Abstract
The mycotoxin citrinin, is produced by several species of Penicillium, Aspergillus and Monascus, and is capable of inducing cytotoxicity, oxidative stress and apoptosis. The aim of the present study was to investigate the effect of citrinin in mouse skeletal muscle cells (C2C12) and to overcome the cellular adverse effects by supplementing green tea extract (GTE) rich in polyphenols. C2C12 myoblasts were differentiated to myotubes and were exposed to citrinin in a dose dependent manner (0–100 µM) for 24 h and IC50 value was found to be 100 µM that resulted in decreased cell viability, increased LDH leakage and compromised membrane integrity. Mitochondrial membrane potential loss, increased accumulation of intracellular ROS and sub G1 phase of cell cycle was observed. To ameliorate the cytotoxic effects of CTN, C2C12 cells were pretreated with GTE (20, 40, 80 µg/ml) for 2 h followed by citrinin (100 µM) treatment for 24 h. GTE pretreatment combated citrinin-induced cytotoxicity and oxidative stress. GTE at 40 and 80 µg/ml significantly promoted cell survival and upregulated antioxidant enzyme activities (CAT, SOD, GPx) and endogenous antioxidant GSH, while the gene and protein expression levels were significantly restored through its effective antioxidant mechanism. Present study results suggested the antioxidant properties of GTE as a herbal source in ameliorating the citrinin-induced oxidative stress.
Keywords: Citrinin, C2C12 cells, Oxidative stress, Cytotoxicity, Green tea polyphenols, Lactate dehydrogenase
Introduction
Citrinin (CTN) is a toxic secondary metabolite of fungi isolated from P. citrinum Thom (Hetherington and Raistrick 1931) and produced by other species of Penicillium, Aspergillus (El-Banna et al. 1987) and Monascus (Blanc et al. 1995). CTN contamination is commonly encountered in crops like wheat, oats, rye, corn, barley, rice and other foods and feeds (Bennett and Klich 2003; Richard et al. 2003). The toxicity of CTN has been implicated in humans as “yellow rice” disease in Japan and Balkan Endemic Nephropathy in South-Eastern European countries (Vrabcheva et al. 2000).
Various cell line studies have shown that CTN mycotoxin has the potential to cause excessive generation of reactive oxygen species (ROS) (Chan 2007), activation of signaling pathway (Chang et al. 2009; Chen and Chan 2009; Liu et al. 2010) and apoptosis (Yu et al. 2006; Liu et al. 2005; Klaric et al. 2012). Further, at the cellular level CTN mediates mitochondrial permeability transition as well as dysfunction of mitochondria with loss of mitochondrial membrane potential (MMP) (Da Lozzo et al. 1998; Chagas et al.1992; Ribeiro et al.1997). Other deleterious properties of CTN include aneuploidogenic, genotoxic (Pfeiffer et al. 1998), embryocidal, fetotoxic, mildly teratogenic effects (Reddy et al. 1982) and cell cycle arrest at G2/M phase in HEK293 cells through interruption of spindle formation and tubulin polymerization (Chang et al. 2011). Jeswal (1996), reported that CTN exposure in mice results in chromosome abnormalities and breakages in bone marrow cells, chromosome aberrations such as gaps, rings, breaks and centric fusions were also observed in mice (Bouslimi et al. 2008).
Green Tea (Camellia sinensis L.,) is one of the most widely consumed beverages in different parts of the world such as China, Japan, India and Sri Lanka and is probably the most consumed beverage that has attracted greater attention in the recent years for its significant effects on health in a variety of disease conditions (Huo et al. 2008). The beneficial effects of green tea are mainly due to the polyphenolic compounds commonly called the catechins, which make up about 30% of the dry weight of green tea leaves (Graham 1992). The major catechins present in green tea are (−) epicatechin (EC), (−) epicatechin-3-gallate (ECG), (−) epigallocatechin (EGC), (−) epigallocatechin-3-gallate (EGCG), (+) catechin and (+) gallocatechin (GC). EGCG, the most abundant catechin in green tea, accounts for 65% of the total catechin content (Zaveri 2006). EGCG, apart from possessing antioxidant activity has also been demonstrated to exhibit health promoting properties against diabetes, Parkinson’s disease, Alzheimer’s disease, obesity and cardiovascular diseases (Khan et al. 2006; Higdon and Frei 2003; Shankar et al. 2007; Velayutham et al. 2008). Tea also contains large amounts of other polyphenolic compounds with remarkable antioxidant properties as well as DNA-damage protective properties (Wiseman et al. 1997; Anderson et al. 2001). Several studies have reported that polyphenols and tea catechins are exceptional electron donors and effective scavengers of physiologically relevant reactive oxygen species in vitro, including superoxide anions (Nakagawa and Yokozawa 2002; Nanjo et al. 1993), peroxyl radicals, and singlet oxygen (Guo et al. 1999; Michalak 2006).
C2C12 myotubes are frequently used as a model for studying muscle cell growth and differentiation and exhibit the characteristics of normal myoblastic cells (Yaffe and Saxel 1977; Salucci et al. 2010). In contrast to being resistant to cell death, apoptosis has been observed in skeletal muscle tissues where it has shown to affect skeletal muscle biology (Salucci et al. 2010). An increased susceptibility to oxidative stress due to elevated ROS production has been reported in a number of muscle disorders such as Duchenne muscular dystrophy (DMD), fibrosis, weakness in dystrophin deficiency etc., (Kozakowska et al. 2015) Therefore, in the present study we investigated the cytoprotective effects of GTE against CTN-induced oxidative stress in C2C12 myotubes.
Materials and methods
Citrinin, Dulbecco’s modified Eagle’s medium (DMEM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), Rhodamine 123, 2′,7′-dichlorofluorescin diacetate (DCFH2-DA), propidium iodide (PI), protease inhibitor cocktail, 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Green tea extract (GTE) was procured from Parry Nutraceuticals (Chennai, Tamil Nadu, India). All other reagents were of the highest purity unless otherwise stated.
DPPH free radical scavenging activity
The free radical scavenging activity of the GTE was determined using the stable radical DPPH (Braca et al. 2001). Briefly, DPPH (0.004% in methanol) solution was mixed with different concentrations of sample and the volume was made up to 3 ml with methanol. The mixture was incubated for 45 min at room temperature in dark and decrease in absorbance was measured at 517 nm. Butylated hydroxyanisole (BHA) was used as a standard. The percentage inhibition was calculated as follows:
where Ac is the absorbance of control and As is the absorbance of the sample. IC50 values represent the concentration of sample needed to scavenge 50% DPPH free radicals.
Determination of total phenolic content
The total polyphenols were estimated using Folin–Ciocalteau method (Singleton and Rossi 1965). To the different concentrations of GTE (50–150 μg), 2.5 ml of Folin-Ciocalteu reagent (1:10) and 2 ml of 7% sodium carbonate (Na2CO3) were added, mixed and incubated at room temperature for 90 min. The absorbance of the sample was measured against the blank spectrophotometrically at 765 nm. Gallic acid was used as standard and the amount of total polyphenolic content was expressed as mg gallic acid equivalent per gram extract (mg GAE/g).
Determination of total flavonoid content
Total flavonoids content of the GTE was determined according to Sakanaka et al. (2005) with minor modifications. Briefly, different concentrations of GTE (50–150 μg) were mixed with 75 µl of 5% sodium nitrite (NaNO2) and 150 µl 10% aluminium chloride (AlCl3) and incubated at room temperature for 5 min. To this, 500 µl of 1 M sodium hydroxide followed by 775 µl of water were added, vortexed and finally the absorbance was measured immediately at 510 nm. Catechin was used as a standard and the amount of total flavonoid was expressed as mg of catechin equivalents per gram extract (mg Catechin/g).
RP-HPLC analysis of green tea extract
RP-HPLC analysis was carried out for identification of phenolic compounds present in GTE using a Jasco HPLC system equipped with a Jasco Intelligent HPLC pump (PU-1580) and a photodiode array detector (JASCO Pu-1580 HPLC system, Gross-Umstadt, Germany) and Waters SunFire™ C18 (5 µM, 4.6 × 250 mm) column (Dublin, Ireland). The mobile phase was 0.1% formic acid (A) and 100% methanol (B) and the total run time was kept to 60 min. The compounds were detected by monitoring at 280 nm. The phenolic compounds were identified by comparing the retention time of the GTE with that of standards.
