Summary
Cardiotoxicity is a well-established complication of oncology therapies. Cardiomyopathy resulting from anthracyclines is a classic example. In the past decade, an explosion of novel cancer therapies, often targeted and more specific than conventional therapies, has revolutionized oncology therapy and dramatically changed cancer prognosis. However, some of these therapies have introduced an assortment of cardiovascular (CV) complications. At times, these devastating outcomes have only become apparent after drug approval and have limited the use of potent therapies. There is a growing need for better testing platforms, both for CV toxicity screening and for elucidating mechanisms of cardiotoxicities of approved cancer therapies. This review discusses the utility of available nonclinical models (in vitro, in vivo, and in silico) and highlights recent advancements in modalities like human stem cell-derived cardiomyocytes for developing more comprehensive cardiotoxicity testing and new means of cardioprotection with targeted anticancer therapies.
Key Words: cardio-oncology, cardiotoxicity, nonclinical model, pre-clinical model
Abbreviations and Acronyms: CML, chronic myeloid leukemia; CRISPR, clustered regularly interspaced short palindromic repeats; CTCAE, Common Terminology Criteria for Adverse Events; CV, cardiovascular; FDA, Food and Drug Administration; hERG, human ether-à-go-go-related gene; NRVM, neonatal rat ventricular myocytes; PSC-CM, pluripotent stem cell-derived cardiomyocyte; TKI, tyrosine kinase inhibitor
In the last decade, there has been a paradigm shift in cancer treatment from the use of nonselective cytotoxic agents toward targeted therapies aimed at cellular pathways that have been hijacked by the cancer (1). Indeed, in 2015, oncology was a natural choice as the initial focus of the U.S. government Precision Medicine Initiative, a $215 million investment for individualized approach to patient care (2). Conventional cancer therapies like radiation can lead to cardiovascular (CV) toxicities due to direct, nonselective myocardial injury (3). Paradoxically, several of the novel targeted oncology therapies are associated with a wide spectrum of CV complications in humans, which were unanticipated based on nonclinical (also known as “preclinical”) safety studies 4, 5. Such discrepancies highlight the limitations of current nonclinical strategies in predicting cardiotoxicities.
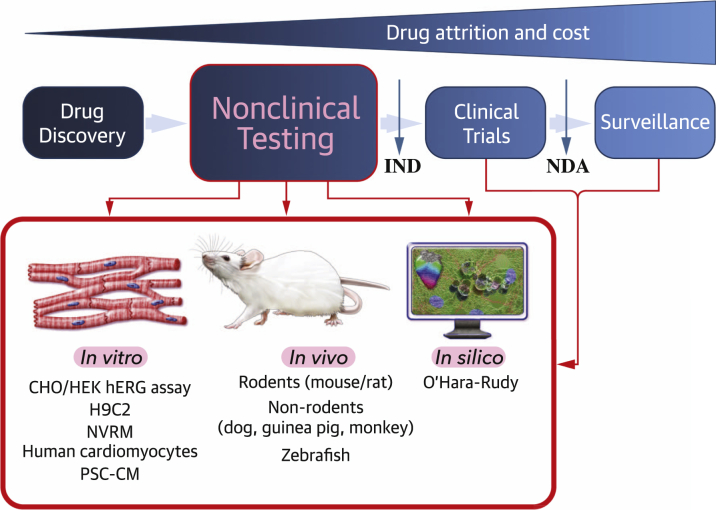
Here, we discuss new insights on CV safety in the development of novel targeted anticancer drugs. Successful and efficient drug development is predicated on establishing nonclinical models that can be high-throughput, cost-effective, and comparable to human physiology for the purposes of clinical efficacy and safety. In addition, these models must help in understanding mechanisms of CV toxicities and strategies for CV toxicity protection. We explore drug-induced cardiotoxicity testing strategies and review the existing nonclinical models (in vitro, in vivo, and in silico), which focus on identifying CV complications with high mortality risk such as sudden cardiac death secondary to arrhythmia and heart failure (Figure 1). In particular, we highlight recent advances in human pluripotent stem cell-derived cardiomyocytes (PSC-CMs) as a revolutionary in vitro model that can improve cardiotoxicity assessment via personalized medicine and discuss the merits of in vivo and in silico models. Combining data from these respective methods will ensure a better translation to improving patient safety. Last, we conclude with a discussion of the clinical implications of monitoring and reducing CV toxicities gleaned from nonclinical studies.
Figure 1.
The Need for More Effective Methods of Nonclinical Screening in Cancer Treatment-Related Cardiotoxicities
There are numerous in vivo, in vitro, and in silico models that can be used for both nonclinical testing of CV toxicities and follow-up investigations of underlying mechanisms, which can be used to develop cardioprotective therapies. IND = investigational new drug; NDA = new drug application.
The Emergence of Cardio-Oncology
Over the past several decades, improved understanding of the cellular and molecular biology underlying various types of cancer has led to rapid advancements in drug discovery and treatment efficacy. From 1991 to 2012, the overall cancer death rate declined by 23% (6). In the United States alone, there were 14.5 million cancer survivors in 2014, with a projected 19 million survivors by 2024 (7). Cardio-oncology (CV and cardiometabolic care of cancer patients), also called oncocardiology, has emerged as a new medical discipline for several reasons. First, cancer survivors are at risk of CV disease because CV disease is prevalent in the general population. Second, both conventional and novel cancer therapies are associated with CV and metabolic toxicities (Table 1). These adverse sequelae include acute and chronic CV toxicities and include a variety of complications such as cardiomyopathy, coronary and peripheral vascular disease, conduction abnormalities, thrombosis, hypertension, and metabolic disorders 4, 8. However, because novel cancer drugs can revolutionize treatment and prolong life, cardiotoxicity risk must be carefully weighed against the overall benefit of cancer treatment.
