wilson 2007). To this end, oxidization of PCL microcarrier surface by UV/O3 enhanced surface wettability and promote higher gelatin immobilization and improved cell adhesion and proliferation.
Acknowledgements
The authors are grateful to the Ministry of Higher Education Malaysia, for financing the research project (PRGS 11-001-0001) under the Prototype Development Research Grant Scheme (PRGS) and to the Department of Biotechnology Engineering, International Islamic University Malaysia for their support.
Contributor Information
Yumi Zuhanis Has-Yun Hashim, Phone: +603-61964442, Email: yumi@iium.edu.my.
Hamzah Mohd. Salleh, Phone: +603-61964495/5767, Email: hamzah@iium.edu.my
References
- Anderson MJ, Whitcomb PJ. DOE simplified: practical tools for effective experimentation. Florida: CRC Press; 2015. [Google Scholar]
- Bock A, Sann H, Schulze-Horsel J, Genzel Y, Reichl U, Möhler L. Growth behavior of number distributed adherent MDCK cells for optimization in microcarrier cultures. Biotechnol Progr. 2009;25:1717–1731. doi: 10.1002/btpr.262. [DOI] [PubMed] [Google Scholar]
- Callen BW, Lowenberg BF, Lugowski S, Sodhi RN, Davies JE. Nitric acid passivation of Ti6Al4V reduces thickness of surface oxide layer and increases trace element release. J Biomed Mater Res. 1995;29:279–290. doi: 10.1002/jbm.820290302. [DOI] [PubMed] [Google Scholar]
- Chen DR, Bei JZ, Wang SG. Polycaprolactone microparticles and their biodegradation. Polym Degrad Stab. 2000;67:455–459. doi: 10.1016/S0141-3910(99)00145-7. [DOI] [Google Scholar]
- Clark JT, Ruiz JD, Fan H, Brinker CJ, Swanson BI, Parikh AN. A new application of UV-ozone treatment in the preparation of substrate- supported mesoporous thin films. Chem Mater. 2000;12:3879–3884. doi: 10.1021/cm000456f. [DOI] [Google Scholar]
- Darain F, Chan WY, Chian KS. Performance of surface-modified polycaprolactone on growth factor binding, release, and proliferation of smooth muscle cells. Soft Mater. 2010;9:64–78. doi: 10.1080/1539445X.2010.520797. [DOI] [Google Scholar]
- Davidson MR, Mitchell SA, Bradley RH. UV-ozone modification of plasma-polymerised acetonitrile films for enhanced cell attachment. Colloids Surf B. 2004;34:213–219. doi: 10.1016/j.colsurfb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007;54:77–83. doi: 10.1007/s10616-007-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh TK-P, Zhang Z-Y, Chen AK-L, Reuveny S, Choolani M, Chan JKY, Oh SK-W. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. BioRes Open Access. 2013;2:84–97. doi: 10.1089/biores.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomathi N, Neogi S. Surface modification of polypropylene using argon plasma: statistical optimization of the process variables. Appl Surf Sci. 2009;255:7590–7600. doi: 10.1016/j.apsusc.2009.04.034. [DOI] [Google Scholar]
- Hinkelmann K, Kempthorne O. Design of experiment: introduction to experimental design. New York: Wiley; 2008. [Google Scholar]
- Hong Y, Gao C, Xie Y, Gong Y, Shen J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials. 2005;26:6305–6313. doi: 10.1016/j.biomaterials.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Jacobson BS, Ryan US. Growth of endothelial and HeLa cells on a new multipurpose microcarrier that is positive, negative or collagen coated. Tissue Cell. 1982;14:69–83. doi: 10.1016/0040-8166(82)90008-8. [DOI] [PubMed] [Google Scholar]
- Kemala T, Budianto E, Soegiyono B. Preparation and characterization of microspheres based on blend of poly(lactic acid) and poly(ε-caprolactone) with poly(vinyl alcohol) as emulsifier. Arab J Chem. 2012;5:103–108. doi: 10.1016/j.arabjc.2010.08.003. [DOI] [Google Scholar]
- Khan W, Kapoor M, Kumar N. Covalent attachment of proteins to functionalized polypyrrole-coated metallic surfaces for improved biocompatibility. Acta Biomater. 2007;3:541–549. doi: 10.1016/j.actbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Kim W-J, Kim S, Lee BS, Kim A, Ah CS, Huh C, Yun WS. Enhanced protein immobilization efficiency on a TiO2 surface modified with a hydroxyl functional group. Langmuir. 2009;25:11692–11697. doi: 10.1021/la901615e. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Coleman MA, Camarero JA. Selective immobilization of proteins onto solid supports through split-intein mediated protein trans-splicing. Angew Chem Int Ed. 2006;45:1726–1729. doi: 10.1002/anie.200503475. [DOI] [PubMed] [Google Scholar]
- Ma Z, Gao C, Ji J, Shen J. Protein Immobilization on the surface of poly-l-lactic acid films for improvement of cellular interactions. Eur Polym J. 2002;38:2279–2284. doi: 10.1016/S0014-3057(02)00119-2. [DOI] [Google Scholar]