UPLC-MS analysis
UPLC-MS analysis of green tea extract was analyzed using Waters Acquity UPLC (CIRF, University of Mysore, Mysuru, India). The UPLC-MS system consisted of MALDI SYNAPT G2 High Definition Mass Spectrometry. Data analysis software was MassLynx SCN781. GTE was separated with an ACQUITY UPLC BEH C18 (1.7 μm 1.0 × 50 mm) column (Waters). Mobile phase A was 0.1% formic acid in water and mobile phase B was acetonitrile, a gradient elution was performed. Flow rate of 0.3 ml/min and 2 μl of sample were injected for each analysis. The column temperature was maintained at 50 °C. The ESI source was operated in positive ion mode with parameters for capillary voltage set at 1.8 kV, desolvation temperature at 200 °C and source temperature at 100 °C. The auxillary and sheath gas were nitrogen and desolvation gas flow was 500 L/Hr. The mass spectra was scanned across the range of 50–700 m/z.
Cell culture and treatments
C2C12 (mouse skeletal muscle cell line) myoblasts (obtained from National Center for Cell Sciences, Pune, India) were cultured in high glucose DMEM supplemented with 10% fetal bovine serum (Hyclone, Invitrogen, Carlsbad, CA, USA), 50 U/ml penicillin and 50 µg/ml streptomycin solution (Sigma-Aldrich), in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. When cells reached approximately 70–80% confluence, the medium was switched to differentiation medium containing DMEM and 2% horse serum to induce myotube differentiation. The medium was changed every day, the differentiated C2C12 myotubes (C2C12 cells) were used for all experiments in serum free medium. The treatment protocol was as follows: for cytotoxicity experiments, C2C12 myotubes were treated with different concentration of CTN (0, 25, 50, 75, and 100 µM) for 24 h. For cytoprotective studies C2C12 myotubes were pretreated with 20, 40, and 80 µg GTE/ml for 2 h followed by with or without CTN treatment for 24 h.
Cell morphology
C2C12 cells were seeded in 25 cm2 flasks (1 × 106 cells) and pre-treated with GTE (20, 40, 80 µg/ml) for 2 h followed by with or without CTN treatment for 24 h. The cellular morphology was observed and photographed using phase contrast microscope (Olympus, Tokyo, Japan) equipped with Cat Cam 200 and Scope photo 3.0.
MTT assay for cell viability
Cell viability was determined by MTT assay by culturing C2C12 cells in a 24 well plate. C2C12 cells were treated as mentioned in the cell culture and treatment section. After the treatment period, MTT (0.5 mg/ml, dissolved in serum free medium) was added to each well and incubated for 2 h at 37 °C. The supernatant was removed and 500 µl of DMSO was added to dissolve the formazan crystals. The absorbance was measured at 570 nm using Plate Chameleon-Multi-technology plate reader, (HIDEX, Turku, Finland) and the percentage viability was calculated (Hanelt et al. 1994).
Lactate dehydrogenase leakage (LDH) assay
LDH leakage assay was carried out using in vitro toxicology assay kit, Lactic Dehydrogenase based (TOX-7, Sigma-Aldrich) according to manufacturer’s instructions. C2C12 cells were treated as mentioned before. The assay is based on the reduction of NAD by LDH and the resulting reduced NAD (NADH) is utilized for the conversion of tetrazolium dye resulting in a colored compound which is measured spectrophotometrically at 490 nm. LDH activity in the medium was expressed as the percentage of the total LDH activity.
Mitochondrial membrane potential (ΔΨm) assay
C2C12 cells were grown in 24-well plates, after treatment cells were loaded with Rhodamine-123 (10 µM) (Buckler and Vaughan-Jones 1998) and incubated for 30 min at 37 °C in a CO2 incubator. The cells were then collected after washing twice with PBS and the fluorescence was measured at an excitation and emission wavelength of 485/535 nm.
Estimation of intracellular ROS
CTN-induced ROS accumulation in C2C12 cells was measured with DCFH2-DA dye according to the method of Wang and Joseph (1999). Briefly, C2C12 cells were seeded in a 24-well plate and treated as described before. After incubation, the cells were washed twice with PBS and loaded with 10 µM DCFH2-DA for 30 min at 37 °C. ROS accumulation was measured at an excitation and emission wavelength of 485/535 nm. The results were expressed as a relative percent of DCF-fluorescence as compared to control cells.
Antioxidant enzyme assays
C2C12 cells were seeded in 75 cm2 flasks and treated as mentioned before, cells were washed with cold PBS and lysed using cell lysis buffer (50 mM sodium phosphate buffer, pH 7.4 containing 2 mM EDTA and 0.1% Triton X-100) in cold conditions, lysate was centrifuged at 12,000 g for 10–15 min at 4 °C. The resulting supernatant was collected and protein concentration estimated by Lowry’s method (1951). The activity of antioxidant enzyme such as superoxide dismutase (SOD), glutathione peroxidase (GPx) was measured using kits as per the manufacturer’s instructions (Cat no. SD125, RS504, Randox, Canada). Catalase (CAT) activity was estimated spectrophotometrically according to the method of Aebi (1984) by measuring the rate of H2O2 (10 mM) decrease at 240 nm at 25 °C for 120 s. Specific activity of catalase was calculated via the following equation:
Estimation of total glutathione (GSH)
Glutathione content was determined by a spectrophotometric method using Ellman’s reagent with minor modifications (Tietze 1969; Aykaç et al. 1985). C2C12 cells were grown in 75 cm2 flasks and treated as described previously. Samples were homogenized in 5% trichloroacetic acid and 5 mM EDTA, centrifuged at 12,000 g for 15 min at 4 °C. For the assay, 0.1 ml of sample supernatant, 2 ml cold phosphate buffer (pH 7.5) and 0.5 ml of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) was added and the absorbance was measured at 412 nm.
Cell cycle analysis by flow cytometry
Flow cytometry was carried out for DNA content analysis by PI staining and apoptotic cells were identified at sub-G1 phase. After treatment, cells were harvested by trypsinization, washed with cold PBS and fixed with 70% ethanol at 4 °C overnight (Krishan 1975; Riccardi and Nicoletti 2006). Cells were re-suspended in PBS and then propidium iodide solution was added. Cell cycle analysis was carried out at a flow rate of 10,000 events per second per sample on a FACS Calibur (Becton–Dickinson, USA) at Central Imaging and Flow Cytometry Facility (National Centre for Biological Sciences, Bengaluru, Karnataka, India), data were analyzed using Cell quest Pro software.
Gene expression analysis by semi-quantitative PCR
RNA extraction was carried out using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. C2C12 cells were grown in 75 cm2 flasks and treated as mentioned before. RNA obtained from C2C12 cells was reverse transcribed to cDNA First Strand cDNA Synthesis Kit (Fermentas) as per the manufacturer’s instructions. 1.5 µg of total RNA was used for cDNA conversion followed by target-gene amplification with PCR. 2 µL of amplified cDNA was subjected to PCR. The sequence of the sense and antisense primers used for semi-quantitative reverse transcription-PCR were designed using Gene Runner software; the NCBI accession number and product length are given in Table 1. Amplification of each gene showed a single band of the expected size (CAT-357 bp, SOD1-139 bp, GPx-431 bp, GAPDH-223 bp). The reaction conditions were an initial denaturation at 94 °C for 3 min, 35 cycles of amplification (94 °C for 30 s, 58 °C for 30 s, and 72 °C for 45 s) were performed followed by 5 min extension at 72 °C for CAT and GPx; 94 °C for 3 min, 35 cycles of amplification (94 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min) were performed followed by a 5 min extension at 72 °C for SOD; 94 °C for 3 min, 35 cycles of amplification (94 °C for 30 s, 60 °C for 30 s, and 72 °C for 45 s) were performed followed by a 7 min extension at 72 °C for GAPDH. The PCR products were separated by electrophoresis on a 1–1.5% agarose gel, visualized by ethidium bromide staining and photographed. The house keeping gene GAPDH was used as reference gene.
Table 1.
