Abstract
Nowadays, human dental pulp stem cells (hDPSCs) became more attractive for therapeutic purposes because of their high proliferation and differentiation potential. Thus, coupling the desired cellular characteristics of hDPSCs with good biomaterial properties of the chitosan scaffolds provide an interesting approach for tissue engineering applications. On the other hand, scaffold surface modification is also needed to promote stem cell adhesion since chitosan lacks adhesion motifs to support direct cell anchorage. In this study, hDPSCs were isolated from third molars of healthy female individuals (aged 16–25) with enzymatic digestion. For cell culture studies, the chitosan scaffolds which have approximately 9 mm diameter and 2 mm thickness with interconnected structure were prepared by freeze-drying. To support cellular attachment the scaffolds were covalently immobilized with either RGD (arginine-glycine-aspartic acid) or fibronectin (Fn) molecules. Cells were seeded on chitosan scaffolds with or without immobilized RGD and fibronectin. Cell attachment, spreading, adhesion behaviors and proliferation capacity were examined by scanning electron microscopy, immunofluorescence staining and PrestoBlue® assays, respectively. In addition, differentiation potential of hDPSCs on Fn immobilized chitosan scaffolds was determined with real time reverse transcriptase polymerase chain reaction analysis. The results showed that chitosan scaffolds were not able to support stem cell attachment. hDPSCs on chitosan scaffolds formed spheroids more quickly and the size of spheroids were smaller than on chitosan-RGD while Fn-immobilized chitosan scaffolds strongly supported cellular attachment but not odontogenic differentiation. The results suggest that the Fn-immobilized chitosan scaffolds may serve as good three-dimensional substrates for dental pulp stem cell attachment and proliferation. In the case of dental regeneration, they must be supported by appropriate biosignals to induce odontogenic differentiation.
Keywords: Chitosan, Dental pulp stem cells, Fibronectin, RGD, Tissue regeneration
Introduction
Stem cell-based regenerative medicine often requires a scaffold or carrier system to deliver cells and/or specific growth factors to the injured site (Collart-Dutilleul et al. 2014). Among the materials used for scaffold fabrication, chitosan has been chosen as the most appropriate candidate compared with other natural polymers due to its excellence in biodegradability and biocompatibility and interest in chemical modifications with considerable and adjustable mechanical properties (Beşkardeş et al. 2015; Dutta et al. 2004; VandeVord et al. 2002). Stem cell-material interactions have been studied on the basis of surface chemistry, topography and molecular changes to achieve optimal and expected cellular responses (Murphy et al. 2014).
Recently, human dental pulp stem cells (hDPSCs) have been considered as more attractive adult stem cells for therapeutic purposes because of their high proliferation and differentiation potential, and easy isolation procedure (Karaoz et al. 2010; Vishwanath et al. 2013). It was shown that, hDPSCs can attach, spread, proliferate and differentiate into different cell populations on polymeric surfaces such as; polycaprolactone (D’Anto et al. 2016; Kim et al. 2014), polylactic acid (Chandrahasa et al. 2011) and collagen (Martens et al. 2014). On the contrary, some other stem cell types such as adipose tissue derived mesenchymal stem cells (MSCs) (Cheng et al. 2012) and human umbilical cord MSCs (Yeh et al. 2014) are able to attach onto chitosan surfaces but are not able to spread and proliferate. This may be attributed to the chemical structure of chitosan, which triggers cell detachment by activating some cell signaling pathways affecting cellular mobility (Huang et al. 2011; Hsu et al. 2012; Yeh et al. 2012, 2014). Cheng et al. (2012) have shown that human adipose tissue derived MSCs on chitosan films form spheroids, which display more stemness profile and better differentiation capabilities than their spread form. This is why, surface modification is needed to support stem cell attachment and proliferation on chitosan scaffolds (Cheng et al. 2012; Hsu et al. 2012; Yeh et al. 2014).
Cellular attachment and adhesion to extracellular matrix (ECM) occur by interactions of transmembrane cell adhesion proteins with the specific amino acid sequences in proteins such as RGD (Arg–Gly–Asp), sequence of fibronectin (Fn) and IKVAV (Ile–Lys–Val–Ala–Val) sequence of laminin (Arnold et al. 2004; Jongpaiboonkit et al. 2009). This is why, ECM proteins or their small peptide sequences immobilized on biomaterials are often used to increase surface bioactivity, cell adhesion and proliferation (Liu et al. 2010; de Mel et al. 2008). Several studies have focused on the immobilization of Fn onto surfaces using covalent coupling chemistries via the amine, thiol and aldehyde groups to obtain stable surface modification (Biran et al. 2001; Custodio et al. 2010). The synergic interactions of peptides such as RGD and PHSRN (Pro–His–Ser–Arg–Asn) of Fn may support cell binding, proliferation and differentiation more effectively. Thus, the use of whole protein is preferred instead of short peptides (Custodio et al. 2010).
In this study, chitosan (Ch) scaffolds were prepared by freeze-drying method. Surface modifications were carried out using RGD sequence or Fn molecule via carbodiimide reaction. Cell culture studies were performed with hDPSCs obtained from third molars of healthy female individuals. In this study, we wanted to compare effectiveness of immobilized RGD and Fn onto chitosan scaffolds for the adhesion and proliferation behaviour of hDPSCs. Cells were seeded on chitosan-based scaffolds with or without 1% (w/v) gelatin solution to investigate the effect of gelatin on the cellular interactions with scaffolds. The attachment and proliferation of hDPSCs on scaffolds were determined by scanning electron microscopy (SEM), proliferation assays and immunofluorescence staining of cell cytoskeleton and nucleus. Also odontogenic differentiation potential of hDPSCs on Fn-immobilized chitosan scaffolds was determined with real time reverse transcriptase polymerase chain reaction (RT-PCR) analysis.
Materials and methods
Materials
Medium molecular weight chitosan (75–85% deacetylation degree), derived from crab shell and acetic acid were purchased from Sigma-Aldrich (Munich, Germany) and Riedel–de–Haen (Seelze, Germany), respectively. Phosphate buffer saline (PBS, pH 7.4) tablets, type A gelatin, Arginine-glycine, aspartic acid (RGD) peptide, fibronectin, 2-(N-morpholino) ethanesulfonic acid (MES) sodium salt, 1-ethyl-3-dimethylaminopropyl carbodiimide (EDC) and ninhydrin were obtained from Sigma. N,N-dimethyl formamide (DMF) and N-hydroxysuccinimide (NHS) were purchased from Merck (Darmstadt, Germany) and Fluka (Buchs, Switzerland), respectively. Paraformaldehyde was obtained from Sigma-Aldrich.
