Abstract
Silibinin is a natural polyphenol with high antioxidant and anticancer properties, which causes cell cycle arrest and apoptosis in most cancer cell types including breast cancer, but the in-line mechanisms, are still unknown. Silibinin significantly downregulated oncomiR miR-21 expression in breast cancer cells. Here the effect of anti-miR-21 on cell viability, apoptotic induction, cell cycle distribution, and the expression levels of downstream targets of miR-21 were investigated in MCF-7 and T47D cells. MiR-21 mimic transfection was also applied in silibinin treated samples to evaluate functional role of miR-21downregulation on silibinin effects. It was found that after anti-miR-21 transfection, no significant changes were detected in cell viability, apoptosis (except early apoptosis), and cell cycle in MCF-7 and T47D cells. Compared to silibinin, miR-21 mimic transfection in combination with silibinin caused a slight modulation in some of the examined silibinin effects including apoptosis, Bcl2 mRNA and PTEN mRNA and protein levels. Silibinin slightly changed luciferase activity from reporters containing the miR-21 recognition elements from PTEN-3′UTR and Bcl2-3′UTR in both cell lines. Together these data demonstrated negligible cancer-progression impact of miR-21 and limited roles of miR-21 downregulation in examined silibinin effects, and strengthened the anti-cancer pathways of silibinin other than miR-21downregulation in MCF-7 and T47D cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-017-0076-5) contains supplementary material, which is available to authorized users.
Keywords: Anti-miR-21, Apoptosis, Breast cancer, MiR-21 mimic, Silibinin, PTEN
Introduction
Accumulating evidence suggests that natural agents open up a new avenue for successful treatment of cancers, especially in combination with conventional therapeutics (Asad et al. 1998). The ideal anticancer agent would exert minimal adverse effects on normal cells with maximal capacity to induce apoptosis in tumor cells. Natural flavonoids are potent anticancer agents because they have the aforementioned outcome. Moreover, they target multiple signaling pathways in cancer cells (Chahar et al. 2011; Sak 2014). Silibinin is a polyphenolic flavonolignan, isolated from milk thistle (Silybum Marianum), that has long been used for its hepatoprotective (antihepatotoxic) properties and protection of liver cells against toxins. It has been well documented that silibinin inhibits growth and proliferation of various cancer cells by inducing apoptosis and cell cycle arrest (Sharma et al. 2003; Deep and Agarwal 2007; Verschoyle et al. 2008; Tiwari et al. 2011). Previous studies have shown that silibinin inhibits the migration and adhesion capacity of MDA-MB-231 breast cancer cells by affecting b1-integrin and downstream molecules; Cdc42, Raf-1 and D4GDI (Mokhtari et al. 2008) and initiates apoptosis and cell cycle arrest that involves the activation of caspase-8, mitochondrial apoptotic pathway, along with the Bcl-2 downregulation and PTEN upregulation (Li et al. 2008; Tiwari et al. 2011).
Cancer is a complex genetic disease, involving the structural and expression abnormalities in both coding and non-coding genes (Ling et al. 2013). MicroRNAs (miRs) are a class of naturally occurring, small, non-coding RNA molecules, distinct from small interfering RNAs (siRNAs). MicroRNAs recognize target RNAs via direct base-pairing and recruit effector complexes to modulate their gene expression in a sequence-specific manner, which can dictate the functional outcome. Perfect base pairing of miRNA to its target can lead to mRNA cleavage and decay, while partial base pairing to target sites can lead to mRNA de-adenylation, translational regulation, primarily mRNA translation silencing, or upregulated expression in response to specific cellular conditions, sequences and co-factors (Bartel 2004; Vasudevan 2012). Aberrant miRNA expression has been linked to different diseases, including breast cancer (Iorio et al. 2005; Shenouda and Alahari 2009; Ranji et al. 2013). miR-21, identified as an ‘oncomiR’, is the most significantly upregulated miRNA in breast tumor biopsies (Mattie et al. 2006; Si et al. 2007), and significantly higher in ERa+ than ERa– breast tumors (Sempere et al. 2007). MCF-7 and T47D cell lines are both ERa+ and have upregulated miR-21 expression (Iorio et al. 2005), suggesting that miR-21 may potentially act as oncogene in these cell lines. MiR-21 inhibits some of the most important tumor suppressors, including PTEN, PDCD4, and TM1 and Bcl2 as an antiapoptotic gene in different tumors (Zhu et al. 2008; Wickramasinghe et al. 2009; Teng et al. 2013). Our previous studies have shown that silibinin caused upregulation of PTEN (one of the inducers of apoptosis and cell cycle arrest (Weng et al. 2001)) in MCF-7 and MCF-10A cells and downregulation of Bcl2 in MCF-7, T47D, and MCF-10A cell lines (Jahanafrooz et al. 2016), and caused downregulation of miR-21 in MCF-7 and T47D cells (Zadeh et al. 2015, 2016). Anti-miR oligonucleotides (ONs), capable of forming complementary base pairs with the guide strand of miRNAs, have proven to be of great value in recent years as tools to understand microRNA action and as potential therapeutics as well (Krützfeldt et al. 2005; Esau 2008; Lee and Dutta 2009; Cheng et al. 2015).
In the present study, we investigated the effect of miR-21 modulation by anti-miR-21 and miR-21mimic oligonucleotide transfection in silibinin-untreated and silibinin-treated samples, respectively, in order to determine whether one of the anti-cancerous pathways of silibinin is via miR-21 inhibition in MCF-7 and T47D cell lines. To specify the effect of silibinin on miR-21 targets, luciferase reporter assay was also performed.
Materials and methods
Cell culture
MCF-7 (human breast adenocarcinoma cell line) and T47D (human ductal breast epithelial tumor cell line) cells were obtained from the National Cell Bank of Iran (Tehran, Iran). These cell lines were cultured in RPMI-1640 medium, supplemented with 10% FBS and 1% penicillin/streptomycin (pen/strep) antibiotics (all three from Gibco by Life Technologies™, Paisley, Scotland, UK) at 37°C in 5% CO2 under 90–95% humidity. MCF-10A (non-cancerous human breast epithelial cell line) cells were cultured in DMEM/F12 (Gibco™, Grand Island, NY, USA), supplemented with 15% FBS, 10 μg/ml insulin (Sigma-Aldrich, St. Louis, USA), 100 μg/ml hydrocortisone (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and 1% pen/strep antibiotics at 37°C in 5% CO2 under 90–95% humidity. Silibinin (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, USA) and the amount of DMSO never exceeded 0.2% (v/v) in the whole experiment.
