Abstract
[Purpose]
The central fatigue hypothesis suggests that exhaustion, or the maximum level of exercise, induces excessive stress and increases serotonin concentrations in the brain, which in turn decreases central nervous system (CNS) function and induces fatigue. Our aim was to determine the effects of colostrum serum on the serotonergic system in the dorsal raphe nuclei during exhaustive exercise.
[Methods]
Animals were randomly divided into five groups: control, exercise, exercise and treatment with 50, 100, and 200 mg/kg of colostrum serum. The rats in the colostrum serum treatment groups were fed colostrum serum at three different doses of 50, 100, and 200 mg/kg per day for seven days. The rats in the control and exercise groups received water by oral gavage once per day for seven days.
[Results]
The time to exhaustion in response to treadmill running increased after treatment with colostrum serum. These results show that exhaustive exercise led to over activation of the serotonergic system in the dorsal raphe nuclei, and that treatment with colostrum serum suppressed of the exercise-induced expression of tryptophan hydroxylase (TPH) and serotonin (5-HT). The results also indicated that exhaustive exercise induced 5-HT1A autoreceptor and serotonin transporter (5-HTT) overexpression in the dorsal raphe nuclei, and that colostrum serum treatment suppressed exhaustive exercise-induced 5-HT1A and 5-HTT expression in the dorsal raphe nuclei. The most effective dose of colostrum serum was 100 mg/kg.
[Conclusion]
Overall, our study suggests that colostrum serum has positive effects on exercise performance and recovery by increasing the resistance to fatigue.
Keywords: Colostrum serum, Central fatigue, Serotonergic system, Exhaustive exercise
INTRODUCTION
The activity in the brain that mediates the desire for optimal exercise performance is influenced by many factors. Such activity affects the cross-bridges of the muscles, which drive muscle contraction. The mechanisms involved in fatigue during exercise are also initiated in the central nervous system (CNS). The causes of the fatigue that occurs during exercise include psychological factors, health conditions, environmental factors, depletion of the energy sources required for muscle activities, the accumulation of metabolites that are produced during exercise, and disruption of the enzyme systems involved in energy metabolism1. Very intense endurance exercise training is associated with symptoms such as a decrease in exercise performance, fatigue, mood disorders, somnipathy, loss of appetite, and anxiety2. The fatigue-related reduction in skeletal muscle power is caused by alterations in efferents from the brain, since the brain does not recruit additional motor units during prolonged exercise because additional recruitment would threaten the capacity to maintain homeostasis, damage various organs, and reduce exercise performance3. The situation predicts that an increased perception of discomfort is produced by exhaustive exercise4. Serotonin (5-hydroxytryptamine or 5-HT), which can act as a cerebral vasoconstrictor, is associated with depression, sleep, and mood disorders. There is growing interest in the role of serotonin as a key factor in the induction of fatigue. Notably, fluctuations in serotoninergic activity during prolonged exercise induce and delay fatigue5-7. Regular and adequate exercise can improve the mood of patients with depression, while excessive exercise may induce mood disorders.
The central fatigue hypothesis suggests that exercising to exhaustion induces excessive stress on the CNS, which then induces an excess of serotonin synthesis and metabolism in the brain leading to fatigue and a reduction in exercise performance in both humans and animals8-10. Serotonin is mainly synthesized in serotonergic neurons in the raphe nuclei of the midbrain and in neurosecretory cells in the pineal gland. Tryptophan hydroxylase (TPH) is an important enzyme that is involved in the synthesis of serotonin11. The modulation of the TPH gene is crucial for the regulation of the serotonergic system. An increase in TPH mRNA levels enhances TPH activity and the effects of serotonin12.
Sports drinks have recently been developed to improve endurance exercise abilities, athletic performance, and recovery from fatigue by enhancing muscle strength13 and suppressing muscle fatigue14. Similar to sports drinks, milk has recently been recognized to be highly effective for rehydration after resistance or endurance exercises15, with some studies showing that milk intake after exercise is as effective as drinks containing both carbohydrates and electrolytes16. Colostrum, which is secreted from a mother during the first 72 h after infant delivery, is particularly abundant in essential nutrients and bioactive factors, including cytokines and various growth factors that promote cell proliferation, collagen and hyaluronic acid production, epithelial cell growth, and angiogenesis17. These molecules play an important role in immunity, growth, and biodefense functions, such as wound healing and antibiotic functions18, 19. Thus, colostrum is an ample source of energy and nutrients for newborns. It also aids in the defense against pathogenic microorganisms and immature intestinal development, and in the support of organ and tissue growth and immune system development20, 21. The present study examines the ergogenic effects of colostrum. Specifically, we investigate whether colostrum delays the central fatigue that is induced by high-intensity exhaustive exercise through activation of the serotonergic system in the dorsal raphe nuclei of the rat midbrain.
