Abstract
In the present study, we showed that in the retina of Drosophila, the expression of the ho gene, encoding haeme oxygenase (HO), is regulated by light but only at the beginning of the day. This timing must be set by the circadian clock as light pulses applied at other time points during the day do not increase the ho mRNA level. Moreover, light-induced activation of HO does not depend on the canonical phototransduction pathway but instead involves cryptochrome and is enhanced by ultraviolet (UV) light. Interestingly, the level of DNA damage in the retina after UV exposure was inversely related to the circadian oscillation of the ho mRNA level during the night, being the highest when the HO level was low and reversed during the day. Accordingly, induction of HO by hemin was associated with low DNA damage, while inhibition of HO activity by SnPPIX aggravated the damage. Our data suggest that HO acts in the retina to decrease oxidative DNA damage in photoreceptors caused by UV-rich light in the morning.
Introduction
Haeme oxygenase (HO) catalyses the degradation of haeme to carbon monoxide (CO), ferrous ions and biliverdin. In mammals, two HO proteins, inducible HO-1 and constitutive HO-2, are encoded by two different genes and have cytoprotective and anti-apoptotic functions by scavenging reactive oxygen species (ROS)1. Drosophila has only one gene encoding HO2 that plays an important role in development3 and in controlling the DNA damage signalling pathway4.
In a previous study, we found that ho in the fly’s retina is cyclically expressed with two peaks, 1 h after lights-on and 4 h after lights-off in a light/dark cycle5. This rhythm must be generated by a circadian clock because it is maintained in constant darkness (DD) and disrupted in the arrhythmic per 01 mutant5. However, HO also has an impact on the molecular mechanism of the clock5.
The molecular mechanism of the circadian clock is based on interlocked transcriptional-translational negative and positive feedback loops. Late in the evening, the CLOCK (CLK) protein, after reaching the appropriate level, forms dimers with CYCLE (CYC)6, and these heterodimers are transported into the nucleus where they bind to the promoter regulatory sequence E-box of per, tim and ccg (clock-controlled gene) genes, inducing their expression. At the end of the night, the amount of these gene products is sufficient to form PERIOD (PER) and TIMELESS (TIM) heterodimers7, and PER/TIM complexes are transported into the nucleus, where they bind to CLK/CYC and repress their activity, thereby inhibiting the transcription of their own genes per and tim 8. This is the main negative feedback loop of the molecular circadian clock. When per and tim transcription is inhibited, transcription of clk is activated, and the CLK protein is synthesized. Next, CLK and CYC form heterodimers involved in the second, positive feedback loop6. In the nucleus, these transcription factors bind to the E-box sequence of vrille (vri) and par domain protein 1 (pdp1) genes, activating their transcription9, 10. Translation and accumulation of the VRI protein in the cytoplasm occur immediately after transcription, and VRI enters the nucleus where it binds to the V/P-box sequence and represses the transcription of the clk gene. At the same time, pdp1 mRNA accumulates in the cytoplasm, and translation occurs. Late at night, PDP1 is transported into the nucleus, where it activates the transcription of the clk gene9, 10. However, the clk mRNA cycles and CLK protein level seems to remain constant, with changes in its phosphorylation state and stability11, 12. Moreover, the interactions of CLK-CYC heterodimers with E-box sequences of target genes are cyclical12. The circadian clock is synchronized to external conditions by light and other environmental cues called Zeitgebers. The circadian clock of D. melanogaster has its own photoreceptor CRYPTOCHROME (CRY)13. After absorption of blue-light photons, CRY changes its conformation and binds the TIM protein. Next, CRY/TIM dimers are ubiquitinated and degraded in proteasomes. When the TIM level is reduced, PER remains in a monomeric form that is unstable and rapidly degraded14.
In addition to CRY, the circadian clock receives photic information from the retina of the compound eye and extraretinal photoreceptors, the H-B eyelet15. The retina of the fruit fly is composed of 800 ommatidia, and each of them comprises 8 types of photoreceptors (R1-R8). Six of them, R1-R6, contain Rh1 opsin, which is sensitive to a broad spectrum of light with the maximum absorption in the spectrum corresponding to green light. These photoreceptors are involved in vision, including motion perception16. R7 contains two different types of opsin, Rh3 and Rh4, both of which are sensitive to UV radiation. The R8 photoreceptor has Rh5 opsin, with the maximum absorption corresponding to blue light, or Rh6, which is sensitive to green light17. R7-R8 photoreceptors are responsible for colour and polarized light detection. The eyelet contains rhodopsin Rh5 and Rh618.
The phototransduction pathway in photoreceptors is based on the visual pigment retinal. Light is absorbed by the retinal chromophore and converts rhodopsin to the active metarhodopsin state. This process catalyses the activation of a heterotrimeric G-protein (encoded by dgq and gbe genes). The GTP-GDP exchange releases the α subunit of the G protein, which activates phospholipase C (PLC), encoded by the norpA gene, generating inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) from the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2). Light-sensitive channels TRP and TRPL, encoded by the trp and trpl genes, respectively, are activated by an unknown mechanism. Activation of TRP causes Ca2+ influx and membrane depolarization. PLC is a key protein in the phototransduction cascade; however, the norpA mutant shows a weak response to light19, 20. This indicates that the classical rhodopsin-dependent phototransduction pathway is not the only one active in the retinal photoreceptors of D. melanogaster.
