Abstract
Interferon gamma (IFNγ) is the major proinflammatory cytokine conferring resistance to the intracellular vacuolar pathogen Toxoplasma gondii by inducing the destruction of the parasitophorous vacuole (PV). We previously identified TRIM21 as an IFNγ-driven E3 ubiquitin ligase mediating the deposition of ubiquitin around pathogen inclusions. Here, we show that TRIM21 knockout mice were highly susceptible to Toxoplasma infection, exhibiting decreased levels of serum inflammatory cytokines and higher parasite burden in the peritoneum and brain. We demonstrate that IFNγ drives recruitment of TRIM21 to GBP1-positive Toxoplasma vacuoles, leading to Lys63-linked ubiquitination of the vacuole and restriction of parasite early replication without interfering with vacuolar disruption. As seen in vivo, TRIM21 impacted the secretion of inflammatory cytokines. This study identifies TRIM21 as a previously unknown modulator of Toxoplasma gondii resistance in vivo thereby extending host innate immune recognition of eukaryotic pathogens to include E3 ubiquitin ligases.
Introduction
Toxoplasmosis is an infectious disease that is caused by an obligate intracellular parasite belonging to the phylum Apicomplexa called Toxoplasma gondii. This unicellular parasite is found throughout the world and is arguably the most successful parasite with a global human infection rate of about 30%1. Toxoplasma is an opportunistic parasite causing chronic infection in humans that remains asymptomatic in many cases. However, complications for the foetus such as abortion, mental abnormalities or ocular disease can develop if an acute infection is acquired during pregnancy. Also, immunocompromised individuals can develop toxoplasmic encephalitis or retinochorioditis2 with the latter being a risk even for immunocompetent adults.
Toxoplasma can infect any nucleated cell in any warm-blooded animal, including humans. Inside the host cell, Toxoplasma resides and replicates within a parasitophorous vacuole (PV) formed during invasion by invagination of the host plasma membrane3. The majority of lipids composing the PV membrane (PVM) are host cell derived, and Toxoplasma regulates the contents of the PVM by preventing host proteins like SNAREs to accumulate at the PVM4. Thus, by never fusing with endo-lysosomes5 and being resistant to acidification6, the PV provides a safe and protective place for the parasite to survive in the host cell, allowing it to persist despite a vigorous immune response.
Type II interferon γ (IFNγ) was identified as the major proinflammatory cytokine that confers resistance against Toxoplasma 7, 8. Upon Toxoplasma infection, IFNγ mediates the deployment of a range of host defence molecules to the PV, ultimately leading to its disruption, autophagic elimination and inflammasome activation9. Central players in this defence mechanism are immunity-related GTPases (IRGs)10–13 and guanylate binding proteins (GBPs)14. These large GTPases recognise vacuoles of intracellular pathogens for destruction and clearance, as well as govern the subsequent activation of the inflammasome15–21. We have recently shown that ubiquitin is another central player in the IFNγ-dependent vacuolar recognition cascade in both mouse22 and human cells23. Ubiquitin is recruited to type II and III (Pru and CEP) Toxoplasma vacuoles in dependence of IRG proteins and the E3 ligase tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6). Removal of the IFNγ-inducible ubiquitination pathway also substantially diminishes the p62-dependent delivery of GBPs to PVs and thus diminishes the host’s ability to restrict Toxoplasma replication22. Ubiquitin undoubtedly serves as a host-induced pattern that marks intracellular structures as immune targets for members of the GBP family of host defense proteins.
Ubiquitin deposition around a pathogen had already been well-established as a central dogma to intracellular defence against bacterial pathogens24, 25. The E3 ubiquitin ligase LRSAM1 has been shown to directly recognise Salmonella, leading to ubiquitination associated with the bacterium. LRSAM1 also participates in the recruitment of LC3-positive autophagosome by binding to the autophagy receptor nuclear dot protein 52 (NDP52)26. The E3 ubiquitin ligase HOIL-1 is a component of the linear ubiquitination chain assembly complex (LUBAC)27. HOIL-1 knockout mice are highly susceptible to Citrobacter rodentium and Toxoplasma gondii infection, exhibiting unchecked parasite burden28. The E3 ubiquitin ligase Parkin is involved in mitophagy and confers susceptibility to Parkinson’s disease29–32. Mice deficient in Parkin succumb during Mycobacterium tuberculosis infection concurrent with a higher bacterial load33. Moreover, some E3 ubiquitin ligases act on both cell-autonomous restriction and immune response regulation during bacterial infection. Listeria monocytogenes infection is fatal in HOIL-1 knockout mice, that cannot control bacterial replication and present an impaired production of protective cytokines by macrophages28. The tripartite motif protein 21 (TRIM21) has been reported to bind to invading antibody-coated adenoviruses as well as Salmonella typhimurium in the cytosol, and target the virus to degradation by the proteasome by virtue of its E3 ligase activity34–36. Following adenovirus and Salmonella infection in mouse embryonic fibroblasts, TRIM21 was suggested to mediate the formation of Lys63-linked chains and upregulate IRF3, IRF5, IRF7, NF-κB and AP-1, thereby inducing the production of proinflammatory cytokines35. Recently, TRIM21 has also been reported to mediate recognition of viral RNA and DNA by the host sensors RIG-I and cGAS, respectively37.
We previously identified TRIM21 as an E3 ligase involved in the deposition of ubiquitin around Chlamydia inclusions22. However, the biological relevance of TRIM21 has only been studied in the context of viral infection in vivo and its role in resistance to bacteria or other pathogens remains unclear37, 38. Here, we demonstrate that TRIM21 knockout mice were highly susceptible to Toxoplasma infection and exhibited decreased levels of proinflammatory cytokines in their serum associated with higher parasite burden in the brain. TRIM21 deficiency led to an enhanced early replication of Toxoplasma without the disrupted vacuole displaying overt morphological differences compared to the wild-type vacuole. We show TRIM21 and GBP1 are being co-recruited to PVs of type II and III (Pru and CEP), but not PruRop16I and CEPRop18I Toxoplasma. Type II and III (Pru and CEP), but not CEPRop18I Toxoplasma, were decorated with ubiquitin in an IFNγ- and TRIM21-dependent manner, with TRIM21 partly mediating Lys63 ubiquitin linkages. Concurrent with our in vivo findings, TRIM21 deficiency led to an enhanced early replication of type II parasites and a disregulated production of proinflammatory cytokines. We define TRIM21 as a novel, crucial intracellular restriction factor during acute Toxoplasma infection acting on both the regulation of innate immune response to the parasite and the restriction of parasite replication.
Results
TRIM21 deficiency increases susceptibility to Toxoplasma infection in vivo
We previously found TRAF6 and TRIM21 are E3 ubiquitin ligases responsible for the deposition of ubiquitin around inclusions of Chlamydia trachomatis and for TRAF6 also around the PV of type II Toxoplasma 22. While TRAF6 is crucially important for ubiquitin deposition at the PV leading to parasite restriction via p62-dependent GBP-mediated control in infected cells22, it is still unclear what the role of TRIM21 is in this process. Additionally, whether these E3 ligases present an important component of the host defence to Toxoplasma in vivo has not been investigated. We thus determined the resistance of TRIM21 knockout mice to infection with type II Pru Toxoplasma. TRIM21-deficient mice inoculated intraperitoneally with 5 × 104 type II Pru Toxoplasma were highly susceptible to infection. At 10 days post-infection, 77% of the TRIM21 knockout mice had succumbed to infection while 83% of the control mice had survived (Fig. 1a). TRIM21 knockout mice exhibited a higher clinical score (characterised by piloerection, hunching and reduced motility) from day 7 post-infection onwards (Fig. 1b). The parasite load assessed by in vivo imaging revealed the high susceptibility of TRIM21-deficient mice to Toxoplasma acute infection is accompanied with a higher parasite burden 5 days post-infection (Fig. 1c). Additionally, TRIM21-deficient mice that presented poor physical condition typical of infection with Toxoplasma exhibited higher tachyzoite parasite counts in their brains compared to healthy wild-type animals on day 7 post-infection (Fig. 1d). Thus, TRIM21 knockout mice are unable to control parasite migration to the brain and/or its replication at this site.
Figure 1.