METHODS
Animals Treatments
Male Sprague-Dawley rats weighing 150.6 ± 10 g (six weeks old) were obtained from a commercial breeder (Orient Co., Seoul, Korea). The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The rats were housed under controlled temperature (20 ± 2°C) and lighting (07:00 to 19:00 h) conditions with food and water available ad libitum. Animals were randomly divided into five groups: control, exercise, exercise and 50 mg/kg of colostrum serum, exercise and 100 mg/kg of colostrum serum, and exercise and 200 mg/kg of colostrum serum (n=10 per group). The colostrum serum solution was diluted in the drinking water at concentrations of 50, 100, and 200 mg/kg, and these doses were made up fresh daily. The rats in the colostrum serum treatment groups received colostrum serum at three different doses (50, 100, and 200 mg/kg of body weight, respectively) each day for seven days. The colostrum serum was administered 60 min prior to the start of exercise, which was conducted as described below. The rats in the control and exercise groups received water by oral gavage once per day for seven days.
Measurement of exercise abilities
To measure the effectiveness of colostrum serum in improving exercise ability, all-out running time was measured on a treadmill (Exer-6M treadmill, Columbus instruments, USA) and used as an index for exercise performance. For treadmill adaptation training, an exercise load of 30 min per day for three days was performed. The 30 min treadmill exercise began at a speed of 10 m/min for the first 10 min, increased to 16 m/min for the next 10 min, and increased further to 21 m/min for the last 10 min. Exhaustion was assessed following the conclusion of the experiment on day seven by making the animals run on a treadmill at a speed of 10 m/min for the first 5 min, after which time the treadmill speed was incrementally increased to 16, 18, 21, 24, 26, 29, 32, 34, and 37 m/min with 5 min intervals. The treadmill speed was then increased to 40 m/min until the animals were exhausted6, 22. The animals were considered to be exhausted at the point when they could no longer maintain their balance running at speed for 3 min. In order to avoid the effects of electric shock stress, we used a sponge as a tactile incentive rather than an electric shock-plate.
Tissue preparation
The animals were sacrificed on the seventh day of the experiment, immediately following the determination of the exhaustion time point. To prepare the brain for sectioning, the animals were fully anesthetized with Zoletil 50® (10 mg/kg, intraperitoneally [i.p.; Vibac, Carros, France), following which the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS) and then fixed with a freshly prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer (PB; pH 7.4). The brains were then removed from the skulls, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections with thicknesses of 40 μm were cut using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry for TPH and 5-HT
To visualize TPH and 5-HT expression, immunohistochemistry for TPH and 5-HT was performed in the dorsal raphe nuclei. The dorsal raphe nuclei, spanning from Bregma -7.20 mm to -8.00 mm, were obtained from each brain. The sections were incubated in PBS for 10 min, and then washed three times in the same buffer. The sections were then incubated in 1% hydrogen peroxide (H2O2) for 30 min. The sections were incubated overnight with a rabbit anti-TPH antibody (1:1000; Oncogene Research Products, Cambridge, UK) and a rabbit anti-5-HT antibody (1:500, Abcam, Cambridge, UK). The sections were subsequently incubated with a biotinylated rabbit secondary antibody (1:200; Vector Laboratories) for another 1 h. The signal was amplified with the Vector Elite ABC kit® (1:100; Vector Laboratories). Antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% DAB, and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount®.
Western blot for 5-HT1A and 5-HTT
The dorsal raphe nuclei were collected and immediately frozen at -70°C. Proteins were extracted from the dorsal raphe nuclei samples. The tissues were homogenized with a lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2·6H2O, 1 mM EGTA, 1 mM PMSF, 1 mM Na2VO4, and 100 mM NaF, and then centrifuged using an ultra-centrifuge at 50,000 rpm for 1 h. The protein content in the samples was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). The samples (30 μg protein) were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels, and then transferred to nitrocellulose membranes. Anti-β actin (1:1000; Santa Cruz), anti-5-HT1A (1:1000; Abcam), and anti-5-HTT (1:1000; Abcam) were used as the primary antibodies. A horseradish peroxidase-conjugated anti-mouse secondary antibody was used for β-actin, and an anti-rabbit secondary antibody was used for 5-HT1A and 5-HTT. Experiments were performed under normal laboratory conditions and at room temperature, except for the transfer of proteins to membranes. Proteins were transferred to the nitrocellulose membranes at 4ºC using a cold pack and pre-chilled buffer. The detection of bands was performed using the enhanced chemiluminescence (ECL) detection kit (GE healthcare, AmershamTM). To compare the relative expression of proteins, densitometry analyses were carried out on the bands detected using Molecular AnalystTM, version 1.4.1 (Bio-Rad).
Colostrum serum extraction method
For the production of colostrum serum, bovine colostrum that was milked on the farm and refrigerated for no more than 72 h was centrifuged at 3000 x g for 30 min, resulting in colostrum free from milk fat. To remove any residual substances, colostrum without fat was collected using filter paper. The colostrum that was collected was mixed with 2N HCl to adjust it to a pH of 4.6, corresponding to the isoelectric point of casein, and this was followed by slow stirring for 1 h to precipitate the casein. For complete separation and precipitation, the colostrum was centrifuged at 8700 x g for 30 min and then the supernatant was collected. The filtrate was filtered through a 0.2 μm diameter membrane filter on a clean bench to remove any pathogenic microorganisms, resulting in the final bovine colostrum extract.