Knowing that the ho mRNA level is especially high in the morning, we hypothesized that it may also be dependent on direct light exposure and that the high level of HO in the morning may protect the retina against ROS and other toxic products of phototransduction. The retina is vulnerable to light damage21–23, and, in mammals, it has already been suggested that HO protects the retina against light-induced degenerative processes24, 25. In the morning, light is rich in blue and UV light, and UV light may induce cellular degeneration26.
In the present study, we tested different lengths and intensities of light pulses applied at different times of the day in constant darkness (DD) and different wavelengths of light on ho expression. Using several clock and phototransduction mutants, we also examined the mechanisms of HO activation by light. Finally, we examined whether HO protects photoreceptors against UV-dependent degeneration.
Results and Discussion
We found that ho expression (measured in isolated retinas using the qPCR SybrGreen RealTime technique) was highest not only in the morning in the light/dark cycle (LD 12:12, 12 h of light and 12 h of darkness) but also after a 1-h light pulse at CT1 (1 h after the beginning of the subjective day) in constant darkness (DD) (Fig. 1). Moreover, the ho mRNA level increased after a white light pulse lasting for at least 45 min of 120–450 lx intensity (Fig. 2) but especially after either blue or UV light (Fig. 3). Blue light was effective at a 25–60 lx intensity, but red, green or white light without UV did not change the level of ho mRNA (Fig. 3). A high irradiance of white light (1000–6000 lx) caused less induction of ho expression than 450 lx. This observation could be explained by the saturation of photoreceptors and degradation of TIM and CRY. TIM degradation (especially in sLNvs) has been shown to depend on the light intensity, and a 10-min light pulse at 7000 nw/cm2 causes stronger TIM degradation than a 600-nw/cm2 light pulse27. A reduction in the TIM level may affect the transcription of clock-controlled genes, i.e., ho when exposed to high light intensities. On the other hand, the saturation of photoreceptors leads to an enormous increase in the calcium concentration inside photoreceptors by opening Ca2+-permeable TRP channels. Ca2+ concentration is a regulator of cell response to light because it modulates phospholipase Cβ (PLC) activity. A Ca2+ concentration from 10 nM to 1 µM augments PLC activity, while a higher concentration (>10 µM) suppresses it28. Short-term light adaptation is based on the termination of the photoresponse by Ca2+-dependent inhibition of TRP channels29. In turn, prolonged high-intensity light exposure significantly elevates the Ca2+ level and, in effect, the saturation. During long-term light adaptation, signalling proteins, Arrestin 2 (Arr2), Gαq and TRPL, are translocated between the cell body and rhabdomere, potentially affecting the physiology of the retina. Long-term intensive light exposure can result in a diminished photoresponse and retinal cell death because of the formation of stable, cytotoxic Rh/Arr2 complexes. In addition, the termination of the response to light is modulated by calmodulin-binding transcription factor (dCAMTA) and its target, dFbx14, which regulate processes such as rhodopsin ubiquitination30. Thus, it is possible that gene expression in the light-saturated retina is similar to that observed in the retina exposed to low light intensities.
Figure 1.
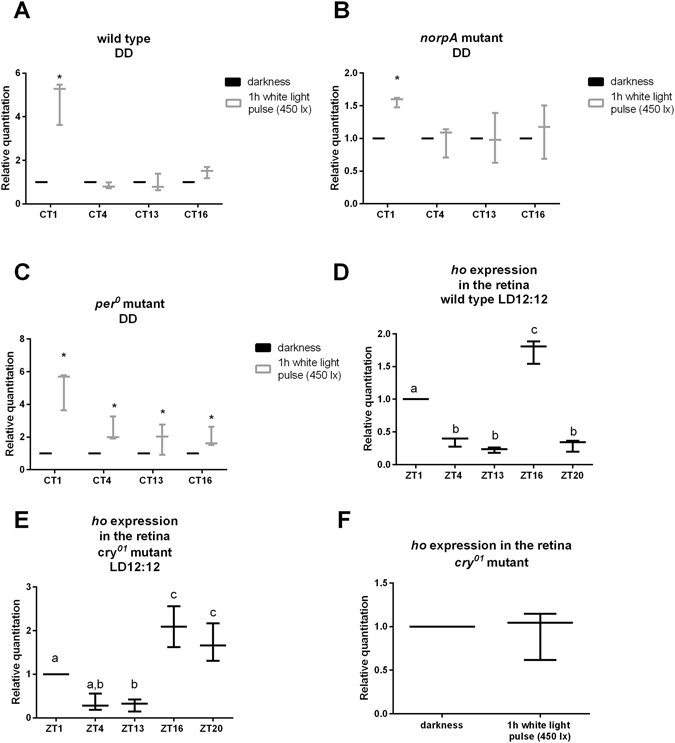
The ho gene mRNA level in the retina of wild type (A), norpA (B) and per 01 (C) mutants after 1 h light exposure in DD at CT1, CT4, CT13 or CT16. Normalization to DD (control) was done at every time point (value = 1.0, horizontal bars). Statistically significant differences are marked with asterisks. (D,E) The ho expression in the retina at different time points in light/dark LD12:12 in Canton S (D) and cry 01 (F). Data are normalized to ZT1. Statistically significant differences are marked with different letters. The same letter above the bars/plots means that there is no statistically significant differences between groups and different letters show significant differences. (F) The ho gene expression in cry 01 mutant 1 h after light pulse at CT0. Data are normalized to the control (DD). No statistically significant differences were found. The ends of the vertical bars for each time point represent the range of findings, with the more centrally located horizontal bars the mean.
