Abstract
The coenzyme nicotinamide adenine dinucleotide (NAD+) has key roles in the regulation of redox status and energy metabolism. NAD+ depletion is emerging as a major contributor to the pathogenesis of cardiac and renal diseases and NAD+ repletion strategies have shown therapeutic potential as a means to restore healthy metabolism and physiological function. The pleotropic roles of NAD+ enable several possible avenues by which repletion of this coenzyme could have therapeutic efficacy. In particular, NAD+ functions as a co-substrate in deacylation reactions carried out by the sirtuin family of enzymes. These NAD+-dependent deacylases control several aspects of metabolism and a wealth of data suggests that boosting sirtuin activity via NAD+ supplementation might be a promising therapy for cardiac and renal pathologies. This Review summarizes the role of NAD+ metabolism in the heart and kidney, and highlights the mitochondrial sirtuins as mediators of some of the beneficial effects of NAD+-boosting therapies in preclinical animal models. We surmise that modulating the NAD+–sirtuin axis is a clinically relevant approach to develop new therapies for cardiac and renal diseases.
The heart and kidneys have the highest numbers of mitochondria and are among the greatest oxygen consumers of all organs in the body1. Energy production is maintained in these organs through the oxidative metabolism of a range of fuels. Substrate metabolism is intricately tied to the pyridine dinucleotide, nicotinamide adenine dinucleotide (NAD+). This essential metabolite serves as a key cofactor in a plethora of mitochondrial metabolic processes that facilitate energy production2 and also has several other important cellular functions3.
NAD+ is a hydride acceptor that forms the reduced dinucleotide NADH. The NAD+/NADH nucleotide pair is vital for driving reduction–oxidation (redox) reactions in energy production. Furthermore, NAD+ is a precursor for the phosphorylated dinucleotide pair NADP+/NADPH, which is required for several cellular biosynthetic pathways and to protect cells from reactive oxygen species (ROS). NAD+ also acts as an enzyme substrate for several non-redox reactions, such as those that occur in signalling pathways. In these reactions, the adenine diphosphate ribose (ADPR) moiety of NAD+ is cleaved and used as a signalling molecule through the enzymatic activity of ADPR cyclases, used to post-translationally modify proteins via the activity of ADP ribosyltransferases, and used to remove post-translational acyl modifications via the activity of sirtuins. The mitochondrial sirtuins control metabolism by coupling NAD+ consumption with deacylation on critical lysine residues of metabolic proteins, thereby regulating flux through cellular metabolism4. The multi-faceted functions of NAD+ uniquely position this metabolite at the regulatory interface of energy metabolism and several cellular processes in metabolically active organs such as the heart and kidneys5.
Consistent with these key roles of NAD+, various cardiac and renal stresses have been associated with decreases in the levels of this metabolite, which contribute to disease pathogenesis1,5. Acute stresses, such as stroke, myocardial infarction, acute kidney injury (AKI) and ischaemia, deplete cellular NAD+ levels and reduce NAD+ synthesis5,6. In the setting of chronic stress, such as ageing, cardiac and renal levels of NAD+ decline and correlate with a general reduction in mitochondrial function7. Thus, elevating intracellular NAD+ levels is a potential therapeutic strategy for cardiac and renal diseases. Several therapeutic strategies to increase NAD+ levels have been established, including exogenous supplementation of NAD+ and its precursors or manipulation of key enzymes in its biosynthetic pathways. A number of studies in model organisms ranging from Caenorhabditis elegans to mice, as well as in humans, have established beneficial effects of boosting NAD+ levels in a wide range of disease states3; however, the mechanisms by which such therapies are efficacious remain incompletely understood.
Here, we describe the primary mechanisms by which NAD+ supplementation might influence cellular processes, and consider the evidence for the contribution of each mechanism to the potentially beneficial effects of NAD+ in the heart and kidney. In particular, we highlight the mitochondrial sirtuins as key mediators of the effects of NAD+ supplementation in preclinical animal models.
NAD+ metabolism in the heart and kidneys
NAD+ synthesis
The heart and kidneys are among the organs with the highest NAD+ levels8 and cellular control of this metabolite is crucial for cardiac and renal metabolic and bioenergetic homeostasis5. In mammals, NAD+ is synthesized from four different biosynthetic precursors in two distinct pathways. De novo synthesis (the deamidated pathway) uses the amino acid tryptophan, which is metabolized to form biosynthetic intermediates. These intermediates ultimately generate nicotinamide (the pyridine moiety of NAD+) and then form NAD+. Dietary vitamin B3 compounds, including nicotinic acid, nicotinamide and nicotinamide riboside, serve as NAD+ biosynthetic precursors and are salvaged from the diet (the amidated pathway) to generate cellular NAD+ (FIG. 1).
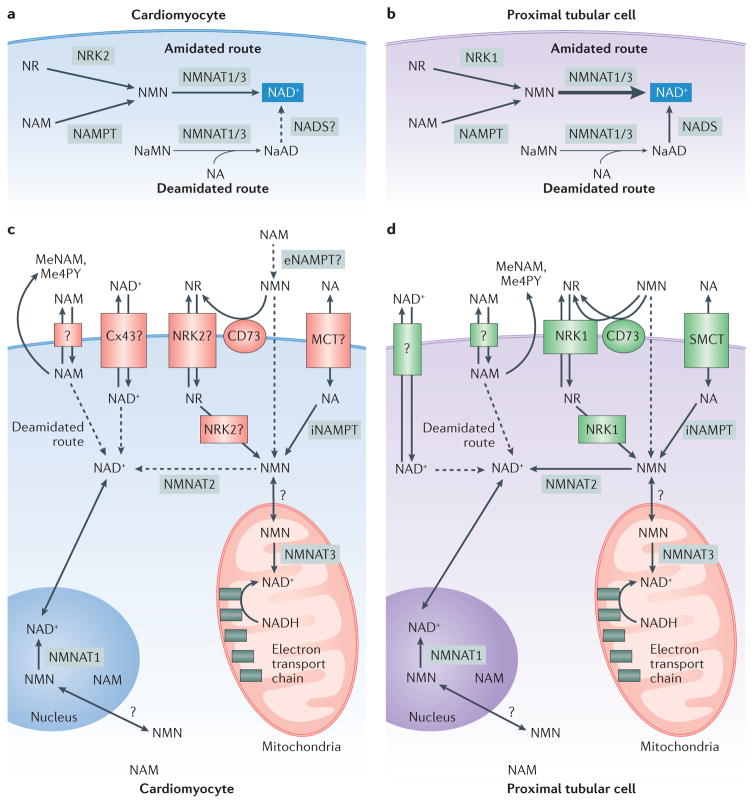
Figure 1. Cardiac and renal biosynthesis and the metabolome of nicotinamide adenine dinucleotide (NAD+).
Two routes of NAD+ synthesis — deamidated (de novo synthesis) and amidated (precursor salvage) — occur in the heart (parts a,c) and the kidney (parts b,d) (REF. 8). In the amidated route, which dominates in both tissues, the activities of nicotinamide riboside kinase (NRK) isoforms (NRK2 in the heart and NRK1 in the kidney) enable generation of nicotinamide mononucleotide (NMN) from the salvage of nicotinamide riboside (NR), whereas nicotinamide phosphoribosyltransferase (NAMPT) activity converts salvaged nicotinamide (NAM) to NMN. Nicotinamide mononucleotide adenyltransferase (NMNAT) isoforms 1 (nuclear) and 3 (mitochondrial) convert NMN to NAD+ in the amidated route and nicotinic acid mononucleotide (NaMN) to nicotinic acid dinucleotide (NaAD) in the deamidated route. The activities of NMNAT1/3 in the amidated route are much greater in the kidney (part b) than in the heart (part a), as indicated by the arrow thicknesses. Nicotinic acid (NA) can also be converted to an intermediate in the deamidated route, and the activity of NAD synthase (NADS) enables conversion of NaAD to NAD+, although the role of NADS in the heart is speculative. Uptake of NAD+ and its precursors occurs through many means in the heart (part c) and kidney (part d). NAD+ can be directly transported into the heart by the connexion 43 hemichannel (Cx43). Renal uptake of NA occurs through the sodium monocarboxylate transporter (SMCT) (REF. 14). NR and NMN require NRK1 for optimal uptake and conversion to NAD+ in the kidney20. NRK2 might serve this role for these NAD+ precursors in the heart. Additionally, extracellular NMN might be converted into NR by membrane bound cluster of differentiation 73 (CD73) (REF. 19) and subsequently taken up by renal NRK1 or cardiac NRK2. Renal NAM uptake is thought to occur through an unidentified transporter14. NAM — generated from exogenous sources or other NAD+ precursors such as NA — is maintained at low levels in the heart and kidney9 through its conversion into the byproducts 1-methylnicotinamide (MeNAM) and N-methyl-4-pyridone-5-carboxamide (Me4PY), which are excreted by the cell. 5′-ectonucleotidase; MCT, monocarboxylate transporter; NADH, reduced NAD.