Data analysis
To confirm the expression of 5-HT1A and 5-HTT proteins, the bands detected were analyzed densitometrically using Molecular AnalystTM software, version 1.4.1. The numbers of TPH and 5-HT positive cells in the dorsal raphe nuclei were counted hemilaterally using a light microscope (Olympus, Tokyo, Japan) and Image-Pro® Plus (Media Cyberbetics Inc.). Values are expressed as the means ± standard error of the means. Data were analyzed using a one-way analysis of variance (ANOVA), followed by a Duncan’s post hoc test using SPSS software (SPSS, Inc., Chicago, IL, USA). Differences with a p-value <0.05 were deemed to be statistically significant.
RESULTS
Effects of colostrum serum on the time to exhaustion during treadmill running
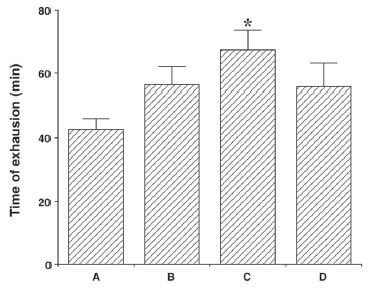
The effects of colostrum serum on the time to exhaustion are presented Fig. 1 The average time to fatigue was 42.33 ± 3.40 min for the exercise group, 56.50 ± 5.79 min for the exercise + 50 mg/kg colostrum serum group, 67.44 ± 6.17 min for the exercise + 100 mg/kg colostrum serum group, and 55.87 ± 7.52 min for the exercise + 200 mg/kg colostrum serum group. These findings indicate that the time to exhaustion in response to treadmill running increased following treatment with colostrum serum (F(3,35)=2.99, p=0.047). Specifically, treatment with 100 mg/kg of colostrum serum significantly increased the time to exhaustion induced by treadmill running when compared with the exercise only group (p=0.027).
Figure 1. Treadmill running time to exhaustion to treatment with colostrum serum. A: exercise group, B: exercise and 50mg/kg of colostrum serum treatment, C: exercise and 100mg/kg of colostrum serum treatment, D: exercise and 200mg/kg of colostrum serum treatment. The data are presented as the mean ± standard error of the mean (S.E.M). * p < 0.05 compared to the exercise group.
Effects of colostrum serum on TPH expression in the dorsal raphe nuclei
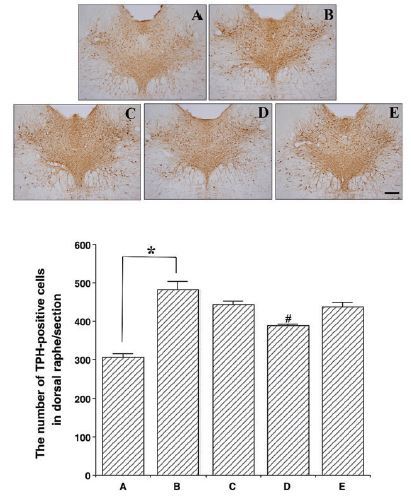
Photomicrographs of the TPH-positive cells in the dorsal raphe nuclei are presented in Fig. 2. The number of TPH-positive cells was 178.22 ± 12.15 for the control group, 359.11 ± 19.68 for the exercise group, 320.33 ± 18.93 for the exercise + 50 mg/kg colostrum serum group, 228.33 ± 11.06 for the exercise + 100 mg/kg colostrum serum group, and 304.33 ± 9.78 for the exercise + 200 mg/kg colostrum serum group. These results indicated that exhaustive exercise induces an overexpression of TPH in the dorsal raphe nuclei, and that treatment with colostrum serum alleviated the exhaustive exercise-induced expression of TPH in the dorsal raphe nuclei (F(4,45)=17.78, p<0.01). Specifically, treatment with 100 mg/kg of colostrum serum significantly suppressed TPH expression in the dorsal raphe nuclei when compared to the exercise group (p<0.01).
Figure 2. Effect colostrum serum on TPH expression in dorsal raphe. Upper: Photomicrograph of TPH-positive cells. The scale bar represents 250 μm (x4). Lower: number of TPH-positive cells in each group. A: control group B: exercise group, C: exercise and 50mg/kg of colostrum serum treatment, D: exercise and 100mg/kg of colostrum serum treatment, E: exercise and 200mg/kg of colostrum serum treatment. The data are presented as the mean ± standard error of the mean (S.E.M). * represents p < 0.05 compared to the control group. # represents p < 0.05 compared to the exercise .