Table 1.
Select Classes of Drugs and Their Reported Cardiotoxicities in Drug Labels
| Class of Anticancer Drug | Example | Initial FDA Approval∗ | Boxed Warning∗ | W and P Label∗ |
|---|---|---|---|---|
| Alkylating agents | Cyclophosphamide | 1959 | Myocarditis, pericarditis, pericardial effusion, arrhythmias, and CHF | |
| Antimetabolites | 5-fluorouracil (5-FU) | 1962 | Myocardial ischemia, angina | |
| Anthracyclines | Doxorubicin | 1974 | CHF | Arrhythmia |
| Liposomal doxorubicin | 1995 | CHF | ||
| Epirubicin | 1999 | CHF | Arrhythmia, thrombophlebitis | |
| Taxanes | Paclitaxel | 1992 | Severe conduction abnormalities, hypotension, bradycardia, and HTN | |
| HER2 inhibitors | Trastuzumab | 1998 | CHF | Cardiac dysfunction, arrhythmia, HTN |
| Pertuzumab | 2012 | Cardiac dysfunction | ||
| Ado-trastuzumab emtansine | 2013 | LV dysfunction | ||
| Tyrosine kinase inhibitors (TKIs) | Imatinib | 2001 | Edema, CHF, hypereosinophilic cardiac toxicity | |
| Dasatinib | 2006 | Cardiac dysfunction, PAH, QT prolongation, fluid retention including pleural and pericardial effusion | ||
| Nilotinib | 2007 | QT prolongation, torsades de pointes, sudden death | Ventricular repolarization abnormalities, cardiac and arterial vascular occlusive events, fluid retention including pleural and pericardial effusion | |
| Crizotinib | 2011 | Bradycardia, QT prolongation | ||
| Ponatinib | 2012 | Arterial thrombosis (fatal MI, stroke) | CHF, HTN, fluid retention, arrhythmia | |
| Cabozantinib | 2012 | Severe hemorrhage | Arterial thromboembolic events (MI, stroke), HTN | |
| Ibrutinib | 2013 | Atrial fibrillation | ||
| VEGF signaling pathway inhibitors | Bevacizumab | 2004 | Severe hemorrhage | MI, stroke, DVT, HTN |
| Sorafenib | 2005 | Ischemia, QT prolongation, HTN | ||
| Sunitinib | 2006 | Ischemia, CHF, QT prolongation, torsades de pointes, HTN | ||
| Pazopanib | 2009 | QT prolongation, torsades de pointes, cardiac dysfunction, HTN, arterial and venous thrombotic events | ||
| Vandetanib | 2011 | QT prolongation, torsades de pointes, sudden deaths | Ischemic cerebrovascular events, hemorrhage, heart failure, HTN | |
| Axitinib | 2012 | HTN, arterial and venous thrombotic events, hemorrhagic events | ||
| Regorafenib | 2012 | Myocardial ischemia, HTN, hemorrhagic events | ||
| mTOR inhibitors | Temsirolimus | 2007 | Hyperglycemia, hyperlipidemia | |
| Everolimus | 2009 | Hyperglycemia, hyperlipidemia, hypertriglyceridemia | ||
| Immunomodulators | Thalidomide | 1998 | DVT, PE | MI, stroke, bradycardia |
| Lenalidomide | 2005 | DVT, PE | ||
| Pomalidomide | 2013 | DVT, PE | ||
| Proteasome inhibitors (PIs) | Bortezomib | 2003 | Hypotension, heart failure, few cases of PAH | |
| Carfilzomib | 2012 | Heart failure, myocardial ischemia, PAH, HTN, venous thrombotic events | ||
| Cancer immunotherapies | Ipilimuab | 2011 | <1% Pericarditis and myocarditis | |
| Nivolumab | 2014 | |||
| Pembrolizumab | 2014 |
CHF = congestive heart failure; DVT = deep vein thrombosis; FDA = Food and Drug Administration; HTN = hypertension; MI = myocardial infarction; NA = not applicable; PAH = pulmonary hypertension; PE = pulmonary embolism; W and P = warnings and precautions.
Data from the U.S. FDA (100). Both boxed warnings and W and P sections of labeling for human prescription drugs are recommended by the FDA as industry guidance to categorize reporting of various adverse reactions. The boxed warnings highlight serious cardiotoxicities (fatal, life-threatening, or permanently disabling), adverse reactions that can be prevented or alleviated, or use with safety restrictions. In addition to the boxed warning, the W and P section describes a discrete set of cardiovascular adverse reactions that are serious or are otherwise clinically significant because they have implications for prescribing decisions or for patient management.
Within the same class of “targeted” therapies, the CV toxicity can be complex. This is illustrated in the case of small molecular inhibitors targeting tyrosine kinase pathways (so-called TKIs or tyrosine kinase inhibitors), used for the treatment of chronic myeloid leukemia (CML). Imatinib, a first-in-class TKI targeting the ABL1 kinase, which is aberrantly activated in CML, revolutionized treatment by roughly doubling the 5-year survival rates of newly diagnosed CML to 89% (9). Subsequently, second- (nilotinib, dasatinib, and bosutinib) and third- (ponatinib) generation TKIs were developed for CML treatment. Initially, these TKIs were developed to overcome imatinib resistance, but given their greater potency against ABL1 kinase, they were positioned for front-line therapy in CML. However, while imatinib carries minimal CV risk, dasatinib is associated with pulmonary hypertension, and nilotinib is associated with hyperglycemia and vascular events (5). Ponatinib held great promise as an ideal TKI for CML treatment given its potent activity in all patients, including those who had developed resistance to other TKIs. Indeed, in late 2012, ponatinib achieved approval through the U.S. Food and Drug Administration (FDA) Accelerated Approval pathway. However, in the fall of 2013, in a subsequent phase 2 study, at a median follow-up of 28 months, 19% of patients had serious vascular events, including cardiovascular (10%), cerebrovascular (7%), and peripheral vascular (7%) events, leading to transient suspension of ponatinib marketing in the United States (10). Nevertheless, given ponatinib’s efficacy in TKI-resistant patients (and specifically, for one “gatekeeper” mutation, BCR-ABL1T315I), the sale of ponatinib resumed, although under narrower indications, with a boxed warning regarding adverse vascular events.