For cell culture studies, collagenase type I was obtained from Biochrom AG (Berlin, Germany). Minimal Essential Medium Alpha Modification (α-MEM) and amphotericin B were purchased from Lonza (Basel, Switzerland). Fetal bovine serum (FBS), trypsin/EDTA solution, penicillin–streptomycin, HEPES, Triton-X 100 and bovine serum albumin (BSA) were obtained from Sigma. Dulbecco’s phosphate buffer solution (DPBS) and isopropanol were purchased from Hyclone (Logan, UT, USA) and Aklar Kimya (Istanbul, Turkey), respectively. Hexamethyldisilazane (HMDS) and hydrochloric acid were obtained from Merck. PrestoBlue® which is used for cell viability analysis, was obtained from Invitrogen (Carlsbad, CA, USA). DAPI (diamidino-2-phenylindole) and Alexa Fluor 488 Phalloidin were purchased from Thermo Scientific (Waltham, MA, USA) and Invitrogen, respectively.
Preparation of chitosan scaffolds
Chitosan scaffolds were prepared by freeze-drying method that has been reported previously (Beşkardeş et al. 2015; Tigli et al. 2007). Briefly, chitosan (2%, w/v) was dissolved in 0.2 M acetic acid solution and poured into 24-well tissue culture polystyrene dishes (TCPS), then they were frozen at −20 °C overnight. Afterwards, scaffolds were transferred into freeze-dryer (Christ, Osterode am Harz, Germany) at −80 °C and lyophilized for 4 days. Once completely dried, scaffolds were placed in 96% (v/v) ethanol overnight and then, stabilized in 70% (v/v) ethanol for 1 h. The scaffolds were cut into 9 mm diameter and 2 mm thickness for cell culture studies.
RGD peptide immobilization
In the present study, chitosan scaffolds were modified with universal cell binding peptide, RGD. Peptides were conjugated onto chitosan surface via carbodiimide reaction, an imide-bond forming between amino groups on chitosan and carboxyl groups on peptides. Immobilization was performed in a similar way as reported in a previous study (Ho et al. 2005). RGD peptide (0.2 mM in DMF), EDC (2.5 mg/mL in DMF) and NHS (5.3 mM in PBS) solutions were prepared and mixed in 25:25:1 (v/v/v) ratio. For immobilization, chitosan scaffolds were immersed into activated peptide solution at 4 °C for 72 h. After the immobilization, scaffolds were washed with PBS (pH 7.4) for several times and used for cell culture studies.
Fibronectin immobilization
Fibronectin was covalently immobilized on chitosan scaffolds via carbodiimide chemistry by using a reported procedure (Custodio et al. 2010). Fibronectin was dissolved in ultrapure water and then, mixed with a solution containing EDC (2 mM in MES buffer) and NHS (5 mM in MES buffer), resulting in 100 µg/mL Fn concentration, in order to pre-activate the Fn by reaction with their carboxyl groups. The activation process was performed at room temperature on a shaker for 20 min. Then, 100 µL Fn solution was added onto each scaffold and the scaffolds were kept at room temperature for 4 h to immobilize Fn with the interactions of carboxyl groups of Fn and amino groups of chitosan.
Ninhydrin assay was used to determine the covalently-linked RGD and Fn on the surface of chitosan scaffolds after immobilization. Briefly, 1.0 M ninhydrin solution was prepared in 96% (v/v) ethanol and scaffolds were immersed into the solution for 1 min. Right after, scaffolds were transferred into dark colour glass bottle and heated at 70 °C in a water bath for 10 min. Then, 2 mL tetrahydrofuran and 2 mL isopropanol solutions were added to the samples and kept in ultrasonic bath for 10 min. The solution was placed in a quartz cuvette and the absorbance was measured at 560 nm by UV–Vis double beam PC (Labomed, Los Angeles, CA, USA) spectrophotometer.
Cell culture studies
Cell isolation and characterization
Cell culture studies were carried out with human dental pulp stem cells (hDPSCs) isolated from third molars of healthy female individuals (aged 16–25). All experiments were done under approval of the Non-invasive Clinical Research Ethic Committee of Hacettepe University (number: GO13/250, Ankara, Turkey). Briefly, the pulp tissue was pulled out with excavator, cut into pieces and digested in 0.075% (w/v) collagenase type I in Ca2+, Mg2+ free PBS for 1 h at 37 °C. Disrupted pulp tissue was collected in PBS and filtered through 70 µm cell strainer (BD Bioscience, Franklin Lakes, NJ, USA) to obtain single cells (Vishwanath et al. 2013). The suspension was washed with PBS and cultured in α-MEM containing 15% (v/v) FBS, 0.4% (v/v) penicillin/streptomycin and 0.4% (v/v) amphotericin B (P/S/AB) at 37 °C in 5% CO2 atmosphere (Heraeus, Hanau, Germany) until passage 5–7.
Flow cytometry
The cells were characterized by flow cytometry using BD FACSAria II Cell Sorter with MSC specific and unspecific surface markers such as, CD90 (Thy-1; FITC), CD166 (activated leukocyte cell adhesion molecule; PE), CD44 (extracellular matrix receptor III; FITC), CD29 (integrin beta 1 chain; APC), CD73 (5′-nucleotidase; PE), CD105 (endoglin; PE), CD14 (monocyte differentiation antigen; APC), CD45 (receptor-type tyrosine-protein phosphatase C; FITC), CD34 (hematopoietic progenitor cell antigen; APC), CD133 (prominin-1; PE) and HLADR (MHC class II surface receptor; PE). CD90 was purchased from BioLegend (USA); CD14, CD29, CD34, CD44, CD45, CD73, CD166 and HLADR were obtained from Becton Dickinson (USA); CD105 and CD133 were purchased from e-Bioscience (USA). Three lineages differentiation capacity of hDPSCs was investigated by osteogenic, chondrogenic and adipogenic differentiation studies and cultures were carried out for 28, 28 and 56 days, respectively. Chondrogenic differentiation was carried out in pellet culture. Adipogenic differentiation was induced by until the lipid droplets were seen in culture. The cells were induced chemically in differentiation medium and analysed by histological stainings given in Table 1.
Table 1.