Effects of colostrum serum on 5-HT expression in the dorsal raphe nuclei
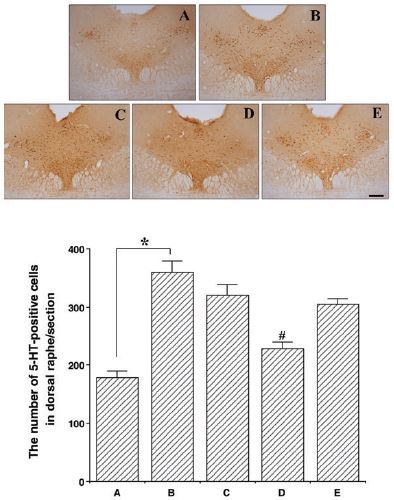
Photomicrographs of 5-HT-positive cells in the dorsal raphe nuclei are presented in Fig. 3. The number of 5-HT-positive cells was 307.11 ± 9.25 in the control group, 483.00 ± 20.32 in the exercise group, 444.22 ± 9.99 in the exercise + 50 mg/kg colostrum serum group, 388.22 ± 4.36 in the exercise + 100 mg/kg colostrum serum group, and 438.00 ± 11.46 in the exercise + 200 mg/kg colostrum serum group. These results indicated that exhaustive exercise induced an overexpression of 5-HT in the dorsal raphe nuclei, and that treatment with colostrum serum alleviated the expression of 5-HT in the dorsal raphe nuclei induced by exhaustive exercise (F(4,45)=15.68, p<0.01). Specifically, treatment with 100 mg/kg of colostrum serum significantly suppressed 5-HT expression in the dorsal raphe nuclei when compared to the exercise group (p<0.001).
Figure 3. Effect colostrum serum on 5-HT expression in dorsal raphe. Upper: Photomicrograph of 5-HT-positive cells. The scale bar represents 250 μm (x4). Lower: number of 5-HT-positive cells in each group. A: control group B: exercise group, C: exercise and 50mg/kg of colostrum serum treatment, D: exercise and 100mg/kg of colostrum serum treatment, E: exercise and 200mg/kg of colostrum serum treatment. The data are presented as the mean ± standard error of the mean (S.E.M). * represents p < 0.05 compared to the control group. # represents p < 0.05 compared to the exercise group.
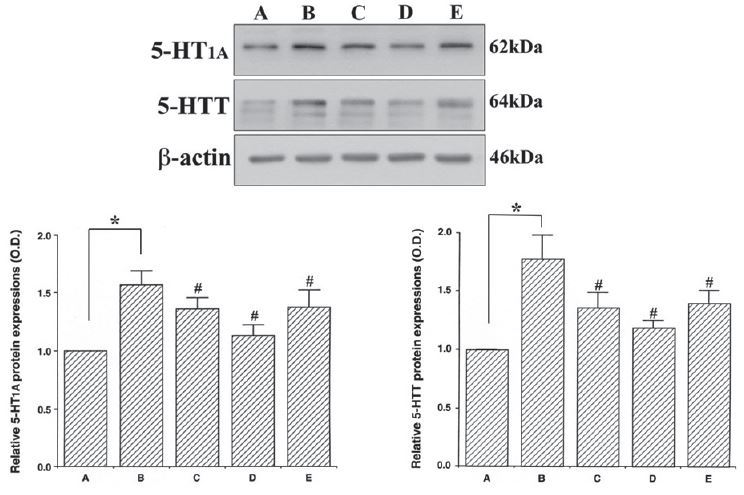
Effects of colostrum serum on 5-HT1A and 5-HTT expression in the dorsal raphe nuclei
The 5-HT1A and 5-HTT protein levels are shown in Fig. 4. When the level of 5-HT1A in the control group was set at 1.00, the level of 5-HT1A was 1.57 ± 0.12 in the exercise group, 1.36 ± 0.10 in the exercise + 50 mg/kg colostrum serum group, 1.13 ± 0.09 in the exercise + 100 mg/kg colostrum serum group, and 1.37 ± 0.15 in the exercise + 200 mg/kg colostrum serum group (F(4,45)=8.48, p<0.01). When the level of 5-HTT in the control group was set at 1.00, the level of 5-HTT was 1.78 ± 0.20 in the exercise group, 1.36 ± 0.13 in the exercise + 50 mg/kg colostrum serum group, 1.19 ± 0.06 in the 100 mg/kg colostrum serum group, and 1.40 ± 0.11 in the 200 mg/kg colostrum serum group (F(4,45)=25.78, p<0.001). These results indicated that exhaustive exercise induces an overexpression of 5-HT1A and 5-HTT in the dorsal raphe nuclei, and that treatment with colostrum serum suppressed the exhaustive exercise-induced expression of 5-HT1A and 5-HTT in the dorsal raphe nuclei. All doses of colostrum serum significantly suppressed 5-HT1A and 5-HTT expression (p=0.012, p<0.001 respectively), with 100 mg/kg being the most effective dose.
Figure 4. Effect colostrum serum on 5-HT1A and 5-HTT expression in dorsal raphe. A: control group B: exercise group, C: exercise and 50mg/kg of colostrum serum treatment, D: exercise and 100mg/kg of colostrum serum treatment, E: exercise and 200mg/kg of colostrum serum treatment. The data are presented as the mean ± standard error of the mean (S.E.M). * represents p < 0.05 compared to the control group. # represents p < 0.05 compared to the exercise group.
DISCUSSION
The fatigue that accompanies exercise is thought to originate from metabolic changes in muscle, such as muscle exhaustion and modifications of the CNS resulting in a decrease in motor neuron impulses to muscles8, 23, 24. The increased brain TPH concentrations and 5-HT synthesis induced by exercise may promote central fatigue and suboptimal exercise performance 24.
Serotonin is mainly synthesized from serotonergic neurons in the raphe nuclei, which regulate the release of serotonin throughout the brain, and from neurosecretory cells in the pineal gland25-27. TPH is a crucial enzyme for the regulation of both serotonin synthesis and levels. The serotonin transporters (5-HTT) and 5-HT1A/5-HT1B autoreceptors also play important roles in the regulation of extracellular levels of serotonin in the raphe nuclei. Increased TPH activity during prolonged exercise increases the activity of serotonin, and this situation may cause a loss of central motivation and drive, and increase the lethargy that underlies fatigue28. Previous studies have shown that excessive exercise can lead to increased serotonin synthesis and TPH expression in the dorsal raphe nuclei, and this was linked to exhaustion during running7, 29.
Our study found that endurance exercise performance was effectively enhanced in the group that received colostrum serum compared to the control group. Furthermore, in addition to the enhancement of exercise performance, the expressions of 5-HT, TPH, 5-HT1A, and 5-HTT were suppressed in the dorsal raphe nuclei of the animals that received colostrum serum. These results suggest that colostrum serum (100 mg/kg) increased exercise performance and inhibited the serotonergic system in the dorsal raphe nuclei.
Colostrum intake for six weeks has been shown to enhance immunity, reduce the concentration of creatine kinase (an indicator of muscle damage), and reduce the concentration of immunoglobulin G (IgG), while also increasing the concentration of cortisol. These effects improve the resistance against stress in marathon athletes30. Moreover, colostrum intake for nine weeks has been shown to increase the resilience of elite female rowing athletes31. Furthermore, a low dose of colostrum enhanced the athletic performance of cycling athletes during high-intensity training32. However, in a study involving two separate tests of physical performance in exercises of gradually increasing intensity to reach exhaustion separated by a 20 min partial recovery, colostrum intake for eight weeks did not induce performance differences during the first trial. However, after 20 min of passive recovery, exercise performance was shown to be increased by 5.2% on average during the second trial33. These results suggest a potential role for colostrum in increasing resilience and the resistance against fatigue. The immunological factors and bioactive substances that are present in colostrum are mainly constituents of colostrum serum. These include immune system molecules, growth factors, antibodies, vitamins, minerals, enzymes, and amino acids34. The major bioactive components of colostrum include IgG, tumor necrosis factor (TNF), interleukin-1,2,6 (IL-1,2,6), lactoferrin (LF), transforming growth factor (TGF), insulin-like growth factor (IGF-1,2), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF)35-37. The molecular weight of these bioactive molecules ranges from 5–10 kDa, and colostrum contains 10 to 500 times more of these bioactive substances than normal milk.
CONCLUSIONS
Our study showed that moderate intake of colostrum serum enhanced exercise performance and suppressed the serotonergic system in the rat dorsal raphe nuclei. These results suggest that the intake of colostrum serum effectively suppressed the central fatigue induced by exhaustive exercise, and possibly alleviated the disruption to the immune system through the suppression of the central serotonergic system. Overall, our study suggests that colostrum serum has beneficial effects on the central fatigue induced by intensity exercise, which may have implications for social, community, and human life.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1A5A8018552).
References
- 1.Brooks GA., Butte NF., Rand WM., Flatt JP., et al. Caballero B. Chronicle of the Institute of Medicine physical activity recommendation: how a physical activity recommendation came to be among dietary recommendations. Am J Clin Nutr. 2004;79:921S–30S. doi: 10.1093/ajcn/79.5.921S. [Brooks GA, Butte NF, Rand WM, Flatt JP, and Caballero B. Chronicle of the Institute of Medicine physical activity recommendation: how a physical activity recommendation came to be among dietary recommendations. Am J Clin Nutr. 2004;79: 921S-30S. ] [DOI] [PubMed] [Google Scholar]
- 2.Davis JM., et al. Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [Davis JM, and Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45-57. ] [DOI] [PubMed] [Google Scholar]
