Abstract
This study investigated the potential of Persian shallot extract as an anticancer agent in HepG2 tumor cell line, an in vitro human hepatoma cancer model system. The inhibitory effect of Persian shallot on the growth of HepG2 cells was measured by MTT assay. To explore the underlying mechanism of cell growth inhibition of Persian shallot, the activity of Persian shallot in inducing apoptosis was investigated through the detection of annexin V signal by flow cytometry and expression of some apoptosis related genes such p21, p53, puma, caspase-8 family-Bcl-2 proteins like bid, bim, bcl-2 and bax were measured by real-time PCR in HepG2 cells. Persian shallot extract inhibited the growth of HepG2 cells in a dose-dependent manner. The IC50 value (inhibiting cell growth by 50%) was 149 μg/ml. The results of real-time PCR revealed a significant up-regulation of bid, bim, caspase-8, puma, p53, p21 and bax genes and a significant downregulation of bcl-2 gene in HepG2 cells treated with Persian shallot extract significantly. Therefore, this is the first report on an increased expression of bid, bim, caspase-8, puma, p53, p21 and bax genes and down regulation of bcl-2 gene indicating that the Persian shallot extract possibly induced the process of cell death through the intrinsic and extrinsic apoptosis pathways and triggers the programmed cell death in HepG2 tumor cell lines by modulating the expression of pro-/anti-apoptotic genes. Furthermore, we showed that Persian shallot extract increased annexin V signal and expression, resulting in apoptotic cell death of HepG2 cells after 24 h treatment. Therefore, according to the results of this study, the Persian shallot extract could be considered as a potential candidate for production of drug for the prevention or treatment of human hepatoma.
Keywords: Allium hirtifolium Boiss, Apoptosis, Gene expression, HepG2 cell line, Persian shallot
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide with prevalent areas in sub-Saharan Africa and Asia. HCC is considered to be the third cause of cancer mortality because of its poor prognosis (Ferlay et al. 2015). There are many different methods for the treatment of HCC such as surgery, liver transplantation, chemotherapy with use of new anti-tumor drugs, interventional therapy etc. Currently, many of medicines used for the treatment of HCC, such as fluorouracil, mitomycin, doxorubicin and cisplatin are typically nonselective cytotoxic molecules that exhibit undesirable adverse effects (Avila et al. 2006; Kaseb et al. 2013). Compounds found in vegetables, pharmaceutical plants and fruits that may help to protect against cancer are attracting a lot of interest in their perceived ability to act as highly effective chemopreventive agents. Nutritional or dietary factors can influence risk for the prognosis after the diagnosis of cancer, development of cancer and quality of life during cancer treatment. It is also considered a reasonable strategy for dietary approaches to prevent cancer. In fact, many efforts are being carried out to isolate the bioactive products from pharmaceutical plants and their use in the treatment of cancer (Al-Fatlawi et al. 2014). In developed countries, the use of herbal medicines is more acceptable than chemical drugs and has attracted special attention to use in alternative and innovative therapies (Omobuwajo et al. 2011). Some kinds of plants can be used as medicine or food. These plants exhibit a variety of pharmacological and biological activities (Khalil et al. 2015).
Persian Shallot is scientifically called Allium hirtifolium Boiss, that is a member of the Liliaceae family. Persian shallot also known as (moosir) is a native plant that grows in some areas of Iran, the bulb and the flower of this plant are applied in the diet nutrition and also used for medical therapy (Azadi et al. 2012). The extract of A. hirtifolium has been demonstrated to have numerous pharmacological activities. For example, it has been used for the treatment of hypertension, rheumatoid, inflammation, and also healing of wounds in traditional medicine. Recently, antibacterial, antifungal, antioxidant, and anticancer activities of A. hirtifolium have been reported. Saponins, sapogenins, flavonoids and sulphur-containing compounds (thiosulfinates) are the most important phytochemicals found in this plant (Asgarpanah and Ghanizadeh 2012).
Persian shallot is different from the common shallot (Allium ascalonicum) in many of its features. For example, common Shallot bulbs are pear-shaped, reddish brown in color and its clusters may contain as many as 15 bulbs, while the Persian shallot bulbs are oval shaped, white in color and typically include one or sometimes two bulbs (Ismail et al. 2013).
Sapogenins, saponins, sulphur compounds (thiosulfinates) and flavonoids, including kaempferol and quercetin are found in various species of Allium genus. Previous studies have shown that the flowers and bulbs of shallot contain high levels of glycosidic flavonoids (Fattorusso et al. 2002; Barile et al. 2005). Disulphide and trisulphide compounds are the most important compounds existing in Allium genus species (Rose et al. 2005). There are many reports indicating that the shallot has pharmaceutical effects such as regulation of immune system (Jafarian et al. 2003), antioxidant (Leelarungrayub et al. 2006), anti-trichomonas (Taran et al. 2006), anticancer (Azadi et al. 2008), hypoglycemic (Hosseini-Zijoud et al. 2012; Mahmoodi et al. 2013) and hepatoprotective (Hosseini et al. 2012) activity.
Previous studies indicated that the Persian shallot extract is able to exert the anti-proliferative effects in cancer cells such as MCF7 (human, caucasion, breast, adenocarcinoma) and HeLa (cervical cancer) cell lines (Azadi et al. 2008), but there is little information about the mechanism of the effect of Persian shallot on cancer prevention. Induction of apoptosis in cancer cells plays an important role in the removal of these cells. Apoptosis is a normal physiological process of programmed cell death that plays a key role in homeostasis of healthy tissues and constant cell density in tissues by removing potentially unwanted cells. However, cancer cells escape apoptosis and increase the cell proliferation rate and will eventually lead to tumor formation. Therefore, the main purpose of cancer treatment is to control the growth of cancer cells and trigger cell death without harming normal cells (Raicht et al. 1980; Hetz et al. 2005).