Differentiation agents and specific stainings
| Differentiation potential | Osteogenic | Chondrogenic | Adipogenic |
|---|---|---|---|
| 50 μg/mL L-ascorbate (Sigma, Germany) |
10 nM dexamethasone | 0.5 μM dexamethasone | |
| 10 nM dexamethasone (Sigma-Aldrich, Germany) |
50 μg/mL L-ascorbate 1% ITS (Sigma, Germany) |
0.25 mM 3-isobutyl-1-1-methylxanthine (IBMX, Sigma, Germany) |
|
| 10 mMβ-glycerophosphate (Sigma-Aldrich, Germany) |
5 ng/mL TGFβ-1 (Sigma-Aldrich, Germany) |
1 μg/mL insulin (Sigma, Germany) |
|
| 5 ng/mL TGFβ-3 (Sigma-Aldrich, Germany) |
50 μM indomethacin (Sigma, Germany) |
||
| Specific stainings | ALP-Von Kossa | Safranin O | Oil Red O |
Cell seeding
Cell culture studies on chitosan scaffolds were carried out in non-treated 24-well culture plates. Before cell seeding, scaffolds were sterilized via soaking in 70% (v/v) ethanol for 2 h and rinsed with PBS for three times. Then, both of their sides were placed under UV light for 45 min. After sterilization, they were immersed in cell culture medium, α-MEM supplemented with 15% (w/v) FBS and P/S/AB, for conditioning overnight.
Human DPSCs (2 × 105 cells/scaffold) were suspended in 30 µL of cell culture medium and suspensions were seeded into the scaffolds. Five hours later, 1 mL cell culture medium was added to wells and cells were incubated at 37 °C in 5% CO2 atmosphere for 9 days.
Cell seeding with gelatine solution
In order to support cell attachment on chitosan scaffolds, cell seeding was also carried out in the presence of gelatine solution (1%, w/v in cell culture medium) with increased cell density allowing more time for cell-material and cell–cell interactions during attachment process. Gelatine solution, 10× (in ultrapure water) concentration, was sterilized with autoclave at 121 °C for 15 min. Then, the solution was diluted in cell culture medium, pH was adjusted to around 7.4, using HEPES buffer. Then, 50 μL of hDPSCs suspension at a concentration of 4 × 105 cells/scaffold in culture medium was added onto each scaffold. Five hours later, 1 mL cell culture medium was added to wells and cells were incubated at 37 °C in 5% CO2 atmosphere for 9 days.
Cell seeding into RGD and Fn modified scaffolds
For the purpose of promoting hDPSCs attachment on chitosan scaffolds, RGD peptide or Fn immobilized chitosan scaffolds were used. Fifty µL of hDPSCs suspension at a concentration of 2 × 105 cells/scaffold were seeded into RGD and Fn immobilized scaffolds. Five hours later, 1 mL cell culture medium was added to wells and cells were incubated at 37 °C in 5% CO2 atmosphere for 9 days.
Scanning electron microscopy (SEM)
The adhesion behaviour and morphology of hDPSCs on scaffolds were observed by SEM (Zeiss Evo 50, Oberkochen, Germany) at desired culture periods. After the culture medium was removed, the cell-scaffold constructs were rinsed with PBS twice and cells were fixed with 2.5% (v/v) glutaraldehyde (diluted in 0.1 M PBS) for 30 min at 4 °C. Then, samples were dehydrated in a series of increasing concentrations of ethanol (30, 50, 70, 90, and 100% v/v) and treated with HMDS for 5 min. After the samples dried completely, scaffolds were coated with a gold–palladium layer and observed under SEM.
Cell viability assay
Proliferation behaviour of hDPSCs on scaffolds was determined by mitochondrial activity using resazurin based PrestoBlue® assay. Instead of other analysis like MTT, cell viability is not affected by PrestoBlue® treatment and the same scaffold can be used during the experimental period. Resazurin is a water-soluble dye and the assay is dependent on the ability of cells to reduce resazurin to resorufin by mitochondrial enzymes that can be detected either colorimetric or fluorometric measurements (Boncler et al. 2014). Briefly, after the culture medium was aspirated, the samples were washed with PBS (pH 7.4) once and 400 µL of PrestoBlue® mix solution (10% PrestoBlue® 10× reagent + 90% culture medium) was added onto samples and incubated at 37 °C for 2 h. Then, the optical density (OD) of 200 μL of the medium was evaluated using a microplate spectrophotometer at a wavelength of 570 nm with the reference to 600 nm. The same procedure was also applied to the control samples, i.e. scaffolds without cells, and OD values were subtracted from the values obtained from scaffolds with cells.
Immunofluorescence staining
Anti-F actin and DAPI staining were performed with the cell-scaffold constructs which cells were seeded with gelatine. Alexa Fluor 488 Phalloidin conjugated anti-F actin and DAPI probe were used for immunostaining. The cell culture medium was removed and the cell-scaffold constructs were fixed with 4% (w/v) paraformaldehyde for 30 min at room temperature. The fixed cells were permeabilized with 0.1% (v/v) Triton-X 100 in PBS (pH 7.4) and washed with PBS (pH 7.4) three times. Then, anti-F actin antibody (1:100) and DAPI probe (1:1000), diluted in 1% (w/v) bovine serum albumin containing PBS (PBS/A), were added onto the scaffolds and incubated for 1 h at room temperature. The samples were then rinsed with PBS/A three times and observed under fluorescent microscope (Olympus IX71, Tokyo, Japan).
Real time reverse transcriptase polymerase chain reaction (RT-PCR) analysis
In order to investigate odontogenic differentiation potential of hDPSCs on Fn-immobilized chitosan scaffolds, collagen I (COLI), Runt-related transcription factor 2 (RUNX2), osteopontin (OPN), osteocalcin (OCN), alkaline phosphatase (ALP) and dentin sialophosphoprotein (DSPP) gene expressions were determined by RT-PCR. Primer sequences are shown in Table 2. To induce odontogenic differentiation potential of hDPSCs, cells were cultured in 10 mM β-glycerophosphate, 50 μg/mL ascorbic acid and 10−8 μg/mL dexamethasone containing medium. After 14 and 21 days, samples were washed with PBS, chopped in Trizol and stored at −80 °C until analysis. RNeasy Mini kit (Qiagen, Valencia, CA, USA) was used for RNA extraction. RNA concentration was determined by measuring optical density at 260 nm using Nanodrop 2000c (Thermo Scientific). High-capacity cDNA kit (Applied Biosystems, Foster City, CA, USA) was used for cDNA synthesis and RT-PCR reactions were performed with 5xHot Fire Pol® Eva Green® qPCR Mix Plus solution (Solis BioDyne, Tartu, Estonia) and LightCycler® Nano Instrument (Roche, Mannheim, Germany). Βeta-actin (ACTB) was used as housekeeping gene and expressions were determined using the 2^ (−ΔΔCt) method. The results were given as fold change relative to the results on day 14.