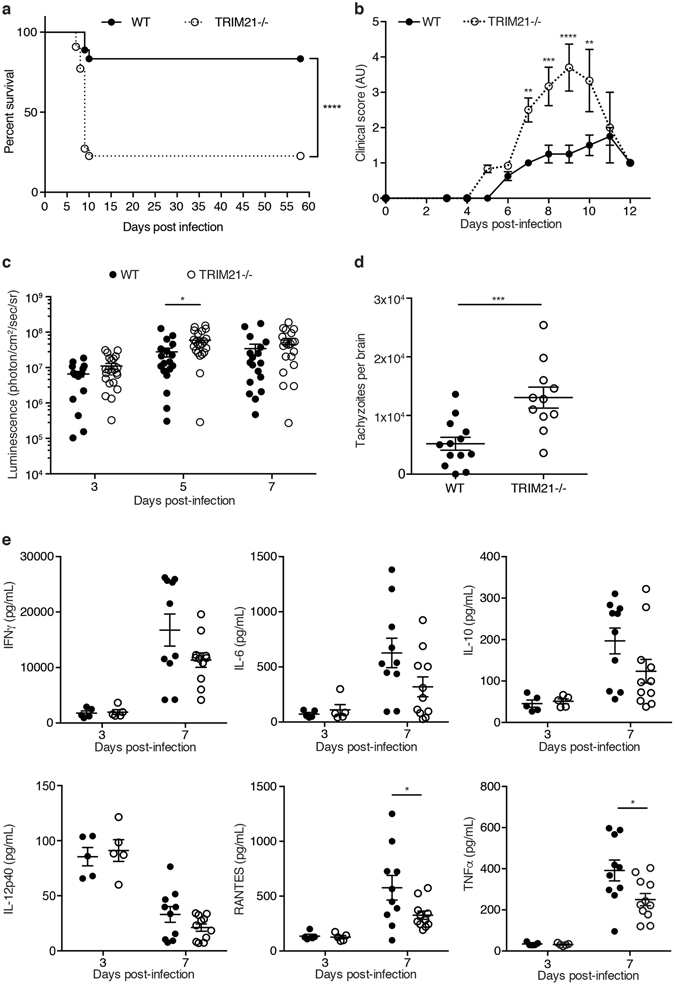
TRIM21-deficient mice are susceptible to Toxoplasma infection correlating with higher parasite burden and decreased levels of proinflammatory cytokines. (a) Survival curves of wild-type versus TRIM21-deficient mice infected intraperitoneally with 5 × 104 type II Toxoplasma (n = 18 wild-type, n = 22 TRIM21 knockout). Curves were compared by log-rank survival analysis of Kaplan-Meier curves, ****p < 0.0001. (b) The clinical score, assessed by piloerection, a hunched position and immobility, was recorded throughout the time of the experiment (n = 18 wild-type, n = 22 TRIM21 knockout). Mean ± SEM, **p < 0.01, ***p < 0.001, ****p < 0.0001, 2-way ANOVA. (c) The evolution of the firefly luciferase-expressing parasite load in wild-type versus TRIM21-deficient mice was assessed by in vivo imaging after injection of luciferin at 3, 5 and 7 days post-infection (n = 18 wild-type, n = 22 TRIM21 knockout). Mean ± SEM, *p < 0.05, 2-way ANOVA. (d) Quantitative analysis of the number of GFP-expressing Toxoplasma tachyzoites in the brain of wild-type and TRIM21-deficient mice at 7d p.i. (n = 14 wild-type, n = 11 TRIM21 knockout). Data pooled from three independent experiments. Mean ± SEM, ***p < 0.001, unpaired t-test. (e) The serum of wild-type and TRIM21-deficient mice infected intraperitoneally with type II Toxoplasma was collected at 3 and 7 days post-infection. Cytokine levels were measured using multiplex. Mean ± SEM, *p < 0.05, unpaired t-test.
Via interaction and ubiquitination of IRF8, TRIM21 mediates the upregulation of IL-12p40 expression, a cytokine important for innate immunity in macrophages and involved in the stimulation of IFNγ production39. TRIM21 has also been shown to downregulate NF-κB signalling in embryonic fibroblasts, leading to lower expression of proinflammatory cytokines40. Therefore, we measured the expression of cytokines and chemokines in the serum at day 3 and 7 post-Toxoplasma infection. At 3 days post-infection corresponding to the time prior to onset of overt disease, no difference was observed in the levels of serum proinflammatory cytokines. However, at day 7 p.i., corresponding to the time when mice start to succumb to the parasitic infection, TRIM21-deficient mice exhibited significantly decreased levels of RANTES and TNFα (Fig. 1e); both are induced by the NF-κB-mediated signalling pathway. The expression levels of the cytokines investigated were all similar in naïve WT and TRIM21 knockout mice (Supplementary Fig. S1). This suggests TRIM21 mediates resistance to Toxoplasma infection in vivo by controlling parasite replication and the overt production of some, but not all inflammatory cytokines.
The E3 ubiquitin ligase TRIM21 localises to GBP1-positive type II Pru vacuoles
Due to the dramatic susceptibility of TRIM21 knockout mice to Toxoplasma infection, we investigated the subcellular localisation of TRIM21 in Toxoplasma-infected cells. Localisation studies revealed TRIM21 is predominantly found at the type II Toxoplasma PV, with up to 26% of vacuoles decorated with the E3 ubiquitin ligase (Supplementary Figs S2a and S3a). As we and others have previously demonstrated, type II Toxoplasma is targeted by IRGs15, 41, 42, GBPs43, 44 (Supplementary Figs S2b and S3b) and ubiquitin22, 23 (Supplementary Figs S3c and S4) in an IFNγ-dependent manner, constituting a host defence system that enables the destruction of the PV and the subsequent elimination of the parasite. Using GBP1 as a marker for the host defence system, we showed that TRIM21 and GBP1 are recruited to the same type II Toxoplasma PVs in IFNγ-stimulated mouse embryonic fibroblasts (MEFs) (Fig. 2a, Supplementary Fig. S5). Quantitative analysis of the PVs found 29.8% positive for both GBP1 and TRIM21, 28.5% PVs were single-positive for GBP1, while only 1.5% PVs were TRIM21 single-positive (Fig. 2b). Interestingly, TRIM21 was only recruited to half of the PVs that are marked by GBP1, but almost always was recruited to GBP1-positive PVs. These results suggest TRIM21 is preferentially recruited to GBP1-positive type II Toxoplasma parasitophorous vacuoles, the same PVs recognised by the host and destined for destruction.
Figure 2.
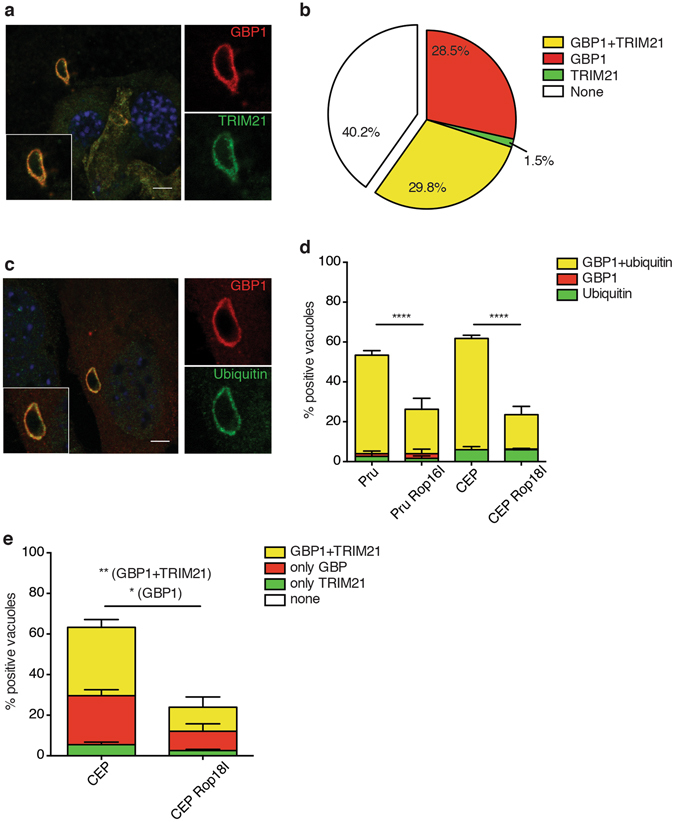
TRIM21 and ubiquitin are targeted to GBP1-positive type II Toxoplasma vacuoles in IFNγ-stimulated cells dependent on parasitic virulence factors. (a) Representative immunofluorescence confocal images of IFNγ-stimulated mouse embryonic fibroblasts infected 1 h with type II Toxoplasma and co-stained for GBP1 and TRIM21. Scale bar is 5 μm. (b) Quantification of GBP1 and TRIM21 co-recruitment to the parasite-containing vacuoles 1 h p.i. Data pooled from three independent experiments. (c) Representative immunofluorescence confocal images of IFNγ-stimulated mouse embryonic fibroblasts infected 1 h with type II Toxoplasma and co-stained for GBP1 and ubiquitin. Scale bar is 5 μm. (d) Quantification of ubiquitin- and/or GBP1-positive parasite-containing vacuoles in IFNγ-stimulated mouse embryonic fibroblasts infected 1 h with the canonical Toxoplasma type II (Pru) and type III (CEP) strains, as well as with Toxoplasma type II strain transgenic for the type I Rop16 (Pru Rop16I), and Toxoplasma type III strain transgenic for the type I version of Rop18 (CEP Rop18I). Data pooled from three independent experiments. Mean ± SEM, ****p < 0.0001, 2-way ANOVA. (e) Quantification of TRIM21- and/or GBP1-positive parasite-containing vacuoles in IFNγ-stimulated mouse embryonic fibroblasts infected 1 h with the canonical Toxoplasma type III (CEP) strain and Toxoplasma type III strain transgenic for the type I version of Rop18 (CEP Rop18I). Data pooled from three independent experiments. Mean ± SEM, *p < 0.05, **p < 0.005, 2-way ANOVA.
Parasitic virulence factors prevent recruitment of ubiquitin and TRIM21 to type II GBP1-positive Toxoplasma vacuoles
Ubiquitin is a small ubiquitously expressed protein that is a central cytosolic host survival molecule in its ability to tag proteins for either degradation45 or to induce immune signalling46. In previous work, we showed that ubiquitin targets type II Toxoplasma in an IFNγ-dependent manner (Supplementary Figs S3c and S4), leading to TRAF6- and p62-dependent recognition of the PV by GBPs and both factors subsequently mediating parasite clearance22. Parasitic virulence factors interfere with this process22, 43. As the total percentage of IFNγ-dependent accumulation of ubiquitin around type II Toxoplasma PVs was strikingly reminiscent of the recruitment percentage we reported for GBP143, we assessed the colocalisation of the proteins during Toxoplasma infection. Both GBP1 and ubiquitin were recruited around the same type II parasites (Fig. 2c). Quantification of GBP1 and ubiquitin recruitment events to type II Toxoplasma vacuoles in IFNγ-stimulated mouse embryonic fibroblasts demonstrated that GBP1-positive vacuoles were always positive for ubiquitin. 49% of type II Pru Toxoplasma and 56% of type III CEP Toxoplasma were decorated with both GBP1 and ubiquitin (Fig. 2d). A minor portion of less than 6% of the PVs was positive for ubiquitin alone, while less than 1% PVs received just GBP1 (Fig. 2d). TRIM21 does not localise to type I Toxoplasma (Supplementary Fig. S2a). We previously showed that the recruitment of GBP1, ubiquitin and TRAF6 to type II Toxoplasma vacuoles can separately be counteracted by type I Toxoplasma ROP16 and ROP18 virulence factors secreted into the host cell cytoplasm22, 43. ROP18 has been reported to phosphorylate and hence inactivate the GTPases Irga6 and Irgb6, thereby preventing their recognition of the vacuole47, 48. ROP18 can mediate the evasion of GBP1 recruitment to vacuoles of type III parasites. ROP16 localises to the host nucleus and activates STAT3 and STAT6, yet also significantly reduces the recruitment of GBP1 to PVs43, 49, 50. In order to determine whether these virulence factors influence the accumulation of the co-recruited GBP1 and ubiquitin in the vicinity of type II Toxoplasma, we used the transgenic type II Pru Toxoplasma strain expressing the type I version of ROP16 (Pru ROP16I), as well as the transgenic type III CEP Toxoplasma strain expressing the type I version of ROP18 (CEP ROP18I). The transgenic strains Pru ROP16I and CEP ROP18I showed significantly reduced accumulation of the co-recruited GBP1 and ubiquitin compared to their respective parental strains, dropping to 22% and 17%, respectively (Fig. 2d). The less than 6% of PVs that were positive for only ubiquitin were not impacted by the virulence factors (Fig. 2d). These results suggest that Toxoplasma virulence factors interfere with the IFNγ-dependent joint recruitment of both GBP1 and ubiquitin to PVs, thus preventing this joint defence machinery from being recruited.
Since GBP1 and ubiquitin colocalisation to type II Toxoplasma vacuoles is impaired by the secretion of virulence factors, we investigated whether parasite virulence factors can also alter the recruitment of TRIM21 and GBP1. Immunofluorescence staining for GBP1 and TRIM21 in IFNγ-stimulated MEFs showed both proteins also colocalised to type III CEP Toxoplasma strain (Fig. 2e). The transgenic strain CEP ROP18I exhibited significantly reduced accumulation of colocalised GBP1 and TRIM21 compared to its parental strain, decreasing from 34% to 18%, as well as single-positive GBP1 PVs, decreasing from 24% to 10% (Fig. 2e). Interestingly, the proportion of Toxoplasma vacuoles decorated with both TRIM21 and GBP1 compared to the proportion of GBP1 single-positive type II vacuoles is approximately conserved (Fig. 2e). This suggests that Toxoplasma virulence factors interfere with the recruitment of GBP1 and the colocalised TRIM21 to the parasitophorous vacuoles.
TRIM21 mediates Lys63-linked ubiquitination recruitment around type II Toxoplasma
TRIM21 and ubiquitin were found to both localise to the parasitophorous vacuole of type II Pru Toxoplasma (Supplementary Figs S2a, S3a, S3a and S6). Next, we investigated the ubiquitination of type II Toxoplasma vacuoles in TRIM21-deficient cells. Distinct ubiquitin linkages have been associated with specific cellular functions. Lys48-Ub substrates have been shown to be targeted for degradation by the proteasome45, 51, while Lys63-Ub substrates have been shown to be involved in DNA repair and activation of immune signalling46, 52. Thus, we examined the ubiquitin linkage present at the vicinity of type II Toxoplasma. Lys48- and Lys-63-linked ubiquitin were observed around type II Toxoplasma in both wild-type and TRIM21-deficient cells (Fig. 3a). Quantitative analysis showed TRIM21-deficiency led to a significant decrease in type II Toxoplasma vacuolar ubiquitination, dropping from 62 to 52% in TRIM21 knockout cells, suggesting TRIM21-mediated ubiquitination accounts at least in part for the presence of ubiquitin in the vicinity of the parasite (Fig. 3b). TRIM21-deficiency did not affect the Lys48-linked ubiquitination of type II Toxoplasma (Fig. 3b). However, Lys63-linked ubiquitination of type II Toxoplasma was decreased by one third in TRIM21 knockout cells compared to wild-type cells as the proportion of Lys63-linked ubiquitination dropped from 68 to 47% (Fig. 3b). When expressing type I ROP18 in type II parasites, K63-linked ubiquitination was diminished by 60% concurring with the previously observed lower targeting of TRIM21 and GBP1 (Figs 2d and 3c).
Figure 3.
