Abstract
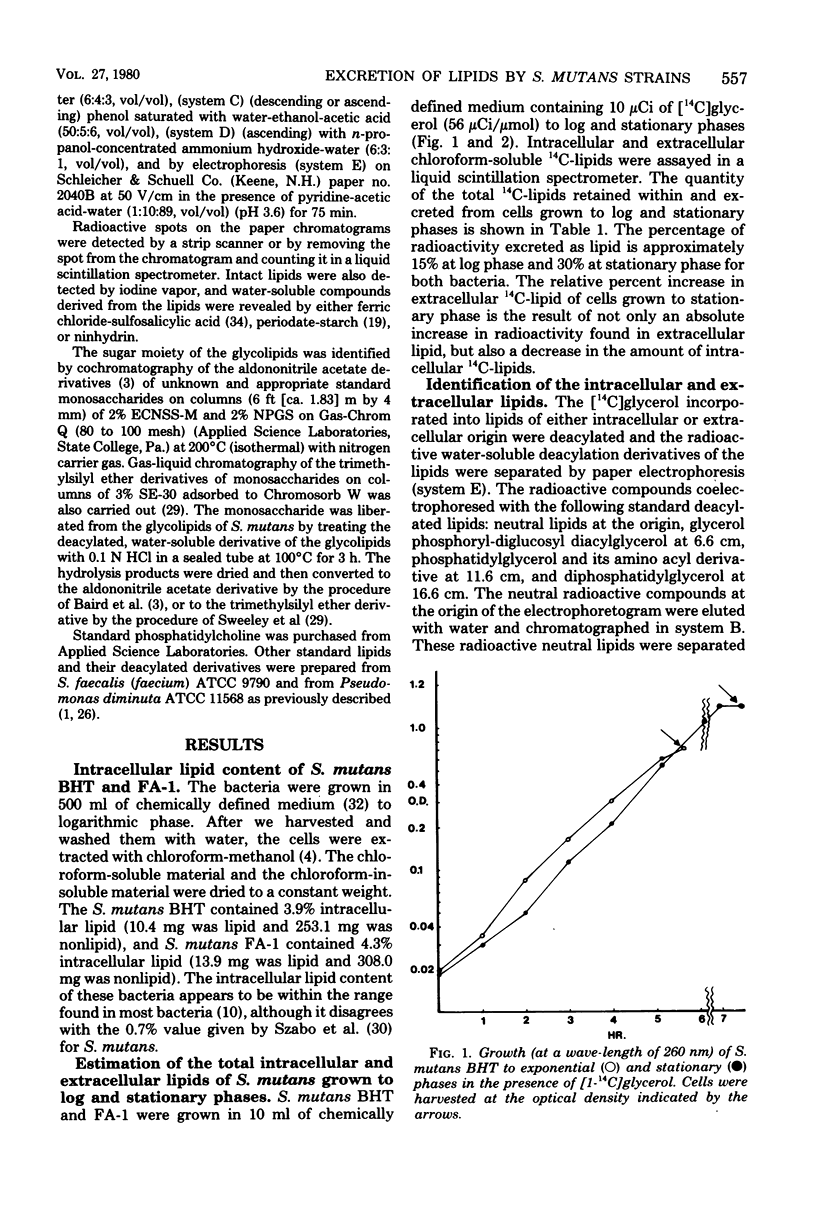
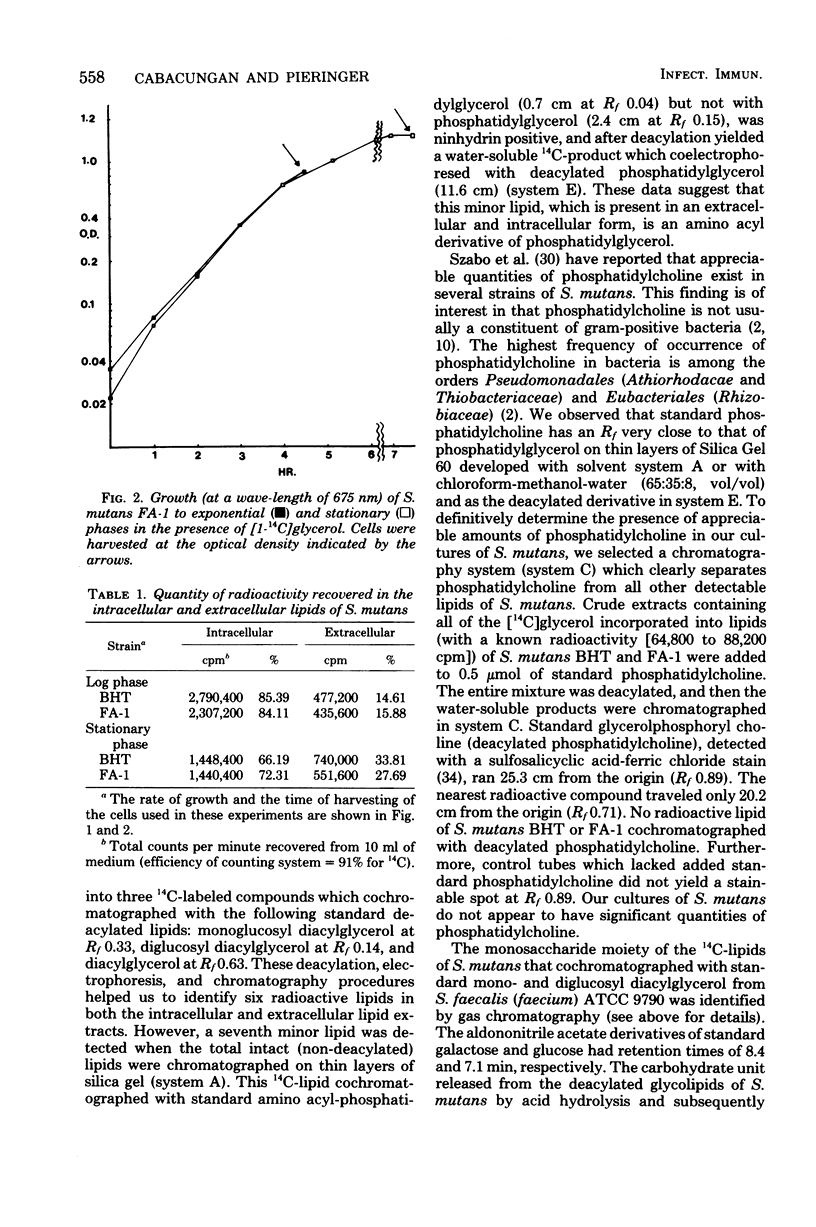
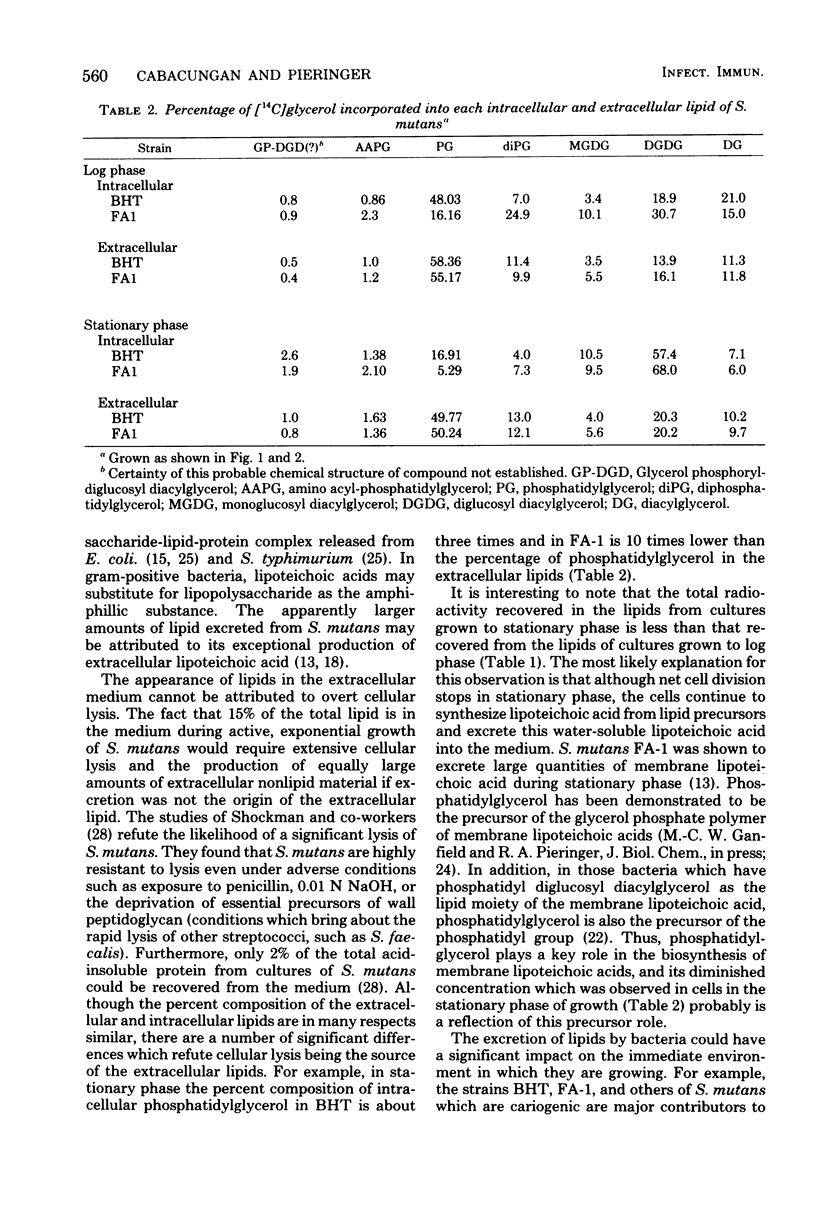
Streptococcus mutans BHT and FA-1, when grown to log phase on chemically defined medium containing [14C]glycerol, excreted 15% of the total biosynthesized 14C-lipid into the medium. When grown to early stationary phase, 28 to 33% of the 14C-lipid was found in the medium. The radioactive lipids of these varieties of S. mutans were identified as diacylglycerol, diglucosyl diacylglycerol (DGD), monoglucosyl diacylglycerol, diphosphatidylglycerol, phosphatidylglycerol (PG), and smaller amounts of two other lipids tentatively were identified as amino acyl-PG and glycerol phosphoryl-DGD. All lipids were found as extracellular and intracellular components from cells grown to either log or stationary phase. However, there were some shifts in the relative percentage of these lipids as the cells changed from log to stationary phase. For example, the intracellular lipid content of log-phase S. mutans BHT was composed of 49% PG and 19% DGD, but these percents shifted to 18% PG and 57% DGD when the cells were grown to stationary phase. However, the extracellular lipids of this organism contained 50 to 60% PG and 20% DGD in both log and stationary phases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Pieringer R. A. The metabolism of glyceride glycolipids. V. Identification of the membrane lipid formed from diglucosyl diglyceride in Streptococcus faecalis ATCC 9790 as an acylated derivative of glyceryl phosphoryl diglucosyl glycerol. J Biol Chem. 1971 Jul 10;246(13):4216–4225. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowfoot P. D., Esfahani M., Wakil S. J. Relation between protein synthesis and phospholipid synthesis and turnover in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1408–1415. doi: 10.1128/jb.112.3.1408-1415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowfoot P. D., Oka T., Esfahani M., Wakil S. J. Turnover of phospholipids in an unsaturated fatty acid auxotroph of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1396–1407. doi: 10.1128/jb.112.3.1396-1407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- Harlander S. K., Schachtele C. F. Streptococcus mutans dextransucrase: stimulation of glucan formation by phosphoglycerides. Infect Immun. 1978 Feb;19(2):450–456. doi: 10.1128/iai.19.2.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Mayo H. E., Tremblay R. Development of dental plaque on epoxy resin crowns in man. A light and electron microscopic study. J Periodontol. 1975 Jan;46(1):10–26. doi: 10.1902/jop.1975.46.1.10. [DOI] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neskovic N., Sarlieve L., Nussbaum J. L., Kostic D., Mandel P. Quantitative thin-layer chromatography of glycolipids in animal tissues. Clin Chim Acta. 1972 Apr;38(1):147–153. doi: 10.1016/0009-8981(72)90220-3. [DOI] [PubMed] [Google Scholar]
- Pieringer R. A., Ambron R. T. A method for the specific labeling of the glycerol in glyceride-containing lipids of Streptococcus faecalis ATCC 9790. J Lipid Res. 1973 May;14(3):370–372. [PubMed] [Google Scholar]