Apoptosis is suppressed in many cancer cells, therefore selective induction of apoptosis in cancer cells is presently one of the most important strategies for cancer therapy (Kim et al. 2014). The mechanisms of apoptosis are highly complex and sophisticated, involving multiple molecular events. There are two main apoptotic pathways: the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway (Gerl and Vaux 2005). The intrinsic pathway is regulated by activation or deactivation of bcl-2 family genes.
bcl-2 is an anti-apoptotic gene which functions to inhibit apoptotic process and is overexpressed in cancer cells. More targeted therapies that induce apoptosis in cancer cells are mainly based on the suppression of the expression of Bcl-2 protein (Kim et al. 2014). Also p53, a tumor suppressor gene is activated in response to signals generated by different genotoxic stress such as DNA damage and its expression is suppressed in many cancer cells, therefore the p53 gene is an appropriate candidate that can be targeted in cancer therapy (Yu 2006). In the present study, we examined the growth inhibitory effect of the Persian shallot extract on human hepatoma cancer (HepG2) cells by MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay and apoptosis was studied using flow cytometry. The expression of some apoptosis genes for example p21, p53, puma, caspase-8, bid, bim, bcl-2 and bax were further assayed by real-time PCR.
Materials and methods
Materials
Fetal bovine serum (FBS), RPMI-1640, trypsin enzyme and penicillin–streptomycin were purchased from Gibco-BRL (Grand Island, NY, USA). MTT and dimethyl sulfoxide (DMSO) were prepared from Roche (Mannheim, Germany). Total RNA extraction Kit and Assay cDNA PCR reverse transcription kit were purchased from PARS Tous (Iran). SYBR Green PCR Master Mix was prepared from GeNet Bio (Chungnam, Korea). The annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit and DNase I were purchased from Ebioscience (San Diego, CA, USA) and Sigma (St. Louis, MO, USA), respectively.
Preparation of hydroalcoholic Persian shallot extract
Persian shallot bulbs were obtained from Kangavar (Kermanshah, Iran). The genus and species of the Persian shallot bulbs were confirmed by the botanists at Department of Botany, Valiasr University Rafsanjan, Iran. Fresh bulbs (100 gr) were well crushed and soaked in 400 ml distilled water/ethanol (75:25 v/v) and the mixture was shaken and stirred. After 48 h incubation, at room temperature the solution was filtered using a filter paper through a Buchner funnel. The filtered solutions concentrated by a vacuum freezedryer and were converted into dry powder and then the desired concentrations were prepared by dissolving the appropriate amounts of powder in water.
Cell culture
Human HCC cell line HepG2 was purchased from Pasteur Institute (Tehran, Iran). HepG2 cells were cultured in RPMI 1640 culture medium supplemented with 10% of fetal bovine serum, 100 IU/ml of penicillin and 100 μg/ml of streptomycin, and incubated in a 5% CO2 incubator at 37 °C. Culture medium was exchanged with fresh medium every 1–2 days, when the cells occupied 70–80% of the flask, the cells were treated with different concentrations of Persian shallot extract.
Human lymphocyte cells were obtained by primary culture. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on lymphocyte cell separation medium (Lymphodex; Inno-Train Diagnostik, Kronberg, Germany) from a heparinized human blood sample. PBMC were washed and resuspended at a concentration of 0.5 × 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 IU/ml/100 μg/ml penicillin/streptomycin at 37 °C with 5% CO2. In addition, phytohemagglutinin (PHA; Sigma) was used as a mitogen to trigger cell division in T lymphocytes at a final concentration of 10 μg/ml. After 24 h, cells were treated with Persian shallot extract at IC50, 1/10 and 1/50 of the IC50 concentrations.
Cell viability assay
Cell viability was determined by measuring the metabolism of a tetrazolium substrate, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT). The HepG2 cells (~2.5 × 103 per well) were cultured overnight in a flat bottom 96 well plate and incubated at 37 °C in a humidified air atmosphere enriched with 5% CO2. HepG2 cells were treated with various concentrations of Persian shallot ranging between 25 and 250 μg/ml for 24 h time points. Then, the medium was replaced by 200 μl of warm RPMI 1640 (without phenol red). After adding 10 μl of 5 mg/ml MTT to each well, the plate was incubated in 37 °C for 3.5 h in the dark until a purple precipitate was visible under the light microscope. Then 100 μl of DMSO was added to each well and the absorbance was read at 570 nm with a reference filter of 620 nm after 15 min. The viability of peripheral blood mononuclear cells (PBMC) as normal cells was determined by trypan blue exclusion (TBE) assay and counting in a hemocytometer after 24 h exposure with Persian shallot extract at IC50, 1/10 and 1/50 of the IC50 concentrations.
Analysis of apoptosis by flow cytometry and inverted microscope
Double staining with Annexin V-FITC and PI for flow cytometry analyses was performed using Annexin V-FITC apoptosis detection kit. The HepG2 cells were cultured for 24 h and incubated with the indicated concentrations (0, 125, 150 and 175 μg/ml) of Persian shallot extract. The treated and/or untreated cells were harvested after 24 h and washed twice with PBS, then re-suspended in the binding buffer (calcium buffer, 200 μl). Annexin V-FITC (5 μl) was added to the cells followed by the addition of 10 μl propidium iodide (PI). The samples were then incubated for 5 min in the dark at 4 °C and were examined under a flow cytometer (BD FACS Calibur, BD Biosciences). BD CellQuest (BD Biosciences, Franklin Lakes, NJ, USA) version 1.0 was used for data analysis.
Morphological changes on HepG2 and PBMC cells after exposure with Persian shallot extract at IC50, 1/10 and 1/50 of the IC50 concentrations were examined with an inverted microscope under 400× magnification.
Gene expression analysis
The mRNA expression of apoptosis regulatory genes, p21, p53, puma, caspase-8, bid, bim, bcl-2 and bax, were detected after treating the HepG2 cells with Persian shallot extract at IC50 concentration for 24 h. Treated and untreated HepG2 cells were harvested and washed with PBS at 4 °C. Total RNA was extracted using total RNA extraction Kit (PARS Tous) according to the manufacturer’s instruction. All RNA samples treated with DNase I at 37 °C for 30 min for removal of any genomic DNA contamination. RNA preparations were also analyzed by agarose gel electrophoresis and with a spectrophotometer, respectively. 3–5 μg of purified total RNA was used for cDNA synthesis using the cDNA PCR reverse transcription kit (PARS Tous) according to the manufacturer’s instructions. cDNA was used for the detection of mRNA expressions of bcl-2 and p53genes using specific oligonucleotide primers (Table 1). ß-actin gene was used as an internal control. The volume of PCR mixture was 20 μl containing 20 ng of cDNA, 10 μl of prime Q-Master Mix with SYBR Green I (GeNet Bio) and 200 nM of the forward and reverse primers according to the manufacturer’s instructions. Real-time polymerase chain reaction (real-time PCR) was performed using a thermal cycler (Bio-Rad CFX96, Hercules, CA, USA).