Cell transfection
For the transfection of anti-has-miR-21 (Exiqon, Woburn, MA, USA), miR-21 mimic (Qiagen Inc., Valencia, CA, USA), and negative control or scrambled miR control (3′-fluorescein labeled, Exiqon, Woburn, MA, USA), 2 × 104 cells were transfected with 25 pmol of anti-has-miR-21 and 5 pmol of miR-21 mimic with 0.75 μl of lipofectamine 2000 (Invitrogen by Life Technologies™, Carlsbad, California, US) in 24-well plates in antibiotics and FBS-free medium. The above-stated amount of each oligonucleotid can yield an oligonucleotid concentration of 50 nM for anti-miR-21 and 10 nM for miR-21 mimic and scrambled, according to manufacturers’ instructions. After 10 h, the medium was completely replaced. Efficiency of transfection in MCF-7 and T47D cells was determined by transfecting scrambled miR control using lipofectamine 2000 (Bakhshandeh et al. 2012). After 10 h of incubation, the cells were visualized by fluorescence microscopy (micros MCXI600, Sankt Veit an der Glan, Austria). Cellular uptakes of lipofectamine/fluorescein labeled-scrambled were also quantified by flow cytometry (BD Biosciences, San Jose, CA, USA). After washing twice with PBS, the cells were trypsinized and centrifuged for 4 min at 1200×g. The resuspended cells were fixed by 4% paraformaldehyde and analyzed using a 2-beam laser FACS Calibur and CellQuest software.
Cell viability investigation
To evaluate miR-21′s role on the cell proliferation and viability, propidium iodide or PI (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) assay was applied after 48 h of incubation. The effect of lipofectamine 2000/scrambled complex on cell viability was also evaluated with PI assay. PI uptake is rejected by viable cells but can penetrate the membranes of dead cells (Riccardi and Nicoletti 2006).The reverse indicator effect of PI on the cell viability was assayed by flow cytometry (BD Biosciences, San Jose, CA, USA) to quantify the exact number of viable cells.
miRNA and RNA extraction and quantitative RT-PCR analysis
At the end of incubation time (48 h), the miRNA extraction and cDNA synthesis of each sample was performed using a slightly modified QIAzol RNA extraction protocol (Qiagen, Austin, TX, USA) to increase the miRNA yield as described previously (Mohammadi-Yeganeh et al. 2013). Total RNA extraction and cDNA synthesis was also performed according the method, described in our previous work for each sample at 48 h (Jahanafrooz et al. 2016). We designed a set of primers of mature miR-21, on the basis of stem loop methodology (Chen et al. 2008; Naderi et al. 2015) with some modifications, and snord-47 was used as a housekeeping gene. The specific primers for cDNA synthesis and RT-PCR of miR-21 and snord-47 are described in Table 1. The specific primers for PTEN, Bcl2, and GAPDH (as an internal control) were the same as in our previous work (Jahanafrooz et al. 2016). To assess the alterations of miR-21, PTEN, and Bcl2 transcriptions for each sample type, the real-time PCR reactions were performed using RealQ PCR 2X Master Mix with Green Dye (A320799, Ampliqon, Odense M, Denmark), in Rotor-Gene 6000 Real-Time Thermal Cycler (Corbett Research, Sydney Australia). Statistical analysis of the primer efficiencies was done by LinReg PCR software (Amsterdam, Netherlands, Version 2012.0). The relative transcriptions were evaluated using method by “Relative Expression Software Tool” (REST 2009, Corbett Research, Australia).
Table 1.
The sequences of Primers
| Name of the primers | Sequence |
|---|---|
| miR-21 (RT) | GTCGTATGCAGAGCAGGGTCCGAGGTATTCGCACTGCATACGACTCAAC |
| miR-21 (F) | ACAGTGCTAGCTTATCAGACT |
| miR-21 (R) AND snord-47 (R) | GAGCAGGGTCCGAGGT |
| Snord-47 (RT) | GTCGTATGCAGAGCAGGGTCCGAGGTATTCGCACTGCATACGACAACCTC |
| Snord-47 (F) | ATCACTGTAAAACCGTTCCA |
RT reverse transcriptase primer, F forward primer, R reverse primer
Western blot
Cells in different treatment groups were homogenized in western blot analysis with the buffer, containing 1 M Tris–HCl (pH 8), 1% (v/v) Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.88% NaCl, 10%(v/v) glycerol and 1 mM PMSF. The homogenate was then centrifuged at 12,000 rpm for 10 min at 4 °C and the supernatant was retained and preserved at −80 °C for later use. Protein concentration was determined using Bradford protein assay. Thirty micrograms of protein from each sample was subjected to electrophoresis on 10% SDS-PAGE gel using a constant electric current flow. Proteins were transferred to nitrocellulose membranes on a semi-dry electrotransferring unit and incubated with PTEN primary antibody (Cell Signaling Technology, Danvers, MA, USA, 1:1000) in Tris-buffered saline containing 0.1% Tween-20 (TBST) and 5% BSA overnight at 4 °C. Then, the membranes were washed and incubated with HRP-labeled second antibody (Cell Signaling Technology, Danvers, MA, USA, 1:3000) in TBST for 2 h. Again the membranes where washed and developed with Amersham™ ECL™ prime western blotting detection reagent (GE HealthcareLife Sciences, Little Chalfont, UK), in accordance with the manufacturer‘s protocol. Chemiluminescence signals were detected with the luminescence-based detection system (Western blot imaging system, SABZ biomedical, Tehran, Iran). The membranes were re-probed with β-actin antibody (Santa Cruz Biotechnology Inc, Dallas, TX, USA, 1:1000) after striping. The signal intensity of primary antibody binding was quantitatively analyzed with Image J software (imagej.nih.gov/ij/) and normalized to a loading control, β-actin.
Dual-luciferase reporter assay
Synthetic DNA oligonucleotides (~35 bp) containing the putative binding site for miR-21 from PTEN-3′UTR and Bcl2-3′UTR and ~5 bp adjacent sequences from each end were annealed and ligated into the XhoI/NotI sites located in the 3′UTR region of the Renilla luciferase reporter (hRluc) in psiCHECK™-2 vector (Promega, Fitchburg, WI, USA) (Supplementary table 1 and figure 1). Each psiCheck-2/PTEN-3′UTR and psiCheck-2/Bcl2-3′UTR construct was transfected using lipofectamine2000 in silibinin treated MCF-7 and T47D cells.
