Abstract
In this work, a recycling and self-healing strategy for polybenzoxazines through both S–S bond cleavage-reformation reaction and supramolecular attractions is described. Both recyclable and self-healable polybenzoxazines can be prepared from low cost chemicals with a simple procedure in only 30 minutes. For this purpose, inverse vulcanization of poly(propylene oxide)benzoxazine (PPOB) and diallybenzoxazine (B-al) with elemental sulfur was performed at 185 °C. The obtained cross-linked polymer films exhibited thermally driven recycling ability up to 5 cycles. Moreover, the self-healing ability of a test specimen was shown. Spectral characterizations, thermal stability and fracture toughness of the films were investigated after each recycling.
Introduction
In the past few decades, polybenzoxazines (PBZs), also known as benzoxazine resins, emerged as a superior alternative to classical resol and novolac type phenolic resins. The main structural difference of PBZs from classical phenolics is the tertiary amine groups in each repeating unit, which generates immense effect especially on hydrogen bonding types and strength. Due to these unique structural characteristics, PBZs exhibit high tensile strength and modulus (100–125 MPa, and 3.8–4.5 GPa, respectively), and high glass transition temperatures (T g) (170–340 °C). Accordingly, these materials, particularly rigid ones or those admixed with transition metals, have high service temperatures, char yields, and are stable under acidic/alkali conditions. Unlike the general nature of phenolics, PBZs have low water adsorption stemming from strong intra-molecular hydrogen bonding. Another important aspect is related to their dimensional stability during synthesis. These resins are obtained by ring-opening polymerization of benzoxazine monomers at temperatures between 160–250 °C, sometimes higher, without any additive (Scheme 1)1–6. It should be emphasized that 1,3-isomers are the only active benzoxazines to undergo such polymerization. According to mechanistic studies, polymerization proceeds via the cationic pathway as oxazine rings have N and O atoms, which are capable of stabilizing cations during polymerization7–9.
The other appealing side of PBZs is the synthesis of corresponding benzoxazine monomers, which can be accomplished using any suitable phenol, a primary amine, and formaldehyde; therefore, the number of possible benzoxazines is large (Fig. 1)10–16. Apparently, this design flexibility brings about a huge molecular diversity and control of structure and properties of PBZs. For example, crosslinking density of the resins can be increased by introducing dimerizable or polymerizable functionalities on benzoxazines17, 18. The toughness of PBZs can be manipulated by using long chain amines and other soft or conversely rigid groups19–22. Gas forming moieties can be attached to benzoxazines causing macroporosity to occur during curing; thus, sponge-like materials can be obtained, etc 23, 24. Such suppleness in the properties of benzoxazine-based materials has led to many patents and patent applications.
Figure 1.
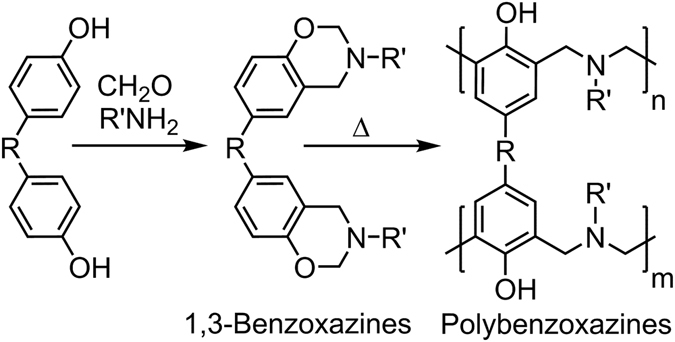
Synthesis of 1,3-benzoxazine monomer and corresponding PBZs.
Apart from monomer synthesis, combining benzoxazines with polymers as main-, side- or end-chains were extensively used as an alternative approach to modifying the properties of PBZs. Many different reactions such as hydrosilylation25, coupling26–28, Diels-Alder29, 30, Huisgen31, 32, Mannich33–39, esterification40, and addition41–44 etc. were successfully applied to obtain curable polymers bearing benzoxazines with designed properties. In recent years, research interest has been directed towards benzoxazine-based smart and advanced materials rather than alternative materials to conventional phenolics, epoxies and their composites. Although, the use of benzoxazines to produce smart materials is relatively premature, several examples such as smart coatings45, 46, shape memory PBZs47, nanofoams48, superhydrophobic surfaces49–51, self-cleaning surfaces52, and electrochromic devices53, 54 were disclosed. Furthermore, self-healing PBZ materials were produced successfully55–57. Adaptability of benzoxazine chemistry was also demonstrated for Li–S battery manufacturing by the reaction of benzoxazines with elemental sulfur (S8)58. Actually, the applied strategy, related to thiol-benzoxazine chemistry, was first reported by Gorodisher et al. and triggered synthesis of several thio-polybenzoxazines59. As known, sulfur is melt processable and can generate radicals on the S atom at temperatures above 160 °C. The industrially employed vulcanization of polybutadienes is based on the ability of thiyl radicals thus formed to react with double bonds. Similarly, inverse vulcanization is the treatment of molten sulfur at temperatures above 160 °C with vinylic monomers affording stable polymeric materials that could be scaled up to kilograms60–62. Several different materials have already been synthesized using this method ranging from IR lenses to mercury sorbents63–67. The process is relatively simple and sulfur can be used as much as 90%-wt. in these materials. Thus, cost effective advanced systems can easily be obtained by a simple melt process. As part of our continuous interest in developing new benzoxazine based materials to expand their applications, we now report the preparation of high sulfur containing tri-component recyclable and self-healable PBZs using inverse vulcanization chemistry. Diallyl functional benzoxazine (B-al) and main-chain PBZs were synthesized and reacted with sulfur at 185 °C. Inverse vulcanization and ring-opening polymerization took place concomitantly to produce poly(benzoxazine-co-sulfide) materials.
Results and Discussions
Designing polymers that respond to temperature, light, ultrasound, pH etc. has been a central endeavor in polymer chemistry. Because, these polymers can significantly alter their properties in a controlled fashion by a stimulus or self-intervention, this is an important aspect for many applications. Self-healable polymers are a branch of the above-mentioned smart materials and have gained interest due to the possibility of prolonging their service life. In general, the damaged zone must have some sort of chain dynamics in a network structure to reform bonds, ensuring the healing. Consequently, a dynamic network equipped with suitable functional groups capable of performing healing reactions are required in a self-mendable material68, 69. Self-healable materials can also be considered as a potential recyclable polymer70, 71. In this study, according to the stated background, polyether amine based PBZ precursor was initially synthesized to provide a dynamic network for PBZs. Thus, poly(propylene oxide) bisamine (Jeffamine, PPOA, M n:2000), bisphenol A (BA) and paraformaldehyde were mixed and refluxed in a toluene-ethanol mixture (2:1, v/v) resulting in PBZs precursors (PPOB, M n: 12200). The other component of the recyclable/self-healing system was independently synthesized using allylamine, BA and paraformaldehyde yielding diallybisbenzoxazine (B-al) (Fig. 2).
Figure 2.
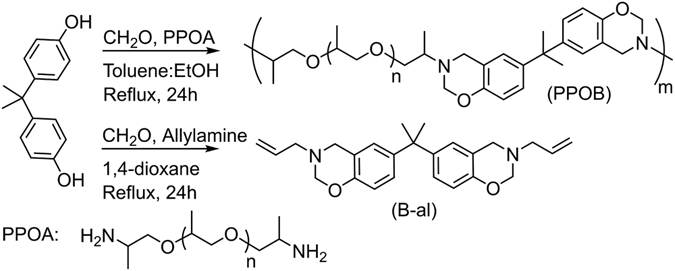
Synthesis of PPOB and B-al starting from bisphenol A.