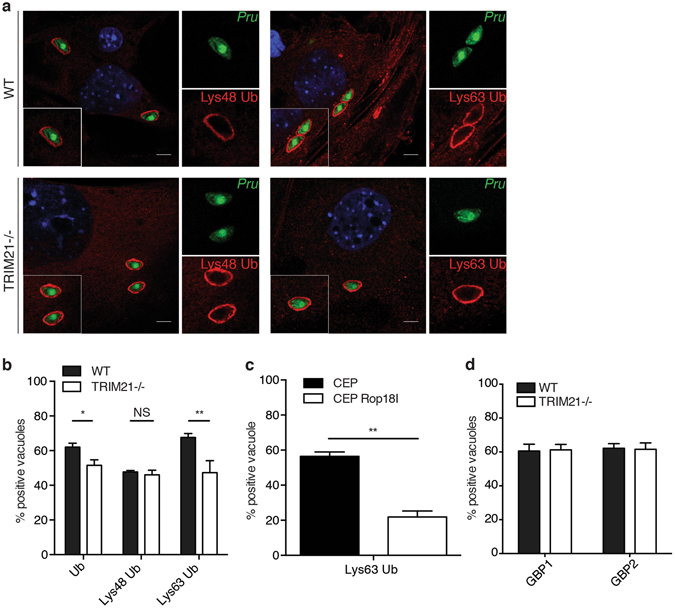
TRIM21 mediates Lys63-linked ubiquitination around type II Toxoplasma. (a) Representative immunofluorescence confocal images of IFNγ-stimulated wild-type (upper panel) or TRIM21 knockout (lower panel) mouse embryonic fibroblasts infected 1 h with type II Toxoplasma expressing GFP and stained for Lys48 ubiquitin (left panel) or Lys63 ubiquitin (right panel). Scale bar is 5 μm. (b) Quantification of ubiquitin- (Ub), Lys48 ubiquitin- and Lys63 ubiquitin-positive parasite-containing vacuoles in IFNγ-stimulated wild-type versus TRIM21 knockout MEFs infected 1 h with type II Toxoplasma. Data were pooled from three independent experiments. Mean ± SEM, *p < 0.05, **p < 0.005, 2-way ANOVA. (c) Quantification of Lys63 ubiquitin-positive parasite-containing vacuoles in IFNγ-stimulated mouse embryonic fibroblasts infected 1 h with the canonical Toxoplasma type III (CEP) strain and Toxoplasma type III strain transgenic for the type I version of Rop18 (CEP Rop18I). Mean ± SEM, **p < 0.005, 2-way ANOVA. (d) Quantification of GBP1 and GBP2 localisation to the parasite-containing vacuoles in IFNγ-stimulated wild-type versus TRIM21 knockout mouse embryonic fibroblasts 1 h p.i. Data pooled from three independent experiments, unpaired t-test.
Since TRIM21 is co-localised to the same PVs as GBP1, we asked whether TRIM21 could influence the level of GBP1 and GBP2 recruitment to these PVs. We found that deletion of TRIM21 had no effect on the recruitment percentage of either GBP1 or GBP2 (Fig. 3d). We had previously defined that deletion of chromosome 3 GBPs had no impact on ubiquitin recognition of the type II Toxoplasma PV22. Thus, our data suggests that the IFNγ-dependent, TRIM21-mediated ubiquitination around the parasites is specific for Lys63-linked polyubiquitin chains.
TRIM21 mediates restriction and the secretion of inflammatory cytokines during Toxoplasma infection
We next investigated the downstream effects of TRIM21-mediated type II Toxoplasma ubiquitination. Mallery et al. showed that TRIM21 binds to invading antibody-coated adenoviruses and targets them to degradation by the proteasome due to its E3 ligase activity34. More recently, it has been shown that TRIM21 also restricts Salmonella intracellular infection36. We thus assessed whether TRIM21-mediated ubiquitination of type II Toxoplasma enables restriction of intracellular parasitic infection. Cell-autonomous restriction of Toxoplasma in IFNγ-stimulated cells is characterised by the blebbing and disruption of the PV15, 16, 53. Some GBPs, including GBP1, are required for this phenomenon17, 44. We thus investigated by transmission electron microscopy the integrity of the vacuolar membrane of type II Toxoplasma in IFNγ-stimulated wild-type or TRIM21 knockout MEFs 2 h after infection. Disruption and blebbing of the vacuolar membrane of type II Toxoplasma was not markedly impaired in TRIM21-deficient cells (Fig. 4a), suggesting TRIM21 is not involved in the IFNγ-induced rupture of Toxoplasma PVs. In vivo, we had observed an increase in parasite load during the acute phase of infection in multiple sites (Fig. 1c,d). We thus asked whether TRIM21 knockout cells would exhibit an increased relative percentage of infected cells in IFNγ-treated versus untreated cells at 24 h p.i. compared to the wild-type cells. Indeed, only control cells showed significantly less infected cells following IFNγ stimulation (Fig. 4b). As another measure of parasite restriction of Toxoplasma replication in TRIM21-deficient cells, enumeration of the parasites per vacuole showed the E3 ubiquitin ligase controled the replication of the parasites (Fig. 4c). At 15 h post-infection, in vitro vacuoles containing 2 or more parasites were significantly more abundant in cells lacking TRIM21 (Fig. 4c). Equally, representative microscopy images demonstrated the increased replicative capacity of type II Toxoplasma in IFNγ-stimulated TRIM21 knockout versus wild-type MEFs (Fig. 4d). These results indicate TRIM21 is involved in early restriction and later elimination of Toxoplasma.
Figure 4.
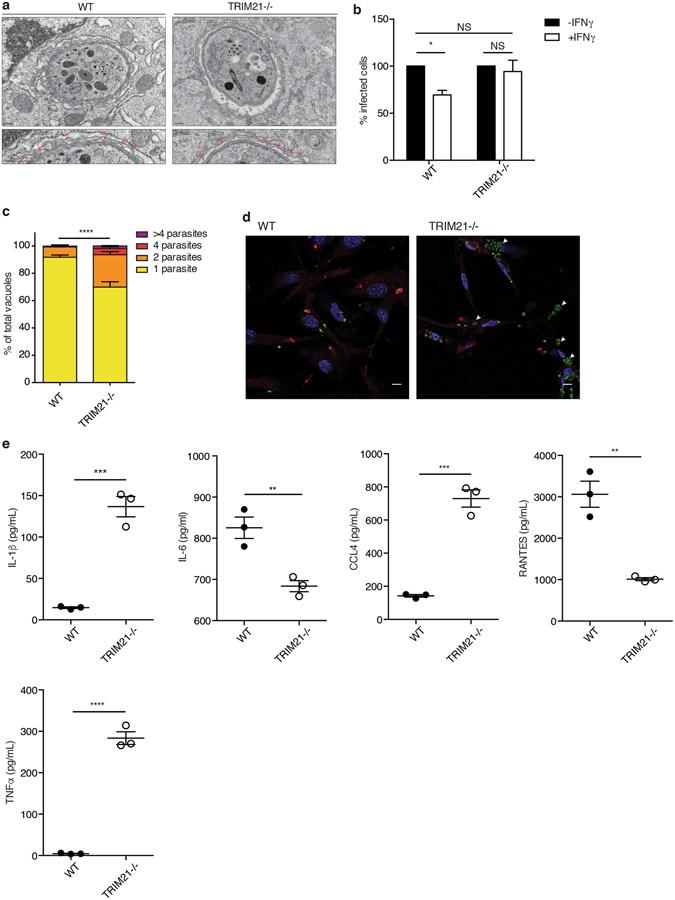
TRIM21 restricts early parasite replication and modulates cytokine production during Toxoplasma infection. (a) Ultrastructural analysis of type II Toxoplasma PVM in IFNγ-stimulated wild-type versus TRIM21 knockout mouse embryonic fibroblasts. The lower images are enlarged views from the upper images. Red arrows indicate blebbing and disruption of the parasitophorous vacuole. Scale bar is 200 nm. (b) Percentage of infection with Toxoplasma in untreated or IFNγ-stimulated TRIM21 knockout and wild-type cells at 1 h p.i.. Data pooled from three independent experiments. Mean ± SEM, p < 0.05, 2-way ANOVA. (c) Quantification of the number of parasites per vacuole in IFNγ-stimulated MEFs infected for 15 h with type II Toxoplasma. Invaded Toxoplasma were assessed for GBP1 recruitment. Data pooled from three independent experiments. Mean ± SEM, ****p < 0.0001, 2-way ANOVA. (d) Representative confocal images of replicating type II Toxoplasma in IFNγ-stimulated MEFs at 15 h post-infection. GBP1 staining in red, type II Toxoplasma in green. White arrows indicate highly replicative Toxoplasma vacuoles. Scale bar is 10 μm. (e) Cytokine production in culture supernatants of IFNγ-stimulated wild-type versus TRIM21-deficient MEFs infected with type II Toxoplasma for 18 h. Data representative of three experiments. Mean ± SEM, **p < 0.01, ***p < 0.005, unpaired t-test.
TRIM21 has also been ascribed innate immune signalling function. TRIM21 mediates the upregulation of IL-12p40 expression via interaction with and ubiquitination of IRF8, a cytokine important for innate immunity in macrophages and involved in the stimulation of IFNγ production39. Additionally, TRIM21 has also been shown to modulate NF-κB signalling in MEFs, leading to differential expression of proinflammatory cytokines, especially IL-635, 40, 54. We hypothesised that TRIM21 in part confers resistance to Toxoplasma infection by controlling cytokine levels to mediate non-cell-autonomous immune responses. Therefore, we measured the expression of cytokines analogous to our in vivo study. We determined a decreased secretion of IL-6, chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL3, leukemia inhibitory factor (LIF) and RANTES, as well as an increased production of IL-1α, IL-1β, chemokine (C-C motif) ligand 3 (CCL3), CCL4, CCL11, granulocyte-macrophage colony-stimulating factor (GM-CSF) and TNFα in INFγ-stimulated TRIM21-deficient cells after infection with type II Toxoplasma (Fig. 4e, Supplementary Fig. S7). The differential cytokine expression observed here could either be solely due to the TRIM21 deficient genotype, but could also be impacted by IFNγ stimulation and/or Toxoplasma infection alone. However, TRIM21 alone (PBS-only) has been shown not to intrinsically have an impact on the production of inflammatory cytokines35 and we observed no difference in cytokine expression in naïve mice (Supplementary Fig. S1). These results demonstrate that TRIM21 contributes to the restriction of Toxoplasma infection, also acting either directly or indirectly as an innate immune modulator that regulates the inflammatory cytokine responses in vitro.
Discussion
Our study has identified TRIM21 as a novel innate immune effector for the eukaryotic pathogen Toxoplasma gondii. To date, only the inflammasome and TLR11/12 are known to recognise Toxoplasma, signal to the immune system and protect against the infection in vivo. TRIM21 expands this concept to E3 ubiquitin ligases and opens the door to studying how distinct ubiquitin-driven cascades signal to activate innate immune defence systems. The balance between innate immune-driven detection and pathogen elimination on one hand and Toxoplasma replication and progression of the infection to chronicity on the other are central to the successful propagation of the parasite.
E3 ubiquitin ligases have emerged as important mediators of resistance against intracellular bacteria by regulating cell-autonomous restriction. LRSAM1 directly recognises Salmonella, leading to ubiquitination of the bacterium and recruitment of LC3-positive autophagosomes via the autophagy receptor NDP5226. TRAF6 partially mediates the decoration of Chlamydia inclusions with ubiquitin, thereby regulating GBP recruitment to the vacuoles for successful bacterial clearance22. TRAF6 is also central for the cell-autonomous restriction of Toxoplasma 22. Activation of this Toxoplasma-restricting E3 ubiquitin ligase is necessary for the effective production of proinflammatory cytokines during the parasitic infection in vitro 31. Regardless, neither the effect of LRSAM1 nor TRAF6 have been investigated during a bacterial or protozoan infection in vivo. In vivo, Parkin knockout mice are highly susceptible to Mycobacterium tuberculosis infection exhibiting a higher bacterial load and HOIL-1 knockout mice succumb to Citrobacter rodentium, Listeria monocytogenes and Toxoplasma infection, exhibiting unchecked parasite and Listeria burden28. Additionally, HOIL-1 knockout mice present an impaired production of protective cytokines by macrophages during Listeria infection, but it is unclear whether this E3 ubiquitin ligase also regulates the innate response to Toxoplasma and whether this E3 ligase directly recognises the parasite28. TRIM21 restricts Salmonella typhimurium growth and targets adenoviruses to degradation by the proteasome34–36 and in vitro mediates the formation of Lys63-linked chains to upregulate IRF3, IRF5, IRF7, NF-κB and AP-1, thereby inducing the production of proinflammatory cytokines35. In vivo, TRIM21 knockout mice are susceptible to adenoviral challenge, a property dependent on the capability of TRIM21 to restrict viral replication and to induce a cytokine response37, 38. Whether TRIM21 restricts bacterial infections in vivo remains to be investigated.
Here, we show that TRIM21 localises to the type II Toxoplasma vacuole and is required for the production of cytokines as well as the parasite restriction during Toxoplasma infection. The modulation of cytokine production following TRIM21 deficiency appears to differ at the cellular level compared to in vivo. This can be explained by both the timing of infection (3–7 days in vivo and 24 h in vitro) as well as the cell types involved in the production of the cytokines in both environments. It is of note that in vitro IFNγ stimulation and/or Toxoplasma infection could impact cytokine production. Importantly, in vivo as seen in naïve mice, TRIM21 deficiency alone does not impact the expression of inflammatory cytokines.
The E3 ubiquitin ligase HOIL-1 is responsible for lowering Toxoplasma burden during in vivo infection, but the intracellular localisation of HOIL-1 during the infection remains unknown28. The role of TRIM21 has only been studied in vivo during viral infections37, 38. The action of E3 ubiquitin ligases during parasitic infection remain unclear. Our studies places TRIM21 as an E3 ubiquitin ligase that mediates both clearance of a eukaryotic pathogen and the successful immune response required for in vivo host survival. The IFNγ-dependent restriction of Toxoplasma replication could be mediated directly by TRIM21, or the E3 ligase could induce other interferon-stimulated genes (such as other members of the TRIM family) to regulate parasitic clearance. As some atypical strains of Toxoplasma 55 and TRIM2156, 57 have been shown to modulate the secretion of type I interferons, which can in turn stimulate the production of TRIMs, it would be of interest to study the production of type I IFNs in TRIM21 deficient cells during the course of Toxoplasma infection. Equally, Toxoplasma-infected TRIM21 deficient cells or mice might present with increased levels of other TRIM family member proteins as demonstrated for poly I:C and LPS-stimulated fibroblasts40.
TRIM21 is one of the molecules operating with GBP1 at the type II and III Toxoplasma vacuole. As with the recruitment of GBP1, the localisation of TRIM21 to the PV can be diminished by the expression of type I Rop16 and Rop1843. As Rop18 has been found at the Toxoplasma PV where it phosphorylates immunity-related GTPases preventing their recruitment58, 59, it is highly likely that TRIM21 is another player in this cohort of host defence molecules22. Rop16 localises to the host nucleus activating STAT3/6, thus its direct effect on TRIM21 localisation is less clear. It is still conceivable that Rop16 may phosphorylate TRIM21 or an as yet unknown host substrate, which then hinders recruitment of the E3 ubiquitin ligase to the PV. Regardless, both type II and III parasites recruit IRGs and GBPs to their vacuoles, and Rop16 is expressed by type I and III Toxoplasma. Thus it is likely that STAT3/6 activation by Rop16 of type I and III Toxoplasma and consequential inhibition of pro-inflammatory cytokine secretion or other STAT3/6-mediated effects decreases the virulence and supersedes interference with the IRG-mediated cell-autonomous immunity.
TRIM21 it is undoubtedly not the sole E3 ubiquitin ligase depositing ubiquitin at the vicinity of the parasite, as can be deduced from the remaining ubiquitination level in TRIM21-deficient cells. Interestingly, TRIM21 deletion does not impact the percentage of PVs recognised by GBP1 and GBP2, however, it does have an impact on Lys63-linked ubiquitination in its vicinity. We have recently identified TRAF6 as another E3 ubiquitin ligases at the PV22. Importantly, TRIM21 and TRAF6 in combination certainly also do not comprise the full set of E3s at the PV and it will be imperative to identify additional ligases acting directly in the vicinity of the parasite. Further studies need to characterise and understand the recruitment kinetics and targets of the ubiquitin ligases at Toxoplasma vacuoles.
Following infection with adenovirus and Salmonella, TRIM21 catalyses the formation of K48 and K63 ubiquitin chains34, 35. The presence of K48 ubiquitin chains targets the virions for degradation by the proteasome in dependence of the AAA ATPase VCP34, 60. The synthesis of K63 ubiquitin chains triggers innate immunity signalling via activation of the NF-κB, IRF3/5/7 and AP-1 pathways, leading to an antiviral state characterised by the production of inflammatory cytokines35. In contrast to bacterial and viral infections, we show that TRIM21 impacts the formation of only K63 and not K48 ubiquitin chains at the vicinity of Toxoplasma PVs, concomitant with the activation of the innate immune signalling characterised by the production of inflammatory cytokines. TRIM21 comprises three N-terminal protein domains: a RING domain involved in protein-protein interactions that confers it with an E3 ligase activity, two B-box domains that are zinc-binding motifs and a helical coiled-coil domain important for protein-protein interactions61. It is tempting to speculate that the E3 ligase activity of TRIM21 directly mediates the ubiquitination at the vicinity of Toxoplasma. TRIM21 ubiquitination substrates are currently unknown and it will be important to define such substrates to assess whether the E3 ubiquitin ligase asserts its effect through host proteins or parasite proteins.
TRIM21 is additionally characterised by a C-terminal B30.2/PRYSPRY domain that was shown to strongly bind to the Fc part of antibodies54, 62, 63. Mallery et al. have disputed the dogma that antibodies do not have an intracellular function by reporting that TRIM21-mediated neutralisation of adenovirus is dependent on the presence of intracellular antibodies coating the invading particles34. In vivo, As far as Toxoplasma is concerned, the presence of antibodies inside the vacuole at the surface of the parasite following invasion remains unclear. It is generally accepted that a fraction of the Toxoplasma-derived surface-exposed proteins are shed or not carried into the cell due to sieving at the Moving Junction during parasite invasion and interestingly antibody-binding of surface AMA1 blocks parasite invasion64, 65. Nevertheless, immunoelectron microscopy of anti-P30 (the major Toxoplasma surface protein) coated parasites left to invade HeLa cells demonstrated that while most of the parasite surface-attached antibody was shed, some escape was noted and a minor fraction of the parasites were faintly labelled with anti-P30 antibody intracellularly66. In terms of viral neutralisation, it has been calculated that 1.6 antibodies per virus particle suffice for TRIM21 activity in the presence of interferon67. It is thus conceivable that very few residual antibodies bound to the parasite could trigger the activity of TRIM21. However, neither the molecular mechanism of antibody-triggered TRIM21 activity has been elucidated, nor do we know how much residual antibody of which specificity the parasite is able to carry into invaded cells in vivo. Recruitment of TRIM21 to the PV is most likely independent of antibody binding as the PV is disrupted at two hours post-infection, while we can localise TRIM21 at the PV already after one hour53. Thus, even though difficult to realise practically, it remains to be investigated if the subsequent effects exerted by TRIM21 during a Toxoplasma infection in vivo are dependent on intracellular antibody-binding.
The requirement of functional intracellular recognition systems -such as the Toll-like receptors (TLRs), inflammasome and autophagy- during the in vivo acute phase of Toxoplasma infection is well-established. Mice lacking TLR12, the TLR-induced adaptor proteins MyD88 or UNC93B1 and the inflammasome proteins NLRP3 or ASC all exhibit a high susceptibility to Toxoplasma infection during the early stage of the infection due to an impaired innate immune response68–71. Moreover, mice deficient in the autophagy proteins ATG5 or ATG16L1 as well as in the E3 ubiquitin ligase HOIL-1 fail to restrict Toxoplasma replication and succumb to acute infection28, 72, 73. Our findings show that TRIM21-deficient mice exhibit death at 7 days post-infection, placing TRIM21 into the category of these major intracellular acute phase mediators of Toxoplasma resistance. Contrary to the E3 ligase HOIL-1, TRIM21 mediates both immune activation and parasite restriction during an in vivo Toxoplasma infection28. We observe a higher parasite burden in the brain of TRIM21 knockout mice, indicating this E3 ligase is also crucial for Toxoplasma restriction. However, our results indicate TRIM21 also plays a role in the induction of proinflammatory cytokines concurrent with protection of infected mice. Thus, E3 ubiquitin ligases are a new category of acute phase resistance mediators during Toxoplasma infection, capable of both parasite restriction and innate immune induction. The ultimate death of TRIM21 knockout mice could be partly mediated by the portion of TRIM21 localised at the PV, but also by an unidentified pool of TRIM21 in the cytoplasm. We speculate that the TRIM21-induced inflammatory response observed both in vitro and in vivo is an important role of TRIM21 during Toxoplasma infection.
In summary, we have identified TRIM21 as a novel intracellular innate immune mediator for the eukaryotic pathogen Toxoplasma. This defines ubiquitination as a key process of innate immune recognition pathways for Toxoplasma. It additionally presents the vital E3 ubiquitin ligase driving not only parasite restriction, but also innate immune activation while localising to the vacuole of an intracellular protozoan. TRIM21 is thus a new key effector molecule in the IFNγ-induced resistance to Toxoplasma acting in concert with the GBP-mediated defence machinery. This complex mechanism between TRIM21 and GBP1 highlights a new host defence mechanism against this intracellular pathogen, where TRIM21 stimulates innate immune defence during Toxoplasma infection. The specific molecular mechanisms underlying the newly discovered Toxoplasma resistance factor TRIM21 in vivo remains to be deciphered.
Methods
Ethics statement
All procedures involving mice were approved by the local ethical committee of the Francis Crick Institute Ltd, Mill Hill Laboratory and are part of a project license approved by the Home Office, UK, under the Animals (Scientific Procedures) Act 1986. All experiments were performed in accordance with relevant guidelines and regulations.
Mice and infection
Wild-type (WT) and TRIM21 deficient40 mice on the C57BL/6 background were bred and intercrossed at the Francis Crick Institute Ltd, Mill Hill Laboratory under specific pathogen-free conditions. To minimise genetic divergence between transgenic mice and their wild-type controls, WT mice were derived from the TRIM21 heterozygous. Experiments were performed on 6- to 8-week-old females. For infections, Toxoplasma type II strain Pru expressing GFP and firefly luciferase6, 43, 74 were harvested from the peritoneum of a 4-day infected C57BL/6 mouse and passaged through a 26 gauge needle (BD). Parasites were washed twice with PBS and mice were injected intraperitoneally (i.p.) with 5 × 104 parasites suspended in PBS. Since TRIM21 has been shown to bind to Fc regions of antibodies, this was to make sure our in vivo infections were performed with Toxoplasma tachyzoites that had a natural coat of antibodies on them. For in vivo imaging, mice were injected i.p. with 3 mg firefly D-luciferin (Perkin Elmer), left for 10 min and imaged with an IVIS Spectrum-bioluminescent and fluorescent imaging system (Xenogen Corporation) under isoflurane anaesthesia (Abbott).
Cell culture and infection
Human foreskin fibroblasts (HFFs, ATCC), Raw 264.7 macrophages (ATCC) and primary mouse embryonic fibroblasts (MEFs, home-made) were cultivated in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) at 37 °C in the presence of 5% CO2. Experiments were performed at a multiplicity of infection (MOI) of 1–10 after induction with 100 U/mL murine recombinant IFNγ (R&D Systems) for 16 h.
Parasite culture
Unless stated otherwise, Toxoplasma gondii type I strain RH wild-type or RH expressing tdTomato, and Toxoplasma type II strain Prugniaud (Pru) wild-type or Pru expressing GFP and firefly luciferase were used (kind gifts from Marc-Jan Gubbels and Jeroen Saeij)43, 74. All strains of Toxoplasma gondii were maintained by serial passage on monolayers of HFFs as described previously75.
Antibodies
Primary antibodies used for immunofluorescence were: rabbit polyclonal anti-GBP117, 43, goat polyclonal anti-TRIM21 (#sc-21365, Santa-Cruz Biotechnology), mouse monoclonal anti-ubiquitin FK2 (#PW8810, Enzo Life Sciences), rabbit monoclonal anti-ubiquitin Lys48 (#05-1307, Merck Millipore), rabbit monoclonal anti-ubiquitin Lys63 (#05-1308, Merck Millipore) and anti-GAPDH (#2118 S, Cell Signaling). Secondary antibodies for immunofluorescence were from Molecular Probes: AlexaFluor®488 goat anti-rabbit (#A11034), AlexaFluor®488 donkey anti-goat (#A11055), AlexaFluor®594 goat anti-rabbit (#A11037) and AlexaFluor®594 donkey anti-goat (#A11058).
Immunofluorescence microscopy
Immunofluorescence microscopy was performed on 12 mm, #1.5 coverslips (Thermo Fisher) in 24-well plates (Nunc). Cells were stimulated with 100 U/mL recombinant murine IFNγ (R&D Systems) overnight and infected with Toxoplasma at an MOI of 5–10. After the indicated infection time, cells were washed 3 times with PBS and fixed in 3% paraformaldehyde (Sigma Aldrich) at RT for 20 min. Cells were permeabilised for 15 min at RT with 50 mM NH4Cl/0.2% saponin/PBS and kept at 4 °C for up to a week in 0.2% fish skin gelatin/0.02% saponin/0.02% NaN3/PBS (PGAS) until antibody staining. Briefly, coverslips were incubated with appropriate primary antibody in a humid chamber at RT for 1 h. Coverslips were then washes 3 times in PGAS and subsequently incubated with appropriate secondary antibody in a humid chamber at RT for 1 h in the dark and washed 3 times in PGAS solution followed by 3 washes in PBS. Nuclei were stained with 1 μg/mL Hoechst 33442 solution (Sigma-Aldrich). Coverslips were mounted on Superfrost® Plus glass slides (Thermo Scientific) with 50 µL Mowiol® 4–88 solution (Sigma-Aldrich) and kept in the dark at 4 °C. The frequency of Toxoplasma-positive vacuoles was determined by counting 100 PVs per replicate using an AxioPlan II fluorescent microscope (Carl Zeiss) equipped with DAPI, GFP and rhodamine filters. Images were captured with a Leica TCS-SP5 inverted confocal microscope (Leica Microsystems) fitted with conventional photomultiplier tubes and hybrid detectors and using a 100x Leica HCX PL APO CS (numerical aperture 1.4) oil immersion objective or with Nikon TI-E inverted epifluorescent microscope fitted with an orca flash 4 camera and a SpectraX light source. Images were processed in Fiji/ImageJ software (National Institute of Health).
Transmission electron microscopy
Ultrastructural analysis of IFNγ-stimulated, Toxoplasma-infected wild-type or TRIM21 knockout MEFs infected with type II Toxoplasma for 2 h. Cells were washed 3x in PBS before 0.05% trypsin-EDTA treatment. Samples were prepared according to details further described in Supplemental Information. Samples were observed with a JEOL 1200 EX transmission electron microscope (JEOL, Tokyo, Japan) equipped with an Orius 1000 CCD camera (Gatan, Pleasanton, CA, USA).
Replication assay
Wild-type or TRIM21 knockout MEFs were seeded on 12 mm, #1.5 coverslips in 24-well plates and induced with 100 U/mL IFNγ 16 h before infection. Cells were infected with type II Toxoplasma for 18 h at an MOI of 5 and subsequently fixed and permeabilised as described before. The number of parasites per vacuoles was determined by counting 50 to 100 PVs per technical replicate using a Nikon TI-E fluorescent microscope. Images were captured using a 60x Nikon oil immersion objective. Images were processed in Fiji/ImageJ software.
Relative infection assay
Wild-type or TRIM21 knockout MEFs unstimulated or stimulated with 100 U/mL IFNγ overnight were infected with Toxoplasma Pru-tdTomato at an MOI of 3 for 24 h. Cells were washed with PBS, 2 mM EDTA, 1%BSA and harvested for analysis by flow cytometry. Uninfected samples were used as a gating control and infected samples without IFNγ-treatment were used as a normalisation control to determine the relative infection (%) of IFNγ-treated samples.
Quantitative polymerase chain reaction
Wild-type or TRIM21 knockout MEFs were stimulated with 100 U/mL IFNγ overnight and infected with type II Toxoplasma for 1 h. Cellular RNA was extracted using the miRNeasy kit (Qiagen) and reverse transcribed into cDNA using the SuperScript® VILOTM cDNA Synthesis kit (Thermo Fischer Scientific). The quantitative PCR was performed using the TaqMan® Real-Time PCR Assay system (Applied Biosystems) with Gbp1 (Mm00657086_m1), Gbp2 (Mm00494576_g1) and Gapdh (Mm99999915_g1) primers.
Quantification of cytokines
For serum cytokines, blood was collected at day 3 and 7 post-infection from the saphenous vein or by terminal cardiac puncture, respectively. Serum was obtained after coagulation at RT for 30 min and centrifugation at 4 °C for 10 min at 1000 g. Serum cytokines and chemokines analysis was performed by mouse 32-plex Discovery Assay® (Eve Technologies, Calgary, Alberta, Canada). For cell supernatant cytokines, wild-type or TRIM21 knockout MEFs were stimulated with 100 U/mL IFNγ overnight and infected with type II Toxoplasma for 18–24 h. Supernatants were collected and analysed by enzyme-linked immunosorbent assay (ELISA) or mouse 32-plex Discovery Assay®. Commercially available kits were used according to the manufacturer’s instructions to quantify the concentration of CCL4 (Insight Bioscience), IL-1β (BD Biosciences), IL-6 (eBioscience), RANTES (eBioscience) and TNFα  .
.
Statistical analysis
All statistical significance analyses were performed using Prism software (GraphPad Software). Comparisons of data were performed with unpaired Student’s t-test or using two-way ANOVA with Sidak multiple comparison correction. Survival rates were compared by log-rank survival analysis of Kaplan-Meier curves.
Electronic supplementary material
Acknowledgements
We would like to thank Jörn Coers, Maximiliano Gutierrez, Jean Langhorne, Katrin Rittinger, Jeroen Saeij and the Frickel and Treeck labs for productive advice and discussion. We are grateful to Jörn Coers for the GBP2 antibody. We thank the Mill Hill microscopy facility, FACS facility and Biological Services for expert technical assistance. This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001076), the UK Medical Research Council (FC001076), and the Wellcome Trust (FC001076). Eva-Maria Frickel was supported by a Wellcome Trust Career Development Fellowship (091664/B/10/Z). Clémence Foltz was the recipient of a Boehringer Ingelheim Fonds PhD Fellowship and a Medical Research Council Studentship (MC_ST_U11033).