- Macmanus LF, Walzak MJ, Mcintyre NS. Study of ultraviolet light and ozone surface modification of polypropylene. J Polym Sci, Part A Polym Chem. 1999;37(14):2489–2501. doi: 10.1002/(SICI)1099-0518(19990715)37:14<2489::AID-POLA23>3.0.CO;2-G. [DOI] [Google Scholar]
- Maia JL, Santana MHA. The effect of some processing conditions on the characteristics of biodegradable microspheres obtained by an emulsion solvent evaporation process. Braz J. 2004;21:1–12. [Google Scholar]
- Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interfac R Soc. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Poulsson HC, Davidson MR, Emmison N, Shard G, Bradley RH. Cellular attachment and spatial control of cells using micro-patterned ultra-violet/ozone treatment in serum enriched media. Biomaterials. 2004;25:4079–4086. doi: 10.1016/j.biomaterials.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Murakami TN, Fukushima Y, Hirano Y. Surface modification of polystyrene and poly (methyl methacrylate) by acti v e oxygen treatment. Colloid Surf B Biointerf. 2003;29:171–179. doi: 10.1016/S0927-7765(02)00189-3. [DOI] [Google Scholar]
- Murakami TN, Fukushima Y, Hirano Y, Tokuoka Y, Takahashi M, Kawashima N. Modification of PS films by combined treatment of ozone aeration and UV irradiation in aqueous ammonia solution for the introduction of amine and amide groups on their surface. Appl Surf Sci. 2005;249:425–432. doi: 10.1016/j.apsusc.2004.12.017. [DOI] [Google Scholar]
- Prockop DJ, Udenfriend S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Ratner DR, Bryant JB. Biomaterial: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- Sabino MA. Oxidation of polycaprolactone to induce compatibility with other degradable polyesters. Polym Degrad Stab. 2007;92:986–996. doi: 10.1016/j.polymdegradstab.2007.03.010. [DOI] [Google Scholar]
- Seland H, Gustafson C-J, Johnson H, Junker JPE, Kratz G. Transplantation of acellular dermis and keratinocytes cultured on porous biodegradable microcarriers into full-thickness skin injuries on athymic rats. Burns J Int Soc Burn Inj. 2011;37:99–108. doi: 10.1016/j.burns.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Shen H, Hu X, Yang F, Bei J, Wang S. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide) Biomaterials. 2007;28:4219–4230. doi: 10.1016/j.biomaterials.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Steynberg T, Visagie M, Mqoco T, Idicula A, Moolman S, Richter W, Joubert A. Qualitative assessment of smooth muscle cells propagated on 2D-and 3D- polycaprolactone polymers via scanning electron microscope. Biomed Res. 2012;23:191–198. [Google Scholar]
- Teare DOH, Emmison N, Bradley RH. Cellular attachment to ultraviolet ozone modified polystyrene surfaces. Langmuir. 2000;16:2818–2824. doi: 10.1021/la9907533. [DOI] [PubMed] [Google Scholar]
- Teare DOH, Emmison N, Ton-That C, Bradley RH. Effects of serum on the kinetics of CHO attachment to ultraviolet-ozone modified polystyrene surfaces. J Colloid Interface Sci. 2001;234:84–89. doi: 10.1006/jcis.2000.7282. [DOI] [PubMed] [Google Scholar]
- van der Velden-de Groot C. Microcarrier technology, present status and perspective. Cytotechnology. 1995;18:51–56. doi: 10.1007/BF00744319. [DOI] [PubMed] [Google Scholar]
- Wu C. Performance of an acrylic acid grafted polycaprolactone/starch composite: characterization and mechanical properties. J Appl Polym Sci. 2002;88:2888–2895. [Google Scholar]
- Yanagisawa K, Murakami TN, Tokuoka Y, Ochiai A, Takahashi M, Kawashima N. Immobilization and enzymatic activity of glucose oxidase on polystyrene surface modified with ozone aeration and UV irradiation in distilled water and/or aqueous ammonia solution. Colloids Surf B Biointerf. 2006;48(1):67–71. doi: 10.1016/j.colsurfb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Yang Y, Porte M, Marmey P, El Hai AJ, Amedee J, Baquey C. Covalent bonding of collagen on poly(L-lactic acid) by gamma irradiation. Nucl Instrum Methods. 2003;207:165–174. doi: 10.1016/S0168-583X(03)00456-7. [DOI] [Google Scholar]
- Yuan S, Xiong G, Roguin A, Choong C. Immobilization of gelatin onto poly(glycidyl methacrylate)-grafted polycaprolactone substrates for improved cell-material interactions. Biointerphases. 2012;7:30. doi: 10.1007/s13758-012-0030-1. [DOI] [PubMed] [Google Scholar]
- Yusilawati AN, Maizirwan M, Hamzah MS, Ng KH, Wong CS. Surface modification of polystyrene beads by ultraviolet/ozone treatment and its effect on gelatin coating. Am J Appl Sci. 2010;7:724–731. doi: 10.3844/ajassp.2010.724.731. [DOI] [Google Scholar]
- Zhou W, Ma G, Su Z. Microspheres for cell culture. In: Ma G, Su Z, editors. Microcapsules in biotechnology. New York: Taylor and Francis; 2013. [Google Scholar]
- Zhu Y, Gao C, Liu X, Shen J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules. 2002;3:1312–1319. doi: 10.1021/bm020074y. [DOI] [PubMed] [Google Scholar]