The experience with TKIs in CML generates several important issues that apply to all new cancer therapies. A TKI with a novel mechanism that demonstrates unprecedented activity in disease areas of highly unmet need has a benefit-to-risk acceptability profile that is different from the second-generation drug in that same class. As other drugs with similar mechanisms are developed for the same cancer type, it is expected that there will be an improvement in the safety profile. To achieve this goal, a more robust CV monitoring plan needs to be implemented during the nonclinical and early clinical trials of newer compounds of the same class (Table 2). Finally, understanding the mechanisms of CV toxicities that do arise will be critical for developing preventive or protective strategies during clinical trials and clinical use. In 2016, better platforms are needed to both screen for and understand mechanistically CV toxicities associated with novel cancer therapies.
Table 2.
Summary of Commonly Used Models of Cardiomyocytes
| Platform | Cell Type (Source) | Utility | Advantages | Disadvantages | (Ref. #) |
|---|---|---|---|---|---|
| In vitro | |||||
| H9C2 | Embryonic BDIX rat heart (primary) | Disease modeling | Homogenous and replicating in culture; preserved cardiac electrophysiology | Morphology similar to immature embryonic cardiomyocytes; different differentiation states | 22, 23, 24, 25, 26 |
| Neonatal rat ventricular myocytes (NRVM) | Neonatal rat ventricular myocyte (primary) | Disease modeling, drug discovery and development | Commercially available, robust in culture; maintain contractility | Sensitivity to experimental conditions and perturbations (e.g., media constituents, duration of drug exposure, timing of post-isolation studies) | 18, 19, 20 |
| Human cardiomyocytes | Human (primary) | Drug discovery and development | Maintain morphologic integrity and electrophysiological properties for a short period; intact mature cardiac ion channels | Lack of tissue availability; long-term culture complicated by dedifferentiation | 14, 15, 16 |
| hERG assay | Chinese hamster ovary and human embryonic kidney cells (culture) | Drug discovery and development | Heterologous expression of single-ion channels; robust assay used ubiquitously for hERG block; high-throughput; cost-effective | Inadequate for multichannel interactions of functional cardiomyocytes; risk of false positives and false negatives | 30, 31, 32 |
| Stem cell-derived human cardiomyocytes | Embryonic and induced pluripotent stem cell-derived cardiomyocytes (culture) | Regenerative medicine, disease modeling, drug discovery and development | Renewable source of cells with robust differentiation; expression of human cardiac-specific sarcomeric proteins and ion channels; spontaneous contractility | Immature cardiomyocytes with varying degrees of sarcomeric organization; heterogeneous mixture of atrial-, ventricular-, and nodal-like subtypes | 51, 52, 53, 54, 55, 56 |
| In vivo | |||||
| Mouse | NA | Disease modeling, drug discovery and development | Xenografted cancer models available; ease of genetic manipulation; efficient breeding; ability to monitor cardiac parameters (e.g., 12-lead ECG, blood pressure, heart rate, cardiac function) and vasculature | Lack of comorbidities (e.g., hypertension, hyperlipidemia, diabetes); multiple compensatory mechanisms; physiologic differences (e.g., 10x faster heart rate); extreme nonphysiologic stressors (e.g., transverse aortic constriction) | 66, 67, 68, 69, 71 |
| Zebrafish | NA | Disease modeling, drug discovery and development | Capacity for high-throughput phenotyping; expression of crucial ion channels similar to other vertebrates; structural transparency; survival for several days in absence of cardiac output and/or presence of major vascular defects | Anatomic differences (2-chamber heart); ability for cardiac regeneration throughout adulthood | 19, 76, 77, 78 |
| In silico | |||||
| O'Hara-Rudy | Human ventricular tissue | Drug discovery and development | High-throughput; accounts for physiologic and genetic influences (e.g., age, gender, ethnicity, drug-drug interactions); assessment of multiple ion channels | Limited data on toxicity screening; lack of established database and standardized parameters (e.g., cell type, experimental conditions) | 80, 81 |
ECG = electrocardiogram; hERG = human ether-à-go-go-related gene; NA = not applicable.
The Current Standard for Nonclinical Testing: A Moving Target in Oncology Drugs
In an effort to achieve greater harmonization in the interpretation and application of technical guidelines, requirements for pharmaceutical product development and approval by regulatory authorities, the International Council for Harmonization (ICH), with representation from the FDA, developed multiple standardized regulatory guidelines. Two such guidelines, ICH S7A and S7B, provide recommendations for nonclinical safety pharmacology studies that assess adverse drug effects on vital organs 11, 12. With regard to CV toxicities, ICH S7A describes evaluations of blood pressure, heart rate, and electrocardiograms in animals. If adverse CV effects are suspected, then follow-up studies may include effects of the drug on such CV parameters as cardiac output, ventricular contractility, and vascular resistance (11). ICH S7B focuses on effects of drugs on the potential for delayed ventricular repolarization via 2 components, an in vitro electrophysiology test measuring drug effects on the human ether-à-go-go-related gene (hERG) potassium channel, which conducts the rapid delayed rectifier current (IKr), and an in vivo QT assay in an animal model (12).
