Abstract
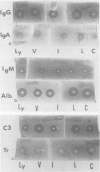
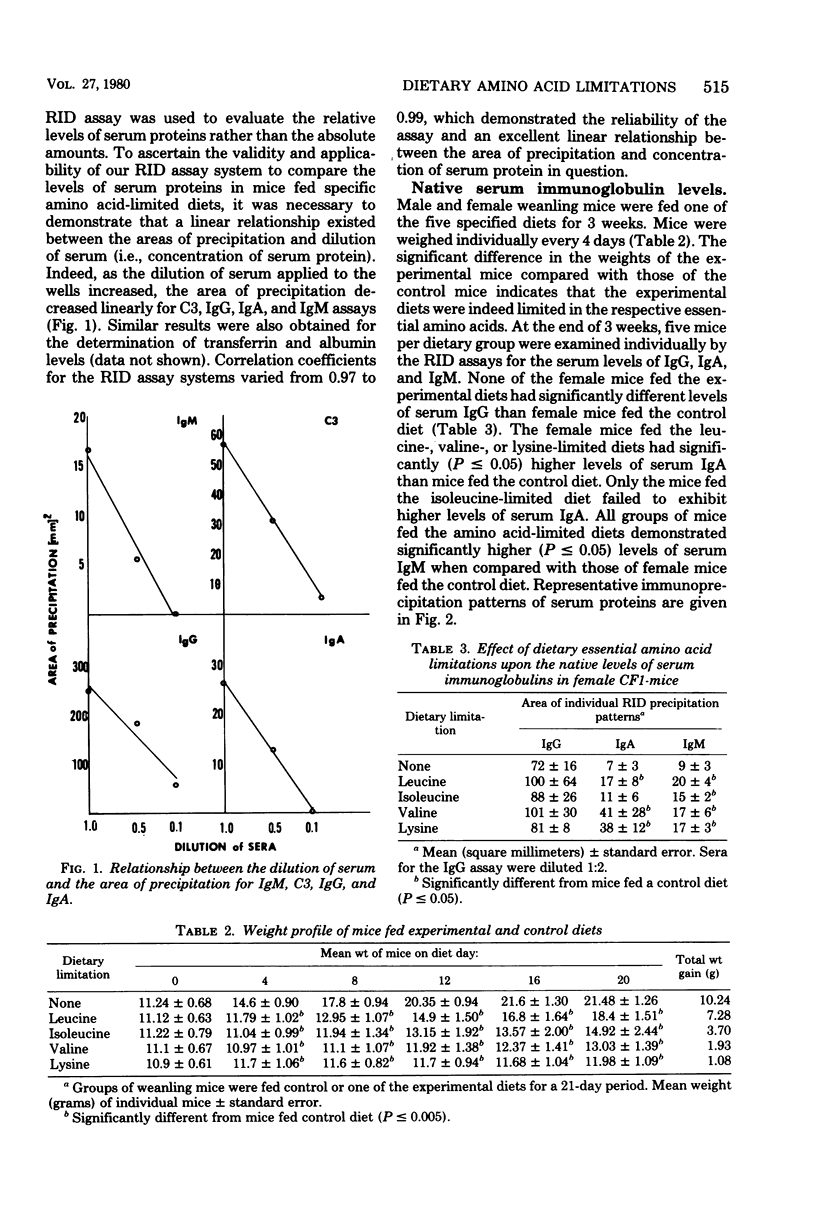
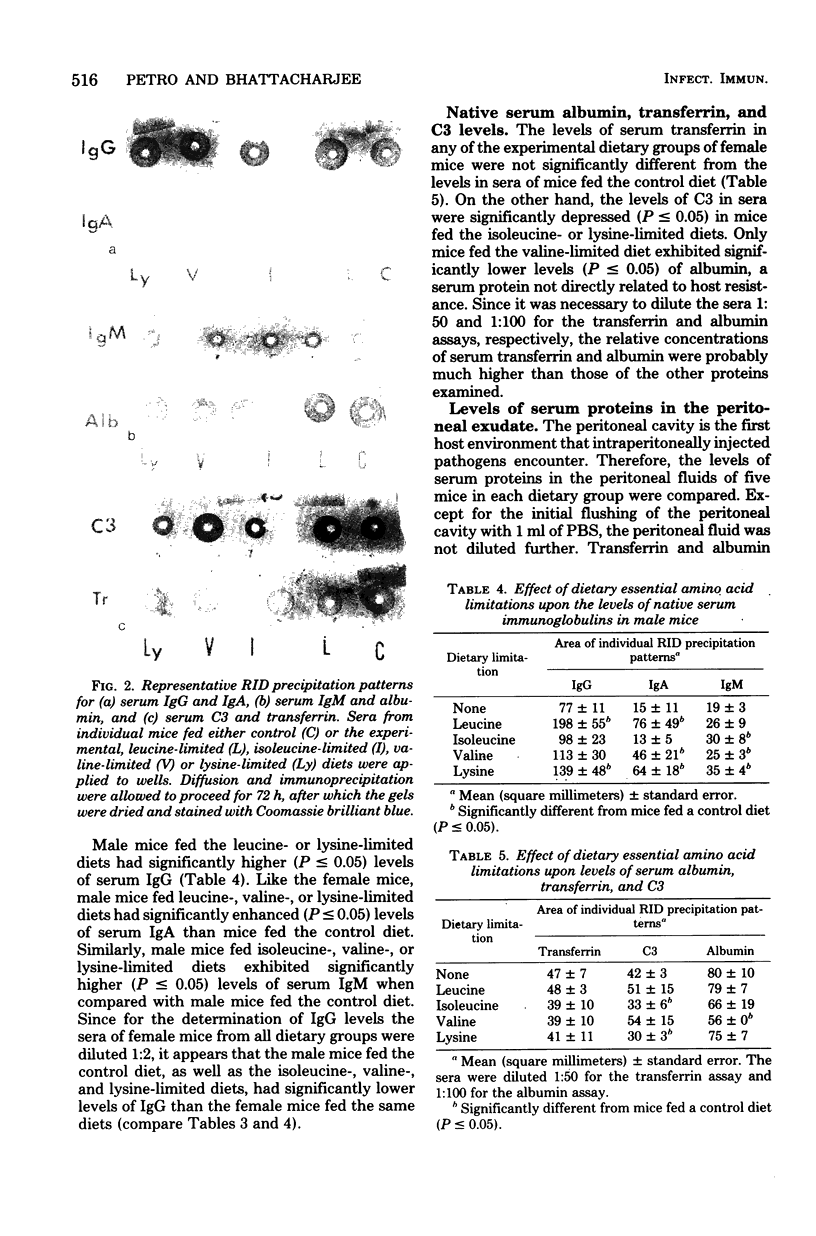
Effects of dietary essential amino acid (leucine, isoleucine, valine, or lysine) limitations upon the native levels of serum immunoglobulin G (IgG), IgM, and IgA, as well as transferrin, the third component of complement (C3), and albumin were determined in CF1 mice by the radial immunodiffusion technique. Male mice fed the control diet and the isoleucine- or valine-limited diets exhibited lower levels of serum IgG than the female mice fed the same diets. Levels of IgG in female experimental mice were not significantly different than those in female control mice. Serum IgM levels in male or female mice fed the experimental diets were higher than those in the control mice. Except for the mice fed the isoleucine-limited diet, serum IgA levels were higher in male and female mice fed the experimental diets than in mice fed the control diet. Serum transferrin levels were not significantly different in mice fed the experimental diets when compared with mice fed the control diet. However, the levels of transferrin found in the peritoneal exudates were lower in mice fed the isoleucine- or lysine-limited diets than in mice fed the control diet. In mice fed the isoleucine- and lysine-limited diets, the levels of serum C3 were significantly lower than those in control mice. These results indicate that the native levels of serum immunoglobulins are increased in some instances and that the levels of serum C3 and the transferrin in the peritoneal fluid are significantly decreased in certain dietary groups of mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTOK L., KEMENES F. THE EFFECT OF VARIOUS EXPERIMENTAL PATHOPHYSIOLOGICAL CONDITIONS ON THE ANTIBODY PRODUCTION. III. INFLUENCE OF THE ALIMENTARY DEFICIENCY OF GLYCINE, ISOLEUCINE OR TRYPTOPHANE. Z Immunitats Allergieforsch. 1963 Dec;125:438–445. [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S. Activation of complement by opportunist pathogens and chemotypes of Salmonella minnesota. Infect Immun. 