Figure 2.

The ho gene mRNA level after different time (15 min, 30 min, 45 min, 60 min) of light exposure (A) and different light intensities (B) in the retina of wild type flies. Data are normalized to constant darkness (value = 1.0). Statistically significant differences are marked as different letters above bars. The ends of the vertical bars for each time point represent the range of findings, with the more centrally located horizontal bars the mean.
Figure 3.
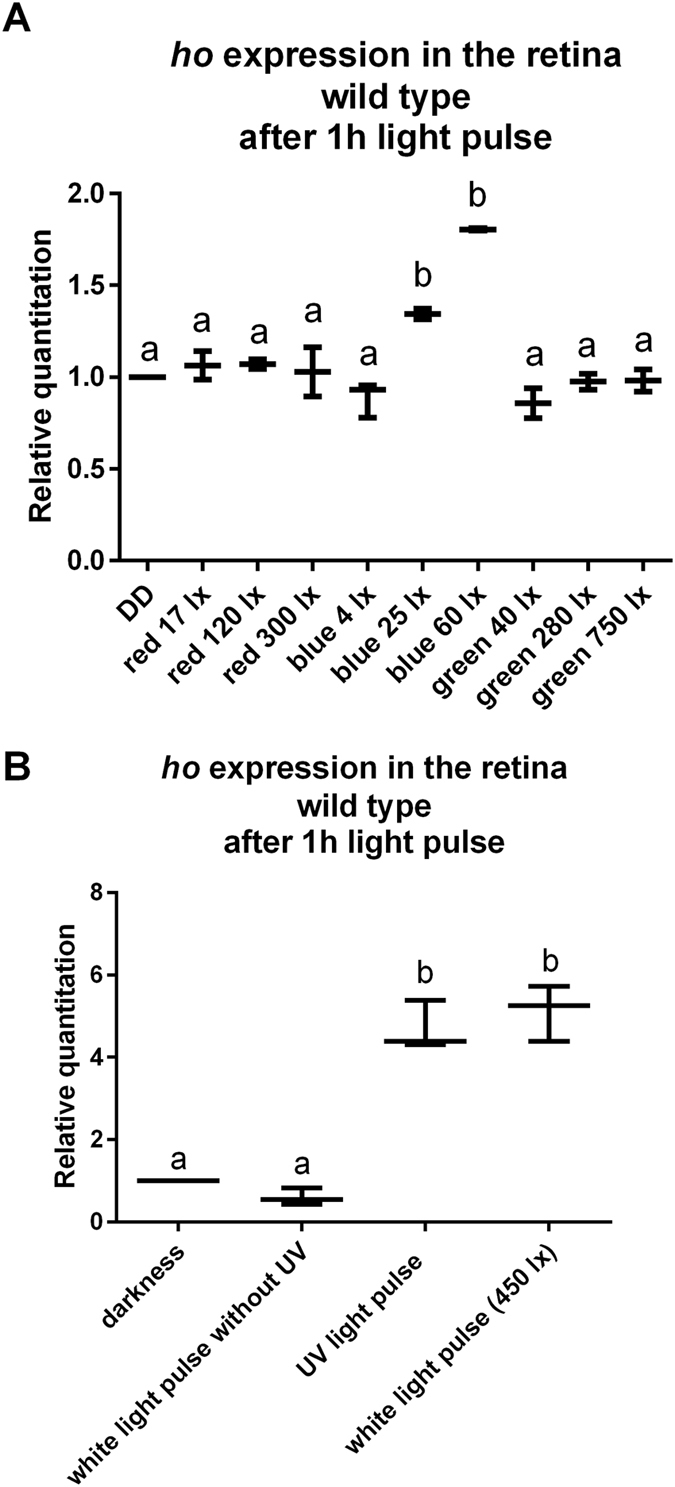
The ho gene mRNA level in the retina of Canton S flies after 1 h exposure to light with different wavelengths. (A) The following intensities were used for red light: 17, 120 and 300 lx, blue light: 4, 25 and 60 lx, green light: 40, 280 and 750 lx. (B) UV light and white light with filtered out UV exposures. Data are normalized to DD (value = 1.0). Statistically significant differences are marked as different letters above bars. The ends of the vertical bars for each time point represent the range of findings, with the more centrally located horizontal bars the mean.