Nonclinical safety studies for oncology drugs often differ from that of drugs for nononcologic indications, given the associated mortality and morbidity in patients with advanced cancer, where there may be limited therapeutic options. The ICH S9 Guidance, “Nonclinical Evaluation for Anticancer Pharmaceuticals,” provides recommendations on the nonclinical testing of anticancer drugs to expedite their development for patients with advanced disease with limited therapeutic options (13). Under ICH S9, stand-alone safety pharmacology studies outlined in ICH S7A and/or S7B are not required to support a first-in-human clinical trial. Cardiovascular measurements can be incorporated into general toxicology studies, with typical durations of 4 weeks to support Phases 1/2 clinical trials and 3 months to support Phase 3 clinical trials and marketing approval. Patients enrolled in Phase 1 clinical trials for anticancer therapies typically have relapsed or refractory disease and limited therapeutic options. The level of acceptable risk of an investigational treatment in this setting does not generally warrant additional assessments of potential CV toxicity. Nevertheless, ICH S9 states that if there are cardiotoxicity concerns about a specific drug, then safety pharmacology studies described in ICH S7A and/or S7B should be considered (13). In practice, decision making is considered on an individual basis for each drug by balancing the potential efficacy in treating a potentially lethal cancer versus acute and/or chronic cardiovascular toxicity. In cases where specific concerns of CV effects are present and the drug is being investigated in a patient population for whom clinical management of these CV toxicities may benefit from further characterization in nonclinical studies, a more comprehensive evaluation of hemodynamics and mechanical and electrical functions may be warranted.
In the following section, we discuss the established and emerging methods to examine potential cardiotoxic effects of cancer drugs (Table 2). We admit that each model system described here has limitations and believe that a combination of the methodology may be necessary to screen for cardiotoxic effects of novel compounds as well as to elucidate mechanisms of cardiotoxicity for existing ones.
In vitro models
Isolated cardiomyocytes, including primary cardiomyocytes established from tissue explants and human PSC-CMs, represent a cost-effective and high-throughput means to assess cardiotoxic effects of novel drugs. These in vitro methods also offer the opportunity to understand on-target and off-target molecular mechanisms in a manner that optimizes the efficacy and safety of new cancer drugs. However, given the complex interactions of novel cancer therapies, not only in the heart but also other systems such as the vasculature, the use of 2-dimensional cultures may be limited by the inability to introduce biomechanical stress like hypoxia and by the absence of intracellular cross talk between cardiomyocytes and other CV cells (endothelial cells, fibroblasts, leukocytes).
Primary cardiomyocytes
The use of primary adult human cardiomyocytes would be most ideal for in vitro toxicity screening for several reasons. These cells maintain their morphological integrity, possess all of the mature cardiac ion channels to detect any multichannel effects, and function electrophysiologically similar to native environment 14, 15, 16. In practice, their utility in nonclinical drug testing has been nonexistent due to a combination of scarcity of human cardiac tissue donors and technical difficulties such as limited number of passages and rapid de-differentiation in culture (17). For this reason, primary cells from other species at various stages of development (neonatal, adult) have been used, such as neonatal rat ventricular myocyte (NRVM). In one recent study, NRVM was used to show that doxorubicin caused cardiotoxicity through mitochondrial iron accumulation, which is reversible by decreasing iron levels through drugs like dexrazoxane (18). NRVMs were also used to show that sorafenib-induced toxicity is mediated through inhibition of the Raf/MEK/ERK pathways (19). NRVMs can be phenotyped for cardiotoxicity by several means, including cell death or indirectly, for example, by measuring cytosolic lactate dehydrogenase release into the medium (20). While NRVMs are commercially available and maintain contractility in culture, a major caveat is that these cells can be overly sensitive to perturbations such as medium and experimental conditions. Also, it is unclear how much these cells recapitulate human cardiomyocytes. In general, the preparation and isolation of primary cells are time-consuming, costly, and technically difficult, as enzymatic digestion disrupts the cell membrane permeability for ion exchange (21).
Cell lines
There are numerous rodent and human cell lines that have been established to further characterize cardiac biology, which overcome the limited proliferative nature of primary cardiomyocytes. One such model is the H9c2 cell line derived from embryonic BDIX rat heart tissue, which has been used to study doxorubicin-induced cardiotoxicity 22, 23, 24 and CV toxicity protection through inhibition of endoplasmic reticulum stress (25). Studies using H9c2 cells have also shown that newer TKI-related cardiac toxicity may be the direct result of functional mitochondrial impairment (26). While these cardiomyoblasts are a homogenous and replicating culture population expressing cardiac ion channels, H9c2 cells are less mature and morphologically distinct from cardiomyocytes (27).
For the purpose of assessing proarrhythmic risk, 2 of the most commonly used cell lines are Chinese hamster ovary and human embryonic kidney cells, which can be genetically modified to overexpress single-ion channels such as hERG and quantify drug effects on these channels 28, 29. However, this is an imperfect system because heterologous expression of single-ion channels cannot adequately recapitulate the complex nature of multichannel interactions within functional cardiomyocytes. Consequently, drugs screening using these hERG-expressing Chinese hamster ovary and human embryonic kidney cells can lead to false positives (e.g., verapamil), resulting in high attrition rate in drug development process and false negatives (e.g., alfuzosin), resulting in market release of potentially hazardous drugs 30, 31, 32, 33. Furthermore, these immortal cell lines lack the intrinsic machinery and physiology of functional cardiomyocytes to detect other cardiac abnormalities.