List of antioxidant gene specific primers used in this study
| Gene | NCBI accession number | Sequence | Size (bp) |
|---|---|---|---|
| GAPDH | XM_001476707.3 | 5′-AACTTTGGCATTGTGGAAGG-3′ 5′-ACACATTGGGGGTAGGAACA-3′ |
223 |
| GPx | NM_008160.6 | 5′-CTCGGTTTCCCGTGCAATCAG-3′ 5′-GTGCAGCCAGTAATCACCAAG-3′ |
431 |
| SOD2 | NM_013671.3 | 5′-GCACCACAGCAAGCACCAC-3′ 5′-TGTCCCCCACCATTGAACT-3′ |
139 |
| Catalase | NM_009804.2 | 5′-TCTGCAGATACCTGTGAACTG-3′ 5′-TAGTCAGGGTGGACGTCAGTG-3′ |
357 |
Western blot analysis
C2C12 cells were seeded in 75 cm2 flasks and treated as mentioned earlier. After treatment, the cells were harvested and washed twice with PBS, lysed using ice-cold RIPA buffer with protease inhibitor cocktail (Sigma-Aldrich). The cell lysates were centrifuged at 12,000 g for 30 min at 4 °C, supernatants were collected as whole cell extracts and protein concentration determined by Lowry’s method (1951). Equal amounts of protein (50 µg) were run on SDS-PAGE followed by electro-blotting on polyvinylidenedifluoride (PVDF, Millipore) membrane. After transfer, the membranes were blocked overnight with 5% (w/v) non-fat dry milk in Tris-buffered saline (10 mM Tris–HCl, 150 mM NaCl, pH 7.5) containing 0.05% Tween-20 (TBS-T) at 4 °C. The blots were incubated with primary antibodies GAPDH (37 kDa, sc-25778), SOD (23 kDa, sc-8637), CAT (64 kDa, sc-34280) and GPx (23 kDa, sc-22146) at 1:1000 dilution for 2 h at 100 rpm in an orbital shaker. The membranes were washed 4 times with TBST for 5 min and incubated with goat anti-rabbit, rabbit anti-goat and goat anti-mouse (DAKO, Glostrup, Denmark) horseradish peroxidase-conjugated secondary antibodies at 1:5000 dilutions, respectively. The membranes were again washed 5 times at 5 min interval and the immunoreactivity was detected using the enhanced chemiluminescence peroxidase substrate kit (CPS-160, Sigma). The obtained signal was developed on a CL-XPosure Film (Thermo Scientific, Waltham, MA, USA) and the band density was analyzed using NIH Image J software.
Statistical analysis
The values were expressed as the mean ± standard deviation (n = 3). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using SPSS statistical software (version 20.0; SPSS, Inc., Chicago, IL, USA). The significant differences were indicated as p < 0.05.
Results
In vitro antioxidant activity of GTE
The antioxidant activity of GTE extract was determined based on the DPPH radical scavenging property and the IC50 value was found to be 7.26 µg/ml. The polyphenolic and flavonoid content was 643.53 mg GAE/g (gallic acid equivalents) and 231.33 mg CE/g of extract, respectively.
Characterization of GTE by RP-HPLC and UPLC-MS analysis
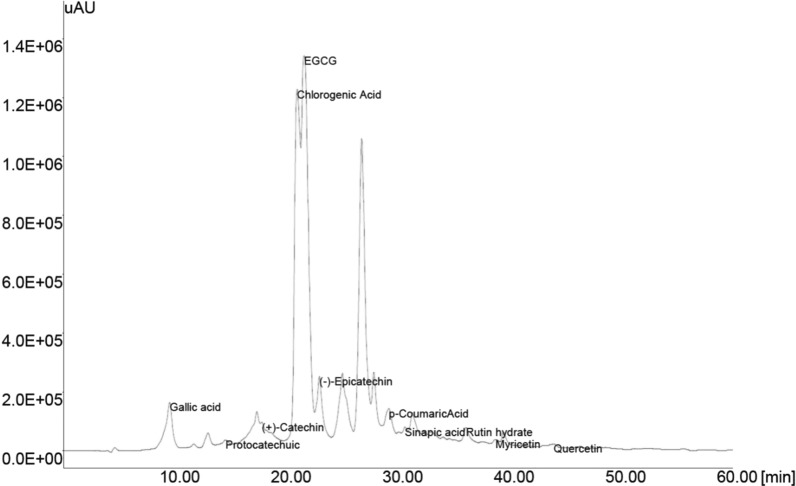
A high performance liquid chromatographic method (HPLC) with UV detection is commonly used for the analysis of tea catechins, identification of the individual phenolic compounds was achieved by comparing the GTE unknowns (Fig. 1) with that of standards (data not shown). The identified phenolic compounds of GTE along with retention time were Gallic acid (9.44), Protocatechuic acid (14.38), (+)-Catechin (17.64), Chlorogenic acid (20.90), (−)-Epigallocatechin gallate (21.40), (−)-Epicatechin (22.81), p-Coumaric acid (29.08), Sinapic acid (30.50), Rutin hydrate (35.96), Myricetin (38.58) and Quercetin (43.82).
Fig. 1.
HPLC chromatogram of phenolic compounds present in green tea extract
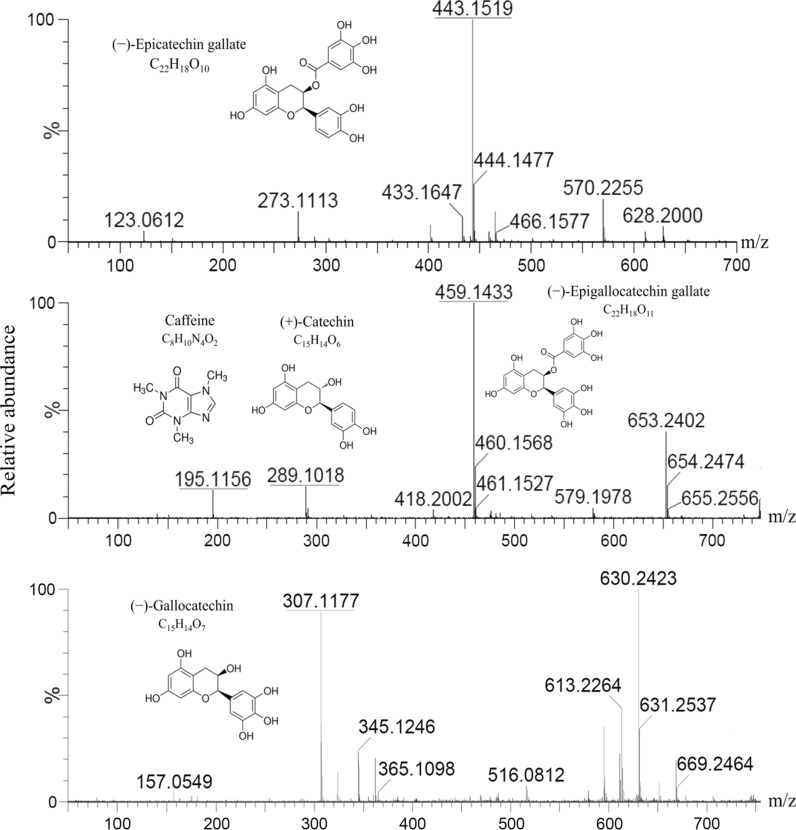
The chromatograms presented in Fig. 2 show the mass spectra of the identified compounds. The ESI–MS method was used for the confirmation of bioactive compounds in GTE. Mass spectra were recorded in positive ionization mode and the method was able to confirm the presence of five phenolic compounds. The presence of these compounds was confirmed based on the comparison of m/z values from the MS2 spectra with the literature values for the standards. The peak at m/z 443.15 corresponds to (−)-Epicatechin gallate, m/z 289.10 represents (+)-Catechin, m/z 307.11 represents (−)-Gallocatechin, (−)-Epigallocatechin gallate gives a peak at m/z 459.14, while caffeine shows a signal at m/z 195.11.
Fig. 2.