- 3.St Clair Gibson A., Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med. 2004;38:797–806. doi: 10.1136/bjsm.2003.009852. [St Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med. 2004;38:797-806. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noakes TD. Linear relationship between the perception of effort and the duration of constant load exercise that remains. J Appl Physiol (1985) 2004;96:1571–2. doi: 10.1152/japplphysiol.01124.2003. [Noakes TD. Linear relationship between the perception of effort and the duration of constant load exercise that remains. J Appl Physiol (1985). 2004;96:1571-2. ] [DOI] [PubMed] [Google Scholar]
- 5.Sung YH., Kim DY., Jung SY., Kim TW., Kim CJ., et al. Seo JH. β-Glucan increases treadmill running time through inhibition of serotonin expression in brains of rats during exhaustive exercise. J Exerc Nutrition Biochem. 2011;15:95–103. [Sung YH, Kim DY, Jung SY, Kim TW, Kim CJ, and Seo JH. β-Glucan increases treadmill running time through inhibition of serotonin expression in brains of rats during exhaustive exercise. J Exerc Nutrition Biochem, 2011;15:95-103. ] [Google Scholar]
- 6.Seo JH., Sung YH., Kim KJ., Shin MS., Lee EK., et al. Kim CJ. Effects of Phellinus linteus administration on serotonin synthesis in the brain and expression of monocarboxylate transporters in the muscle during exhaustive exercise in rats. J Nutr Sci Vitaminol. 2011;57:95–103. doi: 10.3177/jnsv.57.95. [Seo JH, Sung YH, Kim KJ, Shin MS, Lee EK, and Kim CJ. Effects of Phellinus linteus administration on serotonin synthesis in the brain and expression of monocarboxylate transporters in the muscle during exhaustive exercise in rats. J Nutr Sci Vitaminol. 2011;57:95-103. ] [DOI] [PubMed] [Google Scholar]
- 7.Rhim YT., Kim H., Yoou SJ., Kim SS., Chang HK., Lee TH., Lee HH., Shin MC., Shin MS., et al. Kim CJ. Effect of Acanthopanax senticosus on 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphe of exercised rats. J Ethnopharmacol. 2007;114:38–43. doi: 10.1016/j.jep.2007.07.030. [Rhim YT, Kim H, Yoou SJ, Kim SS, Chang HK, Lee TH, Lee HH, Shin MC, Shin MS, and Kim CJ. Effect of Acanthopanax senticosus on 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphe of exercised rats. J Ethnopharmacol. 2007;114:38-43. ] [DOI] [PubMed] [Google Scholar]
- 8.Davis JM., et al. Alderson NL., Welsh RS. Serotonin and central nervous system fatigue: nutritional considerations. Am J Clin Nutr. 2000;72:573S–8S. doi: 10.1093/ajcn/72.2.573S. [Davis JM, and Alderson NL. Welsh RS. Serotonin and central nervous system fatigue: nutritional considerations. Am J Clin Nutr. 2000;72:573S-8S. ] [DOI] [PubMed] [Google Scholar]
- 9.Newsholme EA., Blomstrand E., et al. Ekblom B. Physical and mental fatigue: metabolic mechanisms and importance of plasma amino acids. Br Med Bull. 1992;48:477–95. doi: 10.1093/oxfordjournals.bmb.a072558. [Newsholme EA, Blomstrand E, and Ekblom B. Physical and mental fatigue: metabolic mechanisms and importance of plasma amino acids. Br Med Bull. 1992;48:477-95. ] [DOI] [PubMed] [Google Scholar]
- 10.Strüder HK., et al. Weicker H. Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance: Part I. Int J Sports Med. 2001;22:467–81. doi: 10.1055/s-2001-17605. [Strüder HK, and Weicker H. Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance: Part I. Int J Sports Med. 2001;22:467-81. ] [DOI] [PubMed] [Google Scholar]
- 11.Clark MS., et al. Russo AF. Measurement of tryptophan hydroxylase mRNA levels by competitive RT-PCR. Brain Research Protocols. 1988;2:273–85. doi: 10.1016/s1385-299x(98)00004-x. [Clark MS, and Russo AF. Measurement of tryptophan hydroxylase mRNA levels by competitive RT-PCR. Brain Research Protocols. 1988;2:273-85. ] [DOI] [PubMed] [Google Scholar]
- 12.Chamas F., Serova L., et al. Sabban EL. Tryptophan hydroxylase mRNA levels are elevated by repeated immobilization stress in rat raphe nuclei but not in pineal gland. Neuroscience Letters. 1999;267:157–160. doi: 10.1016/s0304-3940(99)00340-7. [Chamas F, Serova L, and Sabban EL. Tryptophan hydroxylase mRNA levels are elevated by repeated immobilization stress in rat raphe nuclei but not in pineal gland. Neuroscience Letters. 1999;267:157-160. ] [DOI] [PubMed] [Google Scholar]
- 13.Azevedo JL., Tietz E., Two-Feathers T., Paull J., et al. Chapman K. Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PLoS ONE. 2007;2:e927. doi: 10.1371/journal.pone.0000927. [Azevedo JL, Tietz E, Two-Feathers T, Paull J, and Chapman K. Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PLoS ONE. 2007;2:e927. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman JR., Ratamess NA., Ross R., Shanklin M., Kang J., et al. Faigenbaum AD. Effect of a pre-exercise energy supplement on the acute hormonal response to resistance exercise. J Strength Cond Res. 2008;22:874–82. doi: 10.1519/jsc.0b013e31816d5db6. [Hoffman JR, Ratamess NA, Ross R, Shanklin M, Kang J, and Faigenbaum AD. Effect of a pre-exercise energy supplement on the acute hormonal response to resistance exercise. J Strength Cond Res. 2008; 22:874-82. ] [DOI] [PubMed] [Google Scholar]