Author Contributions
Designed experiments: C.F. and E.M.F., conducted experiments: C.F., A.N., R.K., B.C., E.M.H. and E.M.F., wrote the manuscript: C.F., E.M.F., supervised the study: E.M.F.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05487-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunological Review. 2011;240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 3.Lingelbach K, Joiner KA. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell. Sci. 1998;111(Pt 11):1467–1475. doi: 10.1242/jcs.111.11.1467. [DOI] [PubMed] [Google Scholar]
- 4.Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. USA. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones TC, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J. Exp. Med. 1972;136:1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 8.Scharton-Kersten TM, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 9.Liehl P, Zuzarte-Luis V, Mota MM. Unveiling the pathogen behind the vacuole. Nature Reviews Microbiology. 2015 doi: 10.1038/nrmicro3504. [DOI] [PubMed] [Google Scholar]
- 10.Gilly M, Wall R. The IRG-47 gene is IFN-gamma induced in B cells and encodes a protein with GTP-binding motifs. J. Immunol. 1992;148:3275–3281. [PubMed] [Google Scholar]
- 11.Carlow DA, Marth J, Clark-Lewis I, Teh HS. Isolation of a gene encoding a developmentally regulated T cell-specific protein with a guanine nucleotide triphosphate-binding motif. J. Immunol. 1995;154:1724–1734. [PubMed] [Google Scholar]
- 12.Lafuse WP, Brown D, Castle L, Zwilling BS. Cloning and characterization of a novel cDNA that is IFN-gamma-induced in mouse peritoneal macrophages and encodes a putative GTP-binding protein. J. Leukoc. Biol. 1995;57:477–483. doi: 10.1002/jlb.57.3.477. [DOI] [PubMed] [Google Scholar]
- 13.Sorace JM, Johnson RJ, Howard DL, Drysdale BE. Identification of an endotoxin and IFN-inducible cDNA: possible identification of a novel protein family. J. Leukoc. Biol. 1995;58:477–484. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- 14.Cheng YS, Colonno RJ, Yin FH. Interferon induction of fibroblast proteins with guanylate binding activity. J. Biol. Chem. 1983;258:7746–7750. [PubMed] [Google Scholar]
- 15.Martens S, et al. Disruption of Toxoplasma gondii Parasitophorous Vacuoles by the Mouse p47-Resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling YM, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, et al. A cluster of interferon γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012;37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Pilla DM, et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl. Acad. Sci. USA. 2014 doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meunier E, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014 doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 20.Meunier E, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 2015;16:476–484. doi: 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston AC, et al. Human GBP1 does not localize to pathogen vacuoles but restricts Toxoplasma gondii. Cell. Microbiol. 2016;18:1056–1064. doi: 10.1111/cmi.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haldar AK, et al. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc. Natl. Acad. Sci. USA. 2015 doi: 10.1073/pnas.1515966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clough B, et al. K63-Linked Ubiquitination Targets Toxoplasma gondii for Endo-lysosomal Destruction in IFNγ-Stimulated Human Cells. PLoS Pathog. 2016;12:e1006027. doi: 10.1371/journal.ppat.1006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 25.Khaminets A, Behl C, Dikic I. Ubiquitin-Dependent And Independent Signals In Selective Autophagy. Trends Cell Biol. 2016;26:6–16. doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Huett A, et al. The LRR and RING Domain Protein LRSAM1Is an E3 Ligase Crucial for Ubiquitin-Dependent Autophagy of Intracellular Salmonella Typhimurium. Cell Host and Microbe. 2012;12:778–790. doi: 10.1016/j.chom.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirisako T, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDuff, D. A. et al. Phenotypic complementation of genetic immunodeficiency by chronic herpesvirus infection. eLife4 (2015). [DOI] [PMC free article] [PubMed]
- 29.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 30.Greene JC, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C-S, Yuk J-M, Lee Y-H, Jo E-K. Toxoplasma gondii GRA7-induced TRAF6 activation contributes to host protective immunity. Infect. Immun. 2015 doi: 10.1128/IAI.00734-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narendra D, Tanaka A, Suen D-F, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzanillo PS, et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–516. doi: 10.1038/nature12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallery DL, et al. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc. Natl. Acad. Sci. USA. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwan WA, et al. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 2013;14:327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakebrandt N, Lentes S, Neumann H, James LC, Neumann-Staubitz P. Antibody- and TRIM21-dependent intracellular restriction of Salmonella enterica. Pathogens Disease n/a–n/a. 2014 doi: 10.1111/2049-632X.12192. [DOI] [PubMed] [Google Scholar]
- 37.Watkinson RE, McEwan WA, Tam JCH, Vaysburd M, James LC. TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus. PLoS Pathog. 2015;11:e1005253. doi: 10.1371/journal.ppat.1005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaysburd M, et al. Intracellular antibody receptor TRIM21 prevents fatal viral infection. Proc. Natl. Acad. Sci. USA. 2013;110:12397–12401. doi: 10.1073/pnas.1301918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong HJ, et al. Cutting Edge: Autoantigen Ro52 Is an Interferon Inducible E3 Ligase That Ubiquitinates IRF-8 and Enhances Cytokine Expression in Macrophages. J. Immunol. 2007;179:26–30. doi: 10.4049/jimmunol.179.1.26. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimi R, et al. Gene Disruption Study Reveals a Nonredundant Role for TRIM21/Ro52 in NF- B-Dependent Cytokine Expression in Fibroblasts. J. Immunol. 2009;182:7527–7538. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butcher BA, et al. p47 GTPases Regulate Toxoplasma gondii Survival in Activated Macrophages. Infect. Immun. 2005;73:3278–3286. doi: 10.1128/IAI.73.6.3278-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, et al. Virulent Toxoplasma gondii Evade Immunity-Related GTPase-Mediated Parasite Vacuole Disruption within Primed Macrophages. J. Immunol. 2009;182:3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virreira Winter S, et al. Determinants of GBP Recruitment to Toxoplasma gondii Vacuoles and the Parasitic Factors That Control It. PLoS ONE. 2011;6:e24434. doi: 10.1371/journal.pone.0024434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selleck EM, et al. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 2013;9:e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chau V, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 46.Deng L, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/S0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 47.Fentress SJ, et al. Phosphorylation of Immunity-Related GTPases by a Toxoplasma gondii-Secreted Kinase Promotes Macrophage Survival and Virulence. Cell Host and Microbe. 2010;8:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinfeldt T, et al. Phosphorylation of Mouse Immunity-Related GTPase (IRG) Resistance Proteins Is an Evasion Strategy for Virulent Toxoplasma gondii. PLoS Biol. 2010;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saeij JPJ, et al. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2006;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong Y-C, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J. Biol. Chem. 2010;285:28731–28740. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finley D, et al. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol. Cell. Biol. 1994;14:5501–5509. doi: 10.1128/MCB.14.8.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/S0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 53.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii Parasitophorous Vacuole by IFNγ-Inducible Immunity-Related GTPases (IRG Proteins) Triggers Necrotic Cell Death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc. Natl. Acad. Sci. USA. 2008;105:6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melo MB, et al. Transcriptional analysis of murine macrophages infected with different toxoplasma strains identifies novel regulation of host signaling pathways. PLoS Pathog. 2013;9:e1003779. doi: 10.1371/journal.ppat.1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, et al. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat. Immunol. 2013;14:172–178. doi: 10.1038/ni.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manocha GD, et al. Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J Neuroinflammation. 2014;11:24. doi: 10.1186/1742-2094-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor S, et al. A Secreted Serine-Threonine Kinase Determines Virulence in the Eukaryotic Pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 59.Saeij JPJ, et al. Polymorphic Secreted Kinases Are Key Virulence Factors in Toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hauler F, Mallery DL, McEwan WA, Bidgood SR, James LC. AAA ATPase p97/VCP is essential for TRIM21-mediated virus neutralization. Proc. Natl. Acad. Sci. USA. 2012;109:19733–19738. doi: 10.1073/pnas.1210659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Versteeg GA, Benke S, García-Sastre A, Rajsbaum R. InTRIMsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014;25:563–576. doi: 10.1016/j.cytogfr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhodes DA, Trowsdale J. TRIM21 is a trimeric protein that binds IgG Fc via the B30.2 domain. Molecular Immunology. 2007;44:2406–2414. doi: 10.1016/j.molimm.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 63.James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc. Natl. Acad. Sci. USA. 2007;104:6200–6205. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, et al. Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol. Biochem. Parasitol. 2007;151:205–212. doi: 10.1016/j.molbiopara.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Besteiro S, Dubremetz J-F, Lebrun M. The moving junction of apicomplexan parasites: a key structure for invasion. Cell. Microbiol. 2011;13:797–805. doi: 10.1111/j.1462-5822.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- 66.Dubremetz JF, Rodriguez C, Ferreira E. Toxoplasma gondii: redistribution of monoclonal antibodies on tachyzoites during host cell invasion. Exp. Parasitol. 1985;59:24–32. doi: 10.1016/0014-4894(85)90053-0. [DOI] [PubMed] [Google Scholar]
- 67.McEwan WA, et al. Regulation of virus neutralization and the persistent fraction by TRIM21. J. Virol. 2012;86:8482–8491. doi: 10.1128/JVI.00728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koblansky AA, et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scanga CA, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 70.Melo MB, et al. UNC93B1 Mediates Host Resistance to Infection with Toxoplasma gondii. PLoS Pathog. 2010;6:e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorfu, G. et al. Dual Role for Inflammasome Sensors NLRP1 and NLRP3 in Murine Resistance to Toxoplasma gondii. MBio5, e01117–13–e01117–13 (2013). [DOI] [PMC free article] [PubMed]
- 72.Zhao Z, et al. Autophagosome-Independent Essential Function for the Autophagy Protein Atg5 in Cellular Immunity to Intracellular Pathogens. Cell Host and Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi J, et al. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity. 2014;40:924–935. doi: 10.1016/j.immuni.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S-K, Karasov A, Boothroyd JC. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect. Immun. 2007;75:1626–1634. doi: 10.1128/IAI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garnham PC, Baker JR, Bird RG. Fine structure of cystic form of Toxoplasma gondii. Br Med J. 1962;1:83–84. doi: 10.1136/bmj.1.5271.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