Comprehensive profiling of the physiological steady-state levels of components of the NAD+ metabolome (NAD+ precursors and intermediates) and the activities of enzymes involved in NAD+ biosynthesis and salvage in C57BL/6 mice showed that the amidated route accounts for 99.3% and 99.9% of the NAD+ synthesized in the heart and kidneys, respectively8. These data indicate that nearly all cardiac and renal NAD+ is generated from salvage of nicotinic acid, nicotinamide and nicotinamide riboside, although de novo synthesis can contribute to the NAD+ pool under some conditions8,9. This conclusion is corroborated by earlier studies that found low expression and activity of enzymes involved in the deamidated route in these tissues10. Moreover, experiments in perfused rodent kidneys showed slow uptake and use of quinolinic acid, an intermediate in de novo NAD+ synthesis10,11. The amidated salvage route is, therefore, crucial for providing the NAD+ that is required to fulfil the high metabolic demands of the heart and kidneys, as well as for maintaining NAD+ homeostasis in the setting of disease.
Uptake and use of NAD+ and its precursors
Tissue-specific mechanisms of nicotinic acid, nicotinamide, and nicotinamide riboside transport are an emerging area of study. Renal uptake of NAD+ and its salvage precursors occurs at the brush border membrane of the proximal tubules12,13 (FIG. 1d). Nicotinic acid, lactate, glucose, and short-chain fatty acids are rapidly taken up into the kidneys via the sodium-dependent monocarboxylate transporter SLC5A8 (SMCT)14, whereas uptake and transport pharmacokinetics of nicotinamide are much slower9. Uptake of nicotinamide does not occur through the SMCT, but is thought to occur through an unidentified carrier14. In the heart, nicotinic acid is believed to have a direct effect on lactate efflux through the lactate monocarboxylic acid transporter (MCT)15. Specific and/or overlapping mechanisms of nicotinic acid transport, such as active transport via a MCT, is an active area of investigation.
Once taken up by cardiac or renal cells, nicotinamide is converted into nicotinamide mononucleotide (NMN) via the activity of nicotinamide phosphoribosyltransferase (NAMPT) in the amidated pathway, and to nicotinic acid via multiple steps in the deamidated pathway (FIG. 1). The degree to which cardiac cells incorporate nicotinamide or nicotinic acid for NAD+ synthesis seems to depend on precursor availability, but is overall similar for each metabolite16. In the kidneys, both nicotinic acid and nicotinamide are readily metabolized to NAD+; nicotinamide metabolism is tightly regulated to maintain low levels of this precursor9. Radiolabelling studies in perfused rat kidneys showed rapid conversion of nicotinic acid to NAD+ via the deamidated route, as well as rapid consumption of newly synthesized NAD+ into NMN via the amidated route9. In these studies, radiolabelled nicotinamide was converted to NAD+ so quickly that no trace of labelled intermediate NMN was detected. In murine saturation studies, excess nicotinic acid resulted in its decreased conversion to NAD+, by both rate and overall amount incorporated into NAD+ synthesis. The nicotinamide that was generated from the excess nicotinic acid was quickly converted into byproducts that were excreted in the urine9,11,17,18 (FIG. 1d). This tight control of nicotinic acid and nicotinamide is thought to be crucial for regulating the activities of NAD-cleaving enzymes, such as sirtuins, ADP cyclases and ADPR-transferases8. These enzymes generate nicotinamide as a byproduct, which in turn provides feedback inhibition on their activities.
Extracellular NMN is converted to nicotinamide riboside, which is then taken up into cells via membrane transporters such as CD73 (REF. 19) or nicotinamide riboside kinases (NRKs)20. NRKs are required for the intracellular conversion of nicotinamide riboside into NMN, which is then used to synthesize NAD+ by the activity of nicotinamide mononucleotide adenylytransferase (NMNAT). In liver cells, NRK1 activity also seems to be required for the uptake of exogenous nicotinamide riboside and NMN, but not for the uptake of the precursors nicotinamide or nicotinic acid20. In that study, mouse kidney expressed the highest levels of Nmrk1, whereas levels of cardiac Nmrk1 were among the lowest of all the tissues analysed. Moreover, both nicotinamide riboside and NMN supplementation boosted renal NAD+ levels by 150% in a wild-type mouse, but this increase was reduced by two-thirds in an NRK1-knockout mouse. These findings support a key role for NRK1 in nicotinamide riboside and NMN uptake and use in NAD+ synthesis in the kidneys.
NRK1 was not required for increased NAD+ levels in skeletal muscle resulting from either supplementation with nicotinamide riboside or NMN20. Expression of Nrmk2 transcript and NRK2 protein levels were highest in heart and skeletal muscle, suggesting a role for NRK2 in mediating the uptake and metabolism of nicotinamide riboside and NMN in cardiomyocytes. In an NRK1/2-double knockout mouse, however, uptake of NR and NMN and conversation to NAD+ in skeletal muscle was unchanged compared to that in wild-type mice, indicating a non-NRK route of NR and NMN uptake in muscle tissue20. In a parallel study, acute nicotinamide riboside supplementation was highly effective at elevating NAD+ levels in the livers, but not in the hearts of wild-type mice21. Interestingly, intraperitoneal injection of nicotinamide riboside in mice greatly enhanced the cardiac NAD+ metabolome, producing high levels of nicotinamide, which was quickly converted to the byproducts 1-methylnicotinamide and N-methyl-4-pyridone-5-carboxamide. These findings suggest that in the heart, exogenous nicotinamide riboside might have an alternative fate to NAD+ synthesis. By contrast, a plethora of studies have demonstrated that intraperitoneal injection of NMN boosts NAD+ levels in the heart22–24. Future studies are required to elucidate the disparate capacity of NMN and nicotinamide riboside to promote cardiac NAD+ synthesis.
Intracellular fate of NAD+
Once in the cell, NAD+ precursors might have different fates depending on the cellular compartment. In a tissue-wide NAD+ metabolome study, NAD+ biosynthetic rates were reconstructed, and NMNAT isoform expression and activity was found to be a key regulator of the rate of NAD+ biosynthesis in the heart and kidney8 (FIG. 1). NMNAT converts NMN to NAD+ via the amidated route, and converts nicotinic acid mononucleotide to nicotinic acid dinucleotide via the deamidated route; the NMN substrate is an intermediate metabolite of nicotinamide and nicotinamide riboside (FIG. 1). NMNAT has three isoforms: the nuclear isoform, NMNAT1, is ubiquitously expressed and highly active in the heart and kidney3; the cytosolic isoform, NMNAT2, is highly expressed in the heart and poorly expressed in the kidney but has little activity in either tissue; and the mitochondrial isoform, NMNAT3, is robustly expressed in the kidney and weakly expressed in the heart25, but highly active in both tissues25. Interestingly, Nmnat3-knockout mice show no change in steady-state NAD+ levels in the heart or kidney compared with that of wild-type mice, but whether mitochondrial pools were affected by Nmnat3 knockout was unclear26. Together these findings support important functional roles of nuclear NMNAT1 and mitochondrial NMNAT3 in the heart and kidney. Furthermore, they show that the uptake and use of nicotinamide riboside, nicotinamide, and NMN are important for NAD+ synthesis in a cellular compartment-specific manner.
A complex, tissue-specific, and compartment-specific system of NAD+ uptake and homeostasis exists, but understanding the differential bioavailability and use of NAD+ and its precursors requires further study. For example, future investigations will identify cardiac-specific mechanisms of uptake of NAD+ and its precursors, and track cellular compartmental shifts in NAD+ pools to establish flux in the NAD+ metabolome with precursor supplementation. Such research will provide the information necessary to fully understand NAD+ supplementation strategies in the setting of cardiac and renal diseases.
Potential benefits of NAD+ therapy
Homeostasis of the NAD+ metabolome is key for responses to pathological stress in the heart and kidney. Cardiomyopathy studies have shown a critical role of NAMPT in protecting the heart against pathological stress27,28, whereas in the kidneys, NAMPT has an important role in cell growth29 and survival30. Several studies have demonstrated that NAD+ supplementation has a beneficial effect on cardiac function. In a model of pressure-overload-induced left ventricle hypertrophy, supplementation with NMN or genetic manipulation of enzymes in the NAD+ salvage pathways increased NAD+ levels and improved cardiac function23. Similarly, in kidneys subjected to ischaemia, nicotinamide supplementation increased NAD+ levels and decreased the levels of toxic fatty acids that have a critical role in the pathological progression of AKI2.
Several studies have firmly established that NAD+ therapies are efficacious in multiple models of cardiac and renal disease22,23,31–34. Given the re-emerging interest in NAD+ biology and in supplementation strategies for a wide-range of diseases, additional studies will likely identify roles for NAD+ supplementation in mitigating a wide range of cardiac and renal diseases. The challenge moving forward will be to develop a deeper understanding of the mechanisms that mediate the therapeutic benefits of NAD+ supplementation.
NAD+ and sirtuins
A possible mechanism by which NAD+ supplementation might improve outcomes in cardiac and renal diseases is by activating sirtuins (TABLE 1). This family of NAD+-dependent enzymes regulates metabolism by removing acyl-lysine modifications from a variety of proteins35. Sirtuins were initially described as deacetylases that target acetyl-lysine for removal36, but they have now been shown to also target several acyl-lysine modifications, including malonyl-lysine37,38, succinyl-lysine38, and glutaryl-lysine39, as well as long-chain acyl-modifications40.
Table 1.
Effects of manipulating NAD+ on sirtuins in the heart and kidney
| NAD therapy | Murine model and tissue | Physiological effect | Effect on NAD metabolome | Effect on sirtuins | Ref |
|---|---|---|---|---|---|
| Exogenous NAD+ | Cardiac hypertrophy Model | NAD+ blocked cardiac hypertrophic response | NAD+ maintained intracellular NAD | ↑ SIRT3 deacetylase activity | 31 |
| High-glucose-induced mesangial hypertrophy | NAD+ blocked prohypertrophic signaling | NAD+ restored intracellular NAD+:NADH ratio |
|
33 | |
| Genetic manipulation of NAD+: NADH ratio | Cardiac-specific mitochondrial complex I deficiency |
|
↓ NAD+:NADH ratio | SIRT3 overexpression in cardiomyocytes resulted in:
|
22 |
| NMN supplementation | Pressure-overload-induced hypertrophy in cardiac-specific mitochondrial complex I deficiency | NMN improved fractional shortening, left ventricular dilation and hypertrophy via:
|
NMN normalized cardiac NAD+:NADH ratio |
NMN reduced hyperacetylation of two pathological protein targets, which corrected the phenotype: malate aspartate shuttle and regulators of the mPTP | 23 |
| Pharmacological manipulation of NAMPT via AICAR | Cisplatin-induced acute kidney injury | Improved renal function, decreased tubular injury | ↑ NAMPT expression |
|
34 |
AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; mPTP, mitochondrial permeability transition pore; NAD+, nicotinamide adenine dinucleotide; NADH, reduced NAD; NAMPT, nicotinamide phosphoribosyltransferase; NMN, nicotinamide mononucleotide; SIRT, sirtuin.
In mammals, seven sirtuins (SIRT1-7) occupy different cellular compartments. SIRT1, SIRT6, and SIRT7 reside in the nucleus and nucleolus, whereas SIRT2 resides primarily in the cytoplasm. Notably, SIRT6 seems to have a role in mitigating cardiac stress41,42. SIRT3, SIRT4, and SIRT5 are localized in mitochondria and have roles in various metabolic pathways. SIRT3 is a lysine deacetylase that controls several pathways, including lipid metabolism and oxidative stress43. SIRT4 has been described as an ADP-ribosyltransferase44, a deacetylase45, and a lipoadmidase46, but its primary activity has not yet been established. This sirtuin has been shown to influence animo acid and lipid metabolism, as well as the tricarboxylic acid cycle44,47. SIRT5 is a lysine demalonylase, desuccinylase, and deglutarylase, which controls several metabolic pathways, including the urea cycle39. Lysine acetylation is thought to occur nonenzymatically48–50 and to increase in response to elevated levels of acetyl-coenzyme A, such as those that occur in response to disease-associated shifts in metabolism49. Acylation of mitochondrial proteins is generally repressive51, although lysine acylation has been described to activate enzyme activity in a few cases52.
The dependence of sirtuins on NAD+ links energy metabolism to sirtuin function53. Importantly, yeast sirtuins can be activated by manipulating NAD+ biosynthetic pathways or by increasing concentrations of NAD+. For example, increasing the levels of NPT1, which encodes nicotinate phosphoribosyltransferase (an enzyme with a role in NAD+ biosynthesis), increased yeast sirtuin (Sir2, the homologue of mammalian SIRT1) activity and extended lifespan by up to 60% without changing NAD+ levels54. Similar lifespan extension was observed with nicotinamide riboside supplementation, which increased NAD+ levels55. Supplementation of nicotinic acid had no effect on Sir2 activity56, however, demonstrating that different NAD+ precursors elicit different biological effects, perhaps owing to differences in uptake, availability, or fate.
Investigations of NAD+ supplementation and the effects on sirtuin activity in higher organisms have yielded similar results. Exogenous nicotinamide riboside or nicotinamide increased levels of NAD+, improved mitochondrial function, and increased lifespan in C. elegans57. In a mouse model of type 2 diabetes mellitus induced by a high-fat diet, NMN supplementation restored NAD+ levels, an effect that was partially attributed to SIRT1 activation58. Based on these studies and others, supplementation with NAD+ precursors or boosting the activities of enzymes in the NAD+ salvage pathway to activate sirtuins is being explored as a strategy to treat cardiac and renal diseases.
The influence of SIRT1 on ageing and lifespan and the role of this sirtuin in mediating the effects of NAD+ supplementation has been extensively studied59,60. Many studies have linked NAD+ supplementation to SIRT1 activation and shown that SIRT1 protects against cardiac and renal disease. The beneficial effects of SIRT1 in the heart include reduced inflammation, inhibition of cardiomyocyte apoptosis, defence against myocardial oxidative stress, and maintenance of energy metabolism61,62. In the kidney, SIRT1 promotes podocyte function and mitochondrial biogenesis, and inhibits fibrosis, inflammation, and apoptosis63–65. Although strong evidence supports a role for SIRT1 in mediating some of the beneficial effects of NAD+ supplementation, here we focus on the role of mitochondrial sirtuins in cardiac and renal function and the potential effects of NAD+ supplementation on these sirtuins.
Role in cardiac function
SIRT3
SIRT3 has a role in maintaining normal cardiac physiology and potentially in mitigating cardiac pathophysiology. Ablation of SIRT3 in the heart results in hyper-acetylation of metabolic enzymes, hypertrophy, and a marked reduction (>50%) in ATP levels, demonstrating an important role for SIRT3 in regulating cardiac bioenergetics66,67. SIRT3-knockout mice develop spontaneous cardiac hypertrophy with age68 and have increased susceptibility to several pharmacological and non-pharmacological cardiac stressors31,69. In pathophysiological states, levels of NAD+ are reduced, suggesting that SIRT3 activity is decreased23,70. Consistent with this conclusion, hyperacetylation of cardiac mitochondrial proteins has been reported in mouse models of heart failure and in failing human hearts23,70.
Importantly, hyperacetylation of mitochondrial proteins is thought to be a driver of metabolic dysfunction in the heart22,23,43,71. In a model of obesity-induced cardiomyopathy and in a SIRT3-knockout mouse, hyperacetylation increased the activities of cardiac long chain acyl-CoA dehydrogenase and other enzymes involved in fatty acid oxidation (FAO)71. The researchers suggested that the resulting increase in rates of FAO likely reduced cardiac efficiency. By contrast, an earlier study showed that protein hyperacetylation was associated with reduced rates of FAO in the hearts of fasted animals43. This discrepancy suggests that the type of stress might be important in determining the effect of acetylation on protein activity. Future studies are required to elucidate this context-specific regulation of target proteins via acetylation and improve understanding the role of acetylation in various types of cardiac pathology.
SIRT3 mediates some of the beneficial effects of NAD+ therapy in the heart. Early studies showed that treatment of mice or murine embryonic fibroblasts with nicotinamide riboside increased NAD+ levels and activated SIRT1 and SIRT3 (REF. 72). Nicotinamide riboside supplementation also reduced the acetylation of specific targets of SIRT3, including superoxide dismutase 2 (SOD2) and NADH ubiquinone oxidoreductase subunit A9, suggesting that this treatment activates SIRT3. In another murine study, SIRT3 knockout increased the sensitivity of mice to isoproterenol-induced cardiac hypertrophy31. Treatment with exogenous NAD+ blocked isoproterenol-induced hypertrophy in a SIRT3-dependent manner, highlighting an important role of SIRT3 in mediating the therapeutic efficacy of NAD+ supplementation. Finally, NMN supplementation resulted in reduced acetylation of cardiac mitochondrial proteins in a mouse model of complex I deficiency22,23, potentially via activation of SIRT3.
Together, these data show that NAD+ has the capacity to activate SIRT3 in the heart and improve cardiac function. Supplementation with NMN has been shown to modulate cardiac acetylation22 and exogenous NAD+ can activate cardiac SIRT3 (REF. 31). The beneficial effects of NMN in the heart are consistent with efficient transport and conversion to NAD+ in the cardiomyocyte. NMN supplementation seems to be an effective means to reduce mitochondrial protein acetylation and off-set functional cardiac decline during heart failure.
SIRT4
Similar to other mitochondrial sirtuins, SIRT4 is highly expressed in tissues with high metabolic demand, including the heart, kidney, liver and brain44. Little is known about the role of SIRT4 in heart and kidney function. SIRT4 has, however, been shown to attenuate hypoxia-induced apoptosis of cardiomyoblast cells, suggesting a cardioprotective effect73. By contrast, SIRT4 knockout protected mice against angiotensin II-induced cardiac hypertrophy, fibrosis and oxidative stress, suggesting a cardioprotective effect of SIRT4 deficiency and implicating SIRT4 in the pathogenesis of disease74. Although the findings of these studies are conflicting, both highlight a role for SIRT4 in modulating oxidative stress. Clearly, additional studies are required to understand the role of SIRT4 in cardiac physiology and pathophysiology, particularly in the setting of NAD+ precursor supplementation.
SIRT5
The role of SIRT5 in maintaining normal cardiac function is not well understood. An early study found that the heart weight, heart rate, and systolic blood pressure of SIRT5-knockout mice receiving a chronic high-fat diet were similar to those of wild-type controls, suggesting that this mitochondrial sirtuin has little influence over cardiac function75. Stress is often required, however, to elicit a phenotype in sirtuin-deficient animals, and cardiac stress may be needed to study the role of SIRT5 in the heart76. Although SIRT5 targets multiple protein modifications, protein succinylation is uniquely elevated in the hearts of SIRT5-knockout mice77, suggesting that reversible succinylation of metabolic proteins might regulate cardiac metabolism. A large number of proteins involved in metabolic pathways are significantly succinylated in the absence of SIRT5, including those involved in fatty acid metabolism, branched-chain amino acid catabolism, the tricarboxylic acid cycle, oxidative phosphorylation (OXPHOS), stress pathways, ketogenesis, and pyruvate metabolism76.
In mice subjected to cardiac ischaemia, SIRT5-knockout hearts had a greater infarct area post-reperfusion than those of wild-type controls, as well as slightly elevated ROS levels76. Succinylation activated succinate dehydrogenase (SDH) in the SIRT5-knockout hearts, and inhibition of SDH normalized infarct size to wild-type levels76. Together, these data suggest that SIRT5 influences the cardiac stress response by decreasing SDH activity via desuccinylation.
Ageing also elicits a phenotype in the hearts of SIRT5-knockout mice78. The hearts of 39-week-old SIRT5-knockout mice were significantly hypertrophied and had increased fibrosis compared to the hearts of similarly aged wild-type controls. In addition, echocardiograms showed that both the shortening fraction and ejection fraction of the SIRT5-knockout hearts were significantly decreased compared to wild-type levels at 8 weeks and 39 weeks of age. Succinylation and subsequent inhibition of the mitochondrial trifunctional protein α-subunit was a key mechanism that contributed to cardiomyopathy in the hearts of ageing SIRT5-knockout mice.
No studies have directly examined the effects of boosting NAD+ levels on SIRT5, but phenotypic similarities between the hearts of SIRT3-knockout and SIRT5-knockout mice suggest that supplementation with NAD+ precursors in the context of heart failure might activate SIRT5. In this setting, putative SIRT5 activation by NAD+ precursors, such as NMN, would be expected to lead to wide-spread protein desuccinylation, and activation of metabolic pathways that might lead to a decreased pathological response to stresses such as ischaemia–reperfusion injury or ageing. Future studies should be directed at testing the cardiac SIRT5–NAD+ axis.
Role in renal function
SIRT3
In the kidney SIRT3-mediated deacetylation under basal and stress conditions is an emerging area of study. Early work correlated changes in Sirt3 mRNA expression with various renal stresses34,79,80. In general, stress tends to reduce renal expression of Sirt3; however, few studies have measured corresponding changes in SIRT3 activity by monitoring changes in mitochondrial protein acetylation in response to stress. In a model of free fatty acid (FFA)-associated tubulointerstitial inflammation, Sirt3 mRNA expression was significantly decreased, ROS accumulated, and markers of inflammation increased compared to levels in control animals79. Remarkably, retroviral overexpression of Sirt3 alleviated these effects, demonstrating that SIRT3 is an important factor that maintains cellular function in response to FFA-associated tubulointerstitial inflammation. In addition, rats fed a high-fat diet had reduced renal Sirt3 expression and increased oxidative stress, as measured by an increase in malondialdehyde and manganese superoxide dismutase acetylation80.
Mitochondrial function, acetylation, and SIRT3 activity have also been studied in a model of cisplatin-induced AKI34. Cisplatin-treated renal tissue had reduced numbers of mitochondria with several hyperacetylated mitochondrial proteins and decreased Sirt3 mRNA expression. Treatment with an 5′-AMP-activated protein kinase (AMPK) agonist (5-aminoimidazole-4- carboxamide-1-β-d-ribofuranoside) or an antioxidant (acetyl-L-carnitine) together with cisplatin resulted in an increase in Nampt, Ppargc1a, and Sirt3 mRNA and a decrease in mitochondrial protein acetylation. Similarly, exposure of glomerular mesangial cells to high glucose levels resulted in decreases in the levels of Sirt3 mRNA and protein expression33, whereas supplementation with NAD+ prevented the development of high-glucose-induced mesangial hypertrophy and increased SIRT3 mRNA and protein levels. This finding supports a potential protective role of renal SIRT3 in diabetic nephropathy.
Together, the available data support a role of SIRT3 as a therapeutic target for conditions of renal stress, particularly oxidative stress. In models of SIRT3 depletion or activation, however, deacetylase activity and subsequent pathway activation have not yet been directly linked to the observed phenotypes described above34,79,80. Despite this shortcoming, these early studies provide a strong rationale for NAD+ supplementation to activate SIRT3 and improve kidney function in renal disease.
SIRT4
SIRT4 has a potential role in maintaining kidney function after AKI81. In rats, cisplatin-induced nephrotoxicity resulted in decreased expression of NAMPT, SIRT1, SIRT3, and SIRT4. Upon cotreatment with cisplatin and the anti-inflammatory agent curcumin, the reduction in levels of these proteins was partially abrogated and kidney function was improved compared with that of cisplatin-treated controls. The data showed a positive correlation between kidney function, NAD+ metabolism, and SIRT4 levels. The improvement in kidney function cannot be directly attributed to SIRT4, however, because the levels of NAMPT, SIRT1 and SIRT3 also increased in response to cisplatin and circumin treatment. Much work remains to determine the role of SIRT4 in kidney metabolism and physiology.
SIRT5
Ablation of SIRT5 leads to hypersuccinylation of mitochondrial proteins in the kidney and other tissues39,77. The levels of other post-translational modifications that are regulated by SIRT5, namely mal-onylation77 and glutarylation39, also increase in kidneys in the absence of SIRT5. These observations suggest a role for SIRT5 in renal metabolism, but this hypothesis remains largely unexplored.
Some studies have reported a role for SIRT5 in the regulation of circulating ammonia levels. Liver carbamoyl-phosphate synthase 1 (CPS1) is an integral protein in the urea cycle and a target of SIRT5 (REFS 38,39,82). During fasting, SIRT5 deacylates liver CPS1, enabling CPS1 activation and lowering of blood ammonia levels. Following a 48 h fast, blood ammonia levels were increased in mice with global knockout of SIRT5 compared with levels in wild-type controls75. Consistent with a role for SIRT5 in regulation of ammonia handling, activation of glutaminase via SIRT5-mediated desuccinylation of lysine residues resulted in elevated renal ammonia excretion83. Although the effect of AKI or other renal stresses on protein succinylation and SIRT5 expression is not yet known, these studies highlight a key role of SIRT5 in the control of ammonia metabolism, and identify an emerging area for investigation.
Activation by NAD+ supplementation
A wealth of literature demonstrates that pathways affected by NAD+ supplementation converge on pathways affected by sirtuins, which together can be activated by supplementation with NAD+ or NAD+ precursors or by manipulation of NAD+ biosynthesis. In mitochondria, levels of NAD+ are thought to reach a magnitude higher than the Michaelis constants (Km) for SIRT3 and SIRT5, suggesting that sirtuin activity could be rate-limited by mitochondrial NAD+ levels3, and therefore poised for activation.
Given the important role of the mitochondrial sirtuins in cardiac function, these proteins could partly mediate the beneficial effects of NAD+ supplementation. Currently, the strongest evidence exists for a role of SIRT3 in mediating the beneficial effects of NAD+ therapy. Although the effect of NAD+ supplementation on the function of SIRT4 or SIRT5 has not been studied directly, these mitochondrial sirtuins could also protect against cardiac or renal stress. Determining whether the activity of SIRT4 or SIRT5 changes in the setting of NAD+ therapy, for example by measuring changes in sirtuin-specific protein modifications, will be important in future studies.
Several models of renal or cardiac stresses are associated with increases in mitochondrial protein acetylation, which might be due to, or indicative of, depleted intracellular NAD+ and reduced sirtuin activity. Protein acetylation is also observed in human failing hearts23,70, suggesting that these findings have clinical relevance. NAD+ supplementation could activate multiple sirtuins and improve mitochondrial function, thereby improving cardiac and renal function under diseased conditions.
NAD+ and cellular redox
NAD+ supplementation also influences cardiac and renal function by directly influencing the cellular redox state. The heart and kidneys maintain a high degree of metabolic flexibility by consuming various fuel substrates to generate ATP, which is crucial for tissue function. Both organs prioritize fats as the preferred substrate for energy production (fats are thought to provide the greatest output of energy per carbon), but can also use other substrates such as glucose (heart), lactate (heart and kidney), ketones (heart), and amino acids (heart and kidney)5,84. Metabolism of these substrates to generate ATP is achieved predominantly via mitochondrial OXPHOS. This process involves a series of redox reactions whereby free energy released from the catabolism of carbon substrates is captured by exchanges between electron donors and acceptors through the electron transport chain (ETC). Catabolism of mitochondrial substrates generates electrons that are accepted by electron acceptors such as flavin adenine dinucleotide and NAD+. Reduction of NAD+ generates NADH, which is ultimately oxidized by complex I of the ETC to regenerate NAD+ (REF. 3). Ultimately, the exchanges within these redox reactions result in the efficient conversion of chemical energy to ATP, which is a more stable and universal energy-bearing metabolite. The ratio of NAD+ to NADH is a key regulator of several enzymes, including a wide range of oxidoreductases. For example, elevated levels of NADH inhibit several oxidative NAD+-dependent enzymes. NAD+ is an obligatory cofactor of metabolism and its availability controls the overall production of cardiac and renal ATP (REF. 3).
The importance of NAD+/NADH redox exchange as a means to generate energy is underscored by the many cardiac and renal pathologies that are associated with defective OXPHOS resulting from inborn errors in metabolism. Impairment of the OXPHOS machinery contributes to reduced substrate oxidation, decreased ATP production, compromised intracellular Ca2+ flux, generation of ROS, and an altered NAD+ to NADH ratio5,84,85. Various cardiac stressors lead to a switch from oxidative to anaerobic metabolism to reduce ROS damage and salvage ATP. This switch halts OXPHOS and exhausts mitochondrial NAD+ (REF. 24).
In a model of cardiac-specific complex I deficiency (NDUSF4KO), mice had reduced NAD+ to NADH ratio, hyperacetylated mitochondrial proteins, OXPHOS dysfunction, and a sensitivity to cardiac stress. The increased susceptibility to cardiac stress of these mice was mediated by increased acetylation of both the mitochondrial permeability transition pore (mPTP), which sensitized the pore to premature opening, and the malate aspartate shuttle, which inhibited shuttle activity86. Treatment with NMN partially normalized NAD+ levels, reversed hyperacetylation of the mPTP and the malate aspartate shuttle, and attenuated the development of heart failure in NDUFS4KO mice undergoing chronic stress; activation of SIRT3 upon NMN supplementation could have an important role in mediating these beneficial effects. Another provocative mechanistic explanation is that NAD+ supplementation directly shifts the NAD+ to NADH ratio, thereby reactivating cellular oxidoreductases. NDUFS4KO mice had a reduced NAD+ to NADH ratio and possible inhibited SIRT3 (REF. 22). Sirtuins are direct sensors of NAD+, but they do not seem to sense NADH at physiologically relevant levels87; therefore, they are unlikely to sense shifts in the NAD+ to NADH ratio in the same way that several cellular oxidoreductases sense the ratio. If NDUFS4KO mice have NAD+ deficiency and elevated levels of NADH, adding NAD+ might have a direct, sirtuin-independent effect on oxidoreductase activity, which could contribute to the beneficial effects of NMN treatment. Regardless of this alternative hypothesis, SIRT3 and its regulation of acetylation are key mediators of the beneficial effects of NMN in the pathological cardiac remodelling observed in the NDUFS4KO mouse.
In the kidney, the dependence on the NAD+ to NADH ratio and susceptibility to OXPHOS impairment is underscored by the relationship between oxidative metabolism and renal protection against AKI-induced tissue damage2. Peroxisome proliferator-activated receptor γ co-activator 1α (PGC1α) is a key transcriptional regulator of NAD+ biosynthesis that protects against AKI. In PGC1α-deficient mice, supplementation with nicotinamide enhanced fatty acid oxidation and resulting NAD+/NADH redox-driven ATP production, reversed AKI damage, and protected against AKI2. Nicotinamide supplementation seemed to directly influence the NAD+/NADH redox state to enable substrate oxidation, thereby restoring metabolic and OXPHOS activity, and overall ATP production in this model of renal disease.
In summary, under cardiac and renal conditions of impaired OXPHOS or other states associated with a deficiency in electron acceptors (NAD+), supplementation with NAD+ and its precursors might directly relieve inhibition of some OXPHOS enzymes and improve cellular redox. As cellular redox is an important contributor to cardiac and renal metabolism and the response to stress, manipulating redox using NAD+ precursors might have therapeutic efficacy. Future studies are required to test this hypothesis.
NAD+ and cell signalling
In the heart and kidney, NAD+ is also a substrate for enzymes involved in DNA damage repair and calcium signalling pathways, the ADP-ribosyltransferases (ARTDs; also known as PARPs) and the ADPR cyclases, respectively. Substantial efforts have been directed at determining if these enzymes can be manipulated as a strategy to alter intracellular NAD+ levels or, conversely, if they are influenced by NAD+ supplementation therapies.
ARTDs and ADPR cyclases have emerged as major nodes that influence NAD+ levels5,88. Both consume NAD+ and use the ADPR group to relay cellular signals (FIG. 2). Specifically, ARTDs transfer ADPR to accepting molecules to signal DNA damage and induce cell death89. Likewise, ADPR cyclases hydrolyse NAD+ to form ADPR and other signalling metabolites that function as second messengers in calcium signalling90. These enzymatic activities generate nicotinamide as a byproduct, which can feedback to inhibit their activities. Several studies have shown that these enzymes deplete intracellular NAD+ levels under conditions of cardiac and renal pathological stress5. For this reason, manipulating NAD+ by inhibiting ARTDs and ADPR cyclases is currently being explored as a therapy to alter cell viability and function, respectively, in cardiac and renal diseases.
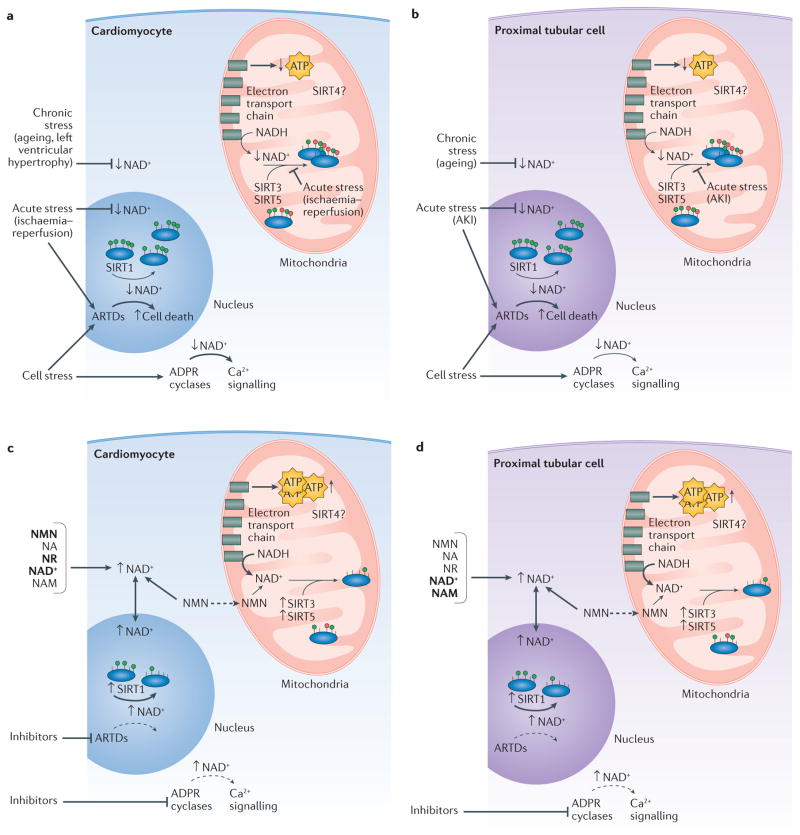
Figure 2. The potential effects of nicotinamide adenine dinucleotide (NAD+) therapy on cardiac and renal pathophysiology.
Chronic cardiac (part a) and renal (part b) stresses such as ageing and left ventricular hypertrophy result in depleted cellular NAD+ levels. Mitochondrial protein hyperacylation accompanies general mitochondrial dysfunction and might contribute to impaired reduction–oxidation (redox) reactions and ATP depletion. Cellular stress causes overactivation of ADP-ribosyltransferases (ARTDs) and adenine diphosphate ribose (ADPR) cyclases, potentially contributing to overall NAD+ depletion. Acute stresses including ischaemia and acute kidney injury (AKI) deplete cellular NAD+ levels by lowering NAD+ synthesis (primarily through the amidated route). Supplementation with NAD+ or NAD+ precursors might boost cellular NAD+ levels and improve cardiac (part c) and renal (part d) function in disease states. The primary mechanism might be activation of mitochondrial sirtuins (SIRTs), which activate protein lysine deacylation and improve mitochondrial function, leading to improved cellular health. Other NAD+ boosting therapies include inhibiting ARTDs and ADPR cyclases to maintain nuclear and cytoplasmic NAD+ levels. In the heart, ARTD inhibition has beneficial effects but the mechanism is unclear. Inhibition of ADPR cyclases substantially increases NAD+ levels in the heart, but only minimally increases NAD+ levels in the kidney. Such inhibition might improve cardiac and renal function in disease states by normalizing calcium signalling. NA, nicotinic acid; NADH, reduced NAD; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside.
ARTDs and NAD+
The ARTD family of enzymes transfer the ADP-ribose group of NAD+ to histones, DNA and proteins as a signal to repair damaged DNA (REF. 89). ARTDs consume NAD+ to synthesize poly(ADP)ribose polymers (PARylation) that covalently attach to accepting molecules (FIG. 2). ARTD1 is the most abundant isoform and serves as a protector of genome stability upon activation by ROS-induced DNA lesions. Activated ARTD1 increases PARylation of enzymes, which is a signal for DNA repair or induction of cell death (parthanatos)3. In the heart and kidneys, persistent oxidative stress triggers over-activation of ARTD1, which contributes to myocardial infarction, ischaemic kidney injury, heart and renal failure, and other cardiovascular and renal pathologies91. Inhibition of ARTD1 protects against several pathological insults in the heart and kidney, such as ischaemia–reperfusion injury, which results in a high level of ROS-induced tissue damage92,93.
Over-activation of ARTD1 can deplete NAD+, resulting in reduced levels of cellular ATP and induction of parthanatos89. Research in skeletal muscle (ARTD1)94, liver (ARTD7), and fat (ARTD5)3 support a role for ARTD-mediated NAD+ depletion as a mediator of cell death. The contribution of NAD+ depletion to cellular death in the heart and kidneys in the setting of various pathological stresses remains to be determined.
By contrast, evidence is mounting that ARTD PARylation rather than NAD+ decline is a direct cause of cellular death in response to pathological stress. For example, direct inhibition of the glycolytic enzyme hexokinase by ARTD1 PARylation reduced glycolysis before NAD+ depletion, increased mitochondrial dysfunction, and led to cortical neuronal death92. Similarly, in the kidney, direct PARylation of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was a key driver of cell death in renal tubules upon ischaemic injury93. ARTD1-mediated PARylation of GAPDH reduced anaerobic respiration, ultimately exacerbating ATP depletion and inducing cell death. These data suggest that the therapeutic benefit of ARTD1 inhibition might be due to altered metabolism at key nodes, independent of altered NAD+ levels under pathological stress. Further research is required to disentangle the role of NAD+ depletion upon ARTD over-activation in renal and cardiomyocyte cell death.
ADPR cyclases and NAD+
Similar to ATRDs during cardiac and renal stress, ADPR cyclases are considered to be important regulators of intracellular NAD+ levels in normal physiology95. These cyclases, primarily CD38 and CD157, serve as important effector molecules for generating Ca2+-mobilizing second messengers that regulate cardiac and renal cell function. In cardiomyocytes, exogenous signals stimulate an initial release of Ca2+, which activates CD38 (REF. 90). Subsequently, CD38 catalyses the conversion of NAD+ to cyclic ADP-ribose (cADPR), which then triggers Ca2+ release from stores in the cardiac sarcoplasmic reticulum96. This Ca2+-induced calcium release serves as the basis of rhythmic contraction of cardiac muscle5. In the kidneys, cADPR signalling is mediated by glomerular mesangial cells97. cADPR stimulates Ca2+ release from the endoplasmic reticulum98, which contributes to renal vasoconstriction in response to various G-protein-coupled receptor agonists99. Mesangial cells and the capillary vasculature form a biomechanical meshwork, whereby Ca2+ signalling regulates mesangial cell contraction and capillary cell surface, ultimately controlling filtration rate and blood volume within the glomerulus99.
Prolonged CD38 activation in response to cardiac stress leads to sustained Ca2+ release and signalling that results in hypertrophy and arrhythmia5,100. Consistent with these findings, male Cd38-knockout mice had enhanced cardiac function, including improved contractility101, and ADPR cyclase inhibitors had anti-arrhythmic effects in models of cellular or cardiac Ca2+ overload100. Several studies support a role of ADPR cyclases other than CD38 in kidney function. For example, inhibition of CD73 has been shown to protect against renal stress such as AKI, and the enzymatic activity of CD39 has been shown to protect against acute renal ischaemic injury102. Kidney-specific compounds that inhibit these ADPR cyclases have been generated, but their influence on kidney function is still under investigation97.
CD38 also alters intracellular NAD+ levels in the heart and kidneys. One study showed that Cd38-knockout mice had ~25-fold higher NAD+ levels in the heart and ~two-fold higher levels in the kidney than did wild-type controls88,95. By contrast, another study showed no changes in NAD+ levels in these tissues in Cd38 knockout mice compared with wild-type controls103. Thus, the extent to which CD38 inhibition or ablation enhances NAD+ levels in the heart and kidney requires further investigation. Despite this gap in knowledge, inhibitors that target ADPR cyclases are currently in development for the treatment of cardiac and renal disease. Thorough characterization of the cellular impact of these inhibitors could identify a role for these enzymes in mediating the therapeutic efficacy of NAD+ supplementation.
Remaining challenges
NAD+ supplementation therapies are efficacious in several animal models of cardiac and renal diseases but two key challenges remain for their continued development and clinical translation. First, elucidating the mechanisms of the therapeutic effects of NAD+ is important to enable their potential to be fully realized in the settings of cardiac and renal pathologies. Disentangling the individual and/or concerted contributions of sirtuin activation, redox, and cellular signalling will be challenging (FIG. 2). Activation of sirtuins, especially SIRT3, via exogenous NAD+ improves cellular bioenergetics, mitochondrial function, and stress resistance. Additionally, NAD+ supplementation seems to correct redox imbalances in the setting of disrupted bioenergetics, which could be mediated partly by directly increasing enzyme redox activity and partly via manipulation of mitochondrial protein acetylation. Finally, increasing NAD+ levels might mitigate the damage induced by overactive ARTDs and ADPR cyclases. Which of these pathways confers the greatest effects on overall cardiac and renal function remains unknown. Specific activators of each pathway, independent of NAD+, might shed light on this question. Alternatively, continued investigation into NAD+ precursor supplementation in animal models lacking relevant pathways (for example NAD+ supplementation in the absence of CD38 (REF. 104) or a sirtuin31) would directly test the role of each pathway in mediating NAD+ therapeutic efficacy.
A second major challenge will be to identify the best pharmacological strategies to alter cellular NAD+ levels (for example precursor supplementation versus inhibition of NAD+ consumers) in patients with various diseases. These strategies have not yet been tested in patients with cardiac or renal diseases. Ongoing clinical trials for other indications are primarily focused on NAD+ precursor supplementation because preclinical studies have demonstrated efficacy of this approach for increasing NAD+ levels and improving a variety of cellular functions. Several clinical trials are underway to determine the pharmacokinetics105–107, bioavailability108, and effect of nicotinamide riboside supplementation in metabolic diseases, with a particular focus on obesity and insulin sensitivity109,110; no adverse effects have been reported with 100–1,000 mg per day. The efficacy of nicotinimide riboside supplementation to improve mitochondrial function, energy metabolism, and parameters of cardiovascular risk is also currently being tested110,111. Interestingly, supplementation with nicotinamide riboside has been reported to increase the NAD+ metabolome in human blood in a dose-dependent manner21. In this study the intermediate nicotinic acid adenine dinucleotide was formed from the recycling of newly synthesized NAD+ and served as a biomarker of enhanced NAD+ levels.
Conclusions
The potential of NAD+ as a therapeutic target has renewed interest in this metabolite, which was discovered over 100 years ago112. NAD+ is a versatile metabolic and redox substrate that is essential for all living cells and has a central role as a co-substrate for sirtuin deacylation reactions, as a coenzyme in OXPHOS reactions, as a substrate in ARTD and ADPR cyclase signalling pathways, and likely also has a role in many as yet undiscovered biological pathways. NAD+ depletion is emerging as a central feature of cardiac and renal disorders that has an important role in the pathogenesis of these diseases2,5. As such, therapeutic approaches to treat these diseases by inhibiting NAD+-consuming enzymes and/or augmenting intracellular NAD+ levels via stimulation of NAD+ biosynthesis or supplementation with NAD+ precursors is an area ripe for discovery.
Key points.
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme with roles in several cardiac and renal metabolic processes
NAD+ depletion is emerging as a major contributor to the pathogenesis of cardiac and renal disease
Preclinical data suggest that NAD+ repletion strategies have the potential to restore healthy renal and cardiac metabolism and physiology
The mitochondrial sirtuins mediate some of the beneficial effects of NAD+ supplementation
NAD+ supplementation can directly enhance metabolism and improve cellular redox reactions in the setting of cardiac and renal disease
NAD+ is also a substrate for enzymes involved in DNA damage repair and calcium signalling pathways; NAD+ supplementation could alter these pathways to influence cell viability, organ function and disease outcomes
Acknowledgments
We acknowledge the scientists whose discoveries were the basis for this review, thank the anonymous peer-reviewers for helpful comments, and apologize to our colleagues whose work we could not cite. We acknowledge funding support from the American Heart Association grants 12SDG8840004 and 12IRG9010008, The Ellison Medical Foundation, Friedreich’s Ataxia Research Alliance, the NIH and the NIA grant R01AG045351, the NIH and the NIAAA grant R01AA022146, the Duke Pepper Older Americans Independence Center (OAIC) Program in Ageing Research supported by the National Institute of Ageing (P30AG028716-01), the Duke O’Brien Center for Kidney Research (5P30DK096493-02). K.A.H. was supported by an NIH/NIGMS training grant to Duke University Pharmacological Sciences Training Program (5T32GM007105-40) and is supported by an NIH pre-doctoral fellowship 1F31HL127959. A.S.M. is supported by an NIH pre-doctoral fellowship 1F31HL123275-31.
Glossary
- Nicotinamide adenine dinucleotide
(NAD+). A pyridine dinucleotide and important metabolic cofactor
- ADPR cyclases
Effector molecules that generate calcium-mobilizing second messengers
- ADP ribosyltransferases
Enzymes that transfer the ADPR group of NAD+ as a signal to repair damaged DNA
- Sirtuins
NAD+-dependent protein deacylases that consume NAD+ to remove post-translational acyl modifications from proteins
- Biosynthetic precursors
The biosynthetic precursors of NAD+ are dietary vitamin B3 compounds, including nicotinic acid, nicotinamide, and nicotinamide riboside. These precursors are recycled from the diet and used by tissues to generate NAD+
- Nicotinamide phosphoribosyltransferase
(NAMPT). An enzyme that converts nicotinamide into NMN in the NAD+ salvage pathway
- Oxidative phosphorylation
(OXPHOS). The electron transport pathway that is used by cells to generate ATP
- Parthanatos
A form of programmed cell death that is induced by accumulation of poly(ADP) ribose and the nuclear translocation of apoptosis-inducing factor from mitochondria.
Footnotes
Author contributions
All authors researched the data, discussed the content, wrote the text and reviewed or edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.Forbes JM. Mitochondria-power players in kidney function? Trends Endocrinol Metab. 2016;27:441–442. doi: 10.1016/j.tem.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Tran MT, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. This study demonstrates that NAM treatment improves renal function in a mouse model of AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantó C, Menzies KJ, Auwerx J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab. 2012;23:467–476. doi: 10.1016/j.tem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Mericskay M. Nicotinamide adenine dinucleotide homeostasis and signalling in heart disease: pathophysiological implications and therapeutic potential. Arch Cardiovasc Dis. 2015;109:207–215. doi: 10.1016/j.acvd.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahimkhani MR, et al. Aag-initiated base excision repair promotes ischemia reperfusion injury in liver, brain, and kidney. Proc Natl Acad Sci USA. 2014;111:E4878–E4886. doi: 10.1073/pnas.1413582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braidy N, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Mori V, et al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS ONE. 2014;9:e113939. doi: 10.1371/journal.pone.0113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin LF, Henderson LM. Pyridinium precursors of pyridine nucleotides in perfused rat kidney and in the testis. J Biol Chem. 1972;247:8023–8030. [PubMed] [Google Scholar]
- 10.Ikeda M, et al. Studies on the biosynthesis of nicotinamide adenine dinucleotide. II A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. J Biol Chem. 1965;240:1395–1401. [PubMed] [Google Scholar]
- 11.Shibata K, Morita N, Shibata Y, Fukuwatari T. Enzymes that control the conversion of L-tryptophan-nicotinamide and the urinary excretion ratio (N 1-methyl-2-pyridone-5-carboxamide + N 1-methyl-4-pyridone-3-carboxamide)/N1-methylnicotinamide in mice. Biosci Biotechnol Biochem. 2013;77:2105–2111. doi: 10.1271/bbb.130467. [DOI] [PubMed] [Google Scholar]
- 12.Eto N, Miyata Y, Ohno H, Yamashita T. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant. 2005;20:1378–1384. doi: 10.1093/ndt/gfh781. [DOI] [PubMed] [Google Scholar]
- 13.Kempson SA, Colon-Otero G, Ou SY, Turner ST, Dousa TP. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest. 1981;67:1347–1360. doi: 10.1172/JCI110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopal E, et al. Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochem J. 2005;388:309–316. doi: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trueblood NA, Ramasamy R, Wang LF, Schaefer S. Niacin protects the isolated heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;279:H764–H771. doi: 10.1152/ajpheart.2000.279.2.H764. [DOI] [PubMed] [Google Scholar]
- 16.Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem. 1972;247:778–783. [PubMed] [Google Scholar]
- 17.Corr PB, May DG. Renal mechanisms for the excretion of nicotinic acid. J Pharmacol Exp Ther. 1975;192:195–200. [PubMed] [Google Scholar]
- 18.Nomura K, et al. Hepatectomy-related hypophosphatemia: a novel phosphaturic factor in the liver–kidney axis. J Am Soc Nephrol. 2014;25:761–772. doi: 10.1681/ASN.2013060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grozio A, et al. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J Biol Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratajczak J, et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trammell SAJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. doi: 10.1038/ncomms12948. The first clinical study of nicotinamide riboside supplementation; shows a dose-dependent increase in NAD+ with nicotinamide riboside, demonstrating the potential of NAD+ boosting therapies in a clinical setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karamanlidis G, et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CF, et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134:883–894. doi: 10.1161/CIRCULATIONAHA.116.022495. This study demonstrates that NMN and cardiac NAMPT increase NAD+, reduce protein hyperacetylation, and improve cardiac function in a mouse model of heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T, et al. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, et al. Nmnat3 is dispensable in mitochondrial NAD level maintenance in vivo. PLoS ONE. 2016;11:e0147037. doi: 10.1371/journal.pone.0147037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CP, Yamamoto T, Oka S, Sadoshima J. The function of nicotinamide phosphoribosyltransferase in the heart. DNA Repair (Amst) 2014;23:64–68. doi: 10.1016/j.dnarep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P, Miao CY. NAMPT as a therapeutic target against stroke. Trends Pharmacol Sci. 2015;36:891–905. doi: 10.1016/j.tips.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Aboud OA, et al. Dual and specific inhibition of NAMPT and PAK4 by KPT-9274 decreases kidney cancer growth. Mol Cancer Ther. 2016;15:2119–2129. doi: 10.1158/1535-7163.MCT-16-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benito-Martin A, et al. Endogenous NAMPT dampens chemokine expression and apoptotic responses in stressed tubular cells. Biochim Biophys Acta. 2014;1842:293–303. doi: 10.1016/j.bbadis.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Pillai VB, et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3–LKB1–AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. Study showing that exogenous NAD+ supplementation blocked agonist-induced hypertrophic responses in cardiomyocytes and mouse models; implicates SIRT3 deacetylase activity in the mechanism of this cardioprotection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuo L, et al. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins–AMPK–mTOR pathway. Cell Physiol Biochem. 2011;27:681–690. doi: 10.1159/000330077. This study shows that exogenous NAD+ maintains SIRT1 and SIRT3 activity in the setting of high-glucose-induced mesangial hypertrophy and provides a mechanism for how sirtuin activity protects against mesangial hypertrophy. [DOI] [PubMed] [Google Scholar]
- 34.Morigi M, et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson KA, Green MF, Huynh FK, Wagner GR, Hirschey MD. SnapShot: mammalian sirtuins. Cell. 2014;159:956–956.e1. doi: 10.1016/j.cell.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denu JM. The Sir2 family of protein deacetylases. Curr Opin Chem Biol. 2005;9:431–440. doi: 10.1016/j.cbpa.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Peng C, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111.012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du J, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288:31350–31356. doi: 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundaresan NR, et al. The sirtuin SIRT6 blocks IGF–Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta M, Samant S, Bao R, Pillai V. The sirtuin SIRT6 represses expression of cachexia-associated cytokine myostatin by blocking its NF-kB-dependent gene transcription. FASEB J. 2016;30:1009.11. [Google Scholar]
- 43.Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haigis MC, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 45.Michishita E, Park JY, Burneskis JM. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathias RA, et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. 2014;159:1615–1625. doi: 10.1016/j.cell.2014.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong SM, et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell. 2013;23:450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner GR, Payne RM. Mitochondrial acetylation and diseases of aging. J Aging Res. 2011;2011:1–13. doi: 10.4061/2011/234875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell. 2014;54:5–16. doi: 10.1016/j.molcel.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghanta S, Grossmann RE, Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: biocehmical and metabolic logic of acetyl-lysine modifications. Crit Rev Biochem Mol Biol. 2013;48:1–42. doi: 10.3109/10409238.2013.838204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chhoy P, et al. In: Sirtuins. Houtkooper R, editor. Vol. 10. Sirtuins; 2016. pp. 105–138. [Google Scholar]
- 52.Park J, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 54.Anderson RM, et al. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- 55.Belenky P, et al. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/ Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 57.Mouchiroud L, et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshino J, Mills KF, Yoon MJ, Imai SI. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet. 2014;30:271–286. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, Li Y. SIRT1: role in cardiovascular biology. Clin Chim Acta. 2015;440:8–15. doi: 10.1016/j.cca.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 62.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong L, et al. Sirtuin 1: a target for kidney diseases. Mol Med. 2015;21:87–97. doi: 10.2119/molmed.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong YJ, et al. Renal protective effect of sirtuin 1. J Diabetes Res. 2014;2014:843786. doi: 10.1155/2014/843786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitada M, Kume S, Koya D. Role of sirtuins in kidney disease. Curr Opin Nephrol Hypertens. 2014;23:75–79. doi: 10.1097/01.mnh.0000437330.85675.ac. [DOI] [PubMed] [Google Scholar]
- 66.Ahn BH, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sundaresan NR, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hafner AV, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Porter G, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol. 2014;306:H1602–H1609. doi: 10.1152/ajpheart.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horton JL, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;1:1–14. doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alrob OA, et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res. 2014;103:485–497. doi: 10.1093/cvr/cvu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cantó C, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu B, et al. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 2013;32:655–662. doi: 10.1159/000354469. [DOI] [PubMed] [Google Scholar]
- 74.Luo Y-X, et al. Sirt4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw138. http://dx.doi.org/10.1093/eurheartj/ehw138. [DOI] [PubMed]
- 75.Yu J, et al. Metabolic characterization of a Sirt5 deficient mouse model. Sci Rep. 2013;3:2806. doi: 10.1038/srep02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boylston JA, et al. Characterization of the cardiac succinylome and its role in ischemia-reperfusion injury. J Mol Cell Cardiol. 2015;88:73–81. doi: 10.1016/j.yjmcc.2015.09.005. The first publication of the cardiac succinylome; the data suggest a role of SIRT5 in the response to ischaemia–reperfusion injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishida Y, et al. SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol Cell. 2015;59:321–332. doi: 10.1016/j.molcel.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadhukhan S, et al. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci USA. 2016;113:4320–4325. doi: 10.1073/pnas.1519858113. This study shows that SIRT5-knockout hearts have impaired fatty acid oxidation that contributes to cardiac hypertrophy with ageing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koyama T, et al. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med. 2011;51:1258–1267. doi: 10.1016/j.freeradbiomed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 80.Yang H, et al. Green tea polyphenols attenuate high-fat diet-induced renal oxidative stress through SIRT3-dependent deacetylation. Biomed Environ Sci. 2015;28:455–459. doi: 10.3967/bes2015.064. [DOI] [PubMed] [Google Scholar]
- 81.Ugur S, et al. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Renal Fail. 2014;37:332–336. doi: 10.3109/0886022X.2014.986005. [DOI] [PubMed] [Google Scholar]
- 82.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Mol Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polletta L, et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy. 2015;11:253–270. doi: 10.1080/15548627.2015.1009778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall AM, Unwin RJ. The not so ‘mighty chondrion’: emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiol. 2007;105:1–10. doi: 10.1159/000096860. [DOI] [PubMed] [Google Scholar]
- 85.Fosslien E. Mitochondrial medicine — molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci. 2001;31:25–67. [PubMed] [Google Scholar]
- 86.Fung Lee C, Garcia-Menendez L, Karamanlidis G, Tian R. Restoration of NAD redox balance ameliorates pressure overload-induced cardiac hypertrophy and dysfunction via regulation of mitochondrial protein acetylation and permeability transition. Free Radic Biol Med. 2013;65:S75. [Google Scholar]
- 87.Madsen AS, et al. Investigating the sensitivity of NAD+-dependent sirtuin deacylation activities to NADH. J Biol Chem. 2016;291:7128–7141. doi: 10.1074/jbc.M115.668699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 89.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malavasi F, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 91.Xiao CY, et al. Poly(ADP-ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 92.Andrabi SA, et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci USA. 2014;111:10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Devalaraja-Narashimha K, Padanilam BJ. PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol. 2009;20:95–103. doi: 10.1681/ASN.2008030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pirinen E, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 96.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 97.Kim SY, Park KH, Gul R, Jang KY, Kim UH. Role of kidney ADP-ribosyl cyclase in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;296:F291–F297. doi: 10.1152/ajprenal.90381.2008. [DOI] [PubMed] [Google Scholar]
- 98.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 99.Thai TL, Arendshorst WJ. Mice lacking the ADP ribosyl cyclase CD38 exhibit attenuated renal vasoconstriction to angiotensin II, endothelin-1, and norepinephrine. Am J Physiol Renal Physiol. 2009;297:F169–F176. doi: 10.1152/ajprenal.00079.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kannt A, Sicka K, Kroll K, Kadereit D, Gögelein H. Selective inhibitors of cardiac ADPR cyclase as novel anti-arrhythmic compounds. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:717–727. doi: 10.1007/s00210-012-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gan L, et al. Disruption of CD38 gene enhances cardiac functions by elevating serum testosterone in the male null mice. Life Sci. 2011;89:491–497. doi: 10.1016/j.lfs.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 102.Rajakumar SV, et al. Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation. 2010;90:1260–1264. doi: 10.1097/TP.0b013e3182003d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD + in the Cd38−/− mouse. Biochem Biophys Res Commun. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 104.Guan XH, et al. CD38 deficiency protects the heart from ischemia/reperfusion injury through activating SIRT1/FOXOs-mediated antioxidative stress pathway. Oxid Med Cell Longev. 2016;2016:7410257. doi: 10.1155/2016/7410257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.US National Library of Medicine. ClinicalTrials.gov. 2015 https://clinicaltrials.gov/ct2/show/NCT02300740.
- 106.US National Library of Medicine. ClinicalTrials.gov. 2014 https://clinicaltrials.gov/ct2/show/NCT02191462.
- 107.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02689882.
- 108.US National Library of Medicine. ClinicalTrials.gov. 2017 https://clinicaltrials.gov/ct2/show/NCT02712593.
- 109.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02303483.
- 110.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02835664.
- 111.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02812238.
- 112.Harden A, Young WJ. The alcoholic ferment of yeast-juice. Part II. — the conferment of yeast-juice. Proc R Soc Lond B. 1906;78:369–375. [Google Scholar]