Table 2.
Primer sequences used in RT-PCR analysis
| Genes | Primers |
|---|---|
| ACTB | Forward primer 5′-TCCTGTGGCATCCACGAAACT-3′ |
| Reverse primer 5′-GAAGCATTTGCGGTGGACGAT-3′ |
|
| ALP | Forward primer 5′-CACCTGCCTTACTAACTCC-3′ |
| Reverse primer 5′-CGTTGGTGTTGAGCTTCTG-3′ |
|
| RUNX2 | Forward primer 5′-TCCCTGAACTCTGCACCA -3′ |
| Reverse primer 5′-ATGCGCCCTAAATCACGTAG -3′ |
|
| COLI | Forward primer 5′-AAGTCTTCTGCAACATGGAG-3′ |
| Reverse primer 5′-TACTCGAACTGGAATCCATC-3′ |
|
| OCN | Forward primer 5′-CAGCCTTTGTGTCCAAGC-3′ |
| Reverse primer 5′-CACAGTCCGGATTGAGCT-3′ |
|
| OPN | Forward primer 5′-GAAGCAGAATCTCCTAGCCC-3′ |
| Reverse primer 5′-ATGTGGTCATGGCTTTCGT-3′ |
|
| DSPP | Forward primer 5′-ACATGCTGTTGGGAAGAG-3′ |
| Reverse primer 5′-TACCTTCGTTGCCTTTCC-3′ |
Statistical analysis
Three similar experiments were performed for each type of assay and numerical values were expressed as mean ± standart deviation. Statistical differences were evaluated by InStat software and t test were used to determine the significant differences among the groups and a statistical significance was considered as * p < 0.05 and ** p < 0.01.
Results and discussion
The aim of this study was to enhance cellular interactions with chitosan scaffolds by investigating hDPSCs attachment, spreading, morphology and proliferation (Fig. 1). Human DPSCs are quite appropriate for bone and tooth tissue engineering applications with their high mineralization capacity. Chitosan has a significant antimicrobial activity, which act on gram-negative, gram-positive bacteria and fungi (Goy et al. 2009). These properties drive chitosan forward as a biomaterial for oral administration. On the other hand, it was reported in few studies that hMSCs form into 3D spheroids when they were cultured on chitosan (Huang et al. 2011; Yeh et al. 2012). This is why, here we decided to investigate the effect of RGD- and Fn-immobilized chitosan scaffolds comparatively on spreading and proliferation of hDPSCs without formation of spheroids.
Fig. 1.
Schematic presentation of the purpose of this study
Immobilization of RGD and Fn on chitosan scaffolds
Fibronectin is a ~450 kDa dimeric glycoprotein and each of its dimers is composed of three different regions; collagen and heparin binding domain and RGD sequence (Vargas Becerril et al. 2013). RGD is one of the most common used cell-binding peptide, found in cell adhesion proteins such as fibronectin and laminin (Tsai et al. 2011). Cell adhesive proteins or peptide sequences can be physically adsorbed or covalently immobilized onto biomaterial surfaces. Physical adsorption is a simple reversible method and lots of proteins can retain their biological activity. However, as a disadvantage, proteins can easily leak from the surface. In case of covalent immobilization, the binding is irreversible, strong and has longer functional stability.
In this study, covalent binding via carbodiimide reaction was used for RGD and Fn immobilization on chitosan-based scaffolds. In brief, carboxyl groups of protein and peptide, which were pre-activated by EDC and NHS, forming intermediate O-acylisourea, conjugate with amino groups of chitosan (Custodio et al. 2010). The results of ninhydrin analysis showed absorbances at 560 nm for chitosan scaffold: 0.82; chitosan-RGD scaffold: 1.10 and for chitosan-Fn: 1.12. Higher absorbance values of RGD and Fn-immobilized scaffolds than that of chitosan scaffolds indicated that RGD and Fn were immobilized on chitosan scaffolds successfully.
Cell culture studies
Characterization of hDPSCs
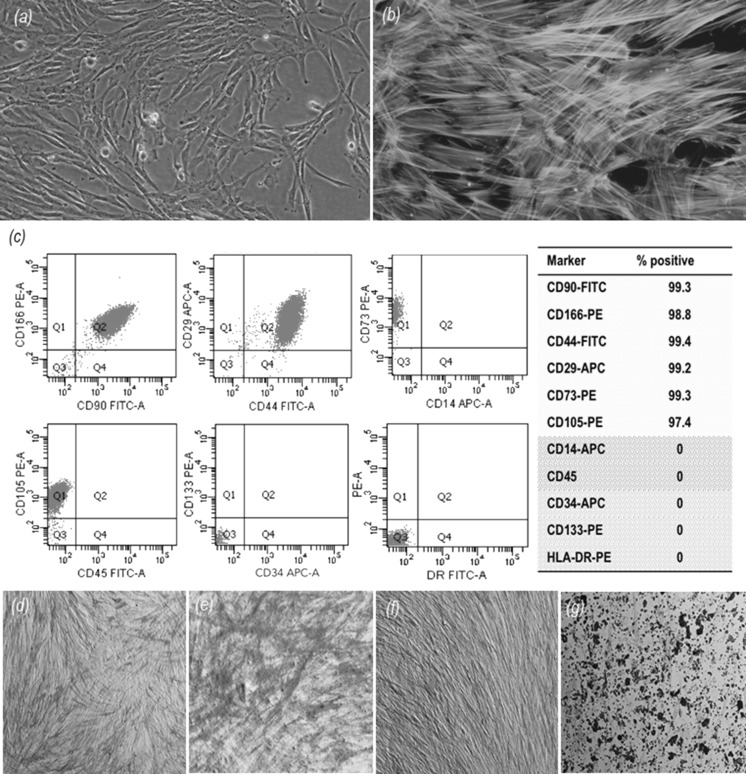
Dental pulp stem cells have similar characteristics to mesenchymal stem cells with their fibroblastic morphology (Fig. 2a, b), solid surface dependency, high proliferation and differentiation capacity (Eslaminejad et al. 2013). Human DPSCs have great importance for regenerative therapy. Detailed in vivo and in vitro studies were previously carried out to examine the proliferation and differentiation capacity of MSCs derived from dental pulp (Atari et al. 2012). They are multipotent and can be easily isolated from human third molars with expressing mesenchymal stem cell markers (Lee et al. 2011).