1977 Jun;16(3):748–753. doi: 10.1128/iai.16.3.748-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounous G., Kongshavn P. A. The effect of dietary amino acids on immune reactivity. Immunology. 1978 Aug;35(2):257–266. [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effect of nutrition on the resistance of mice to endotoxin and on the bactericidal power of their tissues. J Exp Med. 1959 Dec 1;110:935–950. doi: 10.1084/jem.110.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edozien J. C., Niehaus N., Mar M. H., Makoui T., Switzer B. R. Diet-hormone interrelationships in the rat. J Nutr. 1978 Nov;108(11):1767–1776. doi: 10.1093/jn/108.11.1767. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY I. Lysine deficiency and host resistance to anthrax. J Exp Med. 1963 Mar 1;117:497–508. doi: 10.1084/jem.117.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles K. W., Hobday S. M. Improving plant proteins for human needs. World Rev Nutr Diet. 1976;25:217–248. doi: 10.1159/000399573. [DOI] [PubMed] [Google Scholar]
- Gill TJ [. D., Gershoff S. N. The effects of methionine and ethionine on antibody formation in primates. J Immunol. 1967 Nov;99(5):883–893. [PubMed] [Google Scholar]
- John A. M., Bell J. M. Amino acid requirements of the growing mouse. J Nutr. 1976 Sep;106(9):1361–1367. doi: 10.1093/jn/106.9.1361. [DOI] [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Quantitative effects of nutritional essential amino acid deficiency upon immune responses to tumors in mice. J Exp Med. 1973 Jan 1;137(1):1–9. doi: 10.1084/jem.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEY M. A., ARNRICH L., MAR E., RODERUCK C. E. INFLUENCE OF DIETARY PROTEIN ON COMPLEMENT, PROPERDIN, AND HEMOLYSIN IN ADULT PROTEIN-DEPLETED RATS. J Nutr. 1965 Feb;85:213–220. doi: 10.1093/jn/85.2.213. [DOI] [PubMed] [Google Scholar]
- Kelsoe G., Cerny J. Regulation of the immune response. I. Regulatory cell equilibrium in the "virgin" state. Eur J Immunol. 1978 Mar;8(3):176–180. doi: 10.1002/eji.1830080307. [DOI] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Wasynczuk J., McCabe M. A. Effects of injected iron and siderophores on infections in normal and immune mice. Infect Immun. 1978 Nov;22(2):560–567. doi: 10.1128/iai.22.2.