The chemical structure of the PPOB and B-al was confirmed by 1H NMR and FTIR spectral analysis1.H NMR spectra of PPOB and B-al are presented in Supplementary Figures S1 and S2, respectively. The appearance of protons resonating at 4.91, 4.84 ppm (O–CH2–N), and 4.02, 3.65 ppm (Ar–CH2–N) for PPOB and B-al is clear evidence of the benzoxazine ring formations. Moreover, the peak at 1.60 ppm (–CH3) and aromatic protons between 7.05–6.62 ppm disclose the bisphenol A moiety in both structures. Apart from PPOB, allyl protons of B-al are also visible at 5.92 and 5.23 ppm. Moreover, the FTIR spectra of PPOB and B-al are additional evidences for the formation of oxazine rings in both structures (Fig. 3). Allyl C–H and C=C stretching vibrations at 3074 and 1642 cm−1 are clearly visible. The vibration band of tri-substituted benzene ring of benzoxazine for both B-al and PPOB also appears at 1498 and 1510 cm−1, respectively. Besides, the C–O vibration band of PPOB verify the preservation of polyether structure after synthesis. Moreover, the stretching vibrations of aromatic C–H (3004–3062 cm−1) and aromatic C=C (1490–1650 cm−1), the out of plane bending vibrations of aromatic C–H at 930 cm−1 are detected for both structures.
Figure 3.
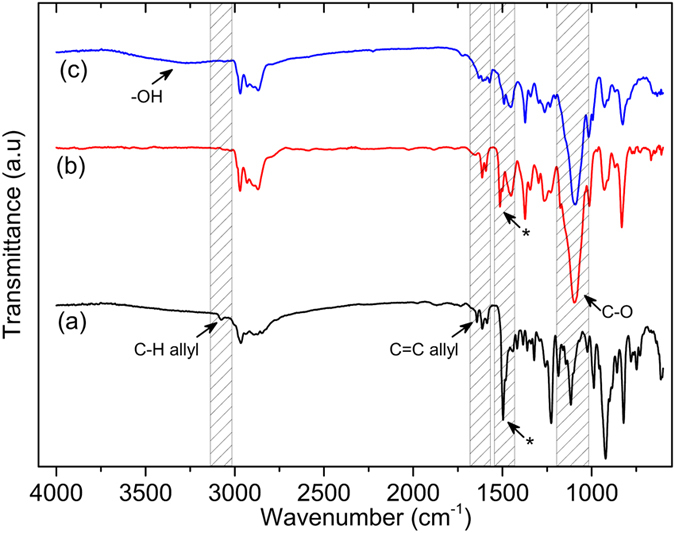
FTIR spectra of B-al (a), PPOB (b), PPOB40-B-al40-S20 (c). (*tri-substituted benzene ring of benzoxazine).
It is well-known that polysulfides are unstable at ambient conditions and degrade to form cyclooctasulfur (S8). However, with the inverse vulcanization approach, reacting S8 with vinylic monomers above 160 °C, yields stable polysulfide copolymers. The stability of such polymers was found to be great even for high sulfur contents such as 90%-wt62. The method is flexible as there are several different vinylic monomers affording advanced materials valuable for many applications. Previously, B-al was used as a replacement for classical vinylic monomers to obtain high sulfur containing benzoxazine resins72. The allyl groups readily react with sulfur radicals forming C-S covalent bonds between the benzoxazine unit and polysulfide moiety. In the meantime, ring-opening polymerization of oxazine takes place producing PBZ. This way, polysulfide is stabilized with PBZ bridges to give a copolymer. As known, the S–S bonds in polysulfide chains are dynamic and upon heating these bonds can be broken and regenerated at a certain temperature. Thus, S–S linkages are useful in designing a dynamic system such as self-healable materials. However, poly(benzoxazine-co-sulfide)s from B-al and S8 are rigid and self-healing was not observed for these samples. Therefore, in order to increase the chain mobility and improve the softness of the material, the above obtained PPOB was mixed with the B-al/S8 system in varying amounts. The mixture was then heated up to 185 °C to realize both curing and inverse vulcanization processes (Fig. 4) yielding copolymers, abbreviated as PPOBx-B-aly-Sz (x, y, z are %-wt).
Figure 4.
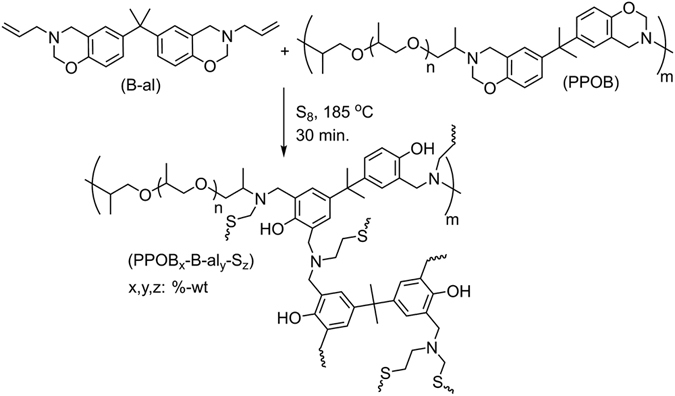
Synthesis of PPOBx-B-aly-Sz by using PPOB, B-al and S8 with various mixing ratios.
The copolymers are soluble at certain mixing ratios especially at high sulfur feeds. As known, even mono functional benzoxazines form insoluble networks upon heating; the observed solubility may be due to the limited collusions of benzoxazine monomers arising from the high content of polysulfide and elemental sulfur. However, copolymers with low amounts of sulfur are insoluble.
The chemical structure of PPOBx-B-aly-Sz copolymers were confirmed using 1H NMR and FTIR analyses. It should be noted that for NMR analysis, soluble copolymers were prepared by increasing sulfur and PPOB content. The molecular weight of this polymer was found to be M n = 32260 as determined by GPC. As seen in Fig. 5, when 1H NMR spectra of B-al, PPOB and PPOB50-B-al20-S30 are overlaid, the allyl protons at 5.9 (–CH=CH) and 5.2 ppm (–CH=CH) disappear after inverse vulcanization evidencing consumption of ally groups by sulfurs. Besides, the peaks that emerge at 2.5 ppm (–S–CH2–) after copolymerization also support the sulfur addition. Moreover, the aromatic and methyl protons of bisphenol A moiety confirm the presence of PBZ in PPOB50-B-al20-S30. Disappearance of N–CH2–O protons also shows the complete ring-opening of oxazines.
Figure 5.
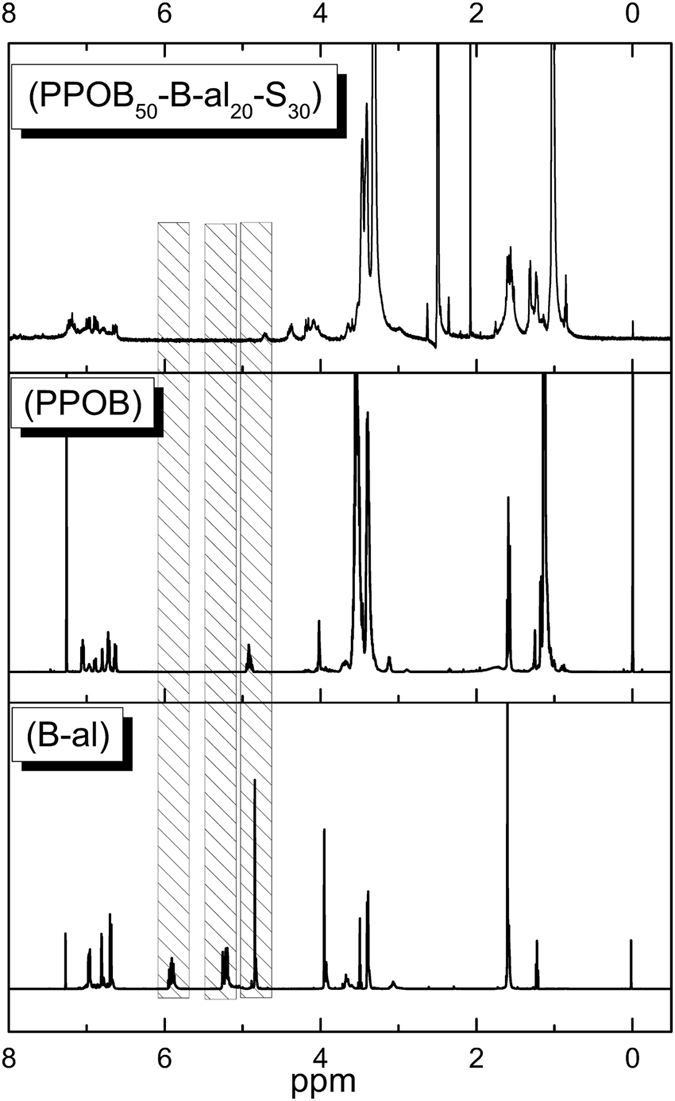
Overlaid 1H NMR spectra of B-al, PPOB and PPOB50-B-al20-S30.