The effect of light on ho expression at CT1 was observed in wild-type flies (Fig. 1A) and in the phototransduction mutant norpA that lacks PLC (Fig. 1B). In arrhythmic per 01 mutants, however, light activated ho expression at every time point studied in DD: CT1, CT4, CT13, and CT16 (CT0 and CT12 are the beginning of the subjective day and the beginning of the subjective night, respectively) (Fig. 1C). In per 01 mutants, ho expression at CT1 was still slightly higher than at other time points as in Canton S, but the differences between CT1 and the other time points were not statistically significant. This indicates that the mechanism that regulates ho expression is light-dependent and clock controlled but does not involve PLC, a protein of the main phototransduction pathway. Moreover, the clock blue-light photoreceptor cryptochrome (CRY)31, 32 is involved, as in cry 01 mutants, the pattern of ho expression oscillation was changed, and instead of two peaks, as observed in LD12:12 at ZT1 and ZT16 (Fig. 1D), the mRNA of ho was high at ZT16, ZT20 and ZT1. In addition, an increase in the ho mRNA level after light exposure was not observed in cry 01 mutants (Fig. 1F). This result suggests that CRY is an important element of the activation of ho expression in the morning. In cry 01 mutants, the impact of the clock on ho expression is maintained; however, ho expression is not induced by light in the morning, and its pattern is changed. The effect of light on ho expression is decreased because of the lack of CRY. As mentioned above, long-term light adaptation is caused by trafficking of the phototransduction proteins between the rhabdomere and the cell body. These proteins, RDGA, NINAC, and INAD, interact with CRY, and the lack of CRY in cry 01 mutants may change their location, function and/or modulation of the TRP channels33.
After determining that only blue light and UV light increase ho mRNA level (Fig. 3), we suggested that blue light activates CRY directly in the retina and that CRY then regulates ho expression. A similar effect of UV light on HO-1 was also observed in mammals34. In addition, we found that two rhodopsins, Rh1 (sensitive to a broad spectrum of light) and Rh3 (UV-sensitive), which are expressed in R1-R6 and R7 photoreceptors, respectively35, contribute to this mechanism (Fig. 4B,C).
Figure 4.
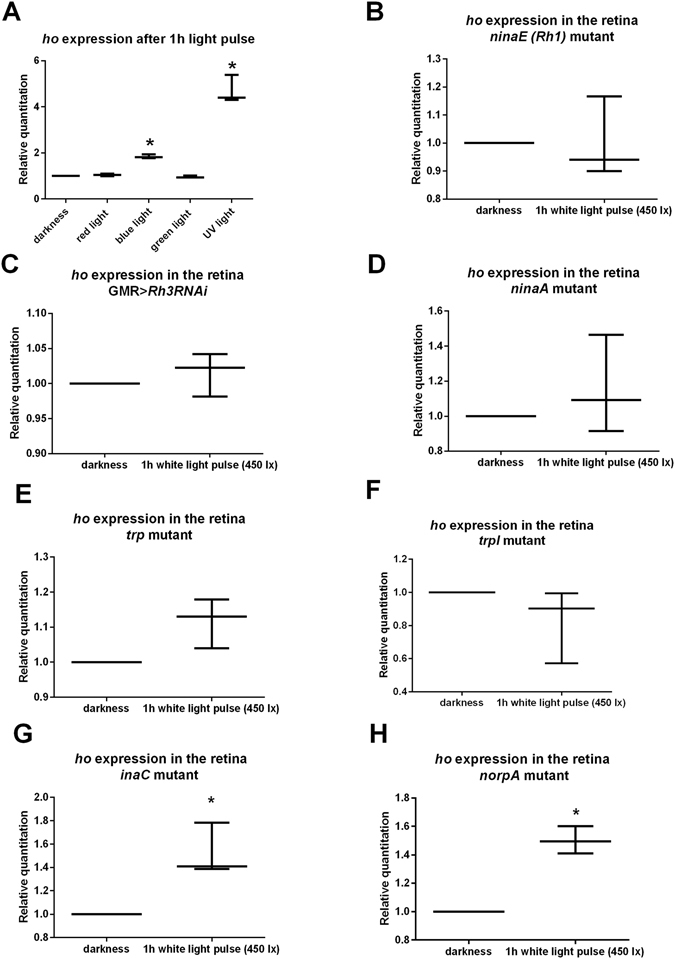
(A) The ho mRNA level in the retina after 1 h exposure to different wavelengths of light. (B–H) The ho mRNA level in the retina after 1 h of white light exposure. Data are normalized to DD (value = 1.0). Statistically significant differences are marked with asterisks. (B) GMR > Rh3RNAi, (C) ninaE, (D) ninaA, (E) trp, (F) trpl, (G) inaC, (H) norpA. The ends of the vertical bars for each time point represent the range of findings, with the more centrally located horizontal bars the mean.
To investigate the phototransduction pathway involved in the regulation of ho expression in addition to CRY, we examined which proteins downstream of rhodopsins are involved in the activation of ho expression. Using phototransduction mutants, we found that the chaperone protein for nascent opsin NINAA, the Drosophila opsin Rh1 protein NINAE, and the transient receptor cationic TRP and TRPL36 channels are important in this process (Fig. 4C–F). However, norpA and inaC, mutants of the main phototransduction pathway proteins PLCβ and protein kinase C (PKC), respectively, do not affect ho expression (Fig. 4G,H). As blue Rh5- and green Rh6-sensitive rhodopsins of R8 photoreceptors, which are involved in colour vision, contribute to phototransduction in the absence of PLC, by using nonretinal PLC or alternative signalling mechanisms37, a similar regulation of ho expression by light is possible. One non-canonical phototransduction pathway is the rhodopsin/Rac2 pathway38; however, ho activation by this pathway has not been studied. Because light-induction of ho expression involves a non-canonical phototransduction pathway, the mechanism must converge on a common input pathway that requires Rh1, Rh3 and CRY.
Because UV and blue light can trigger reactive oxygen species (ROS)39, we examined DNA strand breaks in the retina photoreceptors of flies exposed to UV (1 h, 100 lx) and to high-intensity white light (3 h, 1500 lx). Our preliminary results suggested a protective role of HO in the retina against ROS5. Before the UV exposure, flies were fed a HO activator (hemin), inhibitor (SnPPIX), or glucose only (control 1). In hemin-treated flies, UV caused less DNA damage in the retina than in UV-exposed control flies and no more than in control (control 2) flies not treated with UV (Fig. 5A). Moreover, increased DNA damage was detected in the retina of flies treated with the HO inhibitor than in both controls and in flies treated with hemin. We also compared the DNA damage intensity at different time points and found that there were more DNA strand breaks in the retina during the night, when the HO level was low, than during the day. The most DNA breaks were observed at the beginning of the night (low level of HO) and the fewest at the beginning of the day (peak of HO) (Fig. 5B). Although ho mRNA levels peak in the middle of night in addition to the beginning of the day, this night peak seems to have a different function than the peak at the beginning of the day, or ho mRNA and HO levels increase later at night to prepare the eyes for the day. The high level of HO may also be linked to the higher damage. Degradation of haeme leads to the release of iron, which if not properly sequestered (in ferritin), can react with ROS, leading to further damage40.
Figure 5.
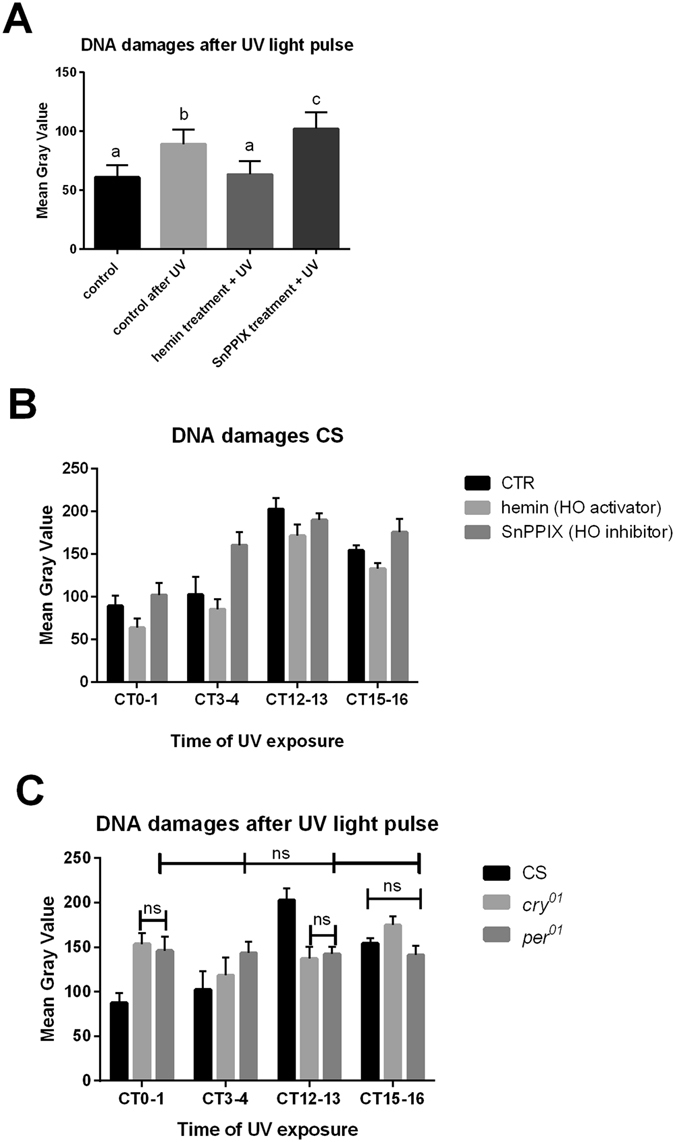
Oxidative DNA damages after UV and intense white light exposure. DNA damages were evaluated as the intensity of labeling with anti-8-hydroxyguanosine, followed by DAB/HRP enzymatic reaction and measured as Mean Gray Value using ImageJ software. (A) Before light exposure wild-type flies were fed a HO inhibitor (SnPPIX), HO activator (hemin) or glucose only (control 1). Flies were exposed to 1 h UV light pulse starting at CT0. One group of flies without UV treatment was used as an additional control (control 2). Statistically significant differences are shown as different letters. (B) Canton S (CS) flies were fed with hemin, SnPPIX or glucose (CTR) and exposed to UV light at different time points. There are statistically significant differences between CTR and experimental flies at every time point as well as between different time points in all groups of flies. (C) Canton S (CS), cry 01 and per 01 flies were exposed for UV light at different time points. There are statistically significant differences between CS at every time point: CT0-1 vs. CT3-4 with p < 0.01, other time points with p < 0.0001. There are statistically significant differences in cry 01 at every time point: CT0-1 vs. CT12-13 with p < 0.01, other time points with p < 0.0001. There are no statistically significant differences in case of per 01 at different time points (labeled as ns). At specific time points there are differences between: CS vs. cry 01 at CT0-1 (p < 0.0001), CS vs. per 01 at CT0-1 (p < 0.0001), CS vs. cry 01 at CT3-4 (p < 0.05), CS vs. per 01 at CT3-4 (p < 0.0001), cry 01 vs. per 01 at CT3-4 (p < 0.0001), CS vs. cry 01 at CT12-13 (p < 0.0001), CS vs. per 01(p < 0.0001) at CT12-13, CS vs. cry 01 at CT15-16 (p < 0.001), cry 01 vs. per 01 at CT15-16 (p < 0.0001).
Feeding flies with hemin and SnPPIX, which were used to activate and inhibit the activity of HO, respectively, caused similar effects at every time point studied (Fig. 5B).
cry 01 mutants had more DNA breaks in the retina when they were exposed to UV during the night and at the beginning of the day, and the lowest amount of DNA damage was detected during the day. There were statistically significant differences between each time point studied (Fig. 5C), suggesting that in the cry mutant, the rhythmic expression pattern of HO was changed, and photoreceptors were not protected against UV light at the beginning of the day. In addition, we observed more damage in cry 01 mutants than in wild-type flies, except at the beginning of the subjective night (CT12-13). High mortality, up to 90%, was observed in per 01 mutants after UV exposure. The few individuals that survived the experiment had more damage in the photoreceptors after UV exposure during the subjective day, less at CT13, and the same amount as control flies at CT16. The level of DNA breaks in per 01 mutants was similar at every time point studied (no statistically significant differences between time points), indicating that arrhythmic flies are not protected against damaging UV light exposure.
In conclusion, our results showed that the retina of Drosophila is protected against blue light and UV-rich sunlight in the morning by HO. A low level of HO in the morning, as a result of the disruption of the circadian clock, leads to an accumulation of DNA oxidative damage in the retina.
Methods
Animals
The following strains of Drosophila melanogaster were used: wild-type Canton S, per 01 (null mutant of the period gene, the main clock gene), cry 01 (null mutant of the cryptochrome gene, the circadian photoreceptor), trp (mutant of the transient receptor potential gene coding calcium channel), trpl (mutant of the transient receptor potential-like gene coding calcium channel), ninaA 1 (mutant of the neither inactivation nor afterpotential A gene coding eye-specific cyclophilin, required for Rh1 biogenesis), ninaE 17 (mutant of the neither inactivation nor afterpotential E gene coding rhodopsin 1), norpA 7 (mutant of the no receptor potential A gene coding phospholipase C), inaC (mutant of the inactivation no afterpotential C gene coding eye-specific protein kinase C), GMR-Gal4 (express Gal4 factor predominantly in photoreceptors), UAS-Rh3RNAi (express dsRNA for the Rh3 gene under the control of a UAS sequence). Flies were maintained under conditions of 12 h of light and 12 h of darkness (LD12:12) or in constant darkness (DD) for 5 days and at a constant temperature of 24 °C.
Experimental procedures
ho mRNA level after light pulses during the subjective day in constant darkness (DD)
Flies were exposed to white light (450 lx) pulses of different durations: 15, 30, 45 or 60 min (Fig. 2A) and to 1-h pulses of different intensities: 6, 60, 120, 170, 300, 450, 1000, 1500, 4000, or 6000 lx (Fig. 2B). Data were normalized to the control (flies kept in darkness, value = 1). In both experiments, 30 wild-type Canton S individuals per group were used, and each experiment was repeated 3 times. For statistical analysis, we used one-way ANOVA, non-parametric Kruskal-Wallis test with multiple comparisons (comparison of the mean rank of each experimental group with that of the control group kept in darkness). Differences were considered statistically significant at p < 0.05.
ho mRNA level after light pulses applied at different times in DD
The effect of light on ho expression at different times of the day in DD was tested in wild-type flies and norpA 7 and per 01 mutants (Fig. 1A–C). One hour before CT1, CT4, CT13 or CT16 (with CT0 being the beginning of the subjective day and CT12 being the beginning of the subjective night), flies were exposed to a white light pulse (1 h, 450 lx). The control group was kept in darkness until the isolation of the retina. At each time point, 30 males were used. Data were normalized to the control kept in DD (value = 1). For statistical analysis, a two-way ANOVA nonparametric test was used, and differences were considered statistically significant at p < 0.05.
Cyclic expression of ho in the retina of wild-type and cry mutant flies in LD12:12. The ho mRNA level was examined in the retina of flies held in the light/dark regime LD12:12 (12 h of light and 12 h of darkness) and fixed at the following time points: ZT1 (Zeitgeber Time), ZT4, ZT13, ZT16 and ZT20 (with ZT0 being the beginning of the light/day phase and ZT12 being the beginning of the night/dark phase). Thirty wild-type Canton S and cry 01 flies per time point were used (Fig. 1D,E). Data were normalized to ZT1 (value = 1). The experiment was repeated 3 times. For statistical analysis, a one-way ANOVA, non-parametric Kruskal-Wallis test with multiple comparisons was used. Differences were considered statistically significant at p < 0.05.
ho mRNA level in the retina of the phototransduction mutants after light pulse in DD
The following strains, cry 01, ninaE 17, ninaA 1, trp, trpl, inaC, norpA 7 and GMR > Rh3RNAi (Figs 1F and 4B–H), were exposed to a 1-h white light pulse (450 lx), and their retina was isolated at CT1. The control group was kept in darkness. Data were normalized to control (value = 1). Thirty individuals per group were examined, and the experiment was repeated 3 times. For statistical analysis, a non-parametric Mann-Whitney test was used to compare two groups. Changes were considered statistically significant at p < 0.05.
ho mRNA level after light pulses of different wavelengths in DD
Flies were exposed to a 1-h red (17, 120, 300 lx), blue (4, 25, 60 lx), green (40, 280, 750 lx) (Fig. 3A), or UV light pulse or to a 1-h white light pulse without UV light (Fig. 3B), whereas the control group was kept in darkness. Heads were fixed at CT1. Data were normalized to the control (value = 1). Thirty individuals per group used, and each experiment was repeated 3 times. For statistical analysis, a one-way ANOVA, non-parametric Kruskal-Wallis test with multiple comparisons was used. Differences were considered statistically significant at p < 0.05.
RNA isolation and qPCR
Males, 7 days old, were decapitated at a particular time point. Heads were fixed in 100% ethanol for 2 h, and retinas were isolated. Total RNA was isolated using TriReagent (MRC Inc.) according to the manufacturer’s protocol. The cDNA for the PCR amplification was prepared from 1 μg total RNA using Superscript II reverse transcriptase (Life Technologies) according to the manufacturer’s protocol. cDNA, diluted 1:10, was used for quantitative PCR. Each experiment was repeated at least three times. The expression of the ho gene was examined using SYBR Green Master Mix (Applied Biosystem) and a 7500 Fast Real-Time PCR System (Applied Biosystems). The following primers were used: ho, forward primer: 5′ACCATTTGCCCGCCGGGATG; reverse primer: 5′ AGTGCGACGGCCAGCTTCCT; rpl32, forward primer: 5′ AGAAGCGCAAGGAGATTGTC; reverse primer: 5′ ATGGTGCTGCTATCCCAATC. Product specificity was assessed by melting curve analysis, and selected samples were run on 1% agarose gels for size assessment.
Data were collected as raw CT values and analysed using the 2−ΔΔCT method. Gene expression was normalized on an arbitrary scale with control (as 1.0).
Enzymatic staining
Flies were kept for 5 days in DD and starved for 6 h with water available ad libitum. Then, they were fed for 6 h (until decapitation) with 6% glucose in water supplemented with the HO activator, 100 μM hemin chloride (Calbiochem), or with the HO inhibitor, 100 μM tin protoporphyrin IX (SnPPIX, Frontier Scientific). The control group was fed with glucose only. All groups were next exposed to 1 h of UV light at CT0 and then to 3 h of intensive white light. After light exposure, they were decapitated and fixed in 4% paraformaldehyde. Cryosections were prepared, and immunodetection with the mouse anti-8-hydroxyguanosine primary antibody (1:500, overnight) (Acris, Cat No AM03160), which labels oxidative DNA damage, was carried out. On the next day, an HRP/DAB (ABC) detection kit was used according to the manufacturer’s protocol (Abcam, Cat No 64264). For CS control, two different groups were used: 1) those fed with glucose and exposed to UV light and intensive white light and 2) those kept in DD (Fig. 5A). The same experimental conditions (5 days in DD, feeding) were used for CS flies exposed to 1 h of UV treatment and then 3 h of white light starting at CT0, CT3, CT12 or CT15 (Fig. 5B). Clock mutants per 01 and cry 01 were exposed to UV and white light at different time points as described previously; they were not fed with the HO activator or inhibitor (Fig. 5C). DNA damage was measured as the mean grey values using ImageJ software.
Statistical analysis
Statistical analysis was performed using two-way ANOVA, one-way ANOVA Kruskal-Wallis test or Mann-Whitney test. The GraphPad Outlier Calculator was used to eliminate outliers. GraphPad Prism 6 software was used for analysis. Differences were considered statistically significant at p < 0.05.
Acknowledgements
We thank Dr. David Dolezel, Biology Centre, CAS, Czech Republic for providing the equipment to expose flies to different light wavelengths. The study was supported by the Polish National Science Centre (NCN) grant No. UMO-2012/07/B/NZ3/02908 to EP and statutory funds for the Department of Medical Biotechnology. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union and the Polish Ministry of Science and Higher Education (grants No.: POIG.02.01.00-12-064/08 and 02.02.00-00-014/08) and is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education.
Author Contributions
M.D. carried out experiments and prepared figures. A.L., A.J., J.D. provided chemicals. E.P. wrote the main manuscript text. M.D., A.L., A.J., J.D. and E.P. discussed results. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Loboda A, et al. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Sato M, Sasahara M, Migita CT, Yoshida T. Unique features of recombinant heme oxygenase of Drosophila melanogaster compared with those of other heme oxygenases studied. Eur. J. Biochem. 2004;271:1713–1724. doi: 10.1111/j.1432-1033.2004.04077.x. [DOI] [PubMed] [Google Scholar]
- 3.Cui L, et al. Relevant expression of Drosophila heme oxygenase is necessary for the normal development of insect tissues. Biochem. Biophys. Res. Commun. 2008;377:1156–1161. doi: 10.1016/j.bbrc.2008.10.133. [DOI] [PubMed] [Google Scholar]
- 4.Ida H, et al. Genetic link between heme oxygenase and the signaling pathway of DNA damage in Drosophila melanogaster. Tohoku J. Exp. Med. 2013;231:117–125. doi: 10.1620/tjem.231.117. [DOI] [PubMed] [Google Scholar]
- 5.Damulewicz, M., Loboda, A., Jozkowicz, A., Dulak, J. & Pyza, E. Interactions between the circadian clock and heme oxygenase in the retina of Drosophila melanogaster. Molecular Neurobiology 1–10, doi:10.1007/s12035-016-0026-9 (2016). [DOI] [PMC free article] [PubMed]
- 6.Rutila J, Suri V, Le M, So W. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/S0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 7.Zerr DM, Hall JC, Rosbash M, Siwicki KK. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J. Neurosci. 1990;10:2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C, Bae K, Edery I. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: a basis for circadian transcription. Mol. Cell. Biol. 1999;19:5316–25. doi: 10.1128/MCB.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyran SA, et al. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/S0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 10.Glossop NRJ, et al. vrille feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron. 2003;37:249–261. doi: 10.1016/S0896-6273(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim EY, et al. Drosophila clock protein is under posttranscriptional control and influences light-induced activity. Neuron. 2002;34:69–81. doi: 10.1016/S0896-6273(02)00639-6. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Zheng H, Houl JH, Dauwalder B, Hardin PE. PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev. 2006;20:723–733. doi: 10.1101/gad.1404406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C, Todo T. The cryptochromes. Genome Biol. 2005;6:220. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 15.Veleri S, Rieger D, Helfrich-Förster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J. Biol. Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- 16.Morante J, Desplan C. Building a projection map for photoreceptor neurons in the Drosophila optic lobes. Semin. Cell Dev. Biol. 2004;15:137–143. doi: 10.1016/j.semcdb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Salcedo E, et al. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J. Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 2002;129:1443–1453. doi: 10.1242/dev.129.6.1443. [DOI] [PubMed] [Google Scholar]
- 19.Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J. Biol. Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- 20.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 21.Smith DP, et al. Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science. 1991;254:1478–1484. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- 22.Harris Wa, Stark WS. Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J. Gen. Physiol. 1977;69:261–91. doi: 10.1085/jgp.69.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iakhine R, et al. Novel dominant rhodopsin mutation triggers two mechanisms of retinal degeneration and photoreceptor desensitization. J. Neurosci. 2004;24:2516–26. doi: 10.1523/JNEUROSCI.5426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutty RK, et al. Induction of heme oxygenase 1 in the retina by intense visible light: suppression by the antioxidant dimethylthiourea. Proc. Natl. Acad. Sci. USA. 1995;92:1177–1181. doi: 10.1073/pnas.92.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun MH, et al. Photoreceptor protection against light damage by AAV-mediated overexpression of heme oxygenase-1. Investig. Ophthalmol. Vis. Sci. 2007;48:5699–5707. doi: 10.1167/iovs.07-0340. [DOI] [PubMed] [Google Scholar]
- 26.Luo X, Puig O, Hyun J, Bohmann D, Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 2007;26:380–90. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinayak, P. et al. Exquisite light sensitivity of Drosophila melanogaster cryptochrome. PLoS Genet. 9 (2013). [DOI] [PMC free article] [PubMed]
- 28.Deer JLR, Hurley JB, Yarfitz SL. G protein control of Drosophila photoreceptor phospholipase C. J. Biol. Chem. 1995;270:12623–12628. doi: 10.1074/jbc.270.21.12623. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Oberwinkler J, Postma M, Hardie RC. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 2005;15:1228–1234. doi: 10.1016/j.cub.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 30.Han J, et al. The Fly CAMTA Transcription factor potentiates deactivation of rhodopsin, a G protein-coupled light receptor. Cell. 2006;127:847–858. doi: 10.1016/j.cell.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 31.Stanewsky R, et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/S0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 32.Emery P, et al. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/S0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 33.Mazzotta G, et al. Fly cryptochrome and the visual system. Proc. Natl. Acad. Sci. USA. 2013;110:6163–8. doi: 10.1073/pnas.1212317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyrrell RM. Solar ultraviolet A radiation: an oxidizing skin carcinogen that activates heme oxygenase-1. Antioxid Redox Signal. 2004;6:835–840. doi: 10.1089/ars.2004.6.835. [DOI] [PubMed] [Google Scholar]
- 35.Montell C, Jones K, Zuker C, Rubin G. A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. J. Neurosci. 1987;7:1558–1566. doi: 10.1523/JNEUROSCI.07-05-01558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung HT, Geng C, Pak WL. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J. Neurosci. 2000;20:6797–6803. doi: 10.1523/JNEUROSCI.20-18-06797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szular J, et al. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-beta signaling. J. Biol. Rhythms. 2012;27:25–36. doi: 10.1177/0748730411431673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elsaesser R, Kalra D, Li R, Montell C. Light-induced translocation of Drosophila visual Arrestin2 depends on Rac2. Proc. Natl. Acad. Sci. 2010;107:4740–4745. doi: 10.1073/pnas.0906386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M-J, Johnson WA. ROS-mediated activation of Drosophila larval nociceptor neurons by UVC irradiation. BMC Neurosci. 2014;15:14. doi: 10.1186/1471-2202-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suttner DM, Dennery Pa. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]