Luciferase assay was performed after 48 h by dual-luciferase activity reporter assay system (Promega, Fitchburg, WI, USA). Renilla luciferase activity was normalized to firefly luciferase (hluc+) expression in each sample and values were normalized by the silibinin-psiCheck-2 control value within that experiment.
Flow cytometric analysis for apoptotic and cell cycle
To investigate the apoptotic phenomenon, the cells were double stained with Annexin V-FITC and PI (EXBIO, Vestec, Czech Republic), following manufacturers’ protocol and analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA). For cell cycle evaluation, all treatments were stained with Ribonuclease I (Sigma-Aldrich, St. Louis, MO, USA)/PI (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) followed by flow cytometry analysis as previously described (Jahanafrooz et al. 2016).
Statistical analysis
All experiments were performed at least thrice. Student’s two-tailed t test was used to compare data between two groups. A P value of <0.05 was considered statistically significant. The results were expressed as mean ± SD.
Results
Silibinin regulated miR-21 expression in breast cancer cells
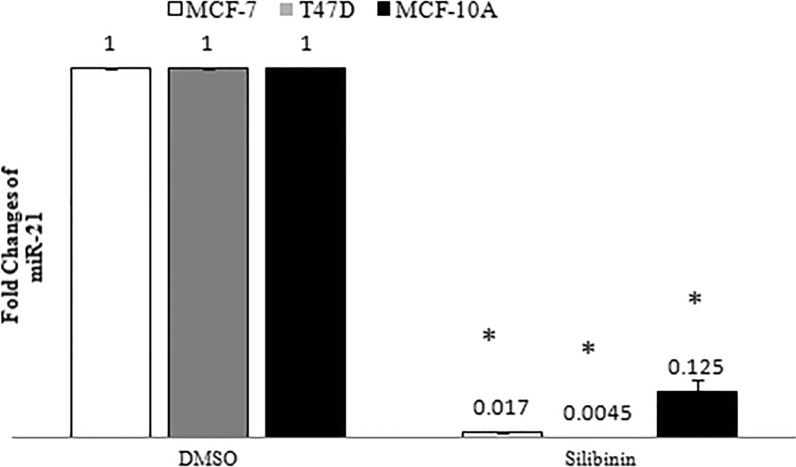
We analyzed alterations of miR-21 expression in MCF-7, T47D and MCF-10A cells by RT-PCR analysis after 48 h of silibinin-treatment. According to the viability assessment in our recent study (Jahanafrooz et al. 2016), an optimum amount of 150 μM silibinin was administered to both cancer cell lines in all experiments because of its possible moderate cytotoxic effects. By using method, RT-PCR results have shown a significant decrease in miR-21 expression in all treated cell types, as compared to the control group (Fig. 1).
Fig. 1.
Effect of silibinin on miR-21 expression in MCF-7, T47D and MCF-10A cell lines. Results are presented as mean ± SD. P < 0.05 was considered as significant difference as compared to control (DMSO)
Cell transfection analysis
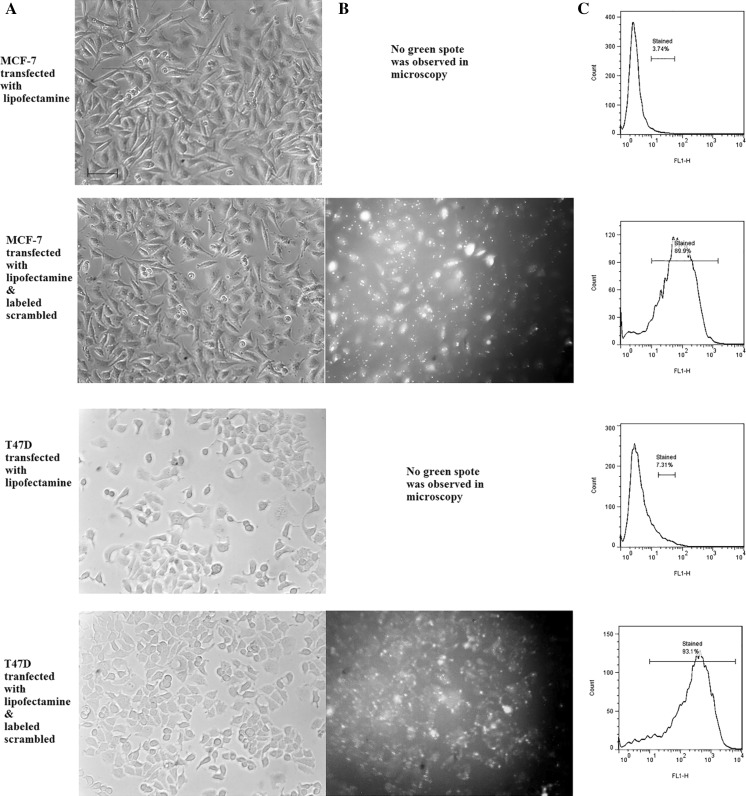
MCF-7 and T47D cells were transfected with fluorescein-labeled scrambled LNA for 10 h and then subjected to fluorescence microscopy and quantified by flow cytometry. The cells having green fluorescence were considered to be transfected successfully. As shown in Fig. 2, cell transfection was successfully effective in the presence of lipofectamine 2000 reagent.
Fig. 2.
Transfection of breast cancer cell lines by fluorescein labeled-scrambled LNA. a Phase contrast microscopy, scale bars 100 μm, b fluorescent microscopy and c flow cytometric analysis of transfected cells, percentages show the transfection efficiencies. The green spots in the fluorescent pictures indicate the cytoplasmic lipofectamine containing fluorescent scrambled. (Color figure online)
Cell viability assessment
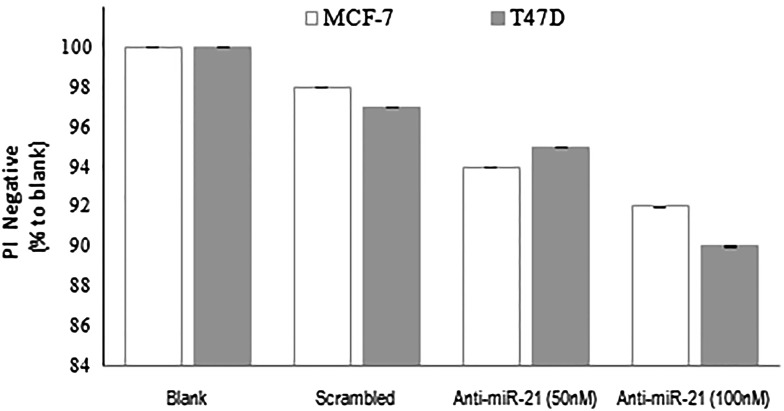
To investigate the effect of downregulation of miR-21 on the viability of MCF-7 and T47D cells, we performed PI assay after anti-miR-21 transfection for 48 h. To exclude the effect of lipofectamine 2000/oligonucleotid complex on cell viability, PI assay was also performed for the cells transfected with lipofectamine 2000/scrambled relative to blank control. As shown in Fig. 3, no significant difference was observed in the average percentage of PI− between blank and lipofectamine 2000/scrambled groups. Down-regulation of miR-21 did not significantly decrease cell viability relative to lipofectamine 2000/scrambled and blank groups in both cancer cell lines.
Fig. 3.
Effect of miR-21 downregulation on the viability of breast cancer cells. Results are expressed as mean viability, as compared to blank (error bars ± 1 SD) There was no significant differences between the different conditions for each cell line
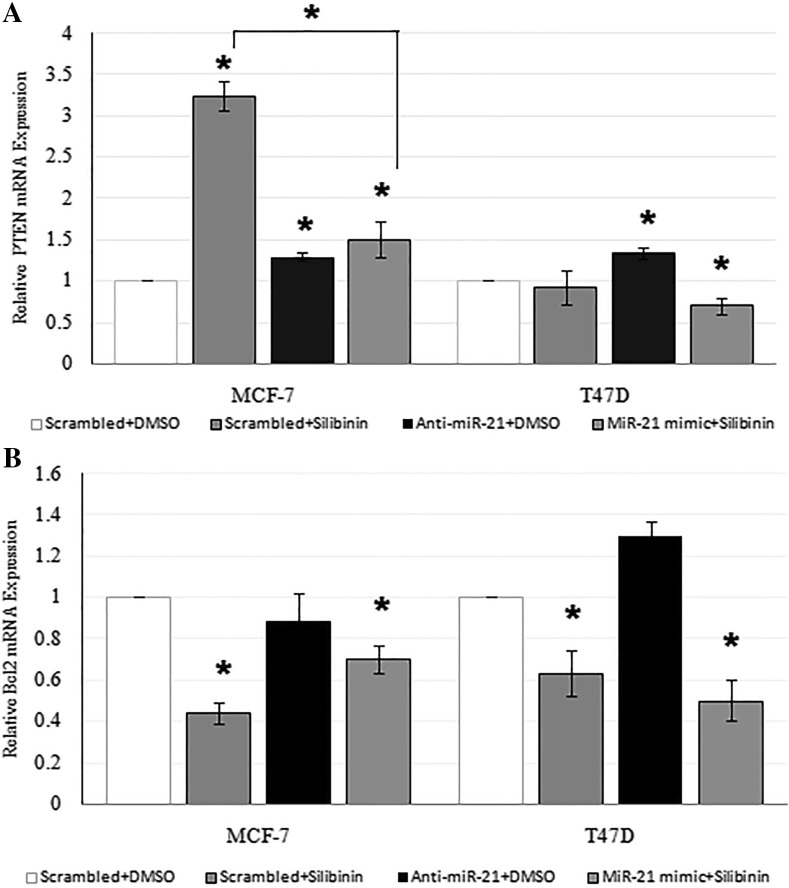
Effect of silibinin and miR-21 downregulation on PTEN and Bcl2 mRNA expression
As also observed in our previous study (Jahanafrooz et al. 2016), silibinin significantly increased PTEN (3.24 ± 0.17, P < 0.05) in MCF-7 cells with no effect on PTEN expression in T47D cells (Fig. 4A) and decreased Bcl2 mRNA expression in both cell lines at 48 h (Fig. 4b). RT-PCR results have shown an increase in the PTEN mRNA expression in anti-miR-21 transfected MCF-7(1.29 ± 0.05, P < 0.05) and T47D (1.34 ± 0.07, P < 0.05) cells, as compared to that in the control cells (Fig. 4a) at 48 h. As shown in Fig. 4b anti-miR-21 reduced Bcl2 expression in MCF-7 cells (0.88 ± 0.14, P > 0.05) and increased it in T47D cells (1.3 ± 0.07, P > 0.05). Co-application of miR-21 mimic and silibinin on cells increased PTEN mRNA expression in MCF-7 cell line (1.5 ± 0.22, P < 0.05 less than 3.24 ± 0.17 in silibinin treatment) and decreased it in T47D cell line (0.7 ± 0.1, P < 0.05) as compared to control samples. That co-application decreased Bcl2 mRNA expression in MCF-7 (0.7 ± 0.07, P < 0.05 more than 0.44 ± 0.05 in silibinin treatment) and T47D cells (0.5 ± 0.1, P < 0.05 less than 0.63 ± 0.11 in silibinin treatment) as compared to control samples.
Fig. 4.
Effect of 150 μM silibinin and miR-21modulation on PTEN (a) and Bcl2 (b) mRNA expression in MCF-7 and T47D cell lines for 48 h. Results are presented as mean ± SD. *P < 0.05 was considered as significant difference as compared to control (scrambled + DMSO) and in MCF-7 cell line, difference was also significant in PTEN mRNA expression in samples transfected with miR-21 mimic and treated with silibinin as compared to silibinin treated samples
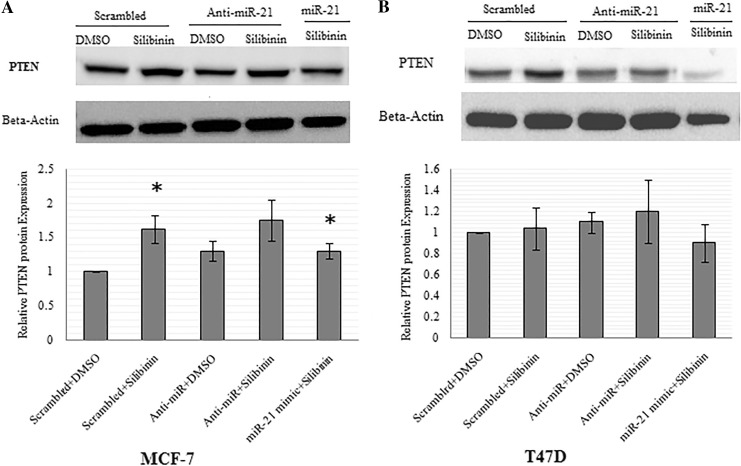
Evaluation of silibinin and miR-21 downregulation effect on PTEN protein expression in MCF-7 and T47D cell lines
Western blotting was used to investigate the alterations in PTEN protein in the cells being treated with silibinin and the silibinin-treated samples after anti-miR-21 and miR-21 mimic transfection. As shown in Fig. 5a, PTEN was upregulated in different amounts post silibinin treatment (1.62 ± 0.2, P < 0.05), after anti-miR-21 transfection (1.3 ± 0.15, P > 0.05), after the combined treatment with silibinin and anti-miR-21 transfection (1.75 ± 0.3, P = 0.05), and finally after the combined treatment of MCF-7 cells with silibinin and miR-21 mimic transfection (1.3 ± 0.12, P < 0.05) as compared to control group. In T47D cells, PTEN was slightly upregulated only by anti-miR-21 transfection in silibinin treated (1.2 ± 0.3, P > 0.05) and untreated samples (1.1 ± 0.1, P > 0.05), while silibinin had no effect on the expression of PTEN protein and slightly decreased it (0.9 ± 0.18, P > 0.05), in combination with miR-21 mimic (Fig. 5b).
Fig. 5.
Effects of silibinin and miR-21modulation on the PTEN protein level. MCF-7 (a) and T47D (b) cells transfected with scrambled, anti-miR-21, and miR-21 mimic and then treated with silibinin. Whole cell lysates were prepared from cells treated for 48 h, and PTEN was analyzed by western blot. The same blot was used for all western blots. Band intensities were analyzed and expressed relative to β-actin, and the values are expressed relative to the control value that was set to 1. Data are expressed as mean ± SD. *P < 0.05 was considered for significant difference as compared to control (scrambled + DMSO)
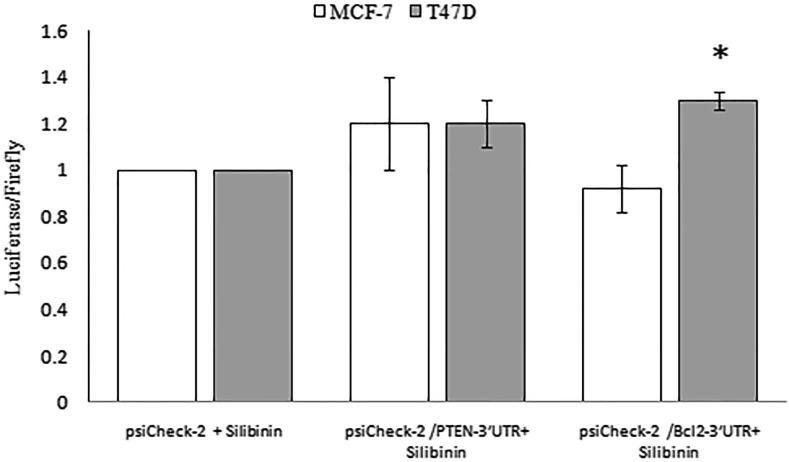
Effect of silibinin on miR-21 targets
To determine whether decrease in miR-21 by silibinin targets PTEN and Bcl2 by their 3′UTR, MCF-7 and T47D cell lines were transiently transfected with psiCheck-2/PTEN-3′UTR and psiCheck-2/Bcl2-3′UTR construct cloned downstream of the Renilla luciferase reporter gene. Luciferase activity with PTEN-3′UTR was marginally enhanced in both cell lines treated with silibinin. Luciferase activity with Bcl2-3′UTR was enhanced in T47D cells (1.3 ± 0.04, P < 0.05) whereas it decreased in MCF-7 cells (0.92 ± 0.1, P > 0.05) after silibinin treatment (Fig. 6).
Fig. 6.
Silibinin gently regulated miR-21 interaction with the 3′UTR of PTEN and Bcl2. MCF-7 and T47D cells were transiently transfected with Renilla luciferase reporter containing the 3′UTR of PTEN and Bcl2 cloned 3′ to Renilla in psiCHECK™-2 vector that contains also firefly gene. Cells were treated with 150 µM silibinnin for 48 h. Relative luciferase activity determined relative to the non recombinant psiCHECK™-2 vector transfected cells. Values are the average of duplicate or triplicate determinations ± SD. *P < 0.05 was considered as significant difference as compared to control (psiCHECK™-2 + silibinin)
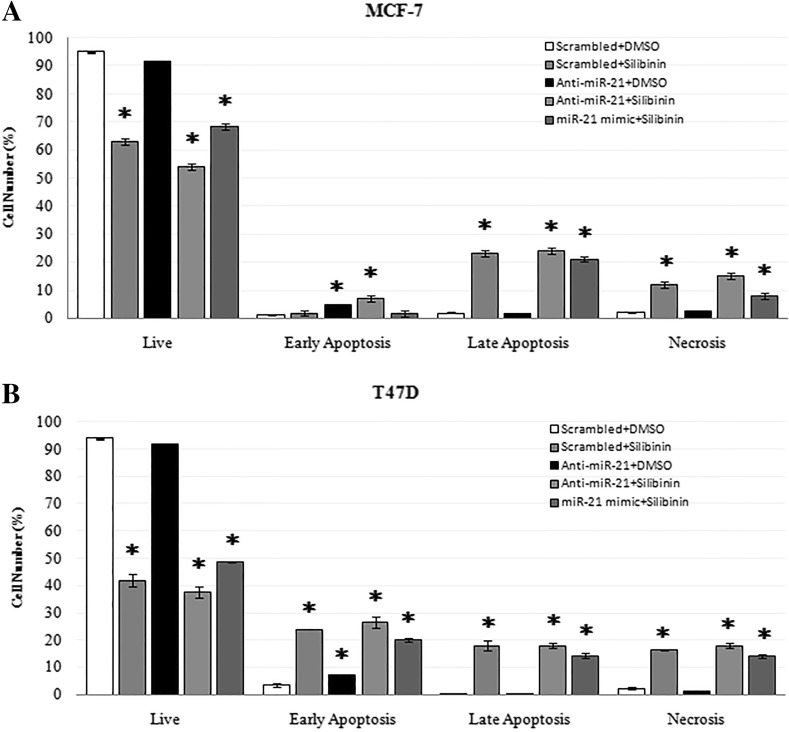
Impact of silibinin and miR-21 downregulation on apoptosis in MCF-7 and T47D cells
In accordance with viability results, anti-miR-21 had little apoptotic effects on breast cancer cell lines, and could cause slight increase in early apoptosis (Annexin V+) as compared to the control cells. Silibinin alone or in combination with anti-miR-21 tranfection had significant apoptotic effects on MCF-7 and T47D cells. Co-application of miR-21 mimic and silibinin on cells showed similar apoptotic results to that in silibinin treatment (Fig. 7).
Fig. 7.
Effect of 150 μM silibinin and miR-21 modulation on the apoptosis of MCF-7 and T47D cells after 48 h. Apoptosis and necrosis percentages in control and treatment samples were determined by Annexin V/PI dual staining in MCF-7 (a) and T47D (b) cells. Data represent the mean ± SD from at least three independent experiments. Results were statistically analyzed with a Student’s t test (*P < 0.05)
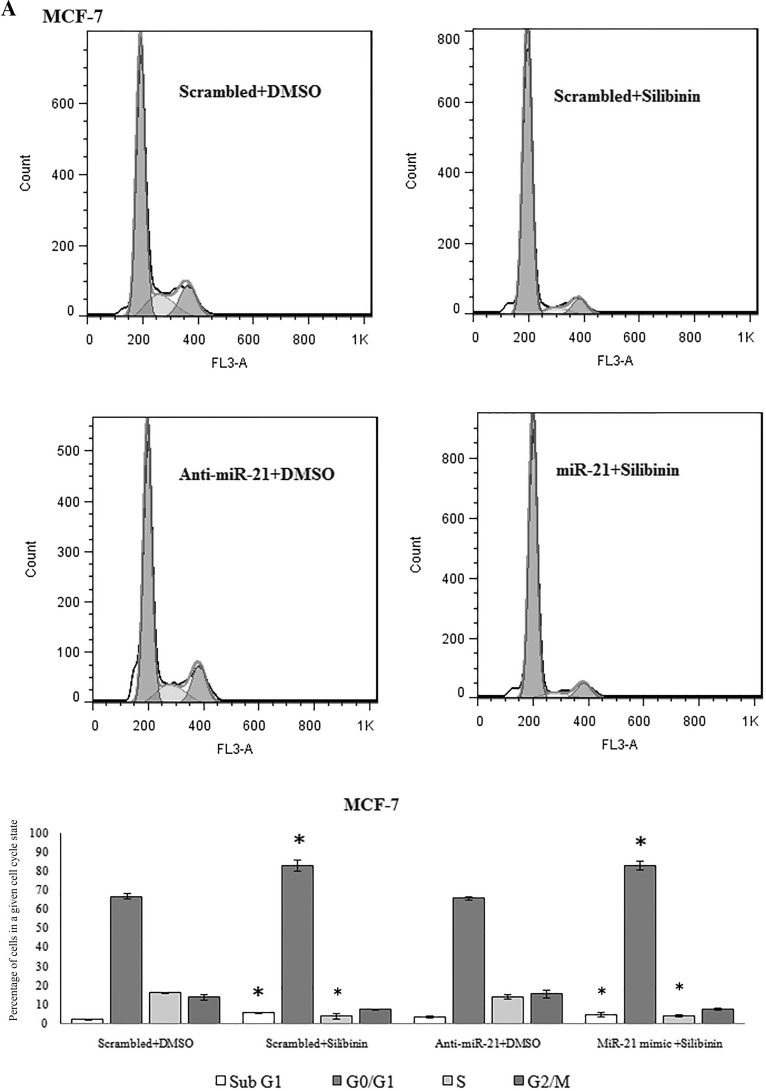
Anti-miR-21 had no effect on cell cycle in MCF-7 and T47D cells
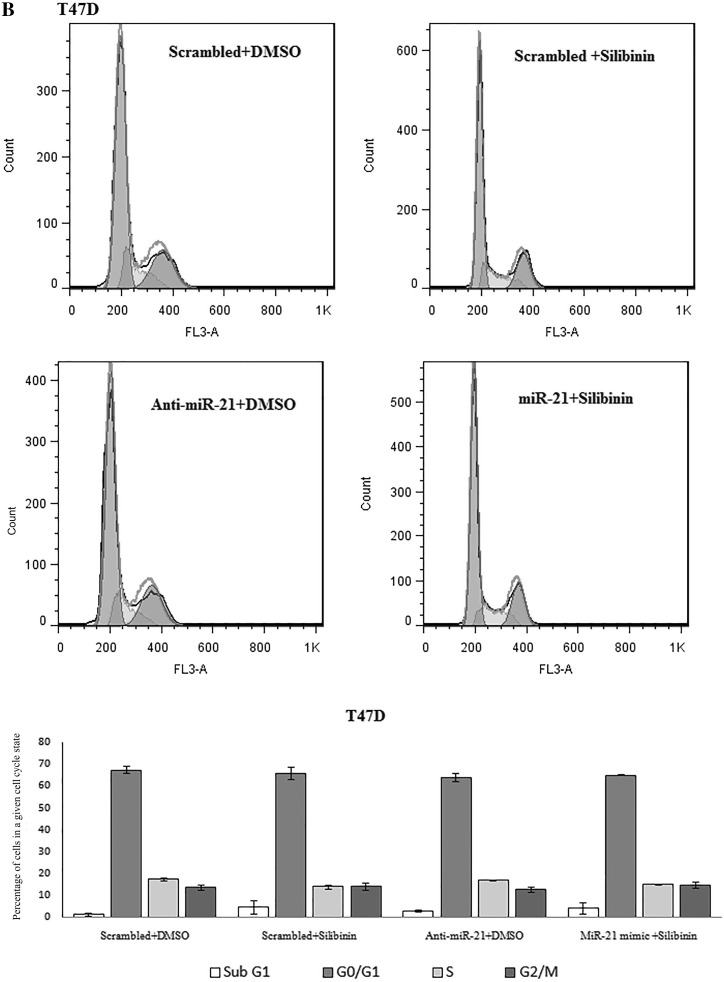
Silibinin caused G1 cell cycle arrest in MCF-7 cells but had no effect on T47D cell cycle progression in our recent study (Jahanafrooz et al. 2016). In this study no slight difference in cell cycle progression was observed after downregulation of miR-21 in MCF-7 and T47D cells as compared to control. Upregulation of miR-21 in silibinin treated samples also was effectless in cell cycle in both cell lines (Fig. 8).
Fig. 8.
Cell cycle analysis by flow cytometry in MCF-7 (a) and T47D (b) cells following miR-21 inhibitor and mimic transfection and treatment with 150 μM dose of silibinin for 48 h. Results were presented as mean (n = 3) ± SD. *P < 0.05, significantly different from control (scrambled + DMSO) by independent t test
Discussion
MiR-21 was the most significantly upregulated miRNA in human breast carcinomas and breast cancer cell lines and its overexpression has been associated with advanced clinical stage, lymph node metastasis, and poor patient prognosis (Volinia et al. 2006; Yan et al. 2008; Shenouda and Alahari 2009; Sicard et al. 2013). Silibinin, as a natural flavonoid, induces various cell functions including growth inhibition, cell cycle arrest, anti-proliferative effect and apoptotic induction and can be applied as anticancer agent (Sharma et al. 2003; Roy et al. 2007; Li et al. 2008; Duan et al. 2011). Our previous report reinforced this point by showing the ability of silibinin to inhibit the growth of both MCF-7 and T47D cells by partially inducing apoptosis and necrosis. Silibinin also induced G1 cell cycle arrest in MCF-7 and MCF-10A cells (Jahanafrooz et al. 2016). The present study demonstrates that silibinin significantly downregulated miR-21 expression in MCF-7, T47D, and MCF-10A cells in accordance with previous ones (Zadeh et al. 2015, 2016).
The aim of this study was to clarify the miR-21 downregulation influence in some anticancer effects of silibinin.
Previous studies showed the negative correlation between miR-21 and its targets for example: (1) downregulation of miR-21 and increase of PTEN in MCF-7 cells under matrin treatment (Li et al. 2012), (2) upregulation of miR-21 and decrease in PTEN and PDCD4 under fludioxonil and fenhexamid treatment (Teng et al. 2013), and (3) downregulation of miR-21 and upregulation of PTEN, PDCD4 and Bcl2 because of estradiol (Wickramasinghe et al. 2009). During the present study, silibinin also downregulated miR-21 expression and increased PTEN expression in MCF-7 cells, but silibinin had no effect on PTEN mRNA and protein expression in T47D cells. In the current study, anti-miR-21 caused small upregulation of PTEN both in T47D and MCF-7 cells and these data were consistent with previous report (Wickramasinghe et al. 2009). In luciferase assay silibinin showed subtle increase in Renilla/Firefly with PTEN-3′UTR for both cell lines in accordance with anti-miR-21 effect. T47D cells exhibited strange behavior, because anti-miR-21 caused increase in PTEN expression; however, silibinin had no effect on PTEN in these cells. This can signify that in T47D cells silibinin upregulated PTEN by its 3′UTR and also downregulated it by another pathway, and these two phenomena neutralize each other. Combined treatment of miR-21 transfection and silibinin administration slightly decreased PTEN mRNA and protein levels and proved this idea in T47D cells. In MCF-7 cells increase in PTEN mRNA in samples with combination of miR-21 mimic transfection and silibinin treatment was less than silibinin treated cells (1.5 ± 0.22, less than 3.24 ± 0.17, P < 0.05), and this shows functional importance of miR-21 downregulation in the silibinin effect on PTEN mRNA in MCF-7 cell line.
Anti-miR-21 caused small decrease in Bcl2 mRNA in MCF-7 cells and this result was in accordance with Si et al. (2007) and Dong et al. (2011) studies (Si et al. 2007; Dong et al. 2011). We observed a partial increase in Bcl2 mRNA expression in T47D cells after anti-miR-21 transfection as expected. Upregulation of miR-21 in silibinin treated samples caused small changes in Bcl2 mRNA expression compared to silibinin treated ones. Therefore miR-21 modulation caused subtle changes in Bcl2 mRNA expression in MCF-7 and T47D cells while silibinin significantly decreased its expression in both cell lines suggesting that regulation of Bcl2 mRNA expression by silibinin was mostly from other pathways (Fig. 6).
Anti-miR-21 transfection had a non-significant role in examined cellular function pathways of silibinin (apoptosis and especially in cell cycle) and miR-21 mimic transfection attenuated apoptotic effect of silibinin in both cell lines with no effect on cell cycle. There are no published data about anti-miR-21 effect on cell cycle so far, but Si et al. (2007) showed that anti-miR-21 induced a significant level of apoptosis in MCF-7 cells as compared to our data (Si et al. 2007). This difference may be because of biological differences among MCF-7 cells from different laboratories (Osborne et al. 1987).
In conclusion, significant downregulation of miR-21 under silibinin treatment had a small role in anticancer effects of silibinin in this study. MiR-21 mimic transfection results in silibinin treated samples partly showed that miR-21 downregulation can be included between functional pathways of silibinin in MCF-7 and T47D cell lines. Anti-miR-21 transfection results of the present study were in favor of consecutive effect of miR-21 overexpression in MCF-7 and T47D cell lines. However, more extensive studies are suggested to be carried out in this direction in the near future, which will more clarify the microRNAs related mechanisms underlying the anticancer effects of silibinin in breast cancer treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our thanks to Prof. Elaheh Elahi for providing psiCHECK™-2 vector, Dr. Ehsan Arefian, for providing dual-luciferase activity reporter assay kit, Mr Babak Saffari, PhD student for constructing psiCheck-2/PTEN-3′UTR and psiCheck-2/Bcl2-3′UTR vectors, Miss Mahfam Shariati, MSc student for vector extraction and Miss Mehraban Mirrahimi, MSc student for bacterial culture, in Tehran University which help us after manuscript revision.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors greatly acknowledge the financial supports of Science and Technology Park, University of Tehran, Tehran, Iran.
Abbreviations
- ERa+
Estrogen receptor alpha positive
- ERa−
Estrogen receptor alpha negative
- PTEN
Phosphatase and tensin homolog
- SNORD-47
Small nucleolar RNA 47
- PDCD4
Programmed cell death protein 4
- TM1
Tropomyosine 1
- PMSF
Phenyl methyl sulfonyl fluoride
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-017-0076-5) contains supplementary material, which is available to authorized users.
Contributor Information
Nasrin Motamed, Phone: +98 (21) 61112472, Email: motamed2@khayam.ut.ac.ir.
Behnaz Bakhshandeh, Phone: +98 (21) 66491622, Email: b.bakhshandeh@ut.ac.ir.
References
- Asad SF, Singh S, Ahmad A, Hadi SM. Flavonoids: antioxidants in diet and potential anticancer agents. Med Sci Res. 1998;26:723–728. [Google Scholar]
- Bakhshandeh B, Soleimani M, Hafizi M, Ghaemi N. A comparative study on nonviral genetic modifications in cord blood and bone marrow mesenchymal stem cells. Cytotechnology. 2012;64:523–540. doi: 10.1007/s10616-012-9430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev. 2011;5(9):1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, Saltzman WM, Slack FJ. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518(7537):107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep G, Agarwal R. Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr Cancer Ther. 2007;6(2):130–145. doi: 10.1177/1534735407301441. [DOI] [PubMed] [Google Scholar]
- Dong J, Zhao YP, Zhou L, Zhang TP, Chen G. Bcl-2 upregulation induced by miR-21 via a direct interaction is associated with apoptosis and chemoresistance in MIA PaCa-2 pancreatic cancer cells. Arch Med Res. 2011;42(1):8–14. doi: 10.1016/j.arcmed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Duan WJ, Li QS, Xia MY, Tashiro S, Onodera S, Ikejima T. Silibinin activated p53 and induced autophagic death in human fibrosarcoma HT1080 cells via reactive oxygen species-p38 and c-Jun N-terminal kinase pathways. Biol Pharm Bull. 2011;34(1):47–53. doi: 10.1248/bpb.34.47. [DOI] [PubMed] [Google Scholar]
- Esau CC. Inhibition of microRNA with antisense oligonucleotides. Methods. 2008;44(1):55–60. doi: 10.1016/j.ymeth.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Ménard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Jahanafrooz Z, Motamed N, Bakhshandeh B. Comparative evaluation of silibinin on cell cycle and apoptosis in human breast cancer MCF-7 and T47D cell lines. Asian Pac J Cancer Prev. 2016;17(5):2661–2665. [PubMed] [Google Scholar]
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Lee YS, Dutta A. MicroRNA in Cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008;272(1):61–69. doi: 10.1016/j.canlet.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang HH, Liu X, Liang DS, Lu YJ, Shan HL, Jiang HC. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem. 2012;30(3):631–641. doi: 10.1159/000341444. [DOI] [PubMed] [Google Scholar]
- Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12(11):847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi-Yeganeh S, Paryan M, Mirab Samiee S, Soleimani M, Arefian E, Azadmanesh K, Mostafavi E, Mahdian R, Karimipoor M. Development of a robust, low cost stem-loop real-time quantification PCR technique for miRNA expression analysis. Mol Biol Rep. 2013;40(5):3665–3674. doi: 10.1007/s11033-012-2442-x. [DOI] [PubMed] [Google Scholar]
- Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol Int. 2008;32(8):888–892. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Naderi M, Abdul Tehrani H, Soleimani M, Shabani I, Hashemi SM. A home-brew real-time PCR assay for reliable detection and quantification of mature miR-122. Appl Immunohistochem Mol Morphol. 2015;23(8):601–606. doi: 10.1097/PAI.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Hobbs K, Trent JM. Biological differences among MCF-7 human breast cancer cell lines from different laboratories. Breast Cancer Res Treat. 1987;9(2):111–121. doi: 10.1007/BF01807363. [DOI] [PubMed] [Google Scholar]
- Ranji N, Sadeghizadeh M, Shokrgozar MA, Bakhshandeh B, Karimipour M, Amanzadeh A, Azadmanesh K. MiR-17-92 cluster: an apoptosis inducer or proliferation enhancer. Mol Cell Biochem. 2013;380(1–2):229–238. doi: 10.1007/s11010-013-1678-7. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1(3):1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007;6(10):2696–2707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8(16):122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, Schwartz G, Wells W, Kauppinen S, Cole CN. Altered microRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67(24):11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649–2655. [PubMed] [Google Scholar]
- Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28(3–4):369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- Sicard F, Gayral M, Lulka H, Buscail L, Cordelie P. Targeting miR-21 for the Therapy of Pancreatic Cancer. Mol Ther. 2013;21(5):986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Manavalan TT, Hu C, Medjakovic S, Jungbauer A, Klinge CM. Endocrine disruptors fludioxonil and fenhexamid stimulate miR-21 expression in breast cancer cells. Toxicol Sci. 2013;131(1):71–83. doi: 10.1093/toxsci/kfs290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P, Kumar A, Balakrishnan S, Kushwaha HS, Mishra KP. Silibinin-induced apoptosis in MCF-7 and T47D human breast carcinoma cells involves caspase-8 activation and mitochondrial pathway. Cancer Invest. 2011;29(1):12–20. doi: 10.3109/07357907.2010.535053. [DOI] [PubMed] [Google Scholar]
- Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev RNA. 2012;3(3):311–330. doi: 10.1002/wrna.121. [DOI] [PubMed] [Google Scholar]
- Verschoyle RD, Greaves P, Patel K, Marsden DA, Brown K, Steward WP, Gescher AJ. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: relationship with silibinin levels. Eur J Cancer. 2008;44(6):898–906. doi: 10.1016/j.ejca.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L, Brown J, Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum Mol Genet. 2001;10(3):237–242. doi: 10.1093/hmg/10.3.237. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37(8):2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh MM, Ranji N, Motamed N. Deregulation of miR-21 and miR-155 and their putative targets after silibinin treatment in T47D breast cancer cells. Iran J Basic Med Sci. 2015;18(12):1209–1214. [PMC free article] [PubMed] [Google Scholar]
- Zadeh MM, Motamed N, Ranji N, Majidi M, Falahi F. Silibinin-induced apoptosis and downregulation of microRNA-21 and microRNA-155 in MCF-7 human breast cancer cells. J Breast Cancer. 2016;19(1):45–52. doi: 10.4048/jbc.2016.19.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18(3):350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.