Abstract
Purpose:
The transformation from volume to value will require communication of outcomes and costs of therapies; however, outcomes are usually nonstandardized, and cost of therapy differs among stakeholders. We developed a standardized value framework by using radar charts to visualize and communicate a wide range of patient outcomes and cost for three forms of prostate cancer treatment.
Materials and Methods:
We retrospectively reviewed data from men with low-risk prostate cancer who were treated with low-dose rate brachytherapy (LDR-BT), proton beam therapy, or robotic-assisted prostatectomy. Patient-reported outcomes comprised the Expanded Prostate Cancer Index Composite-50 domains for sexual function, urinary incontinence and/or bother, bowel bother, and vitality 12 months after treatment. Costs were measured by time-driven activity-based costing for the first 12 months of the care cycle. Outcome and cost data were plotted on a single radar chart for each treatment modality.
Results:
Outcome and cost data from patients who were treated with robotic-assisted prostatectomy (n = 381), proton beam therapy (n = 165), and LDR-BT (n = 238) were incorporated into the radar chart. LDR-BT seemed to deliver the highest overall value of the three treatment modalities; however, incorporation of patient preferences regarding outcomes may allow other modalities to be considered high-value treatment options.
Conclusion:
Standardization and visualization of outcome and cost metrics may allow more comprehensive and collaborative discussions about the value of health care services. Communicating the value framework by using radar charts may be an effective method to present total value and the value of all outcomes and costs in a manner that is accessible to all stakeholders. Variations in plotting of costs and outcomes will require future focus group initiatives.
INTRODUCTION
Value-based health care has been propelled to the forefront of policy discussions as leaders strive to address the underperforming, yet costly, health care system.1,2 As signified by the passage of the Patient Protection and Affordable Care Act,3 the current fee-for-service system is being increasingly phased out in favor of value-based payments.4 Currently, approximately 20% of all Medicare payments tie reimbursement to outcome performance via alternative payment models and multiple federal initiatives, including the Hospital Readmissions Reduction Program and the Hospital Value-Based Purchasing Program. One goal of the US Department of Health and Human Services is to increase the share of payments through alternative models to 30% by the end of 2016 and to 50% by the end of 20184 and another is to tie 85% and 90% of all traditional Medicare payments to quality or value by 2016 and 2018.4
Despite this singular emphasis on value, which can be defined as patient-centered outcomes over the full cycle of care relative to the costs of achieving those outcomes,1,2,5-7 significant debate exists over which metrics to use to measure outcomes and cost. Recent efforts led, in part, by the International Consortium of Health Outcome Metrics have attempted to standardize a set of quality metrics for various disease conditions.8 Although most clinical outcome studies focus on one or a few outcome measures, the outcomes that matter most to patients are typically multiple and range from acute complications to long-term outcomes to patient-reported outcomes. Some patients also value certain outcomes more than others and make health care decisions on the basis of their own individual priorities.
Cost of therapy further differs among patients, providers, and payers. Traditionally, cost metrics have relied on surrogate measurements such as procedure charges and reimbursements, which do not reflect the true underlying cost to the provider to deliver care.9-11 To generate a more accurate assessment of the actual consumption of resources throughout a full treatment cycle, the concept of time-driven activity-based costing (TDABC) has been successfully applied in health care.12-17
Despite initial progress on measurement of outcomes and cost in health care, communication of value to patients, providers, administrators, payers, and policymakers—known collectively as stakeholders—remains a significant challenge. A value-based tool that can visualize the outcomes and cost of various treatments could facilitate decision making. In this proof-of-principle study of prostate cancer therapy at a single tertiary care center, we developed a standardized value framework that involves radar charts to visualize and communicate a wide range of patient outcomes and costs to health care providers.
MATERIALS AND METHODS
Patient Selection Criteria
Retrospectively collected data from The University of Texas MD Anderson Cancer Center were analyzed by comparing the outcomes and costs of three competing treatment modalities—low-dose rate brachytherapy (LDR-BT), proton beam therapy (PBT), and robotic-assisted radical prostatectomy (RARP)—for the treatment of low-risk prostate cancer. Periods of treatment differed among groups, with LDR-BT administered from 1998 to 2009, RARP from 2006 to 2014, and PBT from 2006 to 2012. For the purpose of this study, only patients who received monotherapy for low-risk or very-low-risk prostate cancer were included, and patients who received any form of combination therapy were excluded. Other exclusion criteria included missing domain scores for the Expanded Prostate Cancer Index Composite (EPIC)-50.
Clinical Outcome Measures
Clinical outcome domains are based on the International Consortium of Health Outcome Metrics Standard Set for Localized Prostate Cancer.8 For the purpose of this study, only patient-reported outcomes from the EPIC-50 survey were included in this analysis, as only EPIC-50 scores are collected at MD Anderson Cancer Center. Patients were asked to complete this survey at baseline and at regular intervals after definitive treatment of localized prostate cancer to assess disease-specific health-related quality of life. The EPIC-50 survey is a validated tool that measures patient-reported health-related quality of life in several major domains, including, but not limited, to sexual function, urinary incontinence, urinary bother, bowel bother, and vitality. EPIC-50 scores reported at 12 months after treatment were used in this analysis. Because information on baseline patient comorbid conditions was limited, no risk adjustment was done in this proof-of-principle cohort comparison of men with low-risk prostate cancer. Information on sociodemographics, comorbidities, and clinicopathologic factors were abstracted from the medical record.
Measurement of TDABC
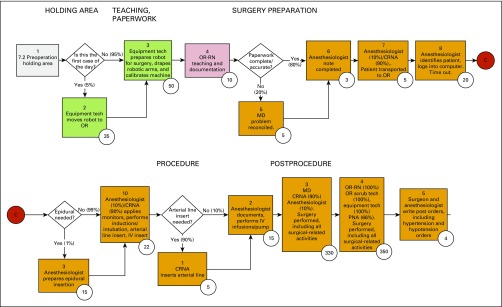
TDABC was used to measure all costs incurred over the full cycle of care for each treatment at MD Anderson for the first 12 months of a typical patient care cycle. TDABC involves developing process maps for every clinical and administrative process used during the full cycle of care, rather than for a particular intervention (Appendix Fig A1, online only), and calculating the capacity cost rate, that is, cost per minute, for every type of staff and equipment involved in a clinical or administrative process. This initial costing algorithm did not include costs of complications during the first 12 months after treatment, as the cost of care provided outside this institution could not be reliably measured. For further details regarding process mapping and calculation of capacity cost rates, see the Appendix (online only) and Appendix Figure A1 and Tables A1, A2, and A3 (online only).
Radar Chart Analysis
The radar chart tool in Microsoft Excel (version 2011) was used to visually display each outcome metric and cost in a single diagram. The radar chart displayed in this manuscript is intended for communication between providers and, therefore, includes the costs (TDABC) and axis labels that are pertinent to providers. Outcome data points for each treatment modality were graphed on separate axes, with all axes being scaled equally from 0 to 100. For instance, EPIC scores are plotted from 0 to 100 according to validated scoring guidelines. Cost data are reported as normalized relative cost ratios anchored to the lowest treatment cost in the study (LDR-BT). Normalized ratios are incorporated into the diagram as their reciprocal (1/Relative Cost), which may allow consistency in the interpretation of the radar chart diagram where improved outcomes or lower costs are indicated by data points farther from the center of the graph. The adjustment algorithm for plotting treatment cost x among costs x, y, z under the reciprocal method is:
Alternative methods for incorporating treatment costs into the radar chart include scaling costs by using a predetermined upper limit (SPUL) or by swapping the minimum and maximum costs (Appendix). These three methods of cost plotting were evaluated by genitourinary radiation oncologists, urologists, and business school professors.
RESULTS
Of 784 patients included in this analysis, 381 had been treated with RARP, 165 with PBT, and 238 with LDR-BT. EPIC health-related quality of life data were abstracted from the 12-month follow-up visit, and the sexual function, urinary incontinence, urinary bother, bowel bother, and vitality domains were used to conform to the International Consortium of Health Outcome Metrics standard set (Table 1). TDABC costs were aggregated over the full cycle of care, which began with consultation and ended 12 months after definitive therapy.
Table 1.
EPIC Domain–Specific Prostate Cancer Scores and Time-Driven Activity-Based Costing at MD Anderson Cancer Center for the First 12 Months of Care for Localized Prostate Cancer
| Treatment | Sexual Function | Urinary Incontinence | Urinary Bother | Bowel Bother | Vitality (hormonal function) |
|---|---|---|---|---|---|
| Brachytherapy | 46.1 | 89.6 | 81.6 | 92.5 | 90.5 |
| Robotic prostatectomy | 38.9 | 84.9 | 88.9 | 97.0 | 90.5 |
| Proton beam therapy | 54.1 | 91.9 | 86.5 | 87.6 | 90.2 |
Abbreviation: EPIC, Expanded Prostate Cancer Index Composite.
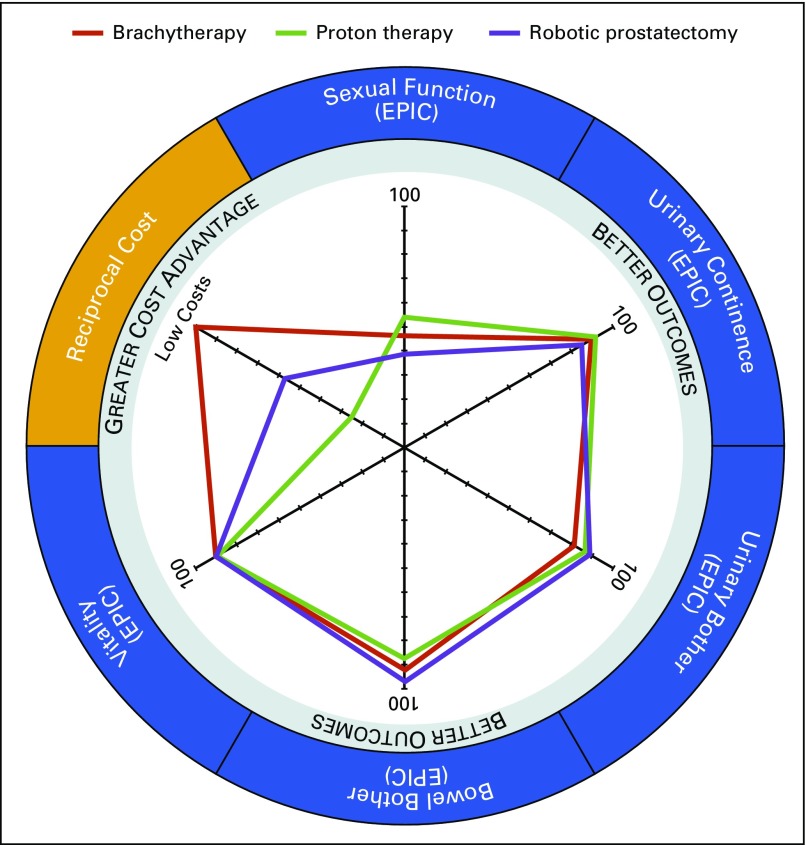
Outcome metric and cost data were plotted on a single radar chart diagram for each treatment modality (Fig 1) for comparative analysis. Each outcome metric or cost was plotted on its own independent axis. Data points that fell further out on any axis indicated better outcomes or lower costs. For instance, in this comparison, bowel bother was better for men who underwent RARP than it was for those who underwent LDR-BT or PBT, but sexual function tended to be better for men who underwent LDR-BT or PBT compared with RARP. Diagrams also reveal dimensions with similar outcomes. Patient-reported vitality was similar across each treatment modality. TDABC costs for PBT were higher than those for RARP, but LDR-BT had the overall lowest cost of all three modalities. Collectively, these findings suggest that treatment with PBT may result in marginally improved sexual functioning, marginally worsened bowel bother, and higher costs than the other two modalities. Of note, these data were aggregated in non–risk-adjusted samples and are used for illustrative purposes rather than as true comparative outcome measures. Statistical comparisons between the three groups were therefore not conducted.
FIG 1.
Radar chart plot of outcome and cost metrics for all three treatment modalities for prostate cancer. Axes for outcomes (Expanded Prostate Cancer Index Composite [EPIC] health-related quality of life domains sexual function, urinary incontinence, urinary bother, bowel bother, and vitality) as well as the reciprocal cost axis are equally scaled from 0 to 100.
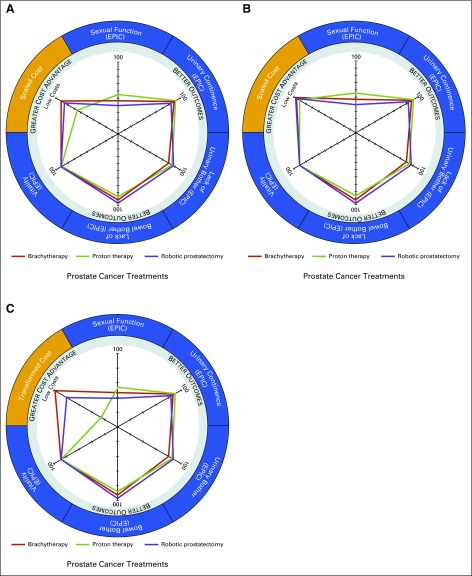
Two other alternative radar chart cost-plotting methods were used for comparison (Appendix). The SPUL method (Figs 2A and 2B) seemed to be susceptible to the arbitrariness of a predetermined upper limit value. As the difference between the predetermined upper limit value and actual costs of treatment increase—a 3.5× difference is illustrated in Figure 2—the plotted points appear more closely together on the costing axis. The minimum-maximum swap method (Fig 2C) seemed to address some limitations from the reciprocal and SPUL methods, but the interpretation of the relative cost difference of the middle cost treatment modality seemed to be less intuitive. However, an advantage of these two alternative cost-plotting methods is the ability to plot costs on an absolute, rather than relative, cost axis.
FIG 2.
Radar chart plots of outcome and cost metrics for all three treatment modalities for prostate cancer under alternative cost plotting methods. (A and B) The scaled to predetermined upper limit (SPUL) cost method. (C) The minimum-maximum (Min-Max) swap cost method. (A and B) In the SPUL cost method, treatment costs are incorporated into the radar chart diagram by being plotted as the difference of the cost from a predetermined upper limit value. In these two examples, the indexed cost axis transformed under the SPUL cost method is determined from a predetermined upper limit value that is censored, given the proprietary nature of medical center costs, with the upper limit in panel (B) set at 3.5 times that set in panel (A). As the difference between the predetermined upper limit value and actual costs of treatment increase, the plotted points appear more closely together on the costing axis (B). (C) The Min-Max swap cost method addresses the limitation of the reciprocal cost plotting method and SPUL cost plotting method by incorporating a linear transformation of the data by swapping the values of the minimum and maximum costs. However, data transformation is more complicated in this method and seemed to be less intuitive when interpreting the radar chart cost axis. Axes for outcome (the Expanded Prostate Cancer Index Composite [EPIC] health-related quality of life domains sexual function, urinary incontinence, urinary bother, bowel bother, and vitality) as well as the scaled cost axis are equally scaled from 0 to 100. See the Appendix for further discussion.
DISCUSSION
In this study, we demonstrated that a visual framework that involves a radar chart can be used to integrate information on outcomes and cost and communicate the value of health care delivery. Value can be enhanced by improving one or more outcomes at the same cost of resources, reducing costs required to achieve the same outcomes, or achieving the ideal of simultaneously improving outcomes and reducing costs. The radar chart therefore allows a unique juxtaposition of outcome metrics and costing data for various forms of prostate cancer treatments and communicates the value of competing treatment modalities across various stakeholders.
The radar chart presents multidimensional metrics in a practical format for analysis, whereas most comparative data are currently communicated in the form of tabular data or other graphical formats, such as box and whisker plots, bar charts, pie charts, and many others. Although these current approaches can help identify the performance of specific metrics, they create difficulty in conceptualizing the overall value delivered. Previous studies that examined performance in prostate cancer treatments have resorted to limiting their analyses to fewer outcome metrics, such as gastrointestinal or genitourinary toxicity, to minimize the complexity of the analysis.18 Accordingly, most clinical trials are powered for a single end point, such as overall survival or toxicity, and may inconsistently record other outcome dimension measures. In a cost-utility study of various prostate cancer treatments, Cooperberg et al10 incorporated several outcome metrics, including those used in this study, and implemented utility weighting to derive a summary quality-adjusted life-year (QALY) outcome score given the complexity of multiple variables. Although this approach can facilitate evaluation of the overall performance and efficiency of cancer treatments, use of QALYs censor individual outcome dimensions, such as erectile dysfunction or urinary incontinence, in the analysis of value.
The radar chart may overcome these challenges by visually depicting outcome and cost data simultaneously, thereby illustrating the total value of each treatment and preserving individual outcome dimension information. Separate axes are devoted to each of the outcome metrics, which allows traditional tabulated data to be captured in a more graphical format and an analysis of each individual factor’s contribution to the overall value of the treatment. For instance, the total value of a specific treatment modality could be reflected by the area formed by connecting the outcome data points across all axes, assuming all axes are weighted equally. The greater the area under the curve, the greater the overall value delivered. Although the three prostate cancer treatment modalities described in this study performed similarly with regard to outcomes—with some possible differences in the EPIC outcomes for sexual function or bowel bother—one can see that the total area for LDR-BT is greater than that of the others given its cost advantage. Conversely, the comparison of the total value of PBT with that of RARP, as reflected by their area under the curve, is not as clear and requires further qualitative decision analysis to justify the increased cost of PBT.
Radar charts can also account for the individualized preference of a specific stakeholder. Frameworks that use QALYs are limited by their reliance on the assumption of a universal weighting for each outcome dimension or cost metric.10,11 An effective value framework must be accessible to various stakeholders, including patients, providers, payers, employers, administrators, and policy makers. Each stakeholder has a different set of priorities and perspectives, which naturally place different weights on each metric. For instance, a policymaker may prioritize the overall value, that is, the area under the curve, of a treatment; the hospital administrator may weigh the cost variation between treatments more heavily; providers may focus on quality metrics and cost-reduction efforts; and patients may prioritize specific outcome measures and out-of-pocket costs rather than TDABC costs.
The visual framework put forth in this study addresses these challenges by communicating data in an accessible manner that remains practical to an audience with heterogeneous priorities. If all metrics were weighted equally, payers and policymakers may deem LDR-BT as perhaps delivering the best overall value, given its cost advantage and relative performance across the outcome dimensions. However, a relatively young patient with a primary concern of avoiding impotence may deem PBT as a greater value. Although PBT may be more costly than LDR-BT, its apparent tendency toward improved sexual function or performance may offset its higher costs and provide a greater value for younger patients. Previous studies have confirmed the preservation of meaningful sexual function after PBT.19 Conversely, an older patient who is concerned about bowel toxicity above all else may ultimately perceive greater value in treatment with RARP. Ultimately, value is not a static measurement but, rather, is shaped by the priorities of the individual patient. By identifying the dimensions that matter to each patient when measuring both the outcomes and costs, a visual framework such as that described here may deliver a more accurate patient-centered approach to care and value. This framework also facilitates comparison of treatments across institutions, such as an academic medical center versus a community ambulatory center, or across multiple satellite facilities within a single institution. At a more granular level, outcomes and costs can also be directly compared between individual or groups of physicians.
Although this proof-of-principle study introduces the visual framework, it does have several important limitations in its current form. First, the three patient cohorts in this study were identified on the basis of convenience samples, with the aim of obtaining initial data for an illustrative, rather than definitive, analysis. All data were retrospectively obtained and did not control for heterogeneities between risk classifications, comorbidities, and cohort sizes. Other treatment modalities, including intensity-modulated radiation therapy, were excluded from the analysis because of gaps in institutionally tracked metrics. Direct statistical comparisons between groups were therefore not conducted but were instead used as a representative example. We are currently collecting and analyzing prospectively obtained treatment modality–specific data, which will allow a more powerful comparison. In addition, data estimates, such as the EPIC scores or treatment costs, are all subject to error. Although the initial version of the radar chart presented in this manuscript does not incorporate error estimates, we anticipate that future iterations of the radar chart will enable visualization of these error estimates by allowing color-coded shading to highlight the lower and upper limits of error on each axis. Such color-coding would therefore allow stakeholders to visually determine the potential extent of end points observed from the calculated data set.
Another potential limitation of our approach is the use of a relative, rather than absolute, scale for the costing axis. Because the purpose of this initial radar chart is to present a basic framework for visually communicating costs and outcomes, the authors initially identified the relative cost-plotting method as an easily reproducible method that obviated the need to publish absolute cost data given the sensitive nature of this internal data. However, as we begin to communicate these data to patients and administrators, we will be considering an absolute cost plotting method, such as the minimum-maximum swap method or SPUL. Although these latter methods could be prone to inconsistency or have the potential to skew the representation of cost—as an upper limit can be arbitrarily chosen for the SPUL plotting method—strict and standardized conditions for these methods can still allow direct comparisons by using an absolute, rather than relative, cost axis. Next, the costing method used in this work does not include the cost of complications, such as urinary retention or infection, that are associated with each modality, given the incomplete data on complications that were treated outside of this institution. We plan to incorporate estimates of these treatment modality–specific complication costs in the future by associating payer data with institutional data.
Our current approach to the radar chart has been evaluated by only a limited group of clinicians and academicians. Our group is currently developing separate focus groups that will include patients, administrators, and clinicians to better refine the visual presentation of outcome and cost data in the radar chart. Future initiatives will also include diverse payer groups as we continue to discuss the utility of the radar chart format. We also anticipate future iterations of this radar chart to be specifically adaptable toward patient-, administrator-, payer-, or even policymaker-directed communication by altering the outcome or costing end points to include only those that are important to the specific stakeholder. For instance, although TDABC has significant advantages over surrogate metrics, such as charges or reimbursements, when measuring the true cost of care delivery to providers, patients are likely more interested in out-of-pocket and premium expenses when weighing costs of different treatment modalities. We would therefore plot these patient-related costs, rather than TDABC costs, on the radar chart when communicating with patients. We would also rename the plot axes to clarify individual axes—rather than “Low costs” we would use “Lowest out-of-pocket cost of treatment options.” Conversely, when communicating with administrators, we would potentially include total charges and total reimbursements in addition to TDABC costs and process measures, such as wait times.
In conclusion, the radar chart is a potentially effective tool that may be used to communicate value in health care by visually representing outcome and cost data. Future initiatives will require detailed feedback from focus groups of patients, administrators, clinicians, payers, and policymakers to ensure that the communication of value is accessible to all stakeholders.
Acknowledgment
N.G.T. and T.N.A. contributed equally to this work. S.J.F. is the principal investigator of a phase II and III randomized clinical trial of intensity-modulated proton therapy versus intensity-modulated radiation therapy for advanced oropharyngeal tumors at The University of Texas MD Anderson Cancer Center.
Appendix
Description of Time-Driven Activity-Based Costing
Process maps.
Process maps were created for all high-level events in the care cycle for low-dose rate brachytherapy, proton beam therapy, and robotic-assisted radical prostatectomy as well as for ancillary clinical and administrative services rendered during the first 12 months of a typical patient care cycle (Fig 1). Each step in the process map was associated with specific personnel, equipment, or facility resources and the time spent by each resource to complete each activity. Owing to the unique circumstances of individual patients, decision and chance nodes were embedded throughout the process maps, which allowed alternative care paths to be followed as appropriate for the patient’s specific circumstances.
Capacity cost rates.
Capacity cost rates were calculated for each staff member and significant equipment involved in the care cycle. The numerator in the capacity cost rate is the total cost incurred by MD Anderson Cancer Center to have each employee productive and available to the patient for some specified period, for example, annually. These personnel costs are derived from compensation data on the basis of job codes that were obtained from the institutional PeopleSoft (Oracle, Redwood Shores, CA) payroll application. In addition to salary and benefits, indirect expenses to support these personnel, including the costs of office space, technology, training, and supervision, were accounted for through an overhead factor. The denominator of the capacity cost rate is the estimated total capacity measured in minutes that each employee is available for productive work, minus the time not available because of nonproductive work time, such as vacation and sick time, and indirect work time, such as orientation, training, and breaks.
For equipment capacity cost rates, costs associated with depreciation of radiation therapy, da Vinci robot, and diagnostic imaging equipment were also embedded into the cost analysis by using a simple depreciation model. Depreciable life for these equipment was estimated on the basis of institutional and manufacturer recommendations. Time capacity for the equipment was calculated as total budgeted time available minus maintenance and scheduled downtime. The direct cost of radiation seed implants was incorporated into the total costs for low-dose rate brachytherapy.
Calculating total costs.
For each process step, activity cost was the product of time elapsed for that step times the capacity cost rate of the resources involved. When multiple resources were potentially involved in a process step, the capacity cost rate was weighted for the number and type of resources.
Description of Three Alternative Cost-Plotting Methods
Three plotting methods, including reciprocal costs, scaling costs with a predetermined upper limit (SPUL) and minimum-maximum swapping method, were explored for the purpose of incorporating treatment costs into the radar chart diagram. The method for graphical representation of costs needed to be standardizable and reproducible; allow for consistency of interpretation, that is, points further out on the axis signifying lower costs; and reflect costs in an intuitive manner. Each of these three plotting methods are discussed in the following paragraphs.
Reciprocal Costs
For illustrative purposes, consider five fictional treatments and associated costs: V, W, X, Y, Z (Appendix Table A1). Under the reciprocal method, treatment costs are first normalized to relative cost ratios anchored to the lowest treatment cost in the study, which, in the case of Appendix Table A1, is treatment V. The reciprocal (1/normalized cost ratio) of each cost is taken to allow for treatments with lower costs to be plotted further out on the axis. Transformation of treatment costs under this methodology are reflected in Appendix Table A1, with each reciprocal cost being indexed to the scale of 0 to 100 to reflect the similar scaling used in this work to match that used for the outcome EPIC score axes. The algorithm for the reciprocal cost transformation for treatment V is: 100*min[V,W,X,Y,Z]/V.
Strengths of the reciprocal method are that it is reproducible and standardized, but still remains intuitive for interpretation with its simple transformations; however, it is limited by its exponential nature where cost differences are skewed at higher levels. This is reflected through the cost differences between treatments X, Y, Z, each separated by $10,000 in cost; however, the equal cost differences are lost with the reciprocal transformation where the difference between X − Y and Y− Z are 2.4 and 1.8, respectively.
SPUL Cost Method
In this method, treatment costs are incorporated into the radar chart diagram by being plotted as the difference of the cost from a predetermined upper limit value, for example $100,000 or $200,000 (Appendix Table A2). Again, this allows us to maintain consistency in the interpretation of the radar chart diagram where, for all axes, improved outcomes or lower costs are indicated by data points farther away from the center of the graph. The algorithm for SPUL cost transformation for treatment V indexed to a scale of 0 to 100, as reflected in Appendix Table A2, is (upper limit – V)/(upper limit/100). The primary strength of SPUL compared with the reciprocal method is that treatment relative cost differences are maintained, even when indexed to a scale of 0 to 100 given the nonexponential transformations. However, a consensus on the predetermined upper limit and, thus, cost scale would be needed to ensure that all cost data points are captured.
Consequently, whereas SPUL has an advantage with its linear transformations, given the nonstandardized approach to setting the upper limit value, cost data points can be visually skewed and may become more clustered, thus visually minimizing their cost differences as the upper limit increases. This is reflected in Appendix Table A2 as the range of indexed treatment costs decreases from 70 to 35 and 14 when the upper limit is increased from $100,000 to $200,000, and $500,000, respectively. This proves to be a further detriment given the often proprietary nature of time-driven activity-based costing and the necessity to censor the upper limit value and cost scales in open publications. This, in turn, complicates interpretation of treatment cost differences and limits the ability to compare value with treatments or institutions outside the given study (Figs 2A and 2B).
Minimum-Maximum Swap Cost Method
The minimum-maximum (Min-Max) swap method addresses the two aforementioned limitations by maintaining cost differences through linear transformations while still maintaining a greater degree of standardization that is missing under SPUL. As reflected in Appendix Table A3, to maintain lower cost plotting furthest out on the cost axis, the costliest treatment—treatment Z—is assigned the lowest cost in the study, treatment V for $10,000. Each of the remaining treatments, treatments V to Y, are assigned a new cost level through adding their cost difference from treatment Z to the transformed cost of treatment Z. As illustrated, treatment X, at a cost of $60,000, has a $20,000 cost difference from treatment Z’s original cost of $80,000. Therefore, treatment X’s transformed cost under the Min-Max swap method is determined by adding $20,000 to Treatment Z’s transformed cost of $10,000, which yields $30,000.
Through the Min-Max swap method, treatment performance is conveyed by allowing lower costs to be plotted further out on the axis while maintaining relative cost differences through nonexponential transformations and under a highly standardized and reproducible method. However given the complicated nature of the transformations, the Min-Max swap method risks the intuitive nature of interpreting the radar chart diagram for its various stakeholders (Fig 2C).
FIG A1.
Representative process maps for robotic-assisted radical prostatectomy. Each box in this excerpted process map reflects a step or activity in the process through which a patient passes. The sequence of each step is numbered at the top of each box, and the numbers circled at the bottom right corner reflect the number of minutes needed to complete the activity described in the box by using the resources listed in the description. Decision nodes with corresponding percentages represent the percentage of patients who pass through each decision tree. CRNA, certified registered nurse anesthetist; OR, operating room; PNA, professional nursing assistant; RN, registered nurse.
Table A1.
Example of Reciprocal Cost Method
| Treatment | Treatment Cost ($) | Normalized to Lowest Cost | Reciprocal Cost | Reciprocal Cost (indexed) |
|---|---|---|---|---|
| V | 10,000 | 1 | 1.000 | 100.0 |
| W | 30,000 | 3 | 0.333 | 33.3 |
| X | 60,000 | 6 | 0.167 | 16.7 |
| Y | 70,000 | 7 | 0.143 | 14.3 |
| Z | 80,000 | 8 | 0.125 | 12.5 |
Table A2.
Example of the Scaling Costs With a Predetermined Upper Limit Cost Method
| Treatment | Treatment Cost ($) | $100,000 Upper Limit (indexed) | $200,000 Upper Limit (indexed) | $500,000 Upper Limit (indexed) |
|---|---|---|---|---|
| V | 10,000 | 90 | 95 | 98 |
| W | 30,000 | 70 | 85 | 94 |
| X | 60,000 | 40 | 70 | 88 |
| Y | 70,000 | 30 | 65 | 86 |
| Z | 80,000 | 20 | 60 | 84 |
Table A3.
Example of the Minimum-Maximum Swap Cost Method
| Treatment | Treatment Cost ($) | Cost Difference from Treatment Z ($) | Min-Max Swap Cost ($) | Indexed Cost (scale 0-100) |
|---|---|---|---|---|
| V | 10,000 | 70,000 | 80,000 | 80 |
| W | 30,000 | 50,000 | 60,000 | 60 |
| X | 60,000 | 20,000 | 30,000 | 30 |
| Y | 70,000 | 10,000 | 20,000 | 20 |
| Z | 80,000 | 10,000 | 10 |
Abbreviation: Min-Max, minimum-maximum
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Nikhil G. Thaker, Tariq N. Ali, Steven J. Frank
Data analysis and interpretation: Nikhil G. Thaker, Tariq N. Ali, Thomas W. Feeley, Robert S. Kaplan, Steven J. Frank
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Communicating Value in Health Care Using Radar Charts: A Case Study of Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Nikhil G. Thaker
No relationship to disclose
Tariq N. Ali
No relationship to disclose
Michael E. Porter
Leadership: Merrimack Pharmaceuticals
Stock or Other Ownership: Merrimack Pharmaceuticals, Sanofi, Merck, Molina Healthcare, Royalty Pharma
Honoraria: Premiere, Medtronic, Sevus, Ontario Hospital Association, Press Ganey, Society of Gynecologic Oncology, Texas Medical Center, Society of Neurologic Surgeons, American College of Radiology, Phillips Health Care Brazil, Nashville Health Care Council, Dell Medical School, Abbott Diagnostics
Consulting or Advisory Role: Molina Healthcare, AllScripts
Speakers' Bureau: Stern Strategy Group
Travel, Accommodations, Expenses: Society of Gynecologic Oncology, Mayo Clinic, Texas Medical Center
Thomas W. Feeley
No relationship to disclose
Robert S. Kaplan
Honoraria: Medtronic, Analogic
Consulting or Advisory Role: Avant-Garde Health
Steven J. Frank
Leadership: C4 Imaging
Stock or Other Ownership: C4 Imaging
Honoraria: Varian Medical Systems, Siemens
Consulting or Advisory Role: Varian Medical Systems
Research Funding: C4 Imaging, Elekta
Patents, Royalties, Other Intellectual Property: C4 Imaging
References
- 1.Porter ME: Value-based health care delivery Ann Surg 248:503–509,2008 [DOI] [PubMed] [Google Scholar]
- 2.Porter ME: What is value in health care? N Engl J Med 363:2477–2481,2010 [DOI] [PubMed] [Google Scholar]
- 3.Skinner D: Defining medical necessity under the Patient Protection and Affordable Care Act Public Adm Rev 73:S49–S59,2013 [Google Scholar]
- 4.US Department of Health and Human Services : Better, Smarter, Healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value http://www.hhs.gov/news/press/2015pres/01/20150126a.html
- 5.Porter ME: A strategy for health care reform--Toward a value-based system N Engl J Med 361:109–112,2009 [DOI] [PubMed] [Google Scholar]
- 6.Porter ME, Teisberg EO: Redefining competition in health care Harv Bus Rev 82:64–76, 136,2004 [PubMed] [Google Scholar]
- 7.Porter ME, Teisberg EO: Redefining Health Care 2006. Boston, MA: Harvard Business School Press [Google Scholar]
- 8.Martin NE Massey L Stowell C, etal: Defining a standard set of patient-centered outcomes for men with localized prostate cancer Eur Urol 67:460–467,2015 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen PL Gu X Lipsitz SR, etal: Cost implications of the rapid adoption of newer technologies for treating prostate cancer J Clin Oncol 29:1517–1524,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR Ramakrishna NR Duff SB, etal: Primary treatments for clinically localised prostate cancer: A comprehensive lifetime cost-utility analysis BJU Int 111:437–450,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodward RM, Menzin J, Neumann PJ: Quality-adjusted life years in cancer: Pros, cons, and alternatives Eur J Cancer Care (Engl) 22:12–19,2013 [DOI] [PubMed] [Google Scholar]
- 12.French K Albright H Frenzel J, etal: Measuring the value of process improvement initiatives in a preoperative assessment center using time-driven activity based costing Healthc (Amst) 1:136–142,2013 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan RS Witkowski M Abbott M, etal: Using time-driven activity-based costing to identify value improvement opportunities in healthcare J Healthc Manag 59:399–412,2014 [PubMed] [Google Scholar]
- 14.Kaplan RS, Anderson SR: Time-driven activity-based costing Harv Bus Rev 82:131–138, 150,2004 [PubMed] [Google Scholar]
- 15.Kaplan RS, Porter ME: How to solve the cost crisis in health care Harv Bus Rev 89:46–52, 54, 56-61 passim,2011 [PubMed] [Google Scholar]
- 16.Thaker N Guzman A Feeley T, etal: Defining the value of proton therapy in an evolving healthcare system using time-driven activity-based costing Oncology Payers, Policy Makers, and Prescribers 1:22–28,2014 [Google Scholar]
- 17.Thaker NG, Frank SJ, Feeley TW: Comparative costs of advanced proton and photon radiation therapies: lessons from time-driven activity-based costing in head and neck cancer J Comp Eff Res 4:297–301,2015 [DOI] [PubMed] [Google Scholar]
- 18.Ciezki J Reddy C Angermeier K, etal: Long-term toxicity and associated cost of initial treatment and subsequent toxicity-related intervention for patients treated with prostatectomy, external beam radiotherapy, or brachytherapy: A SEER/Medicare database study J Clin Oncol 30:2012 (suppl 5, abstr 4) [Google Scholar]
- 19.Pugh TJ Munsell MF Choi S, etal: Quality of life and toxicity from passively scattered and spot-scanning proton beam therapy for localized prostate cancer Int J Radiat Oncol Biol Phys 87:946–953,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]