Identification of phenolic compounds in green tea extract using UPLC-MS
Cytotoxic effect of CTN on C2C12 myotubes and its amelioration by GTE
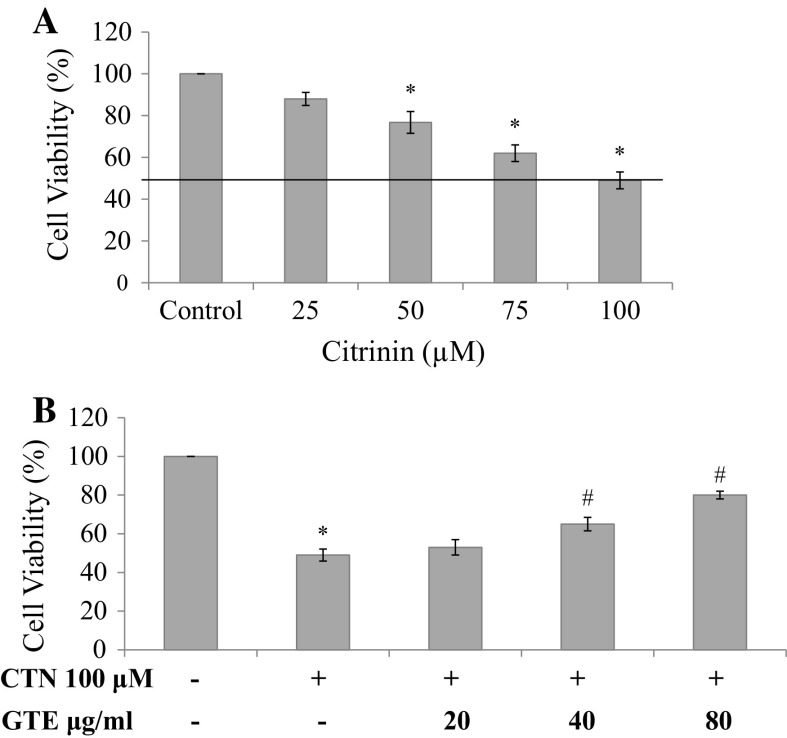
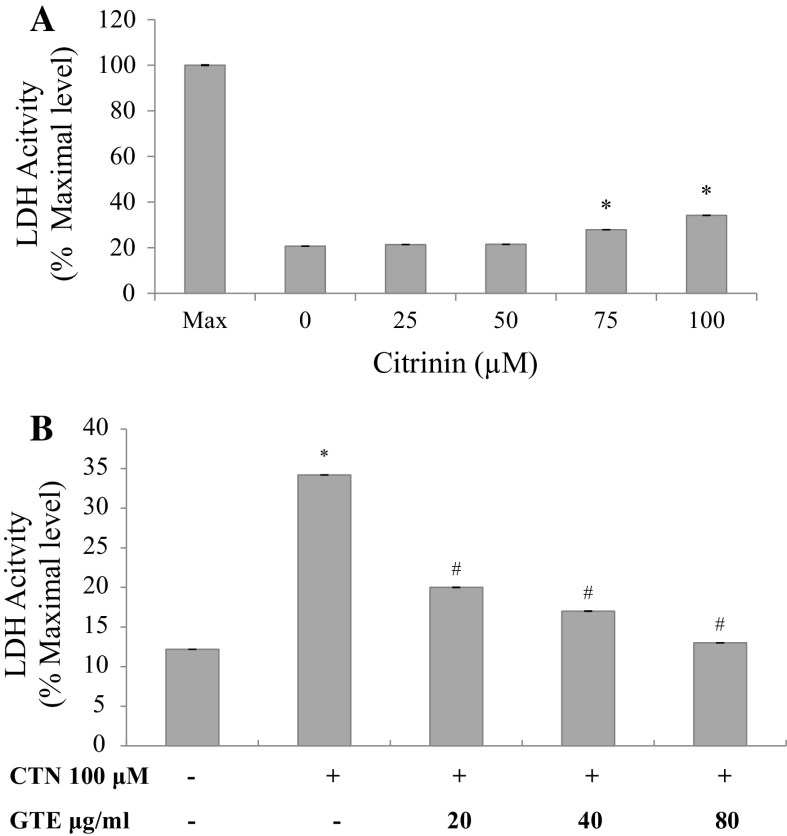
C2C12 cells were exposed to CTN (25–100 µM) for 24 h and cytotoxicity was determined using MTT assay and LDH leakage assay. Cell death was distinctively observed from 75 and 100 µM compared to control cells after 24 h incubation. At the lower concentration of 25 and 50 µM, the effect was found to be insignificant (Fig. 3a). The maximum reduction in cell viability (49%) was observed at the highest dose of 100 µM (p < 0.05 versus control). IC50 was found to be 102.04 µM and is presented as bar graph in Fig. 3a. Further, to determine the protective effect of GTE against CTN-induced myotube damage, C2C12 cells were pre-treated with 20, 40 and 80 µg/ml of GTE for a period of 2 h followed by treatment in presence or absence of CTN for 24 h. Protective effect of GTE was observed to an extent of 65% at the medium dose and 80% at the higher dose of pretreatment, respectively (Fig. 3b). In order to further examine the cytotoxicity of CTN, LDH enzyme activity was carried out to check the membrane damage. Cells were treated with CTN in a dose-dependent manner for 24 h followed by measuring the LDH activity. As shown in Fig. 4a, an increase in LDH release of 27 and 34% was observed at 75 and 100 µM of CTN which was significant (p < 0.05) with respect to control, while, GTE pretreatment inhibited LDH release significantly (p < 0.05) to near basal level compared to CTN only treated cells (Fig. 4b).
Fig. 3.
Cell viability was measured using MTT assay a CTN treatment with 0–100 μM CTN concentration for 24 h, b cells were pretreated with GTE (20, 40, 80 µg/ml) for 2 h followed by with or without CTN (100 µM) treatment for 24 h. Data are expressed as the mean ± standard deviation from three independent experiments, each performed in triplicate. *p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
Fig. 4.
Effect of CTN on LDH leakage in C2C12 cells a CTN treatment with 0–100 μM CTN concentration for 24 h, b cells were pretreated with GTE (20, 40, 80 µg/ml) for 2 h followed by with or without CTN (100 µM) treatment for 24 h. Data are expressed as the mean ± standard deviation from three independent experiments, each performed in triplicate. *p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
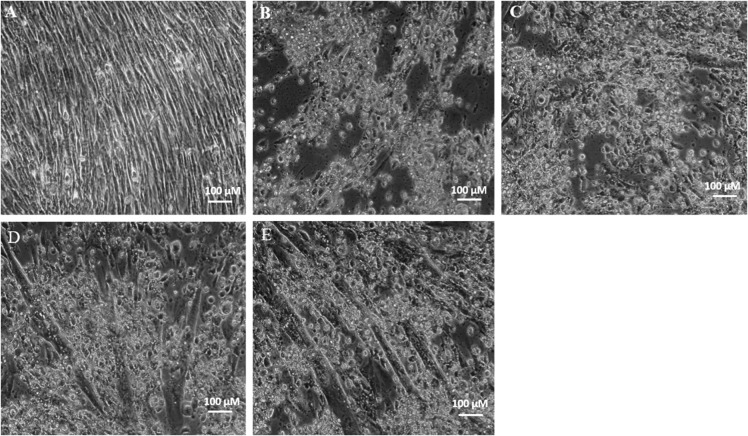
Effect of GTE on cell morphology in CTN treated cells
To evaluate the protective effect of GTE in ameliorating oxidative stress, C2C12 cells were pretreated with three concentrations (20, 40, 80 µg/ml) of GTE for 2 h, followed by treatment with the effective dose of 100 µM CTN for 24 h. The morphology of C2C12 myotubes of both control and CTN treated cells are shown in Fig. 5. Control cells had a clear myotube formation, while 100 μM CTN treatment damaged the natural structures of the myotube leading to cell death. GTE pretreatment for 2 h followed by 100 μM CTN for 24 h protected myotube integrity and cell morphology.
Fig. 5.
