Abstract
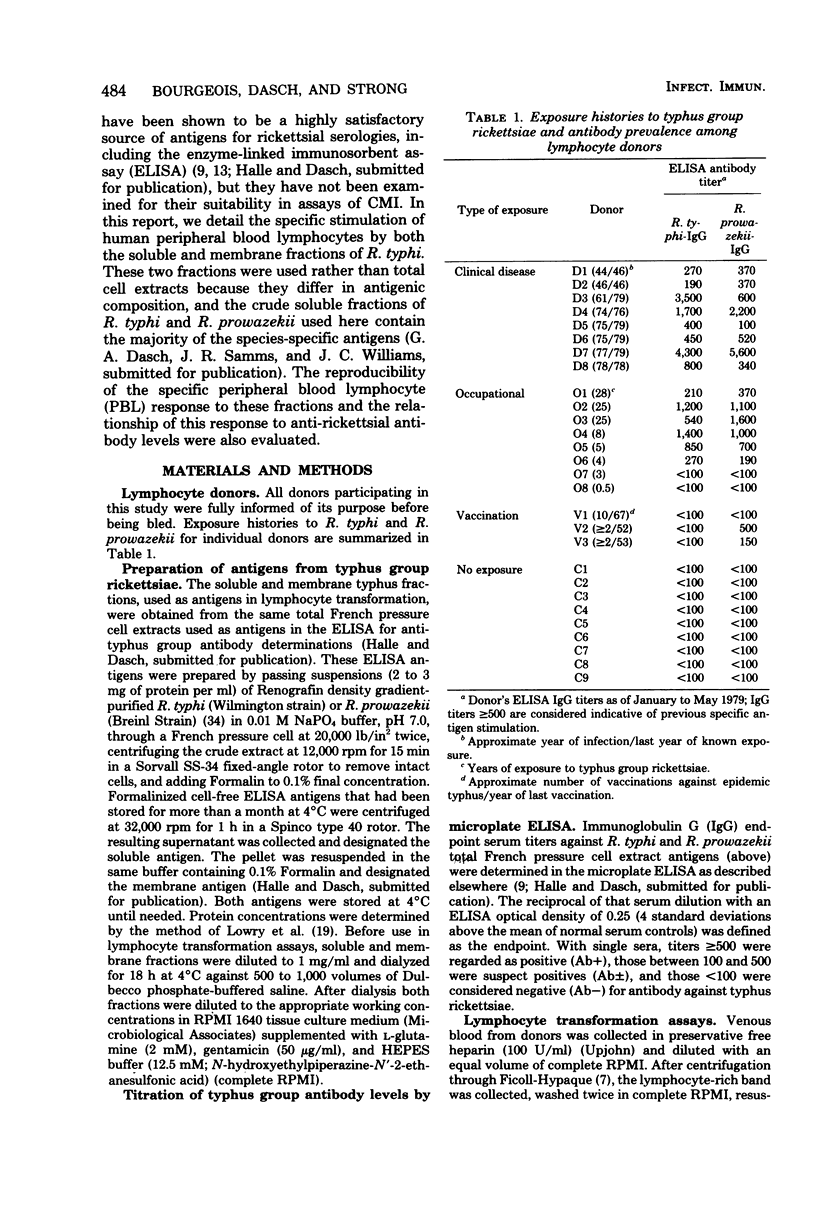
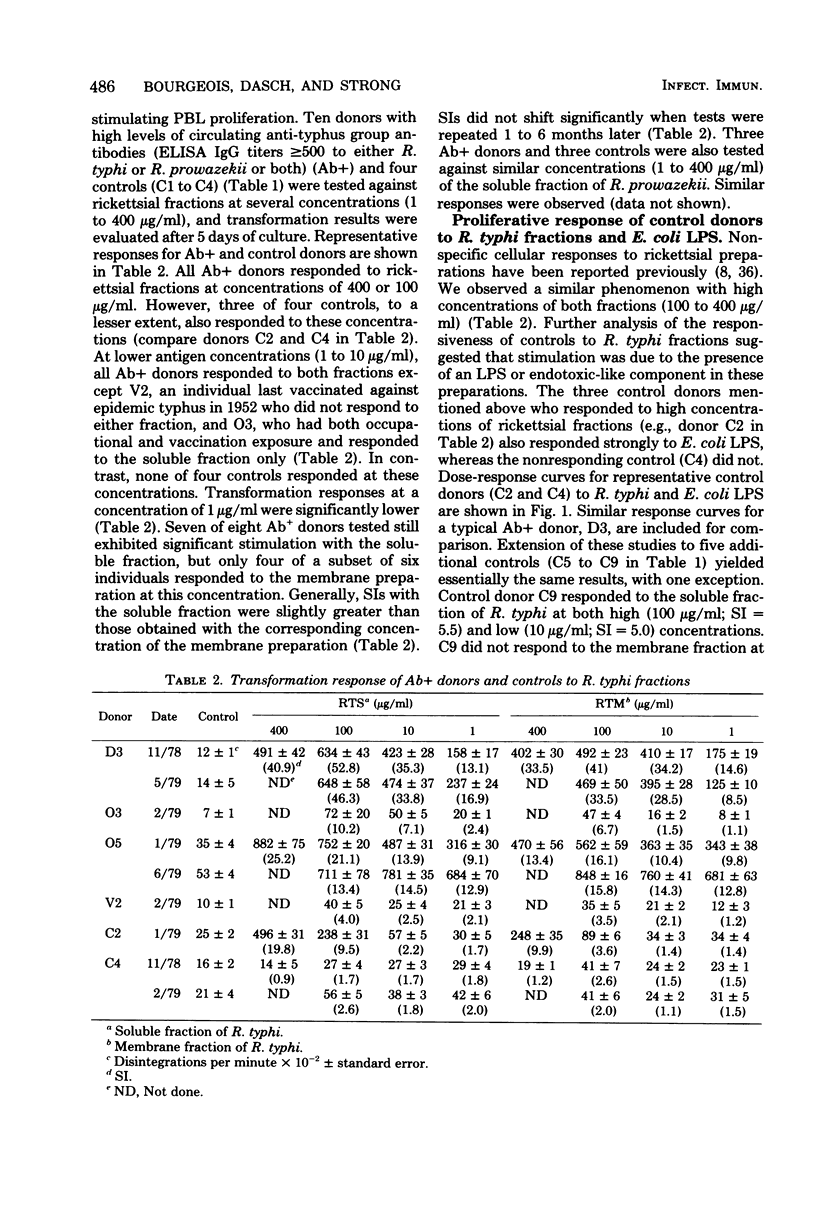
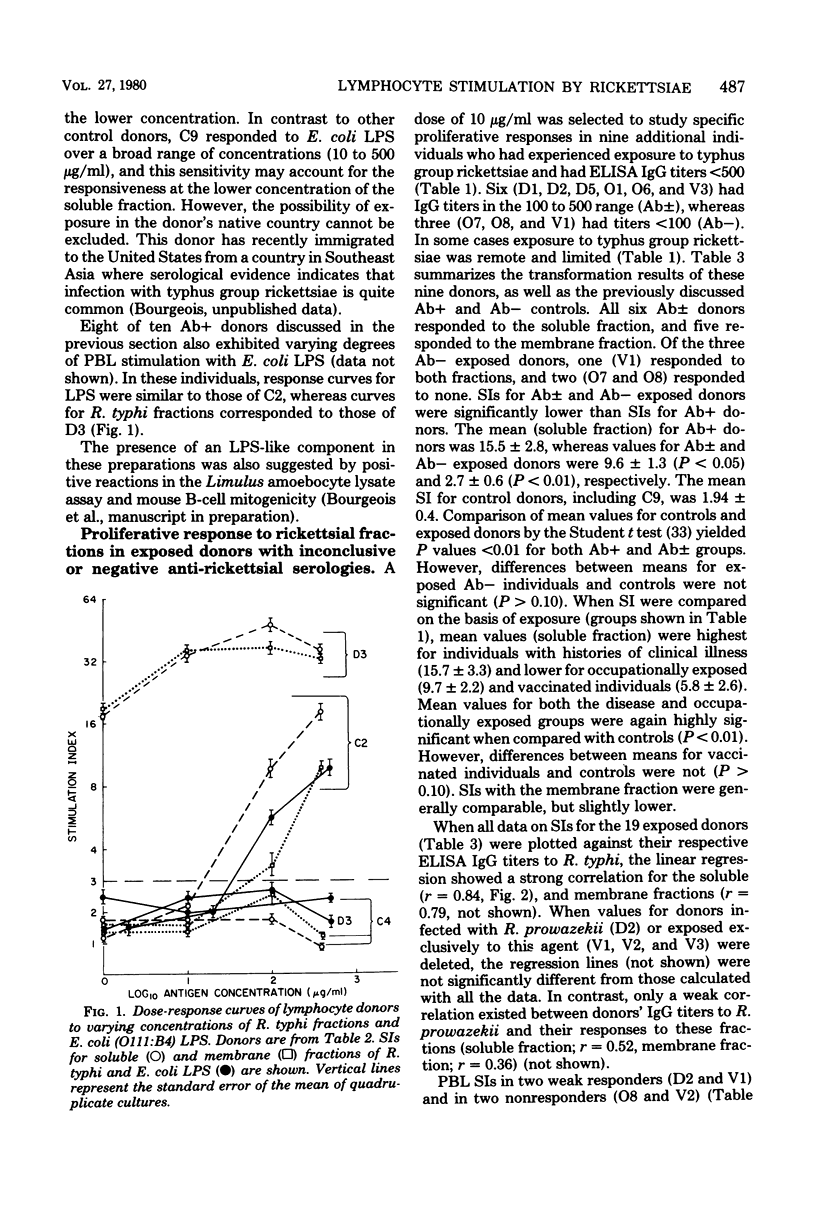
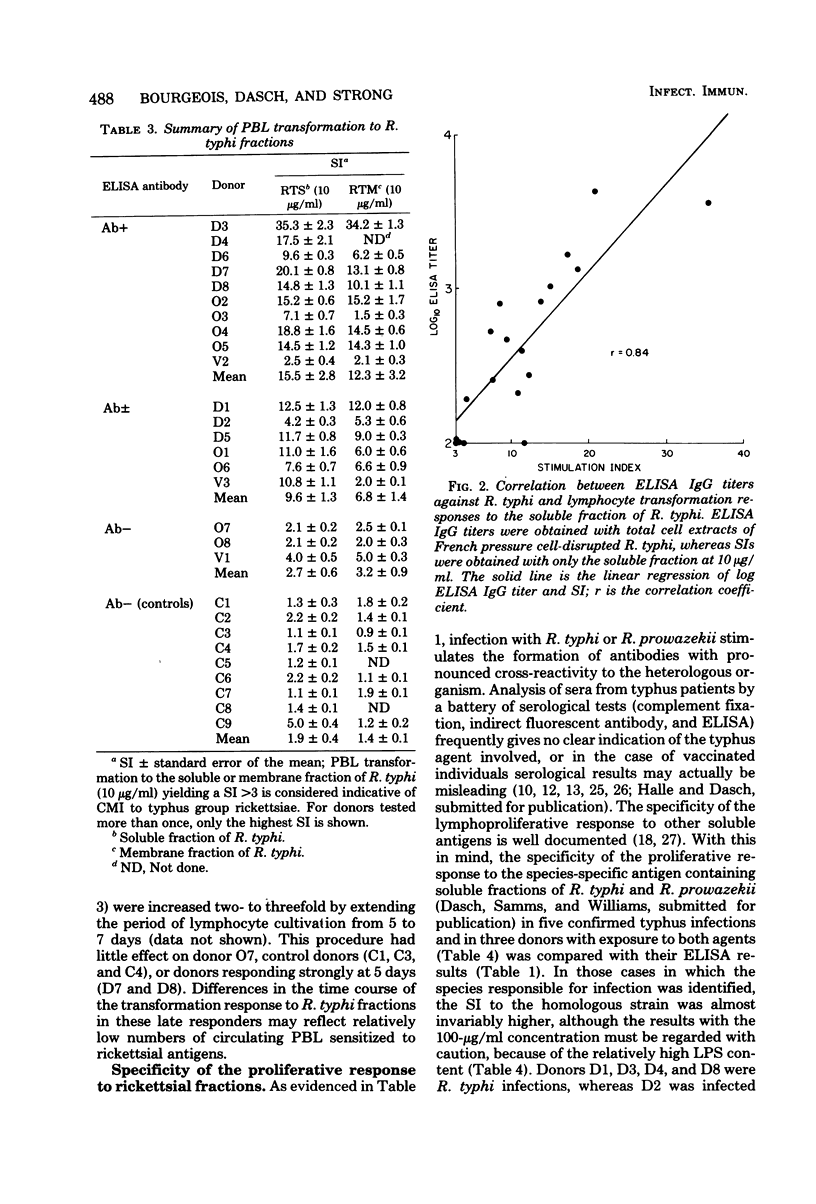
Cell-free extracts of disrupted Renografin-purified Rickettsia typhi and R. prowazekii were evaluated as antigens in lymphocyte transformation assays for cell-mediated immunity to typhus group rickettsiae in 19 individuals with and 9 without histories of exposure to these organisms. Exposure consisted of clinical disease, vaccination with epidemic typhus vaccine, or occupational exposure to these agents. Both the soluble and membrane fractions of disrupted purified rickettsiae were used, and transformation of peripheral blood lymphocytes (PBL) was determined in microcultures by incorporation of [3]thymidine. Of the antigen concentrations tested (1 to 400 μg/ml), 10μg/ml appeared to be the most satisfactory. At this concentration, PBL transformation was highly reproducible and correlated well with donor exposure and the presence of enzyme-linked immunosorbent assay anti-typhus group immunoglobulin G. At higher concentrations, PBL from both exposed and control donors often responded to a lipopolysaccharide-like component present in these preparations. Specific transformation responses to rickettsial fractions were detected in several individuals decades after infection or vaccination, indicating that both fractions contained antigens associated with persisting cell-mediated immunity in humans. Generally, stimulation indexes with the soluble fraction were slightly