- 15.Roy BD. Milk: the new sports drink? J Int Soc Sports Nutr. 2008;2:15. doi: 10.1186/1550-2783-5-15. [Roy BD. Milk: the new sports drink? J Int Soc Sports Nutr. 2008;2:15. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JK., Maughan RJ., Shirreffs SM., et al. Watson P. Effects of milk ingestion on prolonged exercise capacity in young, healthy men. Nutrition. 2008;24:340–7. doi: 10.1016/j.nut.2008.01.001. [Lee JK, Maughan RJ, Shirreffs SM, and Watson P. Effects of milk ingestion on prolonged exercise capacity in young, healthy men. Nutrition. 2008;24:340-7. ] [DOI] [PubMed] [Google Scholar]
- 17.Ramírez-Santana C., Pérez-Cano FJ., Audí C., Castell M., Moretones MG., López-Sabater MC., Castellote C., Franch A. Effects of cooling and freezing storage on the stability of bioactive factors in human colostrum. J Dairy Sci. 2012;95:2319–25. doi: 10.3168/jds.2011-5066. [Ramírez-Santana C, Pérez-Cano FJ, Audí C, Castell M, Moretones MG, López-Sabater MC, Castellote C, Franch A. Effects of cooling and freezing storage on the stability of bioactive factors in human colostrum. J Dairy Sci. 2012;95:2319-25] [DOI] [PubMed] [Google Scholar]
- 18.Ahn JH., et al. Park JK. Effect of supplementation of fermented colostrum on growth and occurrence of diarrhea in holstein calves. J Ani Sci Technol. 2010;52:281–6. doi: 10.5187/jast.2010.52.4.281. [Ahn JH, and Park JK. Effect of supplementation of fermented colostrum on growth and occurrence of diarrhea in holstein calves. J Ani Sci Technol. 2010;52:281-6. ] [DOI] [Google Scholar]
- 19.Uruakpa FO., Ismond MAH., et al. Akoboundu ENT. Colostrum and its benefits: a review. Nutr Res. 2002;22:755–67. doi: 10.1016/s0271-5317(02)00373-1. [Uruakpa FO, Ismond MAH, and Akoboundu ENT. Colostrum and its benefits: a review. Nutr Res. 2002;22:755-67. ] [DOI] [Google Scholar]
- 20.Simmen FA., Cera KR., Maha DC. Stimulation by colostrums or mature milk of gastrointestinal tissue development in newborn pigs. J Ani Sci. 1990;68:3596–603. doi: 10.2527/1990.68113596x. [Simmen FA, Cera KR, and Maha DC. Stimulation by colostrums or mature milk of gastrointestinal tissue development in newborn pigs. J Ani Sci. 1990;68:3596-603. ] [DOI] [PubMed] [Google Scholar]
- 21.Sugisawa H., Itou T., et al. Sakai T. Promoting effect of colostrums on the phagocytic activity of bovine polymorphonuclear leukocytes in vitro. Biol. Neonate. 2001;79:140–4. doi: 10.1159/000047080. [Sugisawa H, Itou T, and Sakai T. Promoting effect of colostrums on the phagocytic activity of bovine polymorphonuclear leukocytes in vitro. Biol. Neonate. 2001;79:140-4. ] [DOI] [PubMed] [Google Scholar]
- 22.Hong H., Kim CJ., Kim JD., et al. Seo JH. β‑glucan reduces exercise‑induced stress through downregulation of c‑Fos and c‑Jun expression in the brains of exhausted rats. Mol Med Rep. 2014;9:1660–6. doi: 10.3892/mmr.2014.2005. [Hong H, Kim CJ, Kim JD, and Seo JH. β‑glucan reduces exercise‑induced stress through downregulation of c‑Fos and c‑Jun expression in the brains of exhausted rats. Mol Med Rep. 2014;9:1660-6. ] [DOI] [PubMed] [Google Scholar]
- 23.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–89. doi: 10.1152/physrev.2001.81.4.1725. [Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725-89. ] [DOI] [PubMed] [Google Scholar]
- 24.Fernstrom JD., Fernstrom MH. Exercise, serum free tryptophan, and central fatigue. J Nutr. 2006;136:553S–9S. doi: 10.1093/jn/136.2.553S. [Fernstrom JD, Fernstrom MH. Exercise, serum free tryptophan, and central fatigue. J Nutr. 2006;136:553S-9S. ] [DOI] [PubMed] [Google Scholar]
- 25.Tao R., Ma Z., et al. Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther. 2000;294:571–9. [Tao R, Ma Z, and Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther. 2000;294:571-9. ] [PubMed] [Google Scholar]
- 26.Hopwood SE., et al. Stamford JA. Multiple 5-HT (1) autoreceptor subtypes govern serotonin release in dorsal and median raphé nuclei. Neuropharmacology. 2001;40:508–19. doi: 10.1016/s0028-3908(00)00192-1. [Hopwood SE, and Stamford JA. Multiple 5-HT (1) autoreceptor subtypes govern serotonin release in dorsal and median raphé nuclei. Neuropharmacology. 2001;40:508-19. ] [DOI] [PubMed] [Google Scholar]
- 27.Adell A., Celada P., Abellán MT., Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–80. doi: 10.1016/s0165-0173(02)00182-0. [Adell A, Celada P, Abellán MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154-80. ] [DOI] [PubMed] [Google Scholar]
- 28.Soares DD., Coimbra CC., Marubayashi U. Tryptophan-induced central fatigue in exercising rats is related to serotonin content in preoptic area. Neurosci Lett. 2007;415:274–8. doi: 10.1016/j.neulet.2007.01.035. [Soares DD, Coimbra CC, and Marubayashi U. Tryptophan-induced central fatigue in exercising rats is related to serotonin content in preoptic area. Neurosci Lett. 2007;415:274-8. ] [DOI] [PubMed] [Google Scholar]
- 29.Min YK., Chung SH., Lee JS., Kim SS., Shin HD., Lim BV., Shin MC., Jang MH., Kim EH., et al. Kim CJ. Red ginseng inhibits exercise-induced increase in 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in dorsal raphe of rats. J Pharmacol Sci. 2003;93:218–21. doi: 10.1254/jphs.93.218. [Min YK, Chung SH, Lee JS, Kim SS, Shin HD, Lim BV, Shin MC, Jang MH, Kim EH, and Kim CJ. Red ginseng inhibits exercise-induced increase in 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in dorsal raphe of rats. J Pharmacol Sci. 2003;93:218-21. ] [DOI] [PubMed] [Google Scholar]
- 30.Hwang MH., Jung HS., et al. Lee CJ. The Effect of Marathoners` 6-week Colostrum Ingestion on Immunogloblin, Cortisol, CK and LDH. Journal of Sport and Leisure Studies. 2008;32:1033–42. [Hwang MH, Jung HS, and Lee CJ. The Effect of Marathoners` 6-week Colostrum Ingestion on Immunogloblin, Cortisol, CK and LDH. Journal of Sport and Leisure Studies, 2008;32:1033-42. ] [Google Scholar]
- 31.Brinkworth GD., Buckely JD., Bourden PC., Gulbin GP., et al. David A. Oral bovine colostrum supplementation enhances buffer capacity but not rowing performance in elite female rowers. Int J Sport Nutr Exer Metabol. 2002;12:349–363. doi: 10.1123/ijsnem.12.3.349. [Brinkworth GD, Buckely JD, Bourden PC, Gulbin GP, and David A. Oral bovine colostrum supplementation enhances buffer capacity but not rowing performance in elite female rowers. Int J Sport Nutr Exer Metabol. 2002;12:349-63. ] [DOI] [PubMed] [Google Scholar]
- 32.Shing CM., Peake J., Suzuki K., Okutsu M., Pereira R., Stevenson L., Jenkins DG., et al. Coombes JS. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J Appl Physiol. 2007;102:1113–22. doi: 10.1152/japplphysiol.00553.2006. [Shing CM, Peake J, Suzuki K, Okutsu M, Pereira R, Stevenson L, Jenkins DG, and Coombes JS. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J Appl Physiol. 2007;102:1113-22. ] [DOI] [PubMed] [Google Scholar]
- 33.Buckley JD., Abbott MJ., Brinkworth GD., et al. Whyte PB. Bovine colostrum supplementation during endurance running training improves recovery, but not performance. J Sci Med Sport. 2002;5:65–79. doi: 10.1016/s1440-2440(02)80028-7. [Buckley JD, Abbott MJ, Brinkworth GD, and Whyte PB. Bovine colostrum supplementation during endurance running training improves recovery, but not performance. J Sci Med Sport. 2002;5:65-79. ] [DOI] [PubMed] [Google Scholar]
- 34.Pakkanen R. Determination of transforming growth factorbeta 2 (TGF-beta 2) in bovine colostrum samples. J Immunoassay. 1998;19:23–37. doi: 10.1080/01971529808005469. [Pakkanen R. Determination of transforming growth factorbeta 2 (TGF-beta 2) in bovine colostrum samples. J Immunoassay. 1998;19:23-37. ] [DOI] [PubMed] [Google Scholar]
- 35.Elfstrand L., Lindmak-Mnsson H., Paulsson M., Nyberg L., et al. kesson B. Immunoglobulins, growth factors and growth hormone in bovine colostrums and the effects of processing. Int Dairy J. 2002;12:879–87. doi: 10.1016/s0958-6946(02)00089-4. [Elfstrand L, Lindmak-Mnsson H, Paulsson M, Nyberg L, and kesson B. Immunoglobulins, growth factors and growth hormone in bovine colostrums and the effects of processing. Int Dairy J. 2002;12:879-87. ] [DOI] [Google Scholar]
- 36.Kang SH. Milk-derived growth factors as neutraceuticals. Korean J Dairy Sci Technol. 2007;25:27–31. [Kang SH. Milk-derived growth factors as neutraceuticals. Korean J Dairy Sci Technol. 2007;25:27-31. ] [Google Scholar]
- 37.Rodriguez C., Castro N., Capote J., Morales-delaNuez A., Moreno-Indias I., Sanchez-Macias D., et al. Arguello A. Effect of colostrum immunoglobulin concentration on immunity in Majorera goat kids. J. Dairy Sci. 2008;92:1696–701. doi: 10.3168/jds.2008-1586. [Rodriguez C, Castro N, Capote J, Morales-delaNuez A, Moreno-Indias I, Sanchez-Macias D, and Arguello A. Effect of colostrum immunoglobulin concentration on immunity in Majorera goat kids. J Dairy Sci. 2008;92:1696-701.] [DOI] [PubMed] [Google Scholar]