Morphological features of C2C12 cells under phase contrast microscope, a control, b CTN 100 µM, c GTE 20 µg/ml + CTN, d GTE 40 µg/ml + CTN, e GTE 80 µg/ml + CTN
Effect of GTE on CTN-induced reduction of MMP (ΔΨm)
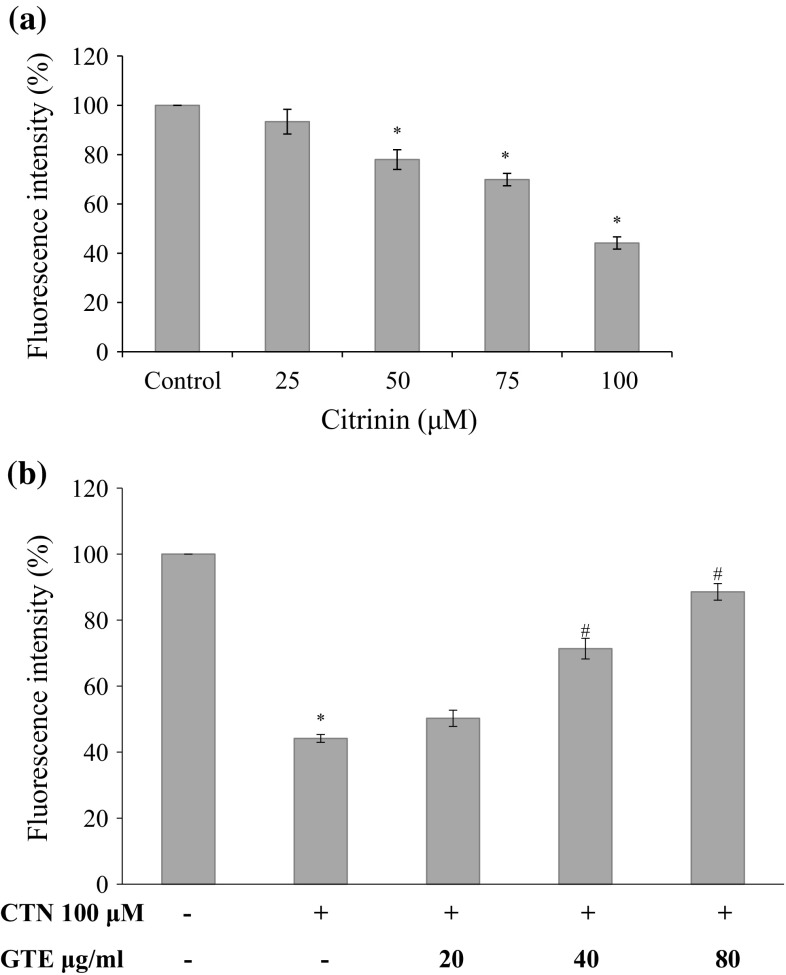
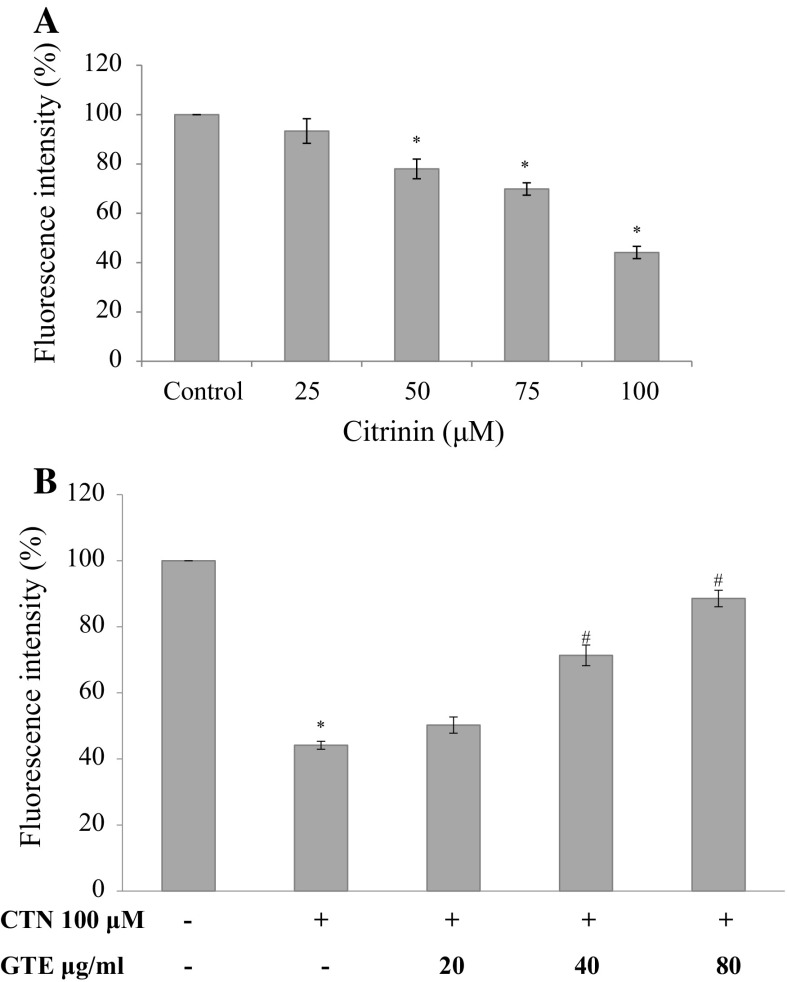
Muscles are rich in mitochondria and healthy C2C12 cells uptake Rhodamine 123 (Rho123) easily. After treatments, C2C12 cell were exposed to Rho 123 for 30 min at 37 °C. Cells showed dose-dependent decrease in MMP (ΔΨm ) as shown in Fig. 6a, by 93.37, 78.0, 69.87, and 44.14% (25, 50, 75 and 100 µM) relative to control cells (p < 0.05 for 50–100 µM) indicating the possibility of CTN in affecting mitochondria and the skeletal muscle cell function. While pretreatment with GTE prevented the mitochondrial damage significantly in a dose dependent manner to 50.25, 71.36 and 88.56% (20, 40, 80 µg/ml of GTE) (Fig. 6b) compared to CTN treated C2C12 cells.
Fig. 6.
Effect of CTN on loss of MMP in C2C12 cells a C2C12 cells were treated with 0–100 µM CTN for 24 h, b cells were pre-treated with GTE (20, 40, 80 µg/ml) for 2 h followed by with or without CTN (100 µM) treatment for 24 h. Data are expressed as the mean ± standard deviation from three independent experiments, each performed in triplicate. *p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
CTN induced ROS generation and its amelioration by GTE treatment
The ability of CTN to induce intracellular ROS accumulation was measured as % DCF fluorescence. DCFH2-DA, a cell permeable non-fluorescent precursor of DCF was used as an intracellular probe for oxidative stress. Figure 7a shows CTN concentration ranging from 0 to 100 µM treatments elevated ROS accumulation in a concentration-dependent manner in C2C12 cells compared to control C2C12 cells (p < 0.05). A 4.4 fold increase in ROS generation was observed at 100 µM and this excessive production of ROS is one of the mechanism by which CTN induces oxidative stress. GTE pretreatment dose-dependently reduced the DCF fluorescence intensity, which is a measure of ROS accumulation. A 3-fold decrease (p < 0.05) in ROS was observed at the higher dose of GTE (80 µg/ml) relative to CTN treated cells (Fig. 7b).
Fig. 7.
Effect of CTN on ROS generation a C2C12 cells were treated with 0–100 µM CTN for 24 h, b ameliorative effect of GTE on CTN-induced ROS generation in C2C12 cells. Data are expressed as the mean ± standard deviation from three independent experiments, each performed in triplicate. *p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
Flow cytometry analysis for hypo-diploid nuclei
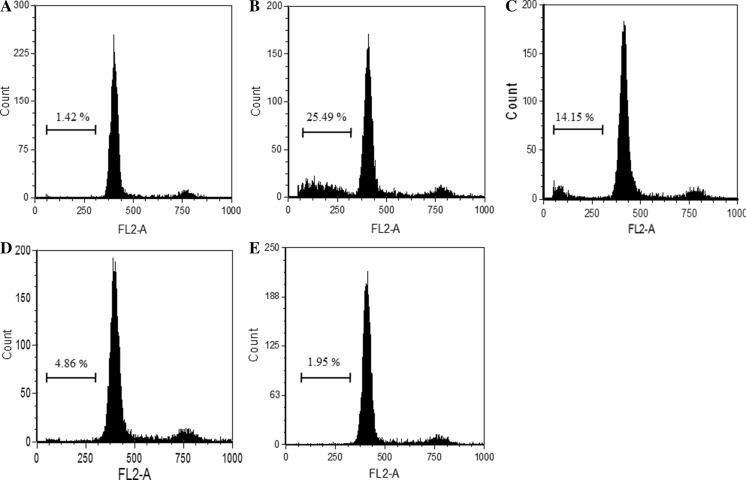
The DNA specific dye PI, identified a distinct hypo-diploid cell population. The results showed that CTN treatment resulted in higher proportion of cells in sub G1 phase (from 1.42 to 25.49%) compared with control cells indicating apoptosis (Fig. 8). The percentage of sub G1 phase cells was reduced after pretreatment with GTE (20, 40, 80 µg/ml) from 25.49 to 14.15, 4.86 and 1.95%, respectively.
Fig. 8.
Flow cytometry analysis of propidium iodide stained C2C12 myotubes after 24 h treatment indicating the percentage of cells at sub-G1 phase. a Control, b CTN 100 µM, c GTE 20 µg/ml + CTN, d GTE 40 µg/ml + CTN, e GTE 80 µg/ml + CTN
GTE ameliorates CTN induced oxidative stress by antioxidative mechanism
The ameliorative properties of GTE polyphenols in counteracting ROS-mediated oxidative stress was studied in terms of activities of antioxidant enzymes such as GPx, CAT and SOD. The treatment with CTN significantly decreased the activities of the antioxidant enzymes GPx, CAT and SOD (p < 0.05) (Table 2). Pretreatment with GTE at the dose of 40 and 80 µg/ml helped in maintaining the enzyme activities at their basal levels by scavenging free radicals.
Table 2.
