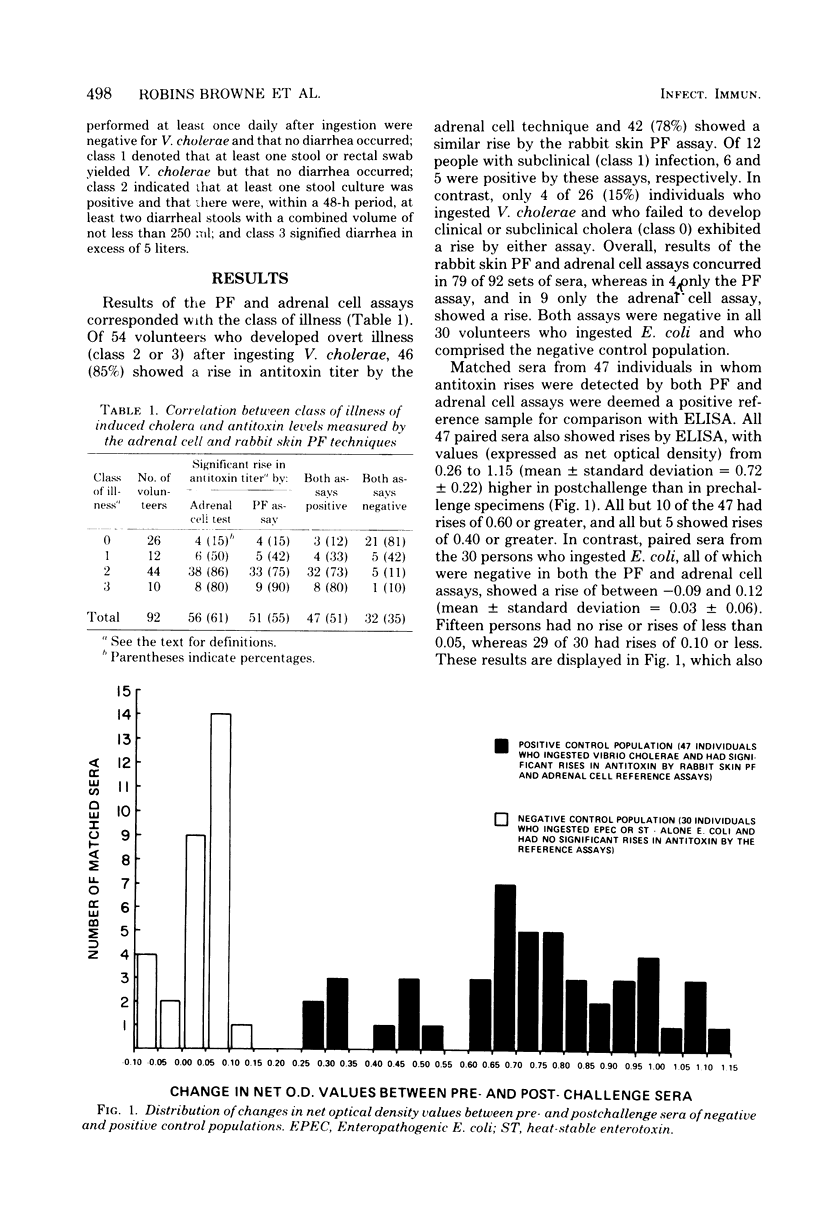
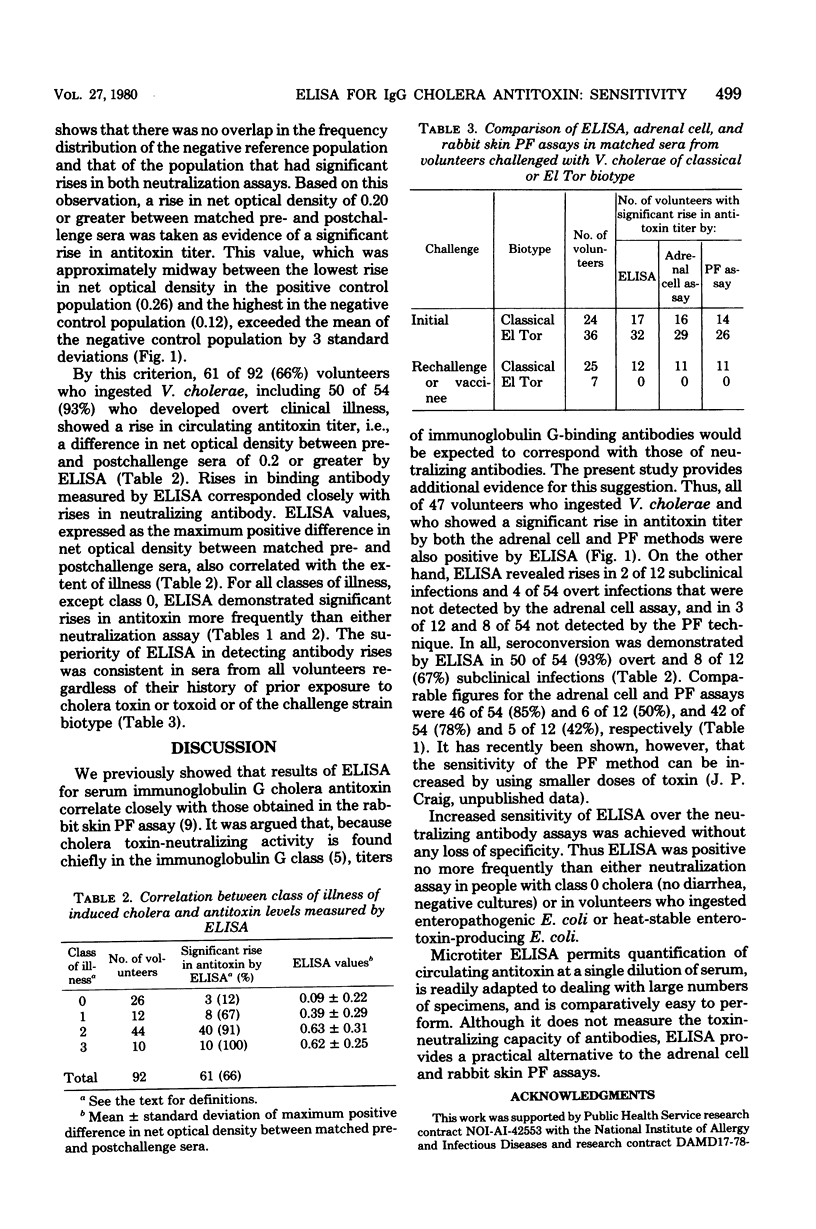
Abstract
Serum samples were obtained from 92 informed, community volunteers before and 10, 21, and 28 days after they ingested 103 to 106Vibrio cholerae of Inaba or Ogawa serotype and classical or El Tor biotype as part of a cholera vaccine development program. Pre- and postchallenge sera were examined for neutralizing antibody to cholera toxin by the rabbit skin permeability factor and adrenal cell techniques. Immunoglobulin G-binding antibodies to cholera toxin were quantitated by enzyme-linked immunosorbent assay (ELISA) in serum diluted 1:200. The results obtained in these cholera volunteers were compared with a negative control population comprising 30 people who ingested enteropathogenic Escherichia coli or E. coli which produced heat-stable but not heat-labile enterotoxin. Although all three antitoxin assays correlated closely with each other in both groups of volunteers, ELISA was more sensitive than either neutralization assay in detecting both subclinical and overt cholera infections. Seroconversion was demonstrated by ELISA in 58 of 66 (88%) volunteers who excreted V. cholerae, including 50 of 54 (93%) with clinical cholera, compared with 47 of 66 (71%) and 52 of 66 (79%) by the rabbit skin permeability factor and adrenal cell techniques, respectively. Although ELISA does not measure the toxin-neutralizing activity of antibodies directly, it provides a practical alternative to the rabbit skin permeability factor and adrenal cell assays.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benenson A. S., Saad A., Mosley W. H., Ahmed A. Serological studies in cholera. 3. Serum toxin neutralization--rise in titre in response to infection with Vibrio cholerae, and the level in the "normal" population of East Pakistan. Bull World Health Organ. 1968;38(2):287–295. [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Snyder M. J., Wenzel R. P., Hornick R. B. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974 Jan;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Eichner E. R., Hornick R. B. Cutaneous responses to cholera skin toxin in man. I. Responses in unimmunized American males. J Infect Dis. 1972 Mar;125(3):203–215. doi: 10.1093/infdis/125.3.203. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Hughes T. P., Young C. R., O'Donnell S., Craig J. P., Holley H. P., Bergquist E. J. Antigenicity of purified glutaraldehyde-treated cholera toxoid administered orally. Infect Immun. 1978 Jul;21(1):158–162. doi: 10.1128/iai.21.1.158-162.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Young C. R., Levine M. M., Craig J. P., Robins-Browne R. Microtiter enzyme-linked immunosorbent assay for immunoglobulin G cholera antitoxin in humans: method and correlation with rabbit skin vascular permeability factor technique. Infect Immun. 1980 Feb;27(2):492–496. doi: 10.1128/iai.27.2.492-496.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