Promising new platform: human stem cell-derived cardiomyocytes, advantages and disadvantages
In recent years, the robust derivations of human cardiomyocytes from embryonic stem cells and induced PSC-CMs among other somatic cells have paved the way for major breakthroughs in drug development and toxicity screening 34, 35, 36, 37, 38. These cardiomyocytes hold great promise because they originate from a renewable source of pluripotent cells, are genetically specific to the donor patient, and can be generated in unlimited quantities. In many ways from structure to function, they are more similar to adult human cardiac physiology than that of nonhuman primary cardiomyocytes. Both types of PSC-CMs express cardiac-specific sarcomeric proteins and ion channels 39, 40. Functionally, stem cell-derived CMs exhibit calcium flux, excitation-contraction coupling, and action potential parameters that are similar to those of human ventricular myocytes 41, 42. Human PSC-CMs offer an in vitro platform that is scalable to meet industrial needs for cardiotoxicity screening.
Despite their scalability, in 2016, stem cell-derived cardiomyocytes remain imperfect for a number of reasons. The cells can be highly variable, and variations in phenotyping, maturity level, and tissue source (atrial, ventricular, and nodal) can alter results and affect reproducibility 41, 43. Morphologically, PSC-CM immaturity is evident by their small cell size and varying degrees of sarcomeric organization, which can influence impulse propagation, action potential depolarization rate, and contractile force (44). PCS-CM do not express all ion channels in the same density or ratio as human ventricular myocytes. This disparity in channel expression may alter PSC-CM responses to proarrhythmic drugs (45). Long-term (1-year) culture of cardiomyocytes enhances phenotypic maturation, and this or similar techniques may eventually provide for an optimized PSC-CM cell substrate that can be used routinely in drug development and safety testing (46).
Revolutionizing personalized medicine
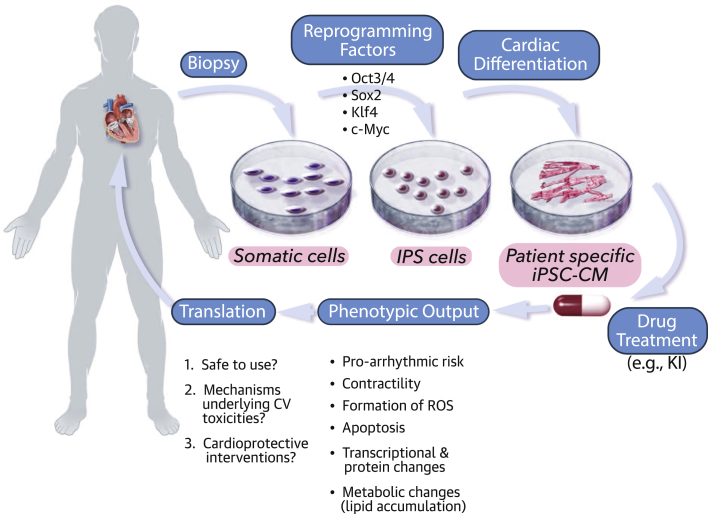
The ability to generate patient-specific PSC-CMs creates the opportunity for a “personalized” approach to characterizing drug-induced toxicities (Figure 2). This personalized PSC-CM approach parallels pharmacogenomic efforts to understand the role of genetics in individual patient drug responses. Because patient-derived PSC-CMs possess the patient-specific genetic variations, cardiotoxicity testing in these cells may allow for in vitro evaluation of drug efficacy or safety for a particular individual (47). Sex and ethnic differences of cancer drug efficacy and safety have been well documented, and use of patient-derived PSC-CMs may enable assessment of the genetic and molecular basis sex- and ethnicity-based variable effects 48, 49, 50.
Figure 2.
Promising Personalized iPSC-CM Model for Assessing Cardiotoxicity
Patient-derived somatic cells can be reprogrammed to induced pluripotent stem (iPS) cells by using Yamanaka’s cocktail of transcription factors and then robustly differentiated into cardiomyocytes (iPSC-CMs) while retaining the individual’s genetic composition. Drug effects on these cells can be assayed through an expanding repertoire of phenotypic outputs to: 1) address whether there are any CV toxicities; 2) if so, their underlying mechanism; and 3) evaluate for potential cardioprotective agents. CV = cardiovascular.
In proof-of-principle investigations, PSC-CM studies detected the cardiotoxicity of drugs that are arrhythmogenic 51, 52, 53, 54, 55, 56. One study demonstrated that PSC-CMs from diseased individuals with long QT syndrome, hypertrophic cardiomyopathy, or dilated cardiomyopathy are more susceptible to known cardiotoxic drugs than those cells of healthy patients (53). They also suggest that in direct comparison, disease-specific PSC-CMs predicted adverse drug responses more accurately than the conventional hERG testing and are able to correctly discriminate drugs like verapamil and alfuzosin as safe and QT prolonging, respectively. Similarly, another study using PSC-CMs to assess cardiotoxicity of 4 TKIs showed that each drug has its own unique toxicity profiles with distinct mechanisms including formation of reactive oxygen species, apoptosis, lipid accumulation, proarrhythmia, and altered contractility (54). The severity of cellular PSC-CM cardiotoxicity seems to correlate with clinical reports of cardiac adverse events. Several PSC-CM safety/screening studies have also used 96-well plates capable of measuring real-time impedance as a primary screen for contractility and arrhythmia 55, 56. This impedance assay, which has higher throughput than conventional patch clamp techniques, showed superior prediction of drug proarrhythmic potential versus standard hERG testing. A recent study of breast cancer patients showed that patient-specific PSC-CMs display a predilection for cardiotoxicity that correlates with the presence of cardiotoxic effects in individual patients. PSC-CMs derived from individuals with breast cancer suffered more doxorubicin-induced toxicity than PSC-CMs derived from patients who did not experience toxicity. The cells from patients who experienced cardiotoxicity had decreased cell viability, impaired mitochondrial function, altered metabolic activity, impaired calcium handling, and increased reactive oxygen species production (57).