Fig. 2.
a Inverted phase-contrast image of human dental pulp stem cells (hDPSCs), original magnification 20X; b immunofluorescence staining images of fibrous actin filaments (green) and cell nuclei (blue) of hDPSCs on tissue culture polystyrene, original magnification ×40; c results of flow cytometry analysis showing % positive expression of surface markers of hDPSCs. d Alkaline Phosphatase and Von Kossa staining of hDPSC: day 14-the control group ×20, e osteogenic differentiation group ×20; f day 28-the control group, ×20, g osteogenic differentiation group, ×20. (Color figure online)
In this study, phenotype positive (CD90, CD166, CD44, CD29, CD73 and CD105) and phenotype negative (CD14, CD34, CD45, CD133 and HLA-DR) cell surface antigens of hDPSCs were characterized by flow cytometry analysis and the results were given in % positiveness in Fig. 2c. Osteogenic differentiation was analyzed with Alkaline Phosphatase and Von Kossa (ALP/Von Kossa) staining (Fig. 2d–g). In the osteogenically induced group, ALP activity of hDPSCs was higher when compared to the control group on day 14. However, it decreased on day 28 with increasing mineralization in the induction group. Chondrogenic differentiation capacity of hDPSCs was shown with Safranin O staining of frozen sections obtained from pellet cultures after 28 days induction. There was much more extracellular matrix synthesis and collagen production in the induction group when compared to unstable pellets in the control group. In addition, Oil Red O staining showed that most of the hDPSCs differentiated into adipogenic cells with red oil droplets (data not shown).
Behaviour of hDPSCs on chitosan scaffolds
Chitosan scaffolds, produced by freeze-drying method, have homogeneous pore size and interconnectivity that are important features for tissue engineering. In addition, the mechanical strength of the scaffolds is one of the crucial parameters for hard tissue engineering. Characterization results of chitosan scaffolds showed that they had appropriate properties for hard tissue engineering in our previous study (Tigli et al. 2007).
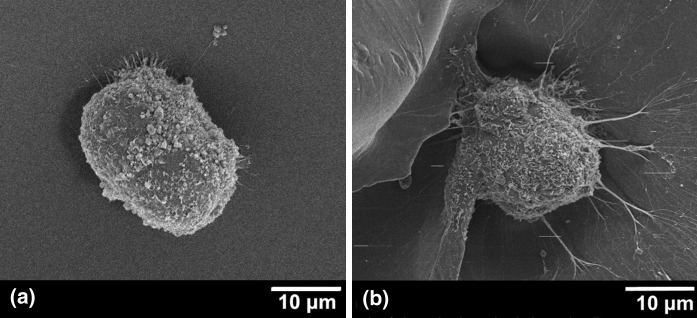
Cellular behaviors of hDPSCs on chitosan scaffolds were investigated for 9 days. SEM images showed that hDPSCs were able to attach on chitosan surface as spheroids, but they were not able to spread and proliferate (Fig. 3). Cells were aggregated very tightly and it was difficult to distinguish the cellular boundary (Fig. 3a) and tiny cellular extensions that were seen in some cells (Fig. 3b). In addition, a number of studies in the related literature indicate that stem cells from different sources present similar characteristics on chitosan surfaces (Huang et al. 2011; Yeh et al. 2014). Yeh et al. (2014) concluded that, depending upon the chemical structure of chitosan, cell mobilization genes of umbilical cord MSCs may be induced, and this could be the reason for the formation of cell spheroids and the detachment of these spheroids from the surfaces. Cheng et al. (2012) studied adipose derived stem cells on chitosan films and they also specified that cells were spontaneously aggregated into small clusters and then, these highly motile structures merged with each other to form bigger spheroids. There are also other experiments with MSC-like populations isolated from human gingival tissue and placenta showed similar results on chitosan (Huang et al. 2011; Hsu et al. 2012). Furthermore, Kim et al. (2009) demonstrated that two types of collagen and gelatin scaffolds supported hDPSCs’ attachment, proliferation and differentiation; however, chitosan did not properly support cell growth. In this study, the same behavior of hDPSCs’ was observed on chitosan scaffolds.
Fig. 3.
SEM images of hDPSCs on chitosan scaffolds on day 1 (a) and day 9 (b)
Seeding of hDPSCs with 1% gelatin solution onto chitosan scaffolds
To overcome problems described above, cell-seeding method was changed. hDPSCs were seeded with 1% (w/v) gelatin to prevent cell–cell contact with increasing viscosity and give more time to cells for attaching onto the chitosan surface. Gelatin is hydrolyzed form of collagen, a biocompatible and frequently used biodegradable material. In some studies it has been shown that hDPSCs seeded gelatin scaffolds have successful results in tissue engineering (Kim et al. 2009; Li et al. 2011).