GTE ameliorates CTN-induced down-regulated antioxidant status antioxidant markers
| Treatment Groups | GPx (µM/mg protein) | Catalase (mM/mg protein) | SOD (U/mg protein) | Total GSH (nM/mg protein) |
|---|---|---|---|---|
| Control | 1.62 ± 0.02 | 0.080 ± 0.01* | 5.99 ± 1.17* | 6.43 ± 0.25* |
| CTN 100 µm | 0.87 ± 0.01 | 0.038 ± 0.01* | 1.81 ± 0.40* | 2.81 ± 0.33* |
| GTE 20 µg/ml + CTN | 0.94 ± 0.01 | 0.038 ± 0.01 | 3.02 ± 0.28 | 2.84 ± 0.53 |
| GTE 40 µg/ml + CTN | 0.94 ± 0.01 | 0.064 ± 0.01# | 3.17 ± 0.33 | 3.63 ± 0.29 |
| GTE 80 µg/ml + CTN | 1.30 ± 0.02 | 0.087 ± 0.01# | 4.05 ± 0.38# | 5.04 ± 0.39# |
Cells were pre-incubated with GTE (20, 40, 80 µg/ml) for 2 h, followed by CTN treatment (100 µM) for 24 h. Data are expressed as the mean ± standard deviation from three independent experiments, each performed in triplicate
* p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
GTE restores depleted GSH
The cellular levels of glutathione (GSH), a tri-peptide antioxidant, decreased significantly (p < 0.05) upon treatment with 100 μM CTN whereas, pre-treatment with GTE evoked a satisfactory increase in intracellular GSH at the highest dose of 80 μg/ml (p < 0.05) compared to CTN alone treated cells (Table 2).
Effect of GTE on gene expression of antioxidant enzymes
Gene expression studies for the antioxidant enzymes such as GPx, CAT and SOD genes were carried out by semi-quantitative RT-PCR. Figure 9 shows that the mRNA levels of GPx, CAT and SOD were found to be decreased in 100 µM CTN treated cells with respect to control. While pretreatment with GTE at the lower and medium dose of 20 and 40 µg/ml slightly increased these mRNA levels and at the higher dose of 80 µg/ml the expression levels were almost restored to that of control.
Fig. 9.
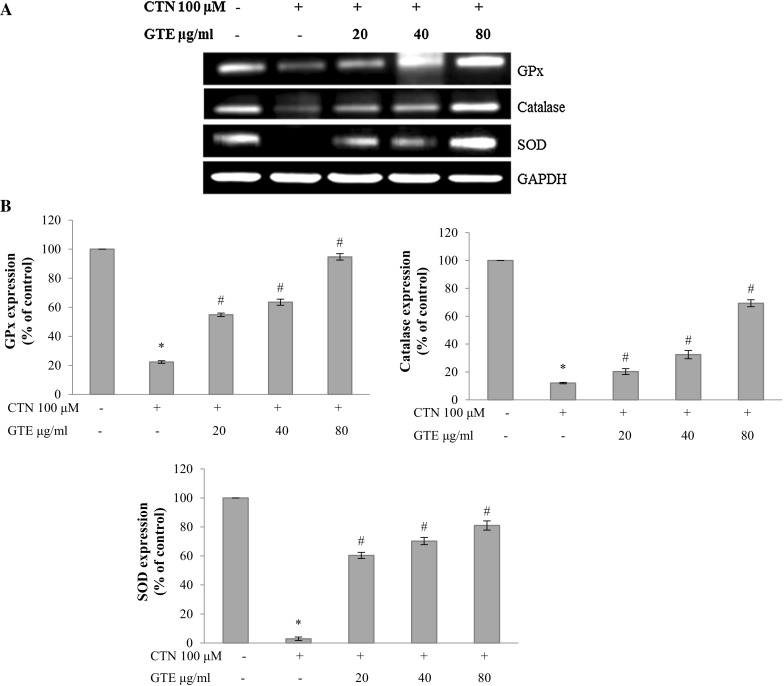
a The protective effect of GTE on CTN-induced expression of oxidative stress marker genes GPx, catalase and SOD by semi-quantitative RT-PCR analysis, b densitometry analysis of GPx, catalase and SOD gene expression (% of control). Data are expressed as the mean ± standard deviation from three independent experiments. *p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
Effect of GTE on antioxidant protein markers
The activity of antioxidant protein markers namely GPx, CAT, SOD were decreased upon CTN treatment, whereas pretreatment with GTE (20, 40, and 80 µg/ml) ameliorated the protein expression levels near to basal levels as observed by western blot analysis (Fig. 10).
Fig. 10.
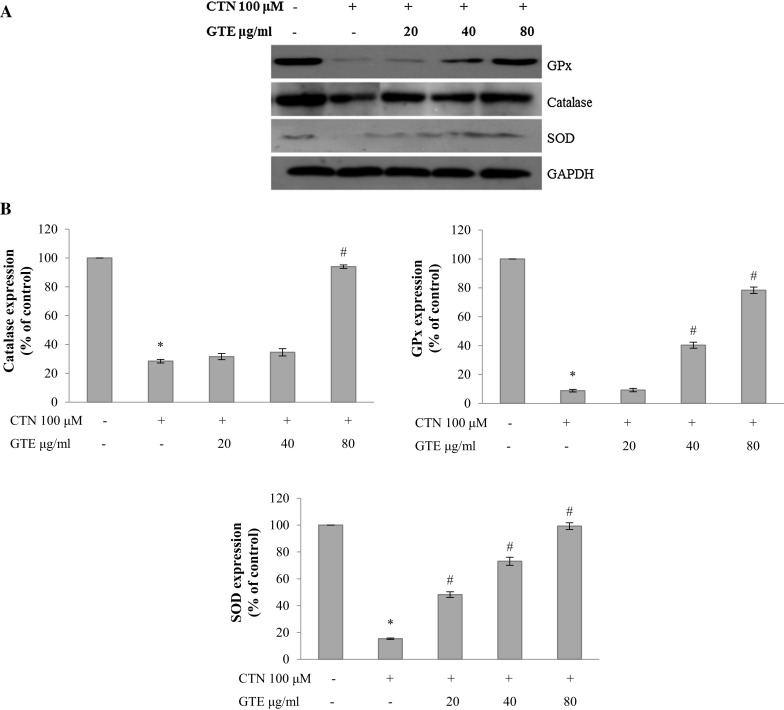
a Western blot analysis of oxidative stress marker proteins GPx, catalase and SOD, b densitometry analysis of GPx, catalase and SOD protein expression (% of control). Data are expressed as the mean ± standard deviation from three independent experiments. *p < 0.05 versus control cells, # p < 0.05 versus CTN treated cells
Discussion
Food and animal feeds are often contaminated with various mycotoxins such as citrinin (CTN), ochratoxin A (OTA), deoxynivalenol (DON), zearlenone, patulin, HT-2 etc. posing serious health problems. As like other toxins, CTN contamination naturally occurs after the harvest by the fungal species and this toxin occur predominantly in stored grains and fruits (Peraica 2012). In addition, phytocompounds such as (−)-Epigallocatechin gallate (EGCG) have also been shown to protect against DON and HT-2 toxin induced cell death suggesting that EGCG could contribute to reducing the toxicities of these mycotoxins (Sugiyama et al. 2011; Kalaiselvi et al. 2013). Similarly, lutein exerts a cytoprotective role in HT-29 cells against DON-induced oxidative stress and cytotoxicity (Krishnaswamy et al. 2010). Rosmarinic acid, a natural phenolic compound was shown to protect against Aflatoxin B1 and Ochratoxin-A-induced cell damage in Hep G2 cells (Renzulli et al. 2004). In another study, resveratrol which possesses antioxidant and anti-tumor properties inhibits CTN-induced ROS generation, activation of JNK and loss of MMP (Chen and Chan 2009).
The present study was aimed to evaluate the cytoprotective role of GTE in ameliorating citrinin-induced oxidative stress in C2C12 myotubes. Although, green tea has been examined intensively over many years for a spectrum of medicinal properties (Higdon and Frei 2003; Nakachi et al. 2003; Nakagawa and Yokozawa 2002), reports on the beneficial effect of GTE in counteracting the mycotoxin like CTN are scanty. The results of the present study showed that GTE contains high levels of phenolic and flavonoid compounds which could be responsible for the remarkable antioxidant activity of the extract. The observed antioxidant activity of the extract could be attributed to the antioxidant compounds present in the extract. Earlier reports on green tea extracts showed a strong antioxidant activity, reducing power and scavenging effects on active oxygen and free radicals (Yen and Chen 1995). The bioactive compounds of GTE exhibit considerable antioxidant activities in vitro. In C2C12 cells, GTE prevented high ROS levels and elevated the stress resistance by its attenuating effect of the oxidative markers and by increasing the survival rate after exposure to mycotoxin CTN.