There are currently several initiatives focused on establishing banks of induced pluripotent stem cell (iPSC) lines from healthy individuals and from patients with cancer diagnoses (58). The Stanford Cardiovascular Institute, for example, is creating a biobank of 1,000 patients with various types of cardiovascular diseases (Stanford Cardiovascular Institute Biobank, Stanford School of Medicine, Stanford, California). Inevitably, there will be substantial heterogeneity in the cells produced due to differences in factors like tissue source and methods of reprogramming and differentiation. While the disease state may contribute to the observed phenotype during drug testing, there needs to be a standardized guideline for characterizing PSC-CMs to differentiate drug-dependent versus drug-independent effects. A sophisticated approach using new genome editing technology, clustered regularly-interspaced short palindromic repeats (CRISPR-Cas9), enables the creation of isogenic iPSCs with any desired mutation in otherwise genetically identical lines (59). Despite their potential as a drug development tool, PSC-CM technology has not matured to the point where it can be routinely incorporated into preclinical drug efficacy or cardiotoxicity testing.
In vivo models
Similar to in vitro assays, animal models, both rodent and nonrodent, are widely used to detect cardiotoxicities, although more highly predictive models are needed because cancer patients often have comorbidities, which cannot be assessed in healthy animals. Animal studies enable examination of complex biological systems such as tumor growth, metastasis, inflammation, and thrombosis 11, 12, 13. By far the most valuable insight an animal model offers over cells in a dish is the full complement of molecular, biochemical, and physiological systems. In addition to monitoring hemodynamics and continuous electrocardiography using jacketed external telemetry, in vivo models can also evaluate many other crucial parameters such as vascular toxicities including hypertension and atherosclerosis. This is particularly relevant in the screening of novel kinase inhibitors, many of which have on- or off-target effects on the CV system. While many of the animal models in cardio-oncology have focused on “myocardial” and “arrhythmogenic” toxicity, better animal models are needed to elucidate vascular and metabolic toxicities with newer agents (such as second-generation TKIs used in CML).
Rodent models
Laboratory studies in rats and mice have been one of the quintessential cornerstones of biology. Rodent models have been widely used due to their relative physiologic similarities to humans, ease for genetic manipulations, and relative efficiency of breeding and maintenance compared with larger mammals like primates. Studies in rats have been used extensively to explore various aspects of anthracycline-induced cardiotoxicity (60), including studies showing that hypertensive rats are more sensitive than normotensive rats to the chronic cardiotoxic effects of doxorubicin (61). Additionally, sexual dimorphism has been reported, with males developing much more significant cardiomyopathy and experiencing higher mortality (62). Small analyses with 39 total anticancer agents suggest that rodents alone can predict a safe Phase I trial starting dose and the majority of toxicities that become dose limiting with treatment 63, 64. In general, rat toxicology studies in conjunction with dog studies have been adequate in defining safety/dosing parameters for clinical trials (65).
Similarly, mice have been used for decades to examine the mechanism of cardiotoxicities of conventional therapies such as anthracyclines and radiation. For example, the use of mice has allowed mechanistic understanding of doxorubicin-induced cardiac injury, implicating the role of free radicals (66), topoisomerase-IIβ (67), and radiation-induced CV injury (68). Crone et al. (69) created a mouse model with ventricular-restricted deletion of HER2 (also called ErbB2) receptor tyrosine kinase, overexpression of which plays an important role in the development and progression of certain aggressive types of breast cancer. This mouse model allowed for better understanding of the cardiomyopathy associated with the breast cancer therapy trastuzumab, a humanized monoclonal antibody specific for the extracellular domain of HER2, implicating HER2 as a previously unappreciated signaling pathway in cardiac biology. Ideally, transgenic mouse strains can provide valuable insights into the specific pathways that lead to CV toxicities. In addition, physiological parameters such as blood pressure, heart rate, and cardiac function can be measured, and biomarker serologic testing may predict risk of risk of actual events.
Despite the utility of preclinical rodent studies, data must be interpreted with caution, because intrastrain mouse genetic differences (or genetic differences from humans) can mask potential side effects or suggest toxicities that may or may not be seen in humans. This pitfall of rodent research may be more accentuated in newer targeted therapies, which act on specific signaling pathways that may vary widely between species. For example, the breast cancer therapy trastuzumab only recognizes human HER2, which negates the ability to study cardiotoxic mechanisms in mice (J. Moslehi, unpublished data, May 2016). A meta-analysis of 16 clinical trials using the breast cancer therapy sunitinib demonstrated increased risk of congestive heart failure (70); however, mouse studies showed minimal changes in left ventricle ejection fraction (71). Several confounders may account for these differences between the preclinical studies and clinical trials. Patients receiving targeted therapies like sunitinib are older with multiple comorbidities such as hypertension, hyperlipidemia, and diabetes. Mouse models to simulate those conditions are often the extremes, including genetic deletion of kinases (as opposed to pharmacological inhibition). Future studies are needed to address the translatability of cardiotoxicity findings in rodents to humans.
Zebrafish
Zebrafish (Danio rerio) is a useful animal model system for studying CV development, genetics, and cardiotoxicity. It was initially popularized for its utility in large-scale forward genetic screens 72, 73. One of its major advantages over existing animal models in cardiotoxicity screening is the capacity for high-throughput phenotyping. Zebrafish are small enough for 384-well plates, and some strains remain transparent throughout adulthood, which enables visualization of cardiovascular phenotypic traits (74). In addition, zebrafish are able to survive in the absence of cardiac output and in the presence of major vascular defects for several days, allowing for enhanced characterization of abnormalities otherwise fatal in mammals. Despite anatomic and physiological differences (zebrafish heart only has 2 chambers and maintains ability to regenerate throughout adulthood), the cardiomyocytes still express crucial ion channels similar to that of other vertebrates including voltage-gated sodium, L-type and T-type calcium, and potassium channels (75). In contrast to other vertebrate models, zebrafish develop rapidly, forming a fully functioning heart within 26 h of fertilization, and can be maintained cost-effectively.