- Pieringer R. A. Biosynthesis of the phosphatidyl diglucosyl diglyceride of Streptococcus faecalis (ATCC 9730) from diglyucosyl diglyceride and phosphatidyl glycerol or diphosphatidyl glycerol. Biochem Biophys Res Commun. 1972 Oct 17;49(2):502–507. doi: 10.1016/0006-291x(72)90439-1. [DOI] [PubMed] [Google Scholar]
- Pieringer R. A., Shaw J. M., Ganfield M. C. The role of phosphatidylglycerol as a donor of phosphatidyl and of sn-glycerol-1-phosphate groups in biosynthetic reactions. Adv Exp Med Biol. 1978;101:279–285. doi: 10.1007/978-1-4615-9071-2_27. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Shaw J. M., Pieringer R. A. Phosphatidylmonoglucosyl diacylglycerol of Pseudomononas diminuta ATCC 11568. In vitro biosynthesis from phosphatidylglycerol and glucosyl diacylglycerol. J Biol Chem. 1977 Jun 25;252(12):4395–4401. [PubMed] [Google Scholar]
- Szabo E. I., Amdur B. H., Socransky S. S. Lipid composition of Streptococcus mutans. Caries Res. 1978;12(1):21–27. doi: 10.1159/000260311. [DOI] [PubMed] [Google Scholar]
- Takazoe I., Matsukubo T., Katow T. Experimental formation of "corn cob" in vitro. J Dent Res. 1978 Feb;57(2):384–387. doi: 10.1177/00220345780570024101. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp J. H. Biochemical changes in Bifidobacterium bifidum var. pennsylvanicus after cell wall inhibition. IX. Metabolism and release of cellular lipids in the presence of antibiotics. Biochim Biophys Acta. 1976 Dec 20;450(3):277–287. doi: 10.1016/0005-2760(76)90001-1. [DOI] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. Detection of phosphate esters on paper chromatograms. Nature. 1953 Mar 21;171(4351):529–530. doi: 10.1038/171529a0. [DOI] [PubMed] [Google Scholar]