Table 1.
Primers sequences used in this study (F, forward; R, reverse)
| Gene | Primer sequences (5′ → 3′) |
|---|---|
| bcl-2 | F: CTTCTTTGAGTTCGGTGGGG R: AAATCAAACAGAGGCCGCAT |
| p53 | F: TGAAGCTCCCAGAATGCCAG R: GCTGCCCTGGTAGGTTTTCT |
| bim | F: GACAAGAATCCGACCAAATGGCAAA R: AAAAGGATCCATGAGAAATCCTTGTGG |
| Caspase-8 | F: ATTAGGGACAGGAATGGAACAC R: GGAGAGGATACAGCAGATGAAG |
| bax | F: TGCCTCAGGATGCGTCCACCAA R: CCCCAGTTGAAGTTGCCGTCAG |
| bid | F: CCTTGCTCCGTGATGTCTTTC R: TCCGTTCAGTCCATCCCATTT |
| puma | F: GACGACCTCAACGCACAGTA R: AGGAGTCCCATGATGAGATTG |
| P21 | F: GGAGACTTCTCAGGGTCGAAAAC R: GGGCTTCCTCTTGGAGAAGATC |
| β-actin | F: GGGCATGGGTCAGAAGGATT R: CGCAGCTCATTGTAGAAGGT |
The PCR reaction conditions were initial denaturation of templates at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 30 s. The amplified products were examined on 2% agarose gel and documented on the Gel-doc system (Bio-Rad) to verify their size and dissociation curve analysis.
Statistical analysis
Data distribution for each of the trials met the requirements of normal distribution (one-sample Kolmogrov-Smirnov) and assumptions of variance homogeneity were checked with Levene’s test.
All groups were compared by using one-way ANOVA. Subsequently, the control group was compared with all test groups using Dunnett’s test, after prior confirmation (with F-test in variance analysis) of statistically significant differences in the analyzed means. Kruskat–Wallis test was used for other variables due to data that were not normally distributed. Mann–Whitney U test was performed for the pair-wise Post-hoc comparison between two groups. All tests were performed with the significance level of p < 0.05, using SPSS 20 software.
Results
Effect of Persian shallot extract on cell viability
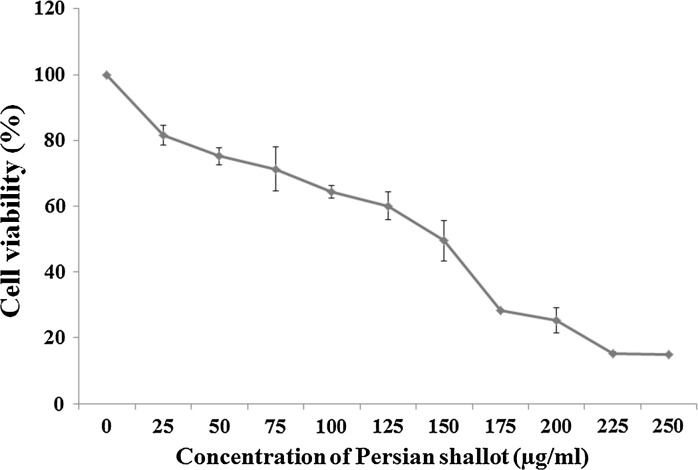
The MTT method was used to evaluate the inhibitory effects of the Persian shallot extract on the growth of HepG2 cells. HepG2 cells were incubated with different concentrations of hydroalcoholic Persian shallot extract (0–250 μg/ml) for 24 h. The results demonstrated that the Persian shallot extract decreased cell viability in HepG2 cells, in a dose-dependent manner (Fig. 1). In MTT assay, the statistical analysis revealed that Persian shallot extract significantly inhibited the proliferation of HepG2 cells. Especially at higher concentrations of Persian shallot (Fig. 1), the number of colony-forming cells was reduced in culture when observed under phase contrast microscope (data not shown).
Fig. 1.
Effect of Persian shallot (Allium hirtifulium Boiss) on cell viability of HepG2 cells. Cells were treated with different concentrations of Persian shallot for 24 h and the cell survival rates were determined by the MTT assay. Each data point is an average of results from three independent experiments performed in triplicate and presented as M ± SD
The cytotoxicity of Persian shallot extract on HepG2 cells was observed with more than 70% at 175 μg/ml and 84.7% at 225 μg/ml growth suppression in 24 h (Fig. 1). The IC50 value (evaluated after 24 h) of Persian shallot extract against HepG2 cells was 149 μg/ml (p ≤ 0.05).
Furthermore, the effect of the extract at 149 μg/ml (the IC50 value) on PBMC cells was investigated. The results showed that the extract decreased the cell viability to 98.6% which was not considered a remarkable cytotoxicity.
The toxicity associated with morphological changes such as decrease in cell volume and rounding (data not shown).
Persian shallot extract induces apoptosis and necrosis in HepG2 cells
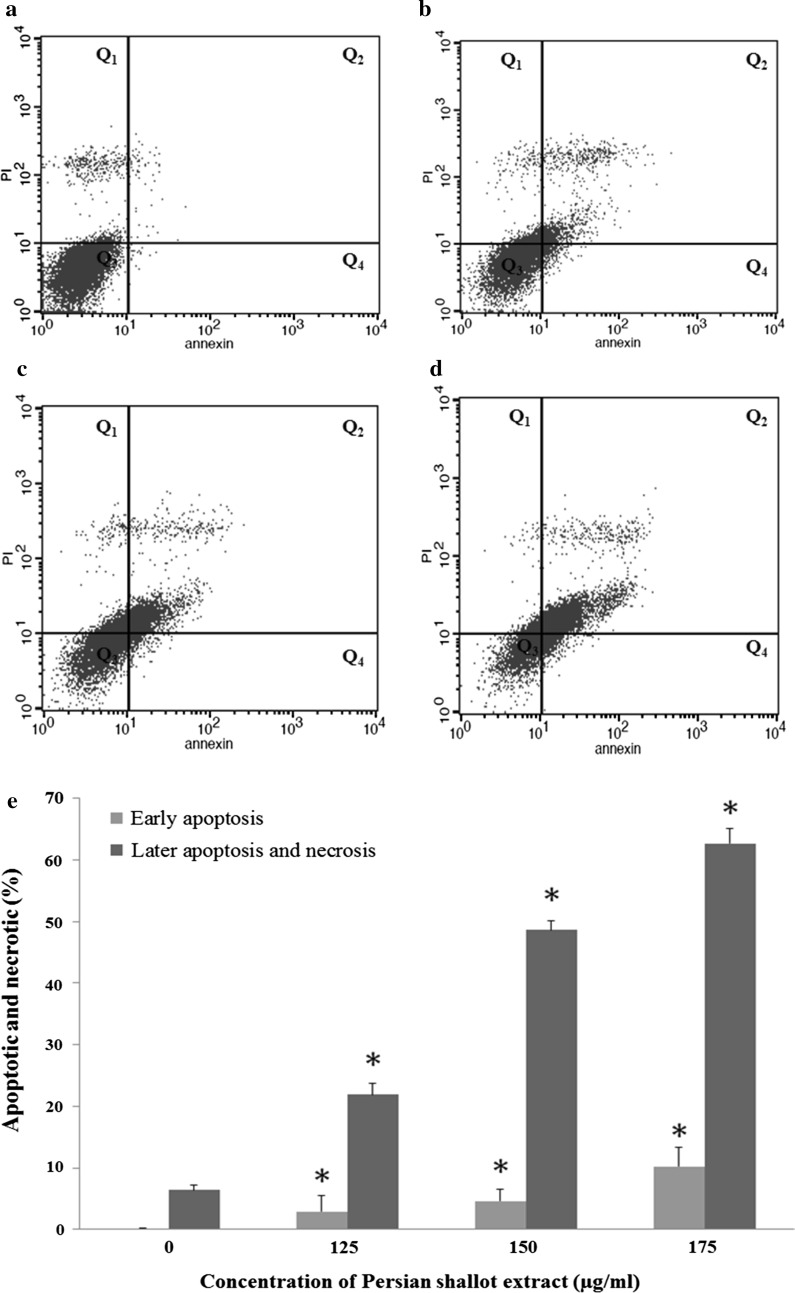
The HepG2 cells were treated by different concentrations of Persian shallot extract (0, 125, 150 and 175 μg/ml) for 24 h. Then the treated cells were stained with annexin V and propidium iodide and the apoptotic effect of Persian shallot extract on HepG2 cells was detected by flow cytometry. The results of flow cytometry analysis revealed that the extract induced apoptosis in treated HepG2 cells compared to control indicating apoptotic cell death is involved in extract induced toxicity (Fig. 2a–d).
Fig. 2.
Induction of apoptosis in HepG2 cells by Persian shallot extract (Allium hirtifulium Boiss). Cells were treated with different concentrations (a control group, b 125 μg/ml, c 150 μg/ml, d 175 μg/ml) of Persian shallot extract for 24 h and stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI). Subsequently, apoptotic and necrotic cells were quantified by flow cytometry. The different subpopulations were defined as Q1 Annexin V-negative but PI-positive, i.e. necrotic cells; Q2 Annexin V/PI-double positive, i.e. late apoptotic cells; Q3 Annexin V/PI-double negative, i.e. normal live cells; and Q4 Annexin V-positive but PI-negative, i.e. early apoptotic cells. *p < 0.05 indicates a significant difference from the control cells. e Comparison of induced early and late apoptosis as well as necrosis in treated HepG2 cells with Persian shallot extract at different concentrations
Normal living cells were observed in Q3 region (Fig. 2a–d). The early apoptotic cells were determined by the sum of the cells in Q4 and late apoptosis and necrosis cells appeared mainly in the Q2 and Q1 regions, respectively (Fig. 2a–d). The percentages of early apoptotic cells following treatment with different extract concentrations (0, 125, 150 and 175 μg/ml) were 0.18 ± 0.03, 2.91 ± 2.5, 4.58 ± 1.97 and 10.25 ± 3.04%. The percentages of necrotic and late apoptotic cells were about 6.39 ± 0.78, 21.58 ± 1.8, 48.62 ± 1.56 and 62.73% ± 2.36, respectively (Table 2; Fig. 2e).
Table 2.
Apoptosis rates of HepG2 cells intervened by different concentrations of Persian shallot extract (Allium hirtifulium Boiss) (x ± s, %)
| Apoptosis rates | Concentrations of Persian shallot (μg/ml) | |||
|---|---|---|---|---|
| 0 | 125 | 150 | 175 | |
| Early apoptosis rate | 0.18% ± 0.03 | 2.91% ± 2.5* | 4.58% ± 1.97* | 10.25% ± 3.04* |
| Necrosis/late apoptosis rate | 6.39% ± 0.78 | 21.58% ± 1.8* | 48.62% ± 1.56* | 62.73% ± 2.36* |
Compared to the control group, * p < 0.05
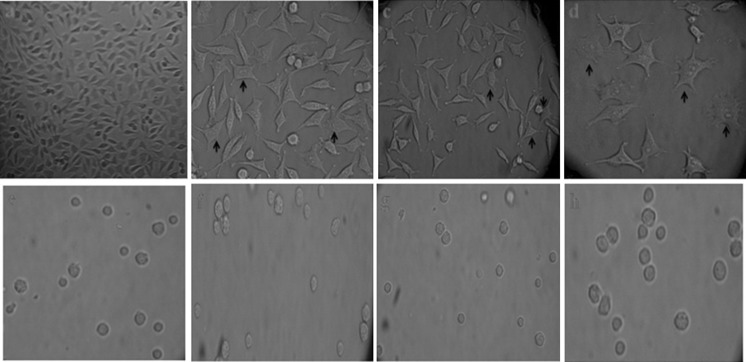
Changes in the cell morphology characteristic of apoptosis was also investigated with an inverted microscope under 400× magnification after 24 h exposure of HepG2 and PBMC cells with Persian shallot extract at IC50, 1/10 and 1/50 of the IC50 concentrations. The typical changes in cellular morphology, including blebbing of the plasma membrane, cytoplasmic swelling and chromatin condensation were observed in HepG2 cells but PBMC cells showed no observable decrease in cell viability and no change in morphology at 24 h after treatment with different concentrations of Persian shallot extract (Fig. 3).
Fig. 3.
Morphological changes on HepG2 (a–d) and PBMC cells (e–h) after exposure with Persian shallot extract at 0 (untreated), 1/50, 1/10 of the IC50 and IC50 concentrations, respectively, that were observed with an inverted microscope under ×400 magnification. Apoptotic cells are indicated by arrows
Effect of Persian shallot extract on the expression of pro-/anti- apoptotic genes
The expression of some apoptotic genes for example p21, p53, puma, caspase-8, bid, bim, bcl-2 and bax was determined at IC50 concentration of Persian shallot-treated HepG2 cells by qPCR.
The mRNA expression of some pro-/anti-apoptotic genes, were examined and the changes in mRNA expression levels were standardized by β-actin expression. The expression level of β-actin was not impacted by Persian shallot extract treatment compared to the other examined genes; therefore it was used as an appropriate housekeeping gene for transcription analysis.
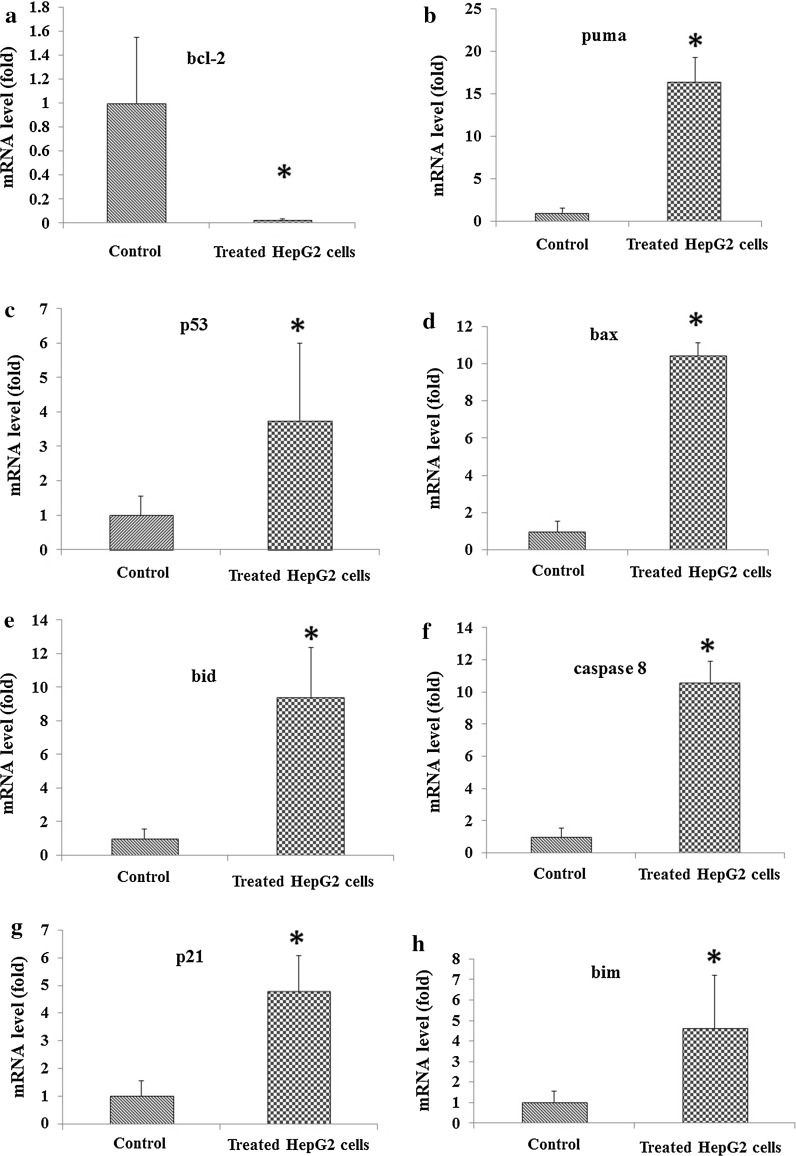
The mRNA expression of bcl-2 showed a decrease of IC50 concentration of Persian shallot (Fig. 4), while the mRNA expression of p53, p21, bid, bim, bax, caspase-8 and puma genes increased significantly compared to untreated cells (Fig. 4).
Fig. 4.
Expression levels of mRNA of some pro-/anti-apoptotic genes in HepG2 cells treated with Persian shallot extract (Allium hirtifulium Boiss). mRNA expression of apoptosis regulatory genes, bcl-2 (a), puma (b), p53 (c), bax (d), bid (e), caspase-8 (f), p21 (g) and bim (h) was detected using real time PCR and normalized to the internal control, β-actin. *p < 0.05 in HepG2 cells after treatment with Persian shallot extract at IC50 concentration (149 μg/ml) for 24 h compared with the control group (untreated HepG2 cells)
The Persian shallot extract treated HepG2 cells showed up-regulation of p53, p21, bid, bim, bax, caspase-8 and puma genes and down-regulation of bcl-2 gene (p ≤ 0.05). At the IC50 (149 μg/ml) of Persian shallot extract, bcl-2 expression was significantly down-regulated about by 0.01-fold (Fig. 4a). The puma (Fig. 4b), p53 (Fig. 4c), bax (Fig. 4d), bid (Fig. 4e), caspase-8 (Fig. 4f), p21 (Fig. 4g) and bim (Fig. 4h) genes were up-regulated about by 16.41, 3.75, 10.43, 9.4, 10.56, 4.77 and 4.62 fold, respectively. The results of this study indicated the biological activity of Persian shallot extract and demonstrated growth inhibition and induction of cell death in HepG2 cells through intrinsic and extrinsic apoptosis pathways with modulation of the expression of pro-/antiapoptotic genes.
Discussion
Cancer is one of the growing diseases threatening global health. For a long time, natural products have been used for prevention and treatment of many diseases; including cancer therefore they are appropriate candidates for the development of anti-cancer drugs (Tavakkol-Afshari et al. 2008).
In the present study, the cytotoxic and apoptotic effects of hydroalcoholic extract of Persian shallot were investigated in HepG2 cell lines. Persian shallot (Moosir) belongs to Allium genus (Alliaceae family) that is a native plant in Iran. The previous reports indicated that the Persian shallot has antibacterial activity (Ismail et al. 2013) and also significant cytotoxic activity on HeLa (cervical cancer) and MCF7 (human, caucasion, breast, adenocarcinoma) cancer cell lines with IC50 values of 20 and 24 μM, respectively, while L929 (mouse, C3H/An, connective), as a normal cell line, did not show any sensitivity to Persian Shallot. However the mechanism of action of this compound has not been elucidated (Azadi et al. 2008).
Allicin is a key molecule in garlic (Allium sativum) and a precursor of many secondary products formed in aged garlic and crushed garlic preparations and presents a lot of biological properties including anti-microbial and anti-tumor activities (Zhang et al. 2008). The presence of Allicin has been shown in bulbs and the extract of Persian shallot that were analyzed by HPLC. Azadi and her co-workers indicated that the inhibitory effect of chloroformic extract of Persian shallot on Hela and MCF7 cancer cells was more elevated than that of Allicin (Azadi et al. 2008).
In the present study, MTT assay was performed for the screening of cell viability and investigation of anti-proliferative activity of Persian shallot extract against HepG2 cells. Persian shallot extract induced HepG2 cell death in a concentration dependent manner with an IC50 value of 149 μg/ml and decreased the viability of HepG2 cells after 24 h.
The apoptotic effects of Persian shallot have received little consideration in the literature. We used flow cytometric method to confirm apoptosis in HepG2 cells treated by Persian shallot extract. The detection of surface exposed phosphatidyl-serine (PS) by Annexin V-FITC has been shown to be a general and early marker of apoptosis as a result of redistribution of the plasma membrane of cells following the occurrence of apoptosis (van Engeland et al. 1998). Consistent with data on morphological changing of cells and result of MTT assay, apoptosis was mostly observed after 24 h of exposure to Persian shallot extract (0, 125, 150, 175 μg/ml). Early apoptosis (Q4 quadrant) and late apoptosis/necrosis (Q2 and Q1 quadrants respectively) were clearly evident in dot plots of Fig. 2a–d.
PBMC cells (normal cells) showed no observable decrease in cell viability and no change in morphology when exposed to different concentrations of Persian shallot extract while the morphological changes such as blebbing of the plasma membrane, cytoplasmic swelling and chromatin condensation were observed in HepG2 cells after treatment with the extract (Fig. 3).
No previous literature was found to report the effect of hydroalcoholic extract of Persian shallot (A. hirtifolium) on the expression of apoptotic and anti-apoptotic genes in any tumor cell line.
This study is the first report of the apoptotic effect of Persian shallot extract on the expression of some pro-/anti-apoptotic genes such as bid, bim, bax, p53, p21, puma, caspase-8 and bcl-2 in HepG2 cells. Apoptosis is a physiological mechanism of cell death. In many tumor cells, the apoptosis process is disrupted; therefore one of the methods for cancer treatment by anticancer drugs is the induction of apoptosis in cancer cells (Shahneh et al. 2013). Understanding apoptosis regulation is important in the development of anticancer drugs on malignant cells.
Apoptosis is regulated by anti and pro-apoptotic genes from bcl-2 family members. The pro13 apoptotic genes (e.g., p53 and bax) and anti-apoptotic genes (e.g., bcl-2) are typically involved in apoptosis and cellular proliferation (Du et al. 2013; Song et al. 2014).
In the present study, we investigated whether apoptosis contributes to the death of HepG2 cells treated by the Persian shallot extract and the expression of pro-/anti-apoptotic genes that are involved in extrinsic and intrinsic apoptosis pathways are affected. All data from the present study indicated the apoptotic effect of Persian shallot extract on HepG2 cells.
The intrinsic apoptotic pathway is mostly regulated by the Bcl-2 family proteins. This family of proteins contains both anti-/pro-apoptotic proteins, including, Bcl-2, Bcl-xL and Bid, Bax, Bim, respectively (Letai et al. 2002; Ming et al. 2006). In this study, the up-regulation of puma, bim, bax, bid, p53 following Persian shallot extract treatment was observed in HepG2 cells.
The pro-apoptotic members could be classified into multi-domain proteins, such as Bak and Bax and BH3-only members, for example Puma and Bid (Desagher et al. 1999; Ming et al. 2006). In general, it has been thought that the BH3-members activate the downstream multi-domain Bax and Bak, which then begin to release mitochondrial apoptogenic agents. Bim is one of the BH3-only members of Bcl-2 family proteins that can directly activate Bax (Ding et al. 2007) while Puma, another important BH3-only member that transcriptionally is up-regulated by P53 (Yu et al. 2001), can activate Bax indirectly by binding to Bcl-xL and dissociate the interaction of Bcl-xL and Bax and trigger the intrinsic apoptotic pathway; then the liberated Bax can release cytochrome c from mitochondria and trigger the apoptosis process (Villunger et al. 2003).
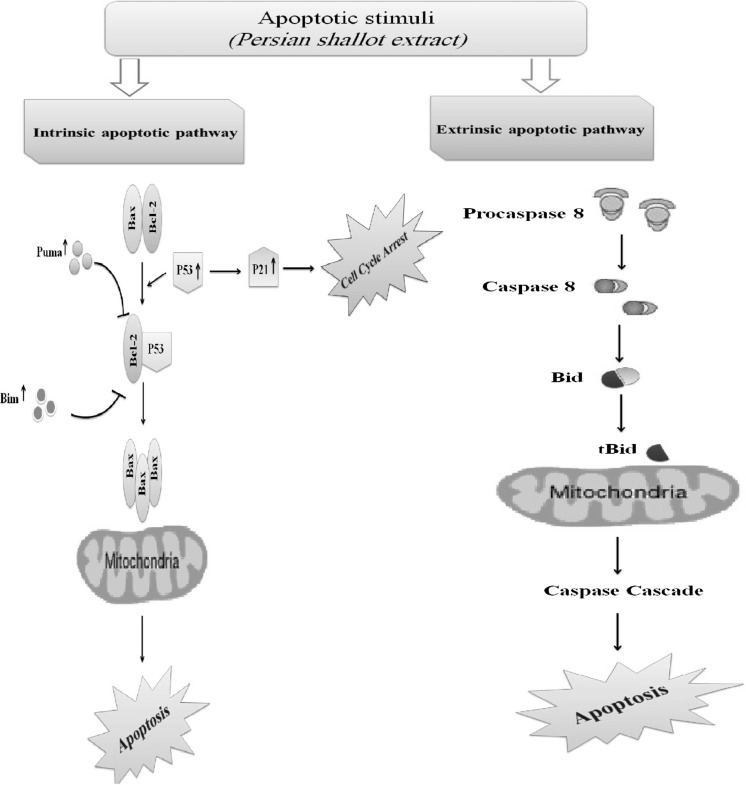
The results of this study indicated that the up-regulation of p53 and puma could be the critical factor for promoting bax dependent mitochondria activation in the hepatoma cancer cells following treatment with Persian shallot extract. On the other hand, suppression of the antiapoptotic genes, such as bcl-2, and up-regulation of pro-apoptotic genes for example bim, bax, puma, p53 and p21 could enhance apoptosis via intrinsic pathway in hepatoma cells after treatment with Persian shallot. Our results indicated that, after treatment of hepatoma cells with Persian shallot extract, the expression of p53 was increased. The p53 gene product is an upstream regulator of the bax gene that binds to the bax promoter and directly activates the transcription of bax gene. bax is probably involved in a p53-regulated pathway that leads either to apoptosis or to cell cycle arrest. The actions of the bax gene product is neutralized when heterodimerized with Bcl-2. As shown in many tumor cells, the Bax/Bcl-2 ratio seems to be important to sensitization of cells to apoptosis (Raisova et al. 2001). After treatment with the extract, the expression of bcl-2 gene was decreased while that of bax was increased and thus apoptosis was induced in HepG2 cells. Additionally, the Persian shallot extract also induced apoptosis through activation of the caspase-8 dependent apoptosis pathway in hepatoma cells because of the increase of the mRNA level of bid and caspase-8 that are involved in extrinsic apoptosis pathway. The expressions of p21, a cyclin-dependent kinase inhibitor involved in cell cycle arrest, was also increased in HepG2 cells treated with Persian shallot extract. These findings are important because this study is the first report indicating that the Persian shallot extract induced apoptosis by different mechanisms in a hepatoma cell line. The probable mechanism of apoptosis induction by Persian shallot extract is illustrated in Fig. 5. As can be seen, this extract may activate both intrinsic and extrinsic apoptotic pathways.
Fig. 5.
The probable mechanisms of apoptosis induction by hydroalcoholic Persian shallot extract in HepG2 cells
The mechanism of action of the Persian shallot extract is related to mitochondrial signaling, to trigger the intrinsic apoptosis pathway because it up-regulates p53, p21 and some pro-apoptotic gene such as bax, bim, puma and down-regulates some anti-apoptotic gene such as bcl-2 that are involved in the intrinsic apoptosis pathway. This extract also up-regulated caspase-8 and bid that are involved in the extrinsic apoptosis pathway. Considering the results of this study, the Persian shallot extract exhibited cytotoxic activities and induced apoptosis via both extrinsic and intrinsic pathways that could be useful in the production of anti-cancer drugs. Therefore, the potential of Persian shallot extract as a candidate for production of anti-cancer drugs in treatment of human hepatocellular carcinoma should be considered.
Acknowledgements
This project was financially supported by a Grant (20/414) from the Rafsanjan University of Medical Sciences. The authors thank the Molecular Medicine Research Center (MMRC) from Rafsanjan University of Medical Sciences (RUMS) of Iran for providing the necessary equipment of this work. The support of vice chancellor to research of RUMS is acknowledged.
Abbreviations
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- FITC
Fluorescein isothiocyanate
- HCC
Hepatocellular carcinoma
- HepG2
Human hepatocellular liver carcinoma cell line
- IC50
Inhibiting cell growth by 50%
- MTT
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate buffered saline
- PI
Propidium iodide
- SPSS
Statistical package for the social sciences
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest.
References
- Al-Fatlawi AA, Al-Fatlawi AA, Irshad M, Zafaryab M, Rizvi M, Ahmad A. Rice bran phytic acid induced apoptosis through regulation of Bcl-2/Bax and p53 genes in HepG2 human hepatocellular carcinoma cells. Asian Pac J Cancer Prev. 2014;15:3731–3736. doi: 10.7314/APJCP.2014.15.8.3731. [DOI] [PubMed] [Google Scholar]
- Asgarpanah J, Ghanizadeh B. Pharmacologic and medicinal properties of Allium hirtifolium Boiss. Afr J Pharm Pharmacol. 2012;6:1809–1814. [Google Scholar]
- Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866–3884. doi: 10.1038/sj.onc.1209550. [DOI] [PubMed] [Google Scholar]
- Azadi HG, Ghaffari SM, Riazi GH, Ahmadian S, Vahedi F. Antiproliferative activity of chloroformic extract of Persian Shallot, Allium hirtifolium, on tumor cell lines. Cytotechnology. 2008;56:179–185. doi: 10.1007/s10616-008-9145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi HG, Fathi B, Kazemi Mehrjerdi H, Maleki M, Shaterzadeh H, Abyazi M. Macroscopic evaluation of wound healing activity of the Persian shallot, Allium hirtifolium in rat. IJVST. 2012;3:31–38. [Google Scholar]
- Barile E, Capasso R, Izzo AA, Lanzotti V, Sajjadi SE, Zolfaghari B. Structure-activity relationships for saponins from Allium hirtifolium and Allium elburzense and their antispasmodic activity. Planta Med. 2005;71:1010–1018. doi: 10.1055/s-2005-873134. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Chen X, Yu J, Zhang L, Yin XM. A coordinated action of Bax, PUMA, and p53 promotes MG132-induced mitochondria activation and apoptosis in colon cancer cells. Mol Cancer Ther. 2007;6:1062–1069. doi: 10.1158/1535-7163.MCT-06-0541. [DOI] [PubMed] [Google Scholar]
- Du P, Cao H, Wu HR, Zhu BS, Wang HW, Gu CW, Xing CG, Chen W. Blocking Bcl-2 leads to autophagy activation and cell death of the HEPG2 liver cancer cell line. Asian Pac J Cancer Prev. 2013;14:5849–5854. doi: 10.7314/APJCP.2013.14.10.5849. [DOI] [PubMed] [Google Scholar]
- Fattorusso E, Iorizzi M, Lanzotti V, Taglialatela-Scafati O. Chemical composition of shallot (Allium ascalonicum Hort.) J Agric Food Chem. 2002;50:5686–5690. doi: 10.1021/jf020396t. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26:263–270. doi: 10.1093/carcin/bgh283. [DOI] [PubMed] [Google Scholar]
- Hetz CA, Torres V, Quest AF. Beyond apoptosis: nonapoptotic cell death in physiology and disease. Biochem Cell Biol. 2005;83:579–588. doi: 10.1139/o05-065. [DOI] [PubMed] [Google Scholar]
- Hosseini J, Hosseini-Zijoud SM, Oubari F, Mahmoodi M, Abbasi Oshaghi E, Rajabi Gilan N, Ghasemi SR, Hashemi B. Hepatoprotective effects of hydroalcoholic extract of Allium hirtifolium (Persian Shallot) in diabetic rats. J Basic Clin Physiol Pharmacol. 2012;23:83–87. doi: 10.1515/jbcpp-2012-0017. [DOI] [PubMed] [Google Scholar]
- Hosseini-Zijoud SM, Hosseini J, Mahmoodi M, Behrooz H. The effects of Persian shallot extract on the levels of some blood biochemical parameters in streptozotocin-induced diabetic rats. Afr J Agric Res. 2012;7:3308–3313. [Google Scholar]
- Ismail S, Jalilian FA, Talebpour AH, Zargar M, Shameli K, Sekawi Z, Jahanshiri F. Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium Boiss. Biomed Res Int. 2013;25:1–9. doi: 10.1155/2013/696835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarian A, Ghannadi A, Elyasi A. The effects of Allium hirtifolium Boiss. on cell mediated immune response in mice. Iran J Pharm Res. 2003;2:51–55. [Google Scholar]
- Kaseb AO, Abaza YM, Roses RE. Multidisciplinary management of hepatocellular carcinoma. Multidiscip Treat Hepatocell Carcinoma Recent Results Cancer Res. 2013;190:247–259. doi: 10.1007/978-3-642-16037-0_16. [DOI] [PubMed] [Google Scholar]
- Khalil MI, Ibrahim MM, El-Gaaly GA, Sultan AS. Trigonella foenum (Fenugreek) induced apoptosis in hepatocellular carcinoma cell line, HepG2, mediated by upregulation of p53 and proliferating cell nuclear antigen. Biomed Res Int. 2015;2015:914645. doi: 10.1155/2015/914645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Li XF, Kang KH, Ryu B, Kim SK. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014;47:433–438. doi: 10.5483/BMBRep.2014.47.8.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelarungrayub N, Rattanapanone V, Chanarat N, Gebicki JM. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition. 2006;22:266–274. doi: 10.1016/j.nut.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/S1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Mahmoodi M, Zarei S, Rezaeian M, Arababadi MK, Ghasemi H, Khoramdelazad H, Rezayati N, Hasanshahi G, Hosseini-Zijoud SM. Persian shallot (Allium hirtifolium Boiss) extract elevates glucokinase (GCK) activity and gene expression in diabetic rats. Am J Plant Sci. 2013;4:1393–1399. doi: 10.4236/ajps.2013.47170. [DOI] [Google Scholar]
- Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- Omobuwajo O, Abdu A, Igbeneghu O, Agboola O, Alade G. Preliminary investigation of a herbal soap incorporating Cassia senna (L) Roxb leaves and Ageratum conyzoides Linn whole plant powders. Cont J Pharm Sci. 2011;5:1–10. [Google Scholar]
- Raicht RF, Cohen BI, Fazzini EP, Sarwal AN, Takahashi M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980;40:403–405. [PubMed] [Google Scholar]
- Raisova M, Hossini AM, Eberle J, Riebeling C, Orfanos CE, Geilen CC, Wieder T, Sturm I, Daniel PT. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–340. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- Rose P, Whiteman M, Moore PK, Zhu YZ. Bioactive S-alk (en) yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- Shahneh FZ, Valiyari S, Azadmehr A, Hajiaghaee R, Bandehagh A, Baradaran B. Cytotoxic activities of Ferulago angulata extract on human leukemia and lymphoma cells by induction of apoptosis. J Med Plants Res. 2013;7:677–682. [Google Scholar]
- Song HY, Deng XH, Yuan GY, Hou XF, Zhu ZD, Zhou L, Ren MX. Expression of bcl-2 and p53 in Induction of esophageal cancer cell apoptosis by ECRG2 in combination with cisplatin. Asian Pac J Cancer Prev. 2014;15:1397–1401. doi: 10.7314/APJCP.2014.15.3.1397. [DOI] [PubMed] [Google Scholar]
- Taran M, Rezaeian M, Izaddoost M. In vitro antitrichomonas activity of Allium hirtifloium (Persian Shallot) in comparison with metronidazole. Iranian J Publ Health. 2006;35:92–94. [Google Scholar]
- Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46:3443–3447. doi: 10.1016/j.fct.2008.08.018. [DOI] [PubMed] [Google Scholar]
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(SICI)1097-0320(19980101)31:1<1::AID-CYTO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53-and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Yu Q. Restoring p53-mediated apoptosis in cancer cells: new opportunities for cancer therapy. Drug Resist Updat. 2006;9:19–25. doi: 10.1016/j.drup.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/S1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yao HP, Huang FF, Wu W, Gao Y, Chen ZB, Liang ZY, Liang TB. Allicin, a major component of garlic, inhibits apoptosis in vital organs in rats with trauma/hemorrhagic shock. Crit Care Med. 2008;36:3226–3232. doi: 10.1097/CCM.0b013e31818f2103. [DOI] [PubMed] [Google Scholar]