The overlaid FTIR spectra of B-al, PPOB and PPOB40-B-al40-S20 also reveal the consumption of both allyl groups and oxazine rings (Fig. 3). The disappearance of the allylic = C–H, C=C stretching vibrations at 3078 and 1643 cm−1, respectively, is clearly noted. Formation of s phenolic hydroxyl peak at 3308 cm−1 supports the ring-opening polymerization during the inverse vulcanization process. Moreover, the stretching vibrations of aromatic C–H (3007–3062 cm−1) and aromatic C=C (1560–1631 cm−1) and C–O ether bands, are detected for PPOB40-B-al40-S20. Consequently, the spectral analysis evidently supports that B-al, PPOB, and S8 reacted to form a network containing PBZ, polyether and polysulfide components.
In additional to spectral analysis, thermal characterization was performed to survey the benzoxazine ring structure before and after inverse vulcanization. As known, the ring-opening polymerization temperatures of benzoxazines are generally between 160–250 °C. In Supplementary Figures S3 and S4, DSC thermograms of B-al and PPOB were sketched. An onset at 203 °C, end-set at 297 °C and the curing maximum at 266 °C are detectable for B-al. Similarly, onset at 222 °C, end-set at 271 °C and a maximum at 253 °C are distinguishable for PPOB. These exotherms are drastically affected by the addition of sulfur. As seen in Fig. 6a and Table 1, the onset and the maximum curing temperatures of the B-al, PPOB, S8 mixture dropped down to 149 and 185 °C, respectively. The admixed S8 reduced the amount of exotherms compared to pristine B-al and PPOB, which can be expected as S8 acts as extra mass without having any exothermic process at these specific temperatures. Moreover, since the melting endotherm of S8 is easily detectable, it was used to track the traces of unreacted S8 in the final PPOBx-B-aly-Sz products. In Fig. 6b–e, after curing, melting endotherm of S8 is not noticeable for PPOB40-B-al40-S20 showing that sulfur is completely consumed by both allyl and oxazine groups. Similarly, curing exotherm of benzoxazines is not visible, indicating a complete curing process.
Figure 6.
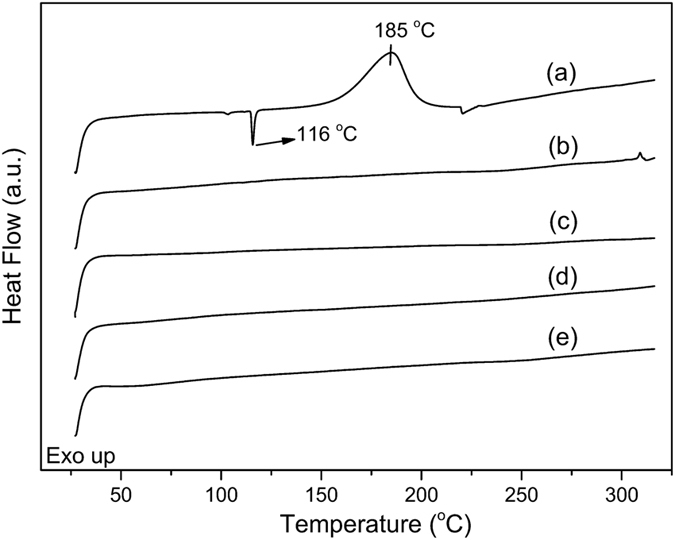
DSC thermographs of B-al, PPOB, S8 mixture (a), PPOB40-B-al40-S20 (b), 1st (c), 3rd (d), 5th (e) healings.
Table 1.
DSCa characteristics of B-al, PPOB and PPOB40-B-al40-S20.
| Compound | On-set of Curing (°C) | End-set of Curing (°C) | Maximum Curing Temperature (°C) | Amount of Exotherm (J/g) |
|---|---|---|---|---|
| B-alb | 203 | 297 | 266 | −280 |
| PPOBb | 222 | 271 | 253 | −20 |
| PPOB40-B-al40-S20 | 149 | 206 | 185 | −105 |
aDSC thermograms were collected under 20 mL.min−1 N2 flow and 10 °C.min−1 heating rate.
bDSC thermograms of B-al and PPOB are presented in supporting information.
In order to utilize S–S bonds incorporated into the network and provide adequate chain mobility for self-healing of PBZs a suitable admixing ratio of PPOB, B-al, and S8 was initially determined. The mixing ratios and copolymer properties are tabulated in Table 2. We have recognized that the S8 mass ratio is more critical than B-al and PPOB ratios to accomplish the ultimate aim. While the addition of excess sulfur resulted in soluble copolymers, conversely a small amount of sulfur yielded brittle, hard and insoluble materials. Moreover, B-al and S8 mixtures without PPOB are non-healable and depending on the S8 amount, these copolymers are either insoluble and rigid, or soluble. The optimum admixing weight ratios are 40% PPOB, 40% B-al and 20% S8 giving the PPOB40-B-al40-S20 copolymer. A sulfur ratio over 20% forms soluble copolymers and below this ratio, yields insoluble but highly rigid copolymers lacking required chain mobility for a self-healing system.
Table 2.
PPOB, B-al and S8 feed ratios for the preparation of PPOBx-B-aly-Sz copolymers.
| Run | PPOB (%-wt) | B-al (%-wt) | S8 (%-wt) | Copolymer Properties |
|---|---|---|---|---|
| 1 | 80 | 10 | 10 | Soft, inhomogeneous, non-healable |
| 2 | 60 | 30 | 10 | Rigid, inhomogeneous, non-healable |
| 3 | 50 | 20 | 30 | Soft, soluble, healable |
| 4 | 50 | 30 | 20 | Semi-soft, partially soluble |
| 5a | 40 | 40 | 20 | Soft, insoluble, healable |
| 6 | 35 | 50 | 15 | Semi-soft, insoluble, partially healable |
| 7 | 30 | 50 | 20 | Semi-soft, partially soluble |
| 8 | 30 | 50 | 0 | Rigid, non-healable |
| 9 | 40 | 40 | 0 | Rigid, non-healable |
aThese mixing ratios yielded insoluble and self-healable material.