The presence of phenolic compounds in GTE was identified by HPLC and confirmed by UPLC-MS analysis. The main bioactive compounds identified by UPLC-MS analysis were (−)-Epicatechin gallate, (+)-Catechin, (−)-Gallocatechin, (−)-Epigallocatechin gallate and Caffeine (Figs. 1, 2). Our mass spectral data are in agreement with the previous results of Savic et al. (2014). In a cellular model, the appropriate dose at which CTN induces the toxicological effects was studied in C2C12 myotubes. C2C12 skeletal muscle cells were chosen as they are one of the susceptible target for ROS injury under physiological condition during contraction due to rapid changes in energy supply and oxygen flux (Chan et al. 1994). When cells were exposed to a graded dose of CTN from 25 to 100 µM for a period of 24 h, cytotoxicity was observed in a dose-dependent manner, accompanied by severe membrane damage with concomitant release of LDH into the growth medium. The released LDH is a direct measure of membrane disintegration and cytotoxicity (Figs. 4, 5). We observed the LDH enzyme release after CTN treatment which served as a marker for myotube damage in this study.
A number of cell line studies have confirmed the potential toxicity of CTN to induce apoptosis, generate ROS and activate the signaling pathways (Chan 2007; Yu et al. 2006). In HL-60 cells, apoptosis was mediated through the intrinsic pathway as well as by the activation of caspases (Yu et al. 2006; Chan 2007). Similarly, CTN also activates signaling pathway in other cells such as HEK293, Hep G2, and MES-13 cells (Chang et al. 2009; Chen and Chan 2009; Liu et al. 2010). In our study, treatments with 100 µM CTN elevated the level of intracellular ROS by 4.4 fold confirming CTN as an inducer of oxidative stress (Fig. 7a). CTN is also known to produce mitochondrial damage and inhibit several enzymes of respiratory chain (Chagas et al.1992; Ribeiro et al. 1997). A change in MMP is considered as an indication of damage to mitochondria which can be generally measured using Rhodamine-123 (Rho-123). Quenching of Rho-123 fluorescence occurs when mitochondrial energization is induced and the rate of fluorescence decay is proportional to the mitochondrial membrane potential (Baracca et al. 2003). In addition, the depolarization of the mitochondrial membrane as an early marker represents the onset of apoptosis. The lipophilic nature of Rho-123 allows it to diffuse through the mitochondrial membrane in response to potential and concentration gradients. Results presented in this paper (Fig. 6a) show that CTN treatment resulted in loss of MMP which in turn might have led to apoptosis (Yu et al. 2006). The effect of CTN on cell cycle distribution and arrest was studied using flow cytometry analysis. Our experimental data (Fig. 8) show that CTN treatment resulted in increased proportion of sub G1 phase cells compared to other phases which might be result of accumulation of hypodiploid cells. Further, changes in cell morphology (Fig. 3) are primary indicators of cell damage and have been regarded as a critical process in apoptosis (Collins et al. 1997; Zhang and Ming 2000). The result presented here might throw a light on involvement of CTN in cytoskeletal function of C2C12 cells which might have been inactivated by cleavage of caspases thus affecting replication, transcription, or translation and pushing the cells to death (Nagata 2000).
Oxidative muscle injuries and their related disease conditions could be overcome by improving the antioxidant status of the cells. This becomes a basic strategy in treatment involving herbal or alternative and complementary medicine. In this regard, the ameliorative effect of GTE against CTN- induced oxidative stress was investigated. GTE supplementation alone did not show any adverse cytotoxic effects on C2C12 cells even after 24 h of treatment and therefore cell viability was similar with respect to control. GTE supplementation (80 µg/ml) into the growth medium for 2 h before the actual exposure of CTN displayed the most protective potential at the highest concentration evaluated (Fig. 4b). The results of cell viability and LDH leakage assay showed that GTE inhibited cytotoxicity significantly (p < 0.05) (Fig. 5b).
Previous in vivo studies have reported that supplementation of GTE decreases the oxidative stress primarily by the multiple-antioxidant mechanism (Buetler et al. 2002). The endogenous-antioxidant enzymes such as GPx, SOD and CAT found in muscle tissue play a vital role in preventing or delaying the oxidative damage. The other non-enzymatic cellular antioxidant include glutathione which is a tripeptide antioxidant synthesized in most organisms. The concentration of reduced GSH is used as an index of non-enzymatic antioxidant since their level critically reflects cellular redox status. Further, CTN-induced depletion of GSH level in C2C12 cells were normalised by the the addition of GTE (Table 2), which may be beneficial in restoring muscle mass in chronic inflammatory disorders (Ardite et al. 2004). Green tea catechins also exhibit antioxidant activity through inhibiting pro-oxidant enzymes and inducing antioxidant enzymes (Velayutham et al. 2008). GPx, SOD and CAT are the major scavenging enzymes that were decreased by CTN insult and upon GTE pretreatment the enzyme activities were restored. Decreased activity of antioxidant enzymes may be due to increased ROS accumulation and our data are consistent with the study by Murphy and Kehrer (1986) where concentrations of antioxidant enzymes were altered in muscles of either DMD patients or mdx mice and cellular antioxidants were found to be lower than normal indicating the involvement of ROS. In another study GTE was demonstrated to improve the muscle health in mdx mice (Buetler et al. 2002).
We also observed that under stress condition of mycotoxin exposure the mRNA expression levels were altered and the gene expression levels of GPx, CAT and SOD were reduced upon CTN insult. Pretreatment with GTE modulated these genes by enhancing their expression level (Fig. 9). The extract further enhanced the expression of these antioxidant protein markers with respect to CTN control group as observed by western blot analysis (Fig. 10). This type of induction and regulation of antioxidant enzymes becomes essential for protecting the myotubes under oxidative stress. The importance of these enzymes were demonstrated in a study by Franco et al. (1999) where they observed that increased sensitivity of myotubes corresponded with decreased antioxidant defenses and as muscle cells differentiated, both transcript and antioxidant enzymes activity levels decreased. The protective effect of GTE as observed from our study is in consistent with the work of Buetler et al. (2002) where GTE protected C2C12 myotubes from tert-butyl hydroperoxide-induced injury (tBHP). Taken together, these findings strongly supported that GTE plays an important role in protecting C2C12 myotubes from oxidative stress-induced by CTN.
Conclusion
Overall, results of the present study showed that GTE as an herbal supplement was effective in scavenging free radicals generated by CTN toxicity and further protected the C2C12 muscle cells from oxidative stress-induced damage in a C2C12 cellular model. The ameliorative effect of GTE may be due to synergistic activities of several phenolic and other constituents of the extract. Further studies on the mechanisms of regulation of antioxidant defenses is needed for the better understanding of muscles disorders in which ROS play a major role.