greater than those obtained with corresponding concentrations of the membrane preparation, and in three individuals transformation was observed only with the soluble fraction. PBL transformation to soluble fractions also appeared to have some species specificity, since PBL from individuals with documented R. typhi infections were more responsive to the homologous soluble preparation than to the soluble fraction of R. prowazekii. PBL transformation also correlated well with homologous but only poorly with heterologous enzyme-linked immunosorbent assay immunoglobulin G titers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. J., Bach F. H. Lymphocyte reactivity in vitro. I. Cellular reconstitution of purified lymphocyte response. Cell Immunol. 1970 Jul;1(2):207–218. doi: 10.1016/0008-8749(70)90008-0. [DOI] [PubMed] [Google Scholar]
- Ascher M. S., Oster C. N., Harber P. I., Kenyon R. H., Pedersen C. E., Jr Initial clinical evaluation of a new Rocky Mountain spotted fever vaccine of tissue culture origin. J Infect Dis. 1978 Aug;138(2):217–221. doi: 10.1093/infdis/138.2.217. [DOI] [PubMed] [Google Scholar]
- Bekker B. V., Dinger J. E., Wolff H. L. Scrubtyphus, an epidemiological study. Acta Leiden. 1968;36:1–8. [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. Macrophage-dependent response of immune human T lymphocytes to PPD in vitro. Influence of HLA-D histocompatibility. Scand J Immunol. 1977;6(8):779–786. doi: 10.1111/j.1365-3083.1977.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Beutner K. R., Morag A., Deibel R., Morag B., Raiken D., Ogra P. L. Strain-specific local and systemic cell-mediated immune responses to cytomegalovirus in humans. Infect Immun. 1978 Apr;20(1):82–87. doi: 10.1128/iai.20.1.82-87.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Dasch G. A., Halle S., Bourgeois A. L. Sensitive microplate enzyme-linked immunosorbent assay for detection of antibodies against the scrub typhus rickettsia, Rickettsia tsutsugamushi. J Clin Microbiol. 1979 Jan;9(1):38–48. doi: 10.1128/jcm.9.1.38-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDWASSER R. A., SHEPARD C. C. Fluorescent antibody methods in the differentiation of murine and epidemic typhus sera; specificity changes resulting from previous immunization. J Immunol. 1959 Apr;82(4):373–380. [PubMed] [Google Scholar]
- Halle S., Dasch G. A., Weiss E. Sensitive enzyme-linked immunosorbent assay for detection of antibodies against typhus rickettsiae, Rickettsia prowazekii and Rickettsia typhi. J Clin Microbiol. 1977 Aug;6(2):101–110. doi: 10.1128/jcm.6.2.101-110.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. H. Blastogenesis of human lymphocytes by endotoxin. Immunol Commun. 1975;4(5):407–417. doi: 10.3109/08820137509057329. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. A., Gartner S., Kaplan H. S. Stimulation of mitogenic responses in human peripheral blood lymphocytes by lipopolysaccharide: serum and T helper cell requirements. J Immunol. 1978 Dec;121(6):2160–2164. [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. G., Jr, Fiset P. Mechanisms of immunity in typhus infection: adoptive transfer of immunity to Rickettsia mooseri. Infect Immun. 1979 May;24(2):387–393. doi: 10.1128/iai.24.2.387-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster C. N., Burke D. S., Kenyon R. H., Ascher M. S., Harber P., Pedersen C. E., Jr Laboratory-acquired Rocky Mountain spotted fever. The hazard of aerosol transmission. N Engl J Med. 1977 Oct 20;297(16):859–863. doi: 10.1056/NEJM197710202971604. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F. Antigen recognition: the specificity of T cells involved in the cellular immune response. Transplant Rev. 1972;10:97–111. doi: 10.1111/j.1600-065x.1972.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Schramek S., Brezina R., Kazár J. Some biological properties of an endotoxic lipopolysaccharide from the typhus group rickettsiae. Acta Virol. 1977 Sep;21(5):439–441. [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Separation of inner and outer membranes of Rickettsia prowazeki and characterization of their polypeptide compositions. J Bacteriol. 1979 Feb;137(2):963–971. doi: 10.1128/jb.137.2.963-971.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D. M., Woody J. N., Factor M. A., Ahmed A., Sell K. W. Immunological responsiveness of frozen-thawed human lymphocytes. Clin Exp Immunol. 1975 Sep;21(3):442–455. [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, el Batawi Y., Wood W. H., Jr, Noriega A. R. Gross and microscopic skin reactions to killed typhus Rickettsiae in human beings. J Immunol. 1967 Jan;98(1):194–209. [PubMed] [Google Scholar]