Typically, a self-healable film was prepared by mixing suitable amounts of PPOB, B-al and S8 in a Teflon mold covered with a Teflon cap tightened with clamps. The mold was placed in an open-air oven and heated up to 185 °C for 60 min. After cooling to room temperature, the cap was removed and the film was carefully separated from the mold. The obtained film was black in color and soft enough to cut into two pieces with scissors. After, cutting the PPOB40-B-al40-S20 film, the parts were kept in contact from the cut edges in a mold. The mold was then covered with a Teflon cap and the material was reheated to heal. After thermal treatment, the film reformed itself successfully (Figure S5). It should be expected that, the prepared film self-healed by S–S bond cleavage and reforming reactions after heat application. The strong hydrogen bonds of the PBZ domain might also contribute to the self-healing, as shown previously73. Briefly, it can be proposed that the healing mechanism is a combination of supramolecular attractions and dynamic S–S bond systems facilitated by soft segments in the structure. Moreover, another experiment was devised with a similar specimen to exhibit the recycling capacity of PPOB40-B-al40-S20. For this experiment, the prepared film was chopped with a knife into many parts. Then, the polymer particles were replaced in a Teflon mold and thermally treated as described for self-healing. Five cycles of chopping and healing were performed and the film successfully reformed itself after each cycle (Fig. 7 and a video as supplementary information).
Figure 7.

Image of chopping and 5th recycling of PPOB40-B-al40-S20. Chopped sample (a), molding (b), after thermal treatment (c).
Apart from visual observations, possible changes in the structure of PPOB40-B-al40-S20 after cycles was monitored by thermal and spectral characterizations. DSC analysis of the film were performed after each cycle and the DSC thermograms are presented in Fig. 6. According to DSC results, polysulfide domains seem to be stable after each healing cycle and do not revert to S8 form which is easily detectable using the melting endotherm of sulfur. Moreover, the network preserved its main components and did not produce small fragments that can be volatile during analysis being detected as evaporation endotherms in DSC thermographs. Moreover, FTIR examinations of PPOB40-B-al40-S20 after each cycle did not show any distinct structural change in the material. In Fig. 8, the overlaid FTIR spectra of B-al and PPOB40-B-al40-S20 after healing cycles are presented. After each cycle C–O stretching vibrations of the polyether domain at 1096 cm−1 are visible, providing evidence that the PPOB part remained in the network after thermal treatments. Moreover, a broad phenolic O–H band at around 3308 cm−1 proves that hydrogen bonding was still present and the main PBZ body did not degrade even after the 5th cycle. The fingerprint region comparison between FTIR spectra of B-al and PPOB40-B-al40-S20 indicates that the component from B-al still remained even after the final thermal treatment. Consequently, thermal and spectral inspections did not reveal any detectable structural changes for PPOB40-B-al40-S20 in the determination limits of DSC and FTIR.
Figure 8.
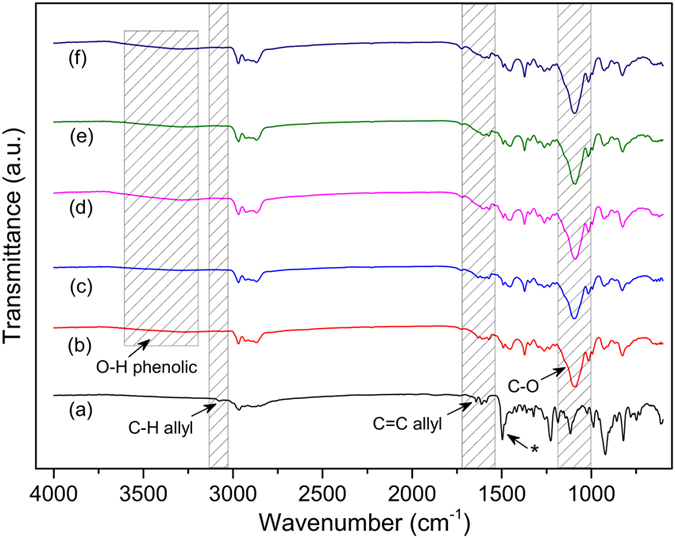
FTIR spectra of B-al (a), PPOB40-B-al40-S20 (b), and after 1st (c), 2nd (d), 3rd (e), 5th (f) recycling processes. (*tri-substituted benzene ring of benzoxazine).
Even though visual, thermal and FTIR analyses provide information about the recovery potential of PPOB40-B-al40-S20, a numeric quantification for recycling was carried out by measuring the recovery of the mechanical properties of the film (Figs 9 and 10). Although, various methods are available to quantify the extent of healing, recovery of properties such as tensile strength, fracture toughness etc. are generally measured. In these measurements, the “healing efficiency” of a self-healing system can be expressed as η (Eq. 1); where K healed and K virgin are the areas of stress-strain curve of the healed and virgin specimens74.
| 1 |
Figure 9.
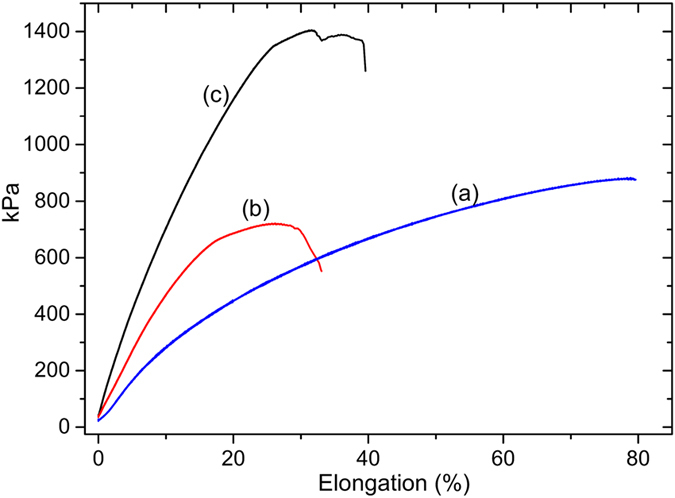
Stress-Strain (%) behavior of PPOB40-B-al40-S20 (a), cut-healed specimen (b), only heat treated specimen (c).
Figure 10.
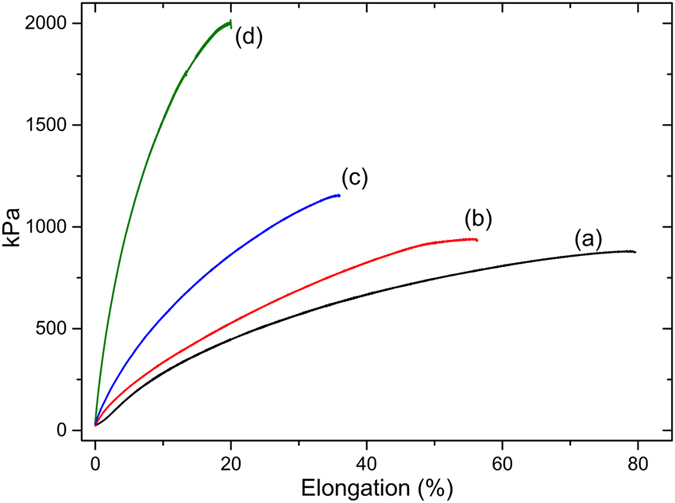
Stress-Strain (%) behavior of PPOB40-B-al40-S20 (a), 1st (b), 3rd (c), 5th (d) healings.
For PPOB40-B-al40-S20 films, fracture toughness of the samples was measured and η was found to be ≈30 for the cut-healed sample. However, η values were found to be better for recycled samples at ≈88% for the one, ≈49% for the three, and ≈34% for the five times chopped-healed specimen. The recovery ratio of the cut-healed film is drastically smaller than the recovery of the 1st recycling. This phenomenon can be explained by the contact surface area of the particles. In the recycling process, the polymer chopped into many pieces and molded. Thus, collusion of S atoms was more likely to form S–S bonds due to the increased surface area. However, for the cut-healed sample only cut edges were kept in contact and the Teflon cap of the mold applied vertical pressure on the mold, which may play a negative effect on the contact strength.