There are several studies that suggest zebrafish can be used to evaluate drug cardiotoxicity. For example, zebrafish can develop signs of cardiomyopathy and electrophysiological abnormalities following treatment with of TKIs. In one study of 100 small molecules, 22 of 23 drugs that caused clinical QT prolongation caused bradycardia in zebrafish by altering the repolarizing potassium current (76). Zebrafish studies also detected drug-drug interactions leading to QT prolongation such as those between erythromycin and cisapride and between cimetidine and terfenadine. A more recent study in zebrafish discriminated between TKIs that caused cardiomyopathy (sunitinib and sorafenib) versus those that do not (gefitinib) (19). The one caveat is that while the zebrafish kinome is very similar to that of human, subtle species differences in amino acid sequence could affect the binding interaction, thus leading to under- or over-estimation of toxicity (77). As shown by the studies cited earlier, quantitative phenotyping of zebrafish illustrates the potential to assess cardiotoxic effects of new classes of targeted therapies.
In addition to screening for toxicity, the high-throughput nature of zebrafish enables chemical screening of large numbers of compounds for efficacy or cardiotoxicity research to explore novel means of cardioprotection and to better elucidate mechanisms of cardiotoxicity. In this regard, zebrafish high-throughput chemical screening in a doxorubicin-induced cardiomyopathy model identified visnagin as a new cardioprotective compound. Visnagin modulates mitochondrial malate dehydrogenase, a key metabolic enzyme during injury responses (78). Despite promising early studies, more research is necessary to correlate establish and validate the translational utility of the zebrafish cardiotoxicity screening.
In silico models
With the increasing availability of large datasets, in silico models, a term for modeling via computer simulations, have garnered more attention and interest from researchers and pharmaceutical industry alike within the past decade as a method of evaluating CV safety (79). In silico models offer the distinct advantages of being high-throughput and testing a wide range of potentially relevant scenarios. Computer simulations could factor in physiologic and genetic influences such as age, gender, and ethnicity, as well as provide an opportunity to explore drug-drug interactions. One established mathematical model, the O’Hara-Rudy model, uses experimental data collected from 140 human hearts to recapitulate a range of physiologic responses to changes in pacing rate and predict arrhythmic behavior with drug blockage on 14 types of outer membrane currents (80). With respect to the risk of torsades, several studies have suggested that voltage clamp data measured from a drug’s effect on multiple ion channels would provide a more accurate assessment of the overall effects on ventricular repolarization that otherwise may not be apparent from analyzing any individual current (i.e., Ikr) 81, 82. One in silico study used a logistic regression approach to examine the cardiotoxicity of 55 drugs (32 torsadogenic and 23 nontorsadogenic) through 3 cardiac channels (IKr, fast sodium, and L-type calcium). That in silico study showed benefit of simulating multiple ion channels and improved the false positives and false rate compared with in silico testing of a single-ion channel (81). However, in silico analyses are only as valid as the dataset used to construct the simulation, and a regulated, open source database with standard testing protocols will need to be developed before in silico testing can be relied on for drug development and safety purposes.
Future Direction of Nonclinical Testing: Emphasis on Mechanistic Understanding
With a mixture of established and emerging models, advancements in nonclinical testing should focus on 2 aspects. The first is establishing better models that allow for more accurate prediction of cardiotoxicities during research and development. For QT prolongation testing, for example, one promising approach under development is the “Comprehensive in vitro Proarrhythmia Assay” (CiPA), which suggests the following: 1) expanding in vitro testing to multi-channel assays; 2) adding in silico simulations to assess proarrhythmic liability; and 3) incorporating human PSC-CM confirmatory studies (83). It remains to be seen whether some of the newer models like PSC-CMs and in silico assays will lead to revisions in cardiotoxicity testing guidelines.
The second component should be focused on better mechanistic characterization of the toxicities using some of the promising new models like PSC-CMs and zebrafish. As new toxicities emerge with the use of TKIs, even during clinical trials, investigators can then conduct focused nonclinical studies to elucidate their underlying mechanisms of action. In the process, zebrafish screening may also provide opportunities for identifying new agents like visnagin that prevents or mitigates CV side effects (78). This will be crucial to pave the path for more detailed clinical studies and to develop best practices of managing these toxicities.
Clinical Monitoring and Prevention of Cardiotoxicities
Novel targeted therapies have revolutionized treatment for various cancers, leading to increased survival in many cancer subtypes, to the point where comorbid CV diseases compete with the cancer as a leading cause of morbidity and mortality (84). As a result, cardio-oncology clinical programs are emerging across the country that serve as an interdisciplinary approach for managing CV comorbidities while treating with necessary life-saving therapies (85). Because CV diseases are common in the general population, it can be hard to dissect treatment-associated cardiotoxicities from treatment-independent CV events. Therefore, a major aim of cancer clinical trials is to identify potential treatment-associated CV toxicities. In this regard, clinical and population cardiotoxicity studies should often accompany and feed nonclinical model systems.
The first and foremost priority to achieving this goal is developing comprehensive standards for assessment of cancer treatment-related CV toxicities. Oncology trials typically use a guideline called the Common Terminology Criteria for Adverse Events (CTCAE), which was developed by the National Cancer Institute to classify undesired effects with criteria for qualitative grading the severity of each event; however, CTCAE often lack standardized quantitative assessment of the event severity. This concept is especially an issue with CV toxicities. Previous studies have varied widely in reported incidence rate of cancer therapy-induced cardiotoxicities between 0% and 57%, depending on the study design and factors such as different classifications, comorbidities, and follow-up length 86, 87, 88, 89, 90. Furthermore, cardiac studies often include independent adjudication committees that help correctly grade a particular CV toxicity, which is not routinely done in oncology trials (4).