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McMurray D. N., Rey H., Casazza L. J., Watson R. R. Effect of moderate malnutrition on concentrations of immunoglobulins and enzymes in tears and saliva of young Colombian children. Am J Clin Nutr. 1977 Dec;30(12):1944–1948. doi: 10.1093/ajcn/30.12.1944. [DOI] [PubMed] [Google Scholar]
- Meléndez M., González M. C., Reid M., Fuentes C., Castillo D. Immunity to antigenically related salmonellae: effects of humoral factors on the bactericidal activity of normal and immune peritoneal exudate cells. Infect Immun. 1978 Dec;22(3):640–643. doi: 10.1128/iai.22.3.640-643.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberne P. M., Hunt C. E., Young V. R. The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br J Exp Pathol. 1968 Oct;49(5):448–457. [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. Effect of dietary proteins and amino acids on the susceptibility of mice to bacterial infections. J Exp Med. 1959 Dec 1;110:921–934. doi: 10.1084/jem.110.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen M. V., Häyry P. O antigen as virulence factor in mouse typhoid: effect of B-cell suppression. Infect Immun. 1978 Jan;19(1):26–28. doi: 10.1128/iai.19.1.26-28.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Ganguly R. Immunity to infections on secretory surfaces. J Infect Dis. 1974 Oct;130(4):419–440. doi: 10.1093/infdis/130.4.419. [DOI] [PubMed] [Google Scholar]
- Watson R. R., Reyes M. A., McMurray D. N. Influence of malnutrition on the concentration of IgA, lysozyme, amylase and aminopeptidase in children's tears. Proc Soc Exp Biol Med. 1978 Feb;157(2):215–219. doi: 10.3181/00379727-157-40024. [DOI] [PubMed] [Google Scholar]
- Wostmann B. S., Pleasants J. R., Bealmear P., Kincade P. W. Serum proteins and lymphoid tissues in germ-free mice fed a chemically defined, water soluble, low molecular weight diet. Immunology. 1970 Sep;19(3):443–448. [PMC free article] [PubMed] [Google Scholar]
- YAEGER R. G., MILLER O. N. EFFECT OF LYSINE DEFICIENCY ON CHAGAS' DISEASE IN LABORATORY RATS. J Nutr. 1963 Oct;81:169–174. doi: 10.1093/jn/81.2.169. [DOI] [PubMed] [Google Scholar]