Although DSC and FTIR analysis did not reveal any structural change in PPOB40-B-al40-S20, the stress-strain test unveils that mechanical properties of the virgin specimen become different after each healing cycle. The toughness of the film gradually decreases and rigidity increases after heat exposure. Accordingly, the stress value for the sample changes from 877 to 2007 kPa and elongation at break reduces from 79% to 20% after the 5th healing cycle. The reduction in toughness is also in accordance with healing efficiencies, since the mobility of the chains is reduced after each cycle. This phenomenon can be explained by the shortening of polysulfide chains by S–S bond cleavage and reformation reactions. Shorter chains increase the crosslinking density and at the same time rigidity which would obviously extinguish self-healing ability at a certain cycle number. On the other hand, thermal treatment of PPOB40-B-al40-S20 reinforces the material that is durable under ≈2000 kPa force. Thermally induced reduction of the toughness and incremental stress on the films was further investigated. In Fig. 9, the difference between PPOB40-B-al40-S20 and its thermal treated state is obvious. As seen, the stress value increased from 877 to 1405 kPa after 1 h thermal treatment. Also, the toughness of the film reduced ≈40% after heating, which is in accordance with the toughness reduction seen in recycled samples. In addition to mechanical analysis, we measured the weight changes of PPOB40-B-al40-S20 for every cycle. A weight change was observed (−1.4%) after the first healing which can be explained as the loss of sulfur presumably in the form of H2S gas. The formation of H2S during inverse vulcanization of benzoxazines was previously reported72. Acidic phenolic –OH groups can still lead to H2S formation in cured samples that are competing with the S–S reforming reaction. This possibility might be an additional explanation for chain shortening of polysulfide domains. The reduction in the amount of sulfur was also measured by elemental analysis of the samples taken from each heal cycle (Table S1). The sulfur content reduced by half at the 5th cycle, compared to the initial sample. These results clearly point out that self-healable materials obtained by inverse vulcanization of acidic compounds might have a number of cycle limitations due to evaporation of sulfur, possibly in the form of H2S.
The thermal stability of PPOB40-B-al40-S20 for each healing cycle was measured using thermo-gravimetric analysis (TGA). TGA traces are presented in Fig. 11 and Table 3; derivative TGA is demonstrated in Supplementary Figure S6. According to TGA data, the thermal stability of PPOB40-B-al40-S20 film gradually enhances towards the 5th healing cycle both in terms of initial degradations and char yields. As can be seen, the PPOB40-B-al40-S20 sample has the lowest T5%, T10% and Tc values at 230, 281 °C, 11% and after 5th cycle, has the highest values at 280, 321 °C, 16%, respectively. There is a 50 °C difference for T5% and 40 °C for T10% showing sulfur evaporation mainly occurs in the range of 200–300 °C. Accordingly, thermal results support the chain shortening of polysulfide domains and sulfur loss in the structure. As the chains shorten and the sulfur amount is reduced, the copolymer becomes more stiff and resistant to heat. However, Tmax is not affected by the length of polysulfide chains and the amount of sulfur in the structures. This value is the same for each cycle at ≈373 °C, indicating that the maximum weight loss is mainly dependent on degradation of PBZ domains. Generally, main weight-loss events between 300–400 °C, are assigned to amine evaporation and is the consequence of the Mannich base cleavage of PBZs. Latter weight loss can be ascribed to phenol decomposition thermal aromatization and crosslinking during degradation will finally lead to char formation.
Figure 11.
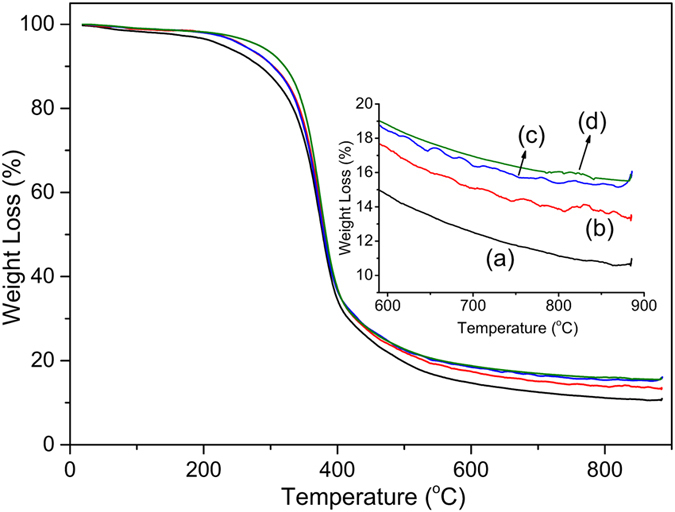
TGA traces of PPOB40-B-al40-S20 (a), 1st (b), 3rd (c) and 5th (d) healing cycles.
Table 3.
Thermal properties of the PPOB40-B-al40-S20 films after each healing cycle.
| Film | T5% (°C) | T10% (°C) | Tmax (°C)a | Tc (%) |
|---|---|---|---|---|
| PPOB40-B-al40-S20 | 230 | 281 | 374 | 11 |
| 1st healing | 257 | 306 | 373 | 14 |
| 3rd healing | 257 | 306 | 373 | 15 |
| 5th healing | 280 | 321 | 374 | 16 |
aThese values extracted from derivative of TGA (Fig. S6) presented in supporting information.
T5%: The temperature for which the weight loss is 5%.
T10%: The temperature for which the weight loss is 10%.
Tmax: The temperature for maximum weight loss.
Tc: The char yield at 800 °C.
Conclusion
The results presented in this work demonstrate that it is possible to use inverse vulcanization of carefully designed benzoxazines to produce poly(benzoxazines-co-sulfide)s with self-healing and recycling property. In the approach, benzoxazine bearing PPOB were prepared through conventional main chain PBZ precursor synthesis methodology using poly(propylene oxide) amines, bisphenol A and formaldehyde to incorporate flexibility to the network. Diallybenzoxazine monomer, PPOB and elemental sulfur were mixed in suitable weight ratios and the films were prepared by thermal treatment of these components at 185 °C. Inverse vulcanization took place between sulfur atoms and allyl groups; ring-opening polymerization of the benzoxazines occurred concomitantly. The synthetic methodology described here, which can be completed within 30 min. by a simple melt process, is relatively fast and easy. The self-healing property of the film was demonstrated and it was shown that cross-linked films can be recyclable and heal themselves successfully up to 5 times. Evidently, the most appealing part of this strategy relies on its simplicity and the low cost of the chemicals that can be converted into advanced materials in one-pot, using the conditions outlined here.
Methods
Materials
4,4′-Isopropylidenediphenol (bisphenol A) (Aldrich, 97%), paraformaldehyde (Acros, 96%), poly(propylene glycol) bis(2-aminopropyl ether) (Mn ~ 2000 Da, Aldrich), allylamine (Aldrich, 98%), sulfur (S8, colloidal powder, reagent grade, Aldrich), sodium hydroxide (Acros, >97%), sodium sulfate (Acros, 99%), 1,4-dioxane (Aldrich, 99%), diethylether (Aldrich, ≥98%,), methanol (MeOH, Aldrich, 99%), ethanol ( ≥99.5%, Aldrich), toluene (Carlo Erba, 99.5%), chloroform (Acros, 99+%), hexane (Aldrich, 95%) were used as received.
Measurements
All 1H NMR spectra were recorded on an Agilent NMR System VNMRS 500 spectrometer at room temperature in CDCl3 or DMSO-d 6 with Si(CH3)4 as an internal standard. FTIR spectra were recorded on a Perkin-Elmer FTIR Spectrum One spectrometer. Differential Scanning Calorimetry (DSC) was performed on Perkin-Elmer Diamond DSC from 30 °C to 320 °C with a heating rate of 10 °C min−1. under nitrogen flow. A typical DSC sample was 2–5 mg in a 30 μL aluminum pan. Thermal gravimetric analysis (TGA) was performed on Perkin-Elmer Diamond TA/TGA with a heating rate of 10 °C min under nitrogen flow. Uniaxial elongation measurements were performed on polymeric film samples (approx. 10 mm length and 2.5 mm2 cross-section area). Measurements were carried out using a Perkin Elmer Pyris Diamond DMA (SII Nanotechnology Inc.) at 25 °C under 50 mN/min. load speed. The tensile strength and percentage elongation at break were recorded.
Synthesis of poly(propylene oxide)benzoxazine (PPOB)
Bisphenol A (5.2 mmol, 1.20 g), paraformaldehyde (20.0 mmol, 0.60 g) and poly(propylene oxide) bisamine (PPO) (5 mmol, 10 g) were dissolved with 100 mL of toluene and 50 mL of ethanol and refluxed for 24 h in a 500 mL round bottomed flask equipped with a condenser. The solvent was evaporated under vacuum and a blondish oily product was precipitated in cold n-hexane. The precipitation process was done two times. The polymer was dried under vacuum for 1 day.
Synthesis of 6,6′-(propane-2,2-diyl)bis(3-allyl-3,4-dihydro-2H-benzo[e][1,3]oxazine) (B-al)
In a 500 mL round bottomed flask, allyamine (30.8 g, 0.54 mol) in 1,4-dioxane (200 mL) was cooled in an ice bath. Paraformaldehyde (32.4 g, 1.08 mol) was added portion-wise to this solution over 10 min. Thereafter, bisphenol A (61.6 g, 0.27 mol) was added to the solution and the mixture was refluxed for 24 h. After this reaction time, the solvent was evaporated using a rotary evaporator. The resulting oily product was dissolved in diethyl ether (250 mL) and extracted with 0.1 N sodium hydroxide various times to remove unreacted bisphenol A. Then, to neutralize the solution, diethyl ether solution washed with distilled water (80 mL) for two times. Afterwards, the diethyl ether layer was dried with anhydrous Na2SO4 and filtered. Solvent was evaporated under vacuum and the remaining oily product was dissolved in MeOH (30 mL). Water was added drop by drop into the MeOH solution until it became turbid, and then refrigerated (4 °C). The precipitated sticky mass was obtained by decantation of MeOH and washing with excess water. The product was dried under vacuum at 60 °C for 24 h. (Yield, 63%)
General procedure for the preparation of poly(benzoxazine-co-sulfide) (PPOBx-B-aly-Sz)
To a 20 mL test tube, equipped with a magnetic stir bar, PBOB, B-al and sulfur were added. The tube was heated up to 185 °C with vigorous stirring in an oil bath. In the first 5 minutes a clear orange solution formed. Then, the color of the solution changed to brown-black. The overall reaction time was 30 min. in total. After cooling the tube to r.t., it was broken and the product was collected. In these reactions, sulfur feed ratios were 10, 15, 20 and 30-wt% while the other components’ feed ratios were 30, 35, 40, 50, 60, 80-wt% (see Table 2). A similar procedure was applied for film preparations; instead of glass tubes, Teflon molds with Teflon covers were used. The mixture dispersed in mold uniformly and the tightly covered mold was placed in an open-air oven and heated up to 185 °C for 60 min. After cooling, the cap was removed and the film was separated from the Teflon mold.
Electronic supplementary material
Acknowledgements
Istanbul Technical University Research Fund is acknowledged for financial support.
Author Contributions
B.K. and Y.Y. wrote the main manuscript text and B.K. prepared all the figures. M.A. performed the experimental studies. All authors have given approval to the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05608-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Baris Kiskan, Email: kiskanb@itu.edu.tr.
Yusuf Yagci, Email: yusuf@itu.edu.tr.
References
- 1.Ghosh NN, Kiskan B, Yagci Y. Polybenzoxazines - New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007;32:1344–1391. doi: 10.1016/j.progpolymsci.2007.07.002. [DOI] [Google Scholar]
- 2.Takeichi T, Kawauchi T, Agag T. High Performance Polybenzoxazines as a Novel Type of Phenolic Resin. Polym. J. 2008;40:1121–1131. doi: 10.1295/polymj.PJ2008072. [DOI] [Google Scholar]
- 3.Nair CPR. Advances in addition-cure phenolic resins. Prog. Polym. Sci. 2004;29:401–498. doi: 10.1016/j.progpolymsci.2004.01.004. [DOI] [Google Scholar]
- 4.Ishida, H. in Handbook of Benzoxazine Resins 3-81 (Elsevier, 2011).
- 5.Arslan, M., Kiskan, B. & Yagci, Y. In Encycl. Polym. Sci. Technol. (John Wiley & Sons, Inc., 2015).
- 6.Wang J, Xu YZ, Fu YF, Liu XD. Latent curing systems stabilized by reaction equilibrium in homogeneous mixtures of benzoxazine and amine. Sci. Rep. 2016;6:38584. doi: 10.1038/srep38584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YX, Ishida H. Cationic ring-opening polymerization of benzoxazines. Polymer. 1999;40:4563–4570. doi: 10.1016/S0032-3861(99)00074-9. [DOI] [Google Scholar]
- 8.Kasapoglu F, Cianga I, Yagci Y, Takeichi T. Photoinitiated cationic polymerization of monofunctional benzoxazine. J. Polym. Sci. Part A: Polym. Chem. 2003;41:3320–3328. doi: 10.1002/pola.10913. [DOI] [Google Scholar]
- 9.Hamerton I, et al. Examining the Initiation of the Polymerization Mechanism and Network Development in Aromatic Polybenzoxazines. Macromolecules. 2013;46:5117–5132. doi: 10.1021/ma401014h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oie H, Sudo A, Endo T. Acceleration effect of N-allyl group on thermally induced ring-opening polymerization of 1,3-benzoxazine. J. Polym. Sci. Part A: Polym. Chem. 2010;48:5357–5363. doi: 10.1002/pola.24338. [DOI] [Google Scholar]
- 11.Wang C, Sun J, Liu X, Sudo A, Endo T. Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine. Green Chem. 2012;14:2799–2806. doi: 10.1039/c2gc35796h. [DOI] [Google Scholar]
- 12.Wang C, et al. Synthesis and thermal properties of a bio-based polybenzoxazine with curing promoter. J. Polym. Sci. Part A: Polym. Chem. 2013;51:2016–2023. doi: 10.1002/pola.26587. [DOI] [Google Scholar]
- 13.Kawaguchi AW, Sudo A, Endo T. Thiol-functionalized 1,3-benzoxazine: Preparation and its use as a precursor for highly polymerizable benzoxazine monomers bearing sulfide moiety. J. Polym. Sci. Part A: Polym. Chem. 2014;52:1448–1457. doi: 10.1002/pola.27131. [DOI] [Google Scholar]
- 14.Andreu R, Reina JA, Ronda JC. Studies on the thermal polymerization of substituted benzoxazine monomers: Electronic effects. J. Polym. Sci. Part A: Polym. Chem. 2008;46:3353–3366. doi: 10.1002/pola.22677. [DOI] [Google Scholar]
- 15.Imran M, Kiskan B, Yagci Y. Concise synthesis and characterization of unsymmetric 1,3-benzoxazines by tandem reactions. Tetrahedron Lett. 2013;54:4966–4969. doi: 10.1016/j.tetlet.2013.07.041. [DOI] [Google Scholar]
- 16.Sini NK, Bijwe J, Varma IK. Renewable benzoxazine monomer from Vanillin: Synthesis, characterization, and studies on curing behavior. J. Polym. Sci. Part A: Polym. Chem. 2014;52:7–11. doi: 10.1002/pola.26981. [DOI] [Google Scholar]
- 17.Agag T, Takeichi T. Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high performance thermosets. Macromolecules. 2003;36:6010–6017. doi: 10.1021/ma021775q. [DOI] [Google Scholar]
- 18.Takeichi T, Nakamura K, Agag T, Muto H. Synthesis of cresol-based benzoxazine monomers containing allyl groups and the properties of the polymers therefrom. Des. Monomers Polym. 2004;7:727–740. doi: 10.1163/1568555042474121. [DOI] [Google Scholar]
- 19.Kiskan B, Koz B, Yagci Y. Synthesis and Characterization of Fluid 1,3-Benzoxazine Monomers and Their Thermally Activated Curing. J. Polym. Sci. Part A: Polym. Chem. 2009;47:6955–6961. doi: 10.1002/pola.23735. [DOI] [Google Scholar]
- 20.Baranek AD, et al. Flexible aliphatic-bridged bisphenol-based polybenzoxazines. Polym. Chem. 2012;3:2892–2900. doi: 10.1039/c2py20498c. [DOI] [Google Scholar]
- 21.Allen DJ, Ishida H. Physical and mechanical properties of flexible polybenzoxazine resins: Effect of aliphatic diamine chain length. J. Appl. Polym. Sci. 2006;101:2798–2809. doi: 10.1002/app.22501. [DOI] [Google Scholar]
- 22.Kudoh R, Sudo A, Endo T. A Highly Reactive Benzoxazine Monomer, 1-(2-hydroxyethyl)-1,3-Benzoxazine: Activation of Benzoxazine by Neighboring Group Participation of Hydroxyl Group. Macromolecules. 2010;43:1185–1187. doi: 10.1021/ma902416h. [DOI] [Google Scholar]
- 23.Zuniga C, et al. Polybenzoxazines from Renewable Diphenolic Acid. J. Polym. Sci. Part A: Polym. Chem. 2011;49:1219–1227. doi: 10.1002/pola.24541. [DOI] [Google Scholar]
- 24.Zúñiga C, Lligadas G, Ronda JC, Galià M, Cádiz V. Self-foaming diphenolic acid benzoxazine. Polymer (United Kingdom) 2012;53:3089–3095. [Google Scholar]
- 25.Aydogan B, Sureka D, Kiskan B, Yagci Y. Polysiloxane-Containing Benzoxazine Moieties in the Main Chain. J. Polym. Sci. Part A: Polym. Chem. 2010;48:5156–5162. doi: 10.1002/pola.24313. [DOI] [Google Scholar]
- 26.Demir KD, Kiskan B, Yagci Y. Thermally Curable Acetylene-Containing Main-Chain Benzoxazine Polymers via Sonogashira Coupling Reaction. Macromolecules. 2011;44:1801–1807. doi: 10.1021/ma1029746. [DOI] [Google Scholar]
- 27.Demir KD, Kiskan B, Latthe SS, Demirel AL, Yagci Y. Thermally curable fluorinated main chain benzoxazine polyethers via Ullmann coupling. Polym. Chem. 2013;4:2106–2114. doi: 10.1039/c2py21029k. [DOI] [Google Scholar]
- 28.Chernykh A, Agag T, Ishida H. Effect of Polymerizing Diacetylene Groups on the Lowering of Polymerization Temperature of Benzoxazine Groups in the Highly Thermally Stable, Main-Chain-Type Polybenzoxazines. Macromolecules. 2009;42:5121–5127. doi: 10.1021/ma900505d. [DOI] [Google Scholar]
- 29.Chou C-I, Liu Y-L. High performance thermosets from a curable Diels–Alder polymer possessing benzoxazine groups in the main chain. J. Polym. Sci. Part A: Polym. Chem. 2008;46:6509–6517. doi: 10.1002/pola.22960. [DOI] [Google Scholar]
- 30.Wang Y-H, Chang C-M, Liu Y-L. Benzoxazine-functionalized multi-walled carbon nanotubes for preparation of electrically-conductive polybenzoxazines. Polymer. 2012;53:106–112. doi: 10.1016/j.polymer.2011.11.040. [DOI] [Google Scholar]
- 31.Nagai A, et al. Synthesis and crosslinking behavior of a novel linear polymer bearing 1,2,3-triazol the main chain by and benzoxazine groups in a step-growth click-coupling reaction. J. Polym. Sci. Part A: Polym. Chem. 2008;46:2316–2325. doi: 10.1002/pola.22558. [DOI] [Google Scholar]
- 32.Ye YS, Huang YJ, Chang FC, Xue ZG, Xie XL. Synthesis and characterization of thermally cured polytriazole polymers incorporating main or side chain benzoxazine crosslinking moieties. Polym. Chem. 2014;5:2863–2871. doi: 10.1039/c3py01432k. [DOI] [Google Scholar]
- 33.Demir KD, Kiskan B, Aydogan B, Yagci Y. Thermally curable main-chain benzoxazine prepolymers via polycondensation route. React. Funct. Polym. 2013;73:346–359. doi: 10.1016/j.reactfunctpolym.2012.04.016. [DOI] [Google Scholar]
- 34.Hanbeyoglu B, Kiskan B, Yagci Y. Hydroxyl Functional Polybenzoxazine Precursor as a Versatile Platform for Post-Polymer Modifications. Macromolecules. 2013;46:8434–8440. doi: 10.1021/ma401888g. [DOI] [Google Scholar]
- 35.Bektas S, Kiskan B, Orakdogen N, Yagci Y. Synthesis and properties of organo-gels by thiol-benzoxazine chemistry. Polymer. 2015;75:44–50. doi: 10.1016/j.polymer.2015.08.026. [DOI] [Google Scholar]
- 36.Chernykh A, Liu JP, Ishida H. Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain. Polymer. 2006;47:7664–7669. doi: 10.1016/j.polymer.2006.08.041. [DOI] [Google Scholar]
- 37.Takeichi T, Kano T, Agag T. Synthesis and thermal cure of high molecular weight polybenzoxazine precursors and the properties of the thermosets. Polymer. 2005;46:12172–12180. doi: 10.1016/j.polymer.2005.10.088. [DOI] [Google Scholar]
- 38.Ates S, et al. Synthesis, characterization and thermally activated curing of polysulfones with benzoxazine end groups. Polymer. 2011;52:1504–1509. doi: 10.1016/j.polymer.2011.01.051. [DOI] [Google Scholar]
- 39.Lin CH, et al. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym. Chem. 2012;3:935–945. doi: 10.1039/c2py00449f. [DOI] [Google Scholar]
- 40.Kiskan B, Yagci Y, Ishida H. Synthesis, characterization,, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain. J. Polym. Sci. Part A: Polym. Chem. 2008;46:414–420. doi: 10.1002/pola.22392. [DOI] [Google Scholar]
- 41.Semerci E, Kiskan B, Yagci Y. Thiol reactive polybenzoxazine precursors: A novel route to functional polymers by thiol-oxazine chemistry. Eur. Polym. J. 2015;69:636–641. doi: 10.1016/j.eurpolymj.2015.02.030. [DOI] [Google Scholar]
- 42.Kawaguchi AW, Sudo A, Endo T. Polymerization–Depolymerization System Based on Reversible Addition-Dissociation Reaction of 1,3-Benzoxazine with Thiol. ACS Macro Lett. 2012;2:1–4. doi: 10.1021/mz3005296. [DOI] [PubMed] [Google Scholar]
- 43.Arslan M, Kiskan B, Yagci Y. Post-Modification of Polybutadienes by Photoinduced Hydrogen Abstraction from Benzoxazines and Their Thermally Activated Curing. Macromolecules. 2016;49:5026–5032. doi: 10.1021/acs.macromol.6b01329. [DOI] [Google Scholar]
- 44.Kiskan B, Yagci Y, Sahmethogilu E, Toppare L. Preparation of conductive polybenzoxazines by oxidative polymerization. J. Polym. Sci. Part A: Polym. Chem. 2007;45:999–1006. doi: 10.1002/pola.21848. [DOI] [Google Scholar]