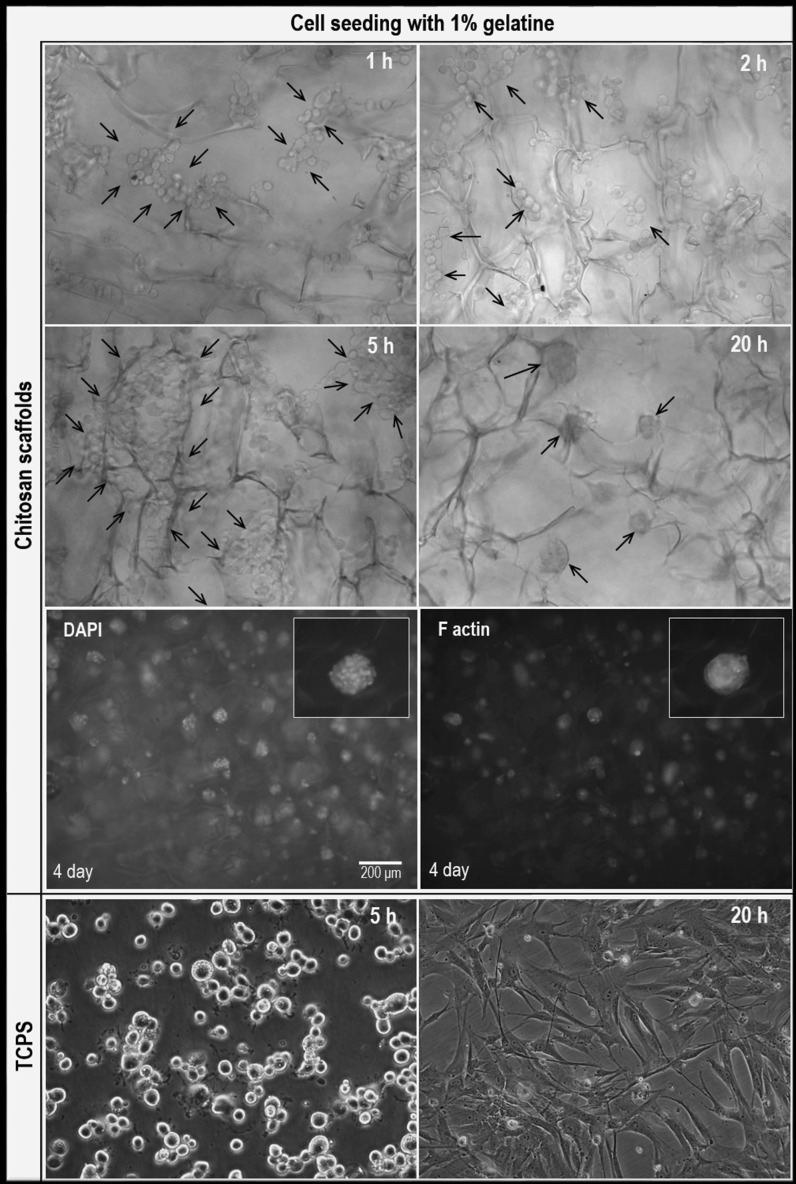
In order to follow the cellular behavior on chitosan scaffolds, inverted phase-contrast images of the cells were taken at specific times (Fig. 4). Human dental pulp stem cells were shown as rounded individual cells on chitosan scaffolds for 5 h. By the time, cell boundaries began to disappear and they came closer to form various sized spheroids at the end of the 20th hour. In addition, cells were seeded with 1% (w/v) gelatin on tissue culture polystyrene (TCPS) surface to follow the effect of gelatin on cellular behavior. Results showed that, cells started to attach on TCPS surface at the 5th hour, completely spread at the 20th hour and cellular viability was not affected by gelatin (Fig. 4).
Fig. 4.
Inverted phase-contrast images of hDPSCs on chitosan scaffolds and TCPS seeded with 1% (w/v) gelatin, ×20. Immunofluorescence staining images of fibrous actin filaments (green) and cell nuclei (blue) of hDPSCs seeded with 1% gelatin on chitosan scaffolds. (Color figure online)
Human dental pulp stem cells on chitosan scaffolds were also monitored by fluorescent microscopy on the 4th day of culture (Fig. 4). It was shown that most of the cells formed spheroids on scaffolds, as before. On the contrary, cells on TCPS expanded, proliferated and coated the entire surface (Fig. 4). Consequently, seeding in 1% (w/v) gelatin solution was not successful to prevent spheroid formation and to enhance cellular adhesion and proliferation. Because gelatin was dispersed in culture medium within the time, cells interacted with chitosan and detached from the surface.
hDPSCs on RGD immobilized chitosan scaffolds
RGD sequence interacts with integrin receptors and enhances cell attachment, growth and differentiation (Clements et al. 2011; Tigli et al. 2007; Tsai et al. 2010, 2013).
The attachment of hDPSCs on RGD-modified and unmodified chitosan scaffolds was carried out for 24 h. It is obvious, that much more hDPSCs attached on RGD-modified chitosan scaffolds as spheroids when compared to unmodified chitosan scaffolds. Spheroids also became larger and more uniform in size on chitosan scaffolds with RGD than chitosan scaffolds without RGD (Fig. 5). The results indicated that, RGD immobilization enhanced the attachment of hDPSCs over this time frame; however, it did not prevent spheroid formation and was not suitable for maintenance of hDPSCs on chitosan scaffolds.
Fig. 5.
SEM images of hDPSCs a without RGD, b with RGD on chitosan scaffolds at 24 h
hDPSCs on Fn immobilized chitosan scaffolds
Fibronectin, one of the most abundant proteins of ECM, plays a crucial role for cellular attachment, proliferation and differentiation (Gao et al. 2006). Interactions of specific Fn domains, for example PHSRN sequences in the 9th type III repeat and RGD motif in the 10th type III repeat together, with clustered integrin receptors is required for prompting cellular processes. Oligopeptides, such as RGD with the lack of complementary and modulatory domains are limited to support cellular attachment (Petrie et al. 2006). Results showed that RGD immobilization on chitosan scaffolds were insufficient to enhance hDPSCs attachment and survival, hence, as a native protein, Fn was immobilized to enable the presentation of multiple cell binding sites on chitosan surfaces.
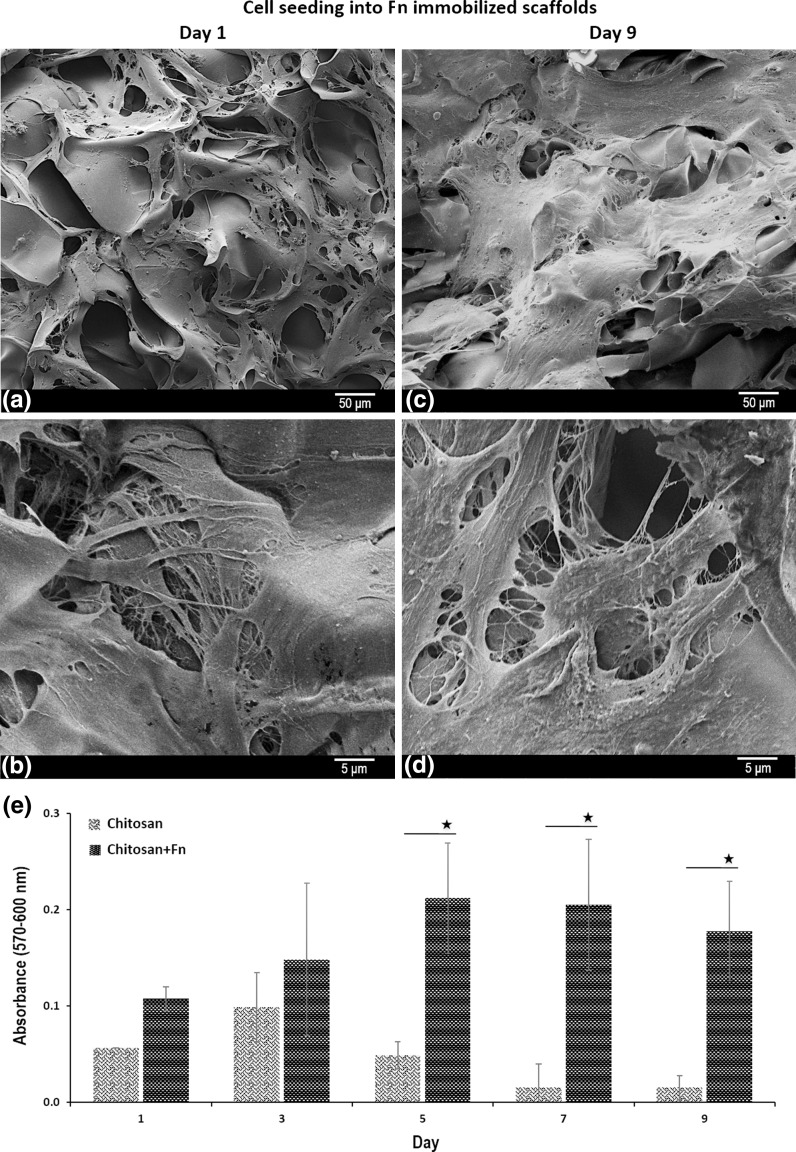
SEM images of hDPSCs seeded on Fn-immobilized chitosan scaffolds after 1 and 9 days are presented in Fig. 6. It is obvious that hDPSCs attached and spread on Fn immobilized scaffolds as star-like phenotype (Fig. 6a, b). On the contrary, they formed 3D spheroids on chitosan scaffolds as mentioned previously (Fig. 3).
Fig. 6.
SEM images of hDPSCs on chitosan+Fn scaffolds on day 1 (a, b) and day 9 (c, d). Proliferation behavior of hDPSCs on chitosan and chitosan+Fn scaffolds were determined by PrestoBlue® analysis for 9 days (*p < 0.5)
After 9 days of culture, the cell morphology on Fn-immobilized chitosan scaffolds was distinctly different from that they were on the first day of cell culture from the viewpoint of cell–cell interactions and ECM formation covering almost the entire surface. They presented sheet like morphology and cellular junctions were intense on day 9 (Fig. 6c, d).
The proliferation of hDPSCs on chitosan and Fn-immobilized chitosan scaffolds was followed by PrestoBlue® analysis through 9 days of culture period (Fig. 6e). The results indicated that proliferation of hDPSCs was much more lower on chitosan scaffolds compared to the proliferation on chitosan+Fn scaffolds due to detaching of cell spheroids from the surface of chitosan scaffolds. Increased cell–polymer interactions play a crucial role in stimulating cell proliferation. Clustered cell adhesive ligands on the surface of the 3D porous polymer scaffolds, mimicking an ECM structure as in tissue, would not only increase cell–polymer interactions, but might also create a favorable environment for facile cell–cell communications. In this study, the results obtained from PrestoBlue® experiment proved that the presence of Fn enhanced the cell attachment which became an advantage for cell proliferation.
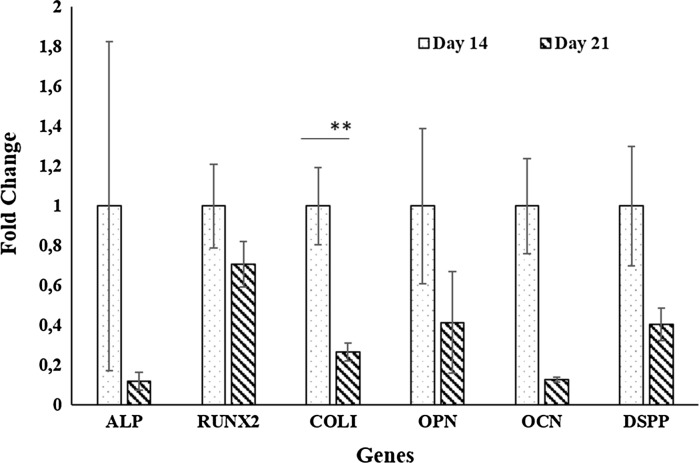
Differentiation ability of cells was investigated by RT-PCR analyses (Fig. 7) and the results showed that expressions of odontogenic markers (COLI, RUNX2, ALP, OPN, OCN and DSPP) were decreased through the culture period as concluded by other researchers (Gronthos et al. 2000; Li et al. 2014; Braut et al. 2003).
Fig. 7.
RT-PCR results of hDPSCs on chitosan+Fn scaffolds (** p < 0.01)
Conclusion
In this study, we have investigated adhesion and proliferation behavior of hDPSCs on chitosan scaffolds with two different surface modifications, RGD immobilization and Fn immobilization. Our major finding was that chemical surface modification strongly influenced the subsequent behavior of hDPSCs that adhered and proliferated on chitosan scaffolds. We have identified, Fn- immobilized chitosan scaffolds were preferable for hDPSCs’ in vitro proliferation and showed an interesting potential for in vivo transplantation.
Acknowledgements
This work was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK), Grant No: SBAG-114S755. The authors are grateful to Selin Gümüşderelioğlu for editing of language in the text.
References
- Arnold M, Cavalcanti-Adam EA, Glass R, Blummel J, Eck W, Kantlehner M, et al. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- Atari M, Gil-Recio C, Fabregat M, García-Fernández D, Barajas M, Carrasco MA, et al. Dental pulp of the third molar: a new source of pluripotent-like stem cells. J Cell Sci. 2012;125:3343–3356. doi: 10.1242/jcs.096537. [DOI] [PubMed] [Google Scholar]
- Beşkardeş IG, Demirtaş TT, Durukan MD, Gümüşderelioğlu M. Microwave-assisted fabrication of chitosan-hydroxyapatite superporous hydrogel composites as bone scaffolds. J Tissue Eng Regen Med. 2015;9:1233–1246. doi: 10.1002/term.1677. [DOI] [PubMed] [Google Scholar]
- Biran R, Webb K, Noble MD, Tresco PA. Surfactant-immobilized fibronectin enhances bioactivity and regulates sensory neurite outgrowth. J Biomed Mater Res. 2001;55:1–12. doi: 10.1002/1097-4636(200104)55:1<1::AID-JBM10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Boncler M, Rozalski M, Krajewska U, Podsedek A, Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J Pharmacol Toxicol Methods. 2014;69:9–16. doi: 10.1016/j.vascn.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M. Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1-2.3-GFP transgene. Int J Dev Biol. 2003;47:281–292. [PubMed] [Google Scholar]
- Chandrahasa S, Murray PE, Namerow KN. Proliferation of mature ex vivo human dental pulp using tissue engineering scaffolds. J Endod. 2011;37:1236–1239. doi: 10.1016/j.joen.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Clements LR, Wang PY, Harding F, Tsai WB, Thissen H, Voelcker NH. Mesenchymal stem cell attachment to peptide density gradients on porous silicon generated by electrografting. Phys Status Solidi A. 2011;208:1440–1445. doi: 10.1002/pssa.201000320. [DOI] [Google Scholar]
- Collart-Dutilleul PY, Secret E, Panayotov I, Deville de Periere D, Martin-Palma RJ, Torres-Costa V, et al. Adhesion and proliferation of human mesenchymal stem cells from dental pulp on porous silicon scaffolds. ACS Appl Mater Interfaces. 2014;6:1719–1728. doi: 10.1021/am4046316. [DOI] [PubMed] [Google Scholar]
- Custodio CA, Alves CM, Reis RL, Mano JF. Immobilization of fibronectin in chitosan substrates improves cell adhesion and proliferation. J Tissue Eng Regen Med. 2010;4:316–323. doi: 10.1002/term.248. [DOI] [PubMed] [Google Scholar]
- D’Anto V, Raucci MG, Guarino V, Martina S, Valletta R, Ambrosio L. Behaviour of human mesenchymal stem cells on chemically synthesized HA-PCL scaffolds for hard tissue regeneration. J Tissue Eng Regen Med. 2016;10:E147–E154. doi: 10.1002/term.1768. [DOI] [PubMed] [Google Scholar]
- de Mel A, Jell G, Stevens MM, Seifalian AM. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9:2969–2979. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- Dutta PK, Dutta J, Tripathi VS. Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res. 2004;63:20–31. [Google Scholar]
- Eslaminejad MB, Bordbar S, Nazarian H. Odontogenic differentiation of dental pulp-derived stem cells on tricalcium phosphate scaffolds. J Dent Sci. 2013;8:306–313. doi: 10.1016/j.jds.2013.03.005. [DOI] [Google Scholar]
- Gao M, Sotomayor M, Villa E, Lee EH, Schulten K. Molecular mechanisms of cellular mechanics. Phys Chem Chem Phys. 2006;8:3692–3706. doi: 10.1039/b606019f. [DOI] [PubMed] [Google Scholar]
- Goy RC, de Britto D, Assis OBG. A Review of the antimicrobial activity of chitosan. Polimeros. 2009;19:241–247. [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MH, Wang DM, Hsieh HJ, Liu HC, Hsien TY, Lai JY, et al. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials. 2005;26:3197–3206. doi: 10.1016/j.biomaterials.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Huang GS, Feng F. Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials. 2012;33:2642–2655. doi: 10.1016/j.biomaterials.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Huang GS, Dai LG, Yen BL, Hsu SH. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials. 2011;32:6929–6945. doi: 10.1016/j.biomaterials.2011.05.092. [DOI] [PubMed] [Google Scholar]
- Jongpaiboonkit L, King WJ, Murphy WL. Screening for 3D Environments That support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng Part A. 2009;15:343–353. doi: 10.1089/ten.tea.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaoz E, Dogan BN, Aksoy A, Gacar G, Akyuz S, Ayhan S, et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010;133:95–112. doi: 10.1007/s00418-009-0646-5. [DOI] [PubMed] [Google Scholar]
- Kim NR, Lee DH, Chung P-H, Yang H-C. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e94–e100. doi: 10.1016/j.tripleo.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Bae WJ, Kim JM, Kim JJ, Lee EJ, Kim HW, et al. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J Biomater Appl. 2014;28:1069–1078. doi: 10.1177/0885328213495903. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Lee D-S, Choung H-W, Shon W-J, Seo B-M, Lee E-H, et al. Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials. 2011;32:9696–9706. doi: 10.1016/j.biomaterials.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Li J-H, Liu D-Y, Zhang F-M, Wang F, Zhang W-K, Zhang Z-T. Human dental pulp stem cell is a promising autologous seed cell for bone tissue engineering. Chin Med J. 2011;124:4022–4028. [PubMed] [Google Scholar]
- Li J-H, Yan M, Wang Z, Jing S, Li Y, Liu G, Yu J, Fan Z. Effects of canonical nf-b signaling pathway on the proliferation and odonto/osteogenic differentiation of human stem cells from apical papilla. BioMed Res Int. 2014;2014:1–12. doi: 10.1155/2014/319651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Che Abdullah CA, Sear RP, Keddie JL. Cell adhesion on nanopatterned fibronectin substrates. Soft Matter. 2010;6:5408–5416. doi: 10.1039/c0sm00201a. [DOI] [Google Scholar]
- Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, et al. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie TA, Capadona JR, Reyes CD, García AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Tigli RS, Karakecili A, Gumusderelioglu M. In vitro characterization of chitosan scaffolds: influence of composition and deacetylation degree. J Mater Sci Mater Med. 2007;18:1665–1674. doi: 10.1007/s10856-007-3066-x. [DOI] [PubMed] [Google Scholar]
- Tsai W-B, Chen RP-Y, Wei K-L, Tan S-F, Lai J-Y. Modulation of RGD-functionalized polyelectrolyte multilayer membranes for promoting osteoblast function. J Biomat Sci Polym E. 2010;21:377–394. doi: 10.1163/156856209X419095. [DOI] [PubMed] [Google Scholar]
- Tsai W-B, Chen Y-R, Liu H-L, Lai J-Y. Fabrication of UV-crosslinked chitosan scaffolds with conjugation of RGD peptides for bone tissue engineering. Carbohydr Polym. 2011;85:129–137. doi: 10.1016/j.carbpol.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Tsai W-B, Chen Y-R, Liu H-L. RGD-conjugated crosslinked chitosan scaffolds for culture and osteogenic differentiation of mesenchymal stem cells. J Taiwan Inst Chem Eng. 2013;44:1–7. doi: 10.1016/j.jtice.2012.09.003. [DOI] [Google Scholar]
- VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59:585–590. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- Vargas Becerril N, Téllez-Jurado L, Rodriguez-Lorenzo LM. Adsorption of fibronectin on hydroxyapatite functionalized with alendronate. J Aust Ceram Soc. 2013;49:112–118. [Google Scholar]
- Vishwanath VR, Nadig RR, Nadig R, Prasanna JS, Karthik J, Pai VS. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: an in vitro study. J Conserv Dent. 2013;16:423–428. doi: 10.4103/0972-0707.117509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HY, Liu BH, Hsu SH. The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials. 2012;33:8943–8954. doi: 10.1016/j.biomaterials.2012.08.069. [DOI] [PubMed] [Google Scholar]
- Yeh HY, Liu BH, Sieber M, Hsu SH. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genom. 2014;15:10. doi: 10.1186/1471-2164-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]