Acknowledgements
The authors are grateful to the Director, DFRL, Mysuru, for providing necessary facilities to conduct the study.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Anderson RF, Fisher LJ, Hara Y, Harris T, Mak WB, Melton LD, Packer JE. Green tea catechins partially protect DNA from OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis. 2001;22:1189–1193. doi: 10.1093/carcin/22.8.1189. [DOI] [PubMed] [Google Scholar]
- Ardite E, Barbera JA, Roca J, Fernández-Checa JC. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-κB activation. Am J Pathol. 2004;165:719–728. doi: 10.1016/S0002-9440(10)63335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykaç G, Uysal M, Yalçin AS, Koçak-Toker N, Sivas A, Öz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36:71–76. doi: 10.1016/0300-483X(85)90008-3. [DOI] [PubMed] [Google Scholar]
- Baracca A, Sgarbi G, Solaini G, Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. BBA-Bioenerg. 2003;1606:137–146. doi: 10.1016/S0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc PJ, Laussac JP, Le Bars J, Le Bars P, Loret MO, Pareilleux A, Prome D, PromeJC Santerre AL, Goma G. Characterization of monascidin A from Monascus as citrinin. Int J Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- Bouslimi A, Bouaziz C, Ayed-Boussema I, Hassen W, Bacha H. Individual and combined effects of ochratoxin A and citrinin on viability and DNA fragmentation in cultured Vero cells and on chromosome aberrations in mice bone marrow cells. Toxicology. 2008;251:1–7. doi: 10.1016/j.tox.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from bauhinia tarapotensis. J Nat Prod. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J ClinNutr. 2002;75:749–753. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- Chagas GM, Oliveira M, Bengna M, Campello AP, Klüppel M, Lúcia W. Mechanism of citrinin-induced dysfunction of mitochondria. II. Effect on respiration, enzyme activities, and membrane potential of liver mitochondria. Cell Biochem Funct. 1992;10:209–216. doi: 10.1002/cbf.290100311. [DOI] [PubMed] [Google Scholar]
- Chan W. Citrinin induces apoptosis via a mitochondria-dependent pathway and inhibition of survival signals in embryonic stem cells, and causes developmental injury in blastocysts. Biochem J. 2007;404:317–326. doi: 10.1042/BJ20061875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KM, Decker EA, Feustman C. Endogenous skeletal muscle antioxidants. Crit Rev Food Sci Nutr. 1994;34:403–426. doi: 10.1080/10408399409527669. [DOI] [PubMed] [Google Scholar]
- Chang CH, Yu FY, Wu TS, Lin YS, Liu BH. Activation of ERK and JNK signaling pathways by mycotoxin citrinin in human cells. Toxicol Appl Pharmacol. 2009;237:281–287. doi: 10.1016/j.taap.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Chang CH, Yu FY, Wu TS, Wang LT, Liu BH. Mycotoxin citrinin induced cell cycle G2/M arrest and numerical chromosomal aberration associated with disruption of microtubule formation in human cells. Toxicol Sci. 2011;119:84–92. doi: 10.1093/toxsci/kfq309. [DOI] [PubMed] [Google Scholar]
- Chen CC, Chan WH. Inhibition of citrinin-induced apoptotic biochemical signaling in human hepatoma G2 cells by resveratrol. Int J Mol Sci. 2009;10:3338–3357. doi: 10.3390/ijms10083338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Schandl CA, Young KK, Vesely J, Willingham MC. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45:923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- Da Lozzo EJ, Oliveira MBM, Gunilla ES, Carnieri EGS. Citrinin-induced mitochondrial permeability transition. J Biochem Mol Toxi. 1998;12:291–297. doi: 10.1002/(SICI)1099-0461(1998)12:5<291::AID-JBT5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- El-Banna AA, Pitt JI, Leistner L. Production of M ycotoxins by Penicillium Species. Syst Appl Microbiol. 1987;10:42–46. doi: 10.1016/S0723-2020(87)80008-5. [DOI] [Google Scholar]
- Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radical Bio Med. 1999;27:1122–1132. doi: 10.1016/S0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W. ESR study on the structure–antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta. 1999;1427:13–23. doi: 10.1016/S0304-4165(98)00168-8. [DOI] [PubMed] [Google Scholar]
- Hanelt M, Gareis M, Kollarczik B. Cytotoxicity of mycotoxins evaluated by the MTT-cell culture assay. Mycopathologia. 1994;128:167–174. doi: 10.1007/BF01138479. [DOI] [PubMed] [Google Scholar]
- Hetherington AC, Raistrick, H (1931) On the production and chemical constitution of a new yellow colouring matter, citrinin, produced from glucose by Penicillium citrinum Thom. Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character 269–295
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Huo C, Wan SB, Lam WH, Li L, Wang Z, Landis-Piwowar KR, Chen D, Dou QP, Chan TH. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacology. 2008;16:248–252. doi: 10.1007/s10787-008-8031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeswal P. Citrinin-induced chromosomal abnormalities in the bone marrow cells of Mus musculus. Cytobios. 1996;86:29–33. [PubMed] [Google Scholar]
- Kalaiselvi P, Rajashree K, Bharathi Priya L, Padma VV. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem Toxicol. 2013;56:110–118. doi: 10.1016/j.fct.2013.01.042. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Klarić MŠ, Želježić D, Rumora L, Peraica M, Pepeljnjak S, Domijan AM. A potential role of calcium in apoptosis and aberrant chromatin forms in porcine kidney PK15 cells induced by individual and combined ochratoxin A and citrinin. Arch Toxicol. 2012;86:97–107. doi: 10.1007/s00204-011-0735-9. [DOI] [PubMed] [Google Scholar]
- Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil. 2015;36:377–393. doi: 10.1007/s10974-015-9438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy R, Devaraj SN, Padma VV. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-κB nuclear localization and down regulation of NF-κB and Cyclo-Oxygenase–2 expression. Free Radical Bio Med. 2010;49:50–60. doi: 10.1016/j.freeradbiomed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wu TS, Su MC, Chung CP, Yu FY. Evaluation of citrinin occurrence and cytotoxicity in Monascus fermentation products. J Agr Food Chem. 2005;53:170–175. doi: 10.1021/jf048878n. [DOI] [PubMed] [Google Scholar]
- Liu BH, Chi JY, Hsiao YW, Tsai KD, Lee YJ, Lin CC, Hsu SC, Yang SM, Lin TH. The fungal metabolite, citrinin, inhibits lipopolysaccharide/interferon-gamma-induced nitric oxide production in glomerular mesangial cells. Int I mmunopharmacol. 2010;10:1608–1615. doi: 10.1016/j.intimp.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 2006;15:523. [Google Scholar]
- Murphy ME, Kehrer JP. Free radicals: a potential pathogenic mechanism in inherited muscular dystrophy Life Sci. 1986;39:2271–2278. doi: 10.1016/0024-3205(86)90657-0. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptotic DNA fragmentation. Exp Cell Res. 2000;256:12–18. doi: 10.1006/excr.2000.4834. [DOI] [PubMed] [Google Scholar]
- Nakachi K, Eguchi H, Imai K. Can tea time increase one’s lifetime? Ageing Res Rev. 2003;2:1–10. doi: 10.1016/S1568-1637(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol. 2002;40:1745–1750. doi: 10.1016/S0278-6915(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Nanjo F, Honda M, Okushio K, Matsumoto N, Ishigaki F, Ishigami T, Hara Y. Effects of dietary tea catechins on alpha-tocopherol levels, lipid peroxidation, and erythrocyte deformability in rats fed on high palm oil and perilla oil diets. Biol Pharm Bull. 1993;16:1156–1159. doi: 10.1248/bpb.16.1156. [DOI] [PubMed] [Google Scholar]
- Peraica M. Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. Eur Food Safe Auth J. 2012;10:1–82. [Google Scholar]
- Pfeiffer E, Gross K, Metzler M. Aneuploidogenic and clastogenic potential of the mycotoxins citrinin and patulin. Carcinogenesis. 1998;19:1313–1318. doi: 10.1093/carcin/19.7.1313. [DOI] [PubMed] [Google Scholar]
- Reddy RV, Maruya K, Hayes AW, Bernd WO. Embryocidal teratogenic and fetotoxic effects of citrinin in rats. Toxicology. 1982;25:151–160. doi: 10.1016/0300-483X(82)90026-9. [DOI] [PubMed] [Google Scholar]
- Renzulli C, Galvano F, Pierdomenico L, Speroni E, Guerra MC. Effects of rosmarinic acid against aflatoxin B1 and ochratoxin-A-induced cell damage in a human hepatoma cell line (Hep G2) J Appl Toxicol. 2004;24:289–296. doi: 10.1002/jat.982. [DOI] [PubMed] [Google Scholar]
- Ribeiro SM, Chagas GM, Campello AP, Kluppel M, Lúcia W. Mechanism of citrinin-induced dysfunction of mitochondria. V. Effect on the homeostasis of the reactive oxygen species. Cell Biochem Funct. 1997;15:203–209. doi: 10.1002/(SICI)1099-0844(199709)15:3<203::AID-CBF742>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Richard JL, Payne GE, Desjardins AE, Maragos C, Norred WP, Pestka JJ. Mycotoxins: risks in plant, animal and human systems. CAST Task Force Report. 2003;139:101–103. [Google Scholar]
- Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:569–575. doi: 10.1016/j.foodchem.2004.03.013. [DOI] [Google Scholar]
- Salucci S, Battistelli M, Burattini S, Squillace C, Canonico B, Gobbi P, Papa S, Falcieri E. C2C12 myoblast sensitivity to different apoptotic chemical triggers. Micron. 2010;41:966–973. doi: 10.1016/j.micron.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Savić IM, Nikolić VD, Savić IM, Nikolić LB, Jović MD, Jović MD. The qualitative analysis of the green tea extract using ESI-MS method. Savremene tehnologije. 2014;3:30–37. doi: 10.5937/savteh1401030S. [DOI] [Google Scholar]
- Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2007;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Sugiyama KI, Kinoshita M, Kamata Y, Minai Y, Sugita-Konishi Y. (−)-Epigallocatechingallate suppresses the cytotoxicity induced by trichothecene mycotoxins in mouse cultural macrophages. Mycotoxin Res. 2011;27:281–285. doi: 10.1007/s12550-011-0105-8. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Velayutham P, Babu A, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabcheva T, Usleber E, Dietrich R, Märtlbauer E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J Agr Food Chem. 2000;48:2483–2488. doi: 10.1021/jf990891y. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Bio Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Crit Rev Food SciNutr. 1997;37:705–718. doi: 10.1080/10408399709527798. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agr Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- Yu FY, Liao YC, Chang CH, Liu BH. Citrinin induces apoptosis in HL-60 cells via activation of the mitochondrial pathway. Toxicol Lett. 2006;161:143–151. doi: 10.1016/j.toxlet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Ming XU. DNA fragmentation in apoptosis. Cell Res. 2000;10:205–211. doi: 10.1038/sj.cr.7290049. [DOI] [PubMed] [Google Scholar]