- 45.Liao C-S, Wang C-F, Lin H-C, Chou H-Y, Chang F-C. Fabrication of Patterned Superhydrophobic Polybenzoxazine Hybrid Surfaces. Langmuir. 2009;25:3359–3362. doi: 10.1021/la900176c. [DOI] [PubMed] [Google Scholar]
- 46.Aydogan C, Kiskan B, Hacioglu SO, Toppare L, Yagci Y. Electrochemical manipulation of adhesion strength of polybenzoxazines on metal surfaces: from strong adhesion to dismantling. RSC Adv. 2014;4:27545–27551. doi: 10.1039/c4ra03763d. [DOI] [Google Scholar]
- 47.Sarawut R, Montha L, Kasinee H, Pornnapa K, Isala D. Shape memory polymers from benzoxazine-modified epoxy. Smart Mater. Struct. 2013;22:075033. doi: 10.1088/0964-1726/22/7/075033. [DOI] [Google Scholar]
- 48.Cheng H, et al. Super flame-retardant lightweight rime-like carbon-phenolic nanofoam. Sci. Rep. 2016;6:33480. doi: 10.1038/srep33480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C-F, et al. Low-Surface-Free-Energy Materials Based on Polybenzoxazines. Angew. Chem. Int. Edit. 2006;45:2248–2251. doi: 10.1002/anie.200503957. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Lu X, Xin Z, Zhou C. Synthesis and Surface Properties of Low Surface Free Energy Silane-Functional Polybenzoxazine Films. Langmuir. 2013;29:411–416. doi: 10.1021/la303730m. [DOI] [PubMed] [Google Scholar]
- 51.Kuo S-W, Wu Y-C, Wang C-F, Jeong K-U. Preparing Low-Surface-Energy Polymer Materials by Minimizing Intermolecular Hydrogen-Bonding Interactions. J. Phys. Chem. C. 2009;113:20666–20673. doi: 10.1021/jp9059642. [DOI] [Google Scholar]
- 52.Zhang W, Lu X, Xin Z, Zhou C. A self-cleaning polybenzoxazine/TiO2 surface with superhydrophobicity and superoleophilicity for oil/water separation. Nanoscale. 2015;7:19476–19483. doi: 10.1039/C5NR06425B. [DOI] [PubMed] [Google Scholar]
- 53.Lin L-C, et al. Novel near-infrared and multi-colored electrochromic polybenzoxazines with electroactive triarylamine moieties. J. Mater. Chem. C. 2014;2:7796–7803. doi: 10.1039/C4TC01469C. [DOI] [Google Scholar]
- 54.Shih H-K, Chu Y-L, Chang F-C, Zhu C-Y, Kuo S-W. A cross-linkable triphenylamine derivative as a hole injection/transporting material in organic light-emitting diodes. Polym. Chem. 2015;6:6227–6237. doi: 10.1039/C5PY00882D. [DOI] [Google Scholar]
- 55.Kiskan B, Yagci Y. Self‐healing of poly (propylene oxide)‐polybenzoxazine thermosets by photoinduced coumarine dimerization. J. Polym. Sci. Part A: Polym. Chem. 2014;52:2911–2918. doi: 10.1002/pola.27323. [DOI] [Google Scholar]
- 56.Taskin OS, Kiskan B, Yagci Y. Polybenzoxazine Precursors As Self-Healing Agents for Polysulfones. Macromolecules. 2013;46:8773–8778. doi: 10.1021/ma4019153. [DOI] [Google Scholar]
- 57.Arslan M, Kiskan B, Yagci Y. Benzoxazine-Based Thermosets with Autonomous Self-Healing Ability. Macromolecules. 2015;48:1329–1334. doi: 10.1021/ma5025126. [DOI] [Google Scholar]
- 58.Ghosh A, Shukla S, Khosla GS, Lochab B, Mitra S. Sustainable Sulfur-rich Copolymer/Graphene Composite as Lithium-Sulfur Battery Cathode with Excellent Electrochemical Performance. Sci. Rep. 2016;6:25207. doi: 10.1038/srep25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorodisher, I., DeVoe, R. J. & Webb, R. J. in Handbook of Benzoxazine Resins (ed Hatsuo IshidaTarek Agag) 211-234 (Elsevier, 2011).
- 60.Lian Q, Li Y, Li K, Cheng J, Zhang J. Insights into the Vulcanization Mechanism through a Simple and Facile Approach to the Sulfur Cleavage Behavior. Macromolecules. 2017;50:803–810. doi: 10.1021/acs.macromol.6b02335. [DOI] [Google Scholar]
- 61.Griebel JJ, Li G, Glass RS, Char K, Pyun J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A: Polym. Chem. 2015;53:173–177. doi: 10.1002/pola.27314. [DOI] [Google Scholar]
- 62.Chung WJ, et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013;5:518–524. doi: 10.1038/nchem.1624. [DOI] [PubMed] [Google Scholar]
- 63.Griebel JJ, et al. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015;4:862–866. doi: 10.1021/acsmacrolett.5b00502. [DOI] [PubMed] [Google Scholar]
- 64.Griebel JJ, et al. New Infrared Transmitting Material via Inverse Vulcanization of Elemental Sulfur to Prepare High Refractive Index Polymers. Adv. Mater. 2014;26:3014–3018. doi: 10.1002/adma.201305607. [DOI] [PubMed] [Google Scholar]
- 65.Simmonds AG, et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li–S Batteries. ACS Macro Lett. 2014;3:229–232. doi: 10.1021/mz400649w. [DOI] [PubMed] [Google Scholar]
- 66.Arslan M, Kiskan B, Cengiz EC, Demir-Cakan R, Yagci Y. Inverse vulcanization of bismaleimide and divinylbenzene by elemental sulfur for lithium sulfur batteries. Eur. Polym. J. 2016;80:70–77. doi: 10.1016/j.eurpolymj.2016.05.007. [DOI] [Google Scholar]
- 67.Crockett MP, et al. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Angew. Chem. Int. Edit. 2016;55:1714–1718. doi: 10.1002/anie.201508708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wool RP. Self-healing materials: a review. Soft Matter. 2008;4:400–418. doi: 10.1039/b711716g. [DOI] [PubMed] [Google Scholar]
- 69.Wu DY, Meure S, Solomon D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008;33:479–522. doi: 10.1016/j.progpolymsci.2008.02.001. [DOI] [Google Scholar]
- 70.Xu WM, Rong MZ, Zhang MQ. Sunlight driven self-healing, reshaping and recycling of a robust, transparent and yellowing-resistant polymer. J. Mater. Chem. A. 2016;4:10683–10690. doi: 10.1039/C6TA02662A. [DOI] [Google Scholar]
- 71.Xiang HP, Rong MZ, Zhang MQ. Self-healing, Reshaping, and Recycling of Vulcanized Chloroprene Rubber: A Case Study of Multitask Cyclic Utilization of Cross-linked Polymer. ACS Sustainable Chemistry & Engineering. 2016;4:2715–2724. doi: 10.1021/acssuschemeng.6b00224. [DOI] [Google Scholar]
- 72.Arslan M, Kiskan B, Yagci Y. Combining Elemental Sulfur with Polybenzoxazines via Inverse Vulcanization. Macromolecules. 2016;49:767–773. doi: 10.1021/acs.macromol.5b02791. [DOI] [Google Scholar]
- 73.Kim HD, Ishida H. A study on hydrogen-bonded network structure of polybenzoxazines. J. Phys. Chem. A. 2002;106:3271–3280. doi: 10.1021/jp010606p. [DOI] [Google Scholar]
- 74.Brown EN, Sottos NR, White SR. Fracture testing of a self-healing polymer composite. Exp. Mech. 2002;42:372–379. doi: 10.1007/BF02412141. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