The discrepancies in the CV toxicity assessment of clinical trials not only obfuscate clinicians’ ability to identify treatment-associated cardiotoxicities, but they also compromise any effective assessment of cardioprotective interventions. In retrospective analyses with potential for misclassification bias, TKIs like sunitinib are associated with increased risk of congestive heart failure (RR: 1.81) (70). Such unexpected CV side effects need to be validated in well-designed clinical trials that prospectively follow patients for adverse CV outcomes. As investigators work to develop improved preclinical cardiotoxicity screening strategies, we will need to rely on more rigorous assessment of CV events clinical trial evaluation of novel cancer drugs.
Better strategies should be implemented for post-marketing surveillance and vigilance, especially due to the novelty and chronic administration of many therapies. For instance, emerging evidence from long-term studies like the Childhood Cancer Survivors Study suggest that the risk of morbidity and mortality among survivors continues to increase decades later (91). Due to the lack of an established protocol for surveillance, many patients may be lost to follow-up, and true incidence of cardiotoxicities is probably underestimated. Many approaches are under investigation for utility in clinical monitoring such as cardiac imaging and biomarker studies, including measurements of left ventricle ejection fraction and natriuretic peptides, respectively. Strain measurements on echocardiography appear to be promising for early detection of myocardial changes and prediction of cardiotoxicity in patients receiving cancer treatment (92). In the future, genetic screening may help to identify at-risk cardiotoxicity patients, as evidence by the fact that single nucleotide polymorphisms associated with protection from or susceptibility to anthracycline CV toxicity 93, 94, 95. Ultimately, well-designed epidemiologic studies from prospective trials will be essential to determine the true incidence, severity, and natural history of various CV toxicities.
Another emerging model to predict potential cardiotoxicity of the ever-expanding pipeline of targeted cancer therapeutics, especially TKIs, is the use of human genetic information coupled to electronic health records. For instance, Vanderbilt University Medical Center’s BioVU, a large, human DNA repository linked to de-identified electronic health records within the Synthetic Derivative database can be used to predict both on-target therapeutic effects as well as adverse outcomes in man 96, 97, 98. Using BioVU as a human genome-phenome analysis platform, one can carry out a phenome-wide association study (PheWAS) to determine what clinical phenotypes were associated with genetic variations in the genes targeted by drugs. As an example, such analysis identified a rare nonsynonymous variant in a kinase gene that is strongly associated with osteoporosis in patients, suggesting that pharmacological inhibition of this kinase could lead to osteoporosis in patients (C. C. Hong, personal communication, April 2016). One can easily envision search for potential associations of drug target genes with cardiovascular outcomes such as myocardial infarction, sudden cardiac death, and heart failure. In summary, the emerging marriage of human genetic data and electronic medical records can be leveraged to gain early human biological insights to potential adverse cardiovascular effects of new therapeutics.
Conclusions
Moving forward, there is no doubt that both preclinical testing and clinical detection of cardiotoxicity will continue to improve. As the focus of anticancer therapies shifts from a broadly cytotoxic approach to more targeted molecular treatments, there is increasing concern for unexpected CV toxicities that have been reported through case reports and retrospective studies 9, 70, 91, 99. Historically, preclinical safety testing has focused on in vitro hERG-centric assays and QT monitoring, and this has resulted in unexpected cardiotoxicity during clinical trials or in post-market drug surveillance. In time, new drugs may be able to harness emerging methods such as in silico, PSC-CMs, and zebrafish testing to identify potent candidate agents that have good safety profiles. Developing more accurate and comprehensive assessment of cardiotoxicity in nonclinical models may ultimately reduce costs through early target optimization. In the future, advances in preclinical testing methods should be combined with heightened assessment of CV events in oncology trials; these synergistic initiatives will help to maximize therapeutic impact while also helping to quantify and minimize CV risk for burgeoning classes of life-saving cancer therapies.
Footnotes
The views expressed in this article do not necessarily represent an official position of U.S. Food and Drug Administration. Dr. Wu has received funding from Sanofi; and sits on the scientific advisory board of Stem Cell Theranostics. Dr. Force has consulted for Ariad and Bristol-Myers Squibb. Dr. Moslehi has consulted for Novartis, Pfizer, Bristol-Myers Squibb, Takeda, Ariad, Acceleron, Vertex, Incyte, Rgenix, and Verastem. Dr. Croce has received funding from Ariad; and has consulted for Ariad, Bristol-Myers Squibb, The Medicines Company, Abbott Vascular, and St. Jude Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Gerber D.E. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311–319. [PubMed] [Google Scholar]
- 2.Collins F.S., Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groarke J.D., Nguyen P.L., Nohria A., Ferrari R., Cheng S., Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2013;35:612–623. doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W., Croce K., Steensma D.P., McDermott D.F., Ben-Yehuda O., Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol. 2015;66:1160–1178. doi: 10.1016/j.jacc.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Moslehi J.J., Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;62:4718. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 7.DeSantis C.E., Lin C.C., Mariotto A.B. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 8.Ky B., Vejpongsa P., Yeh E.T.H., Force T., Moslehi J.J. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113:754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druker B.J., Guilhot F., O'Brien S.G. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H.M., Kim D.-W., Pinilla-Ibaz J. Ponatinib (PON) in patients (pts) with Philadelphia chromosome-positive (Ph plus) leukemias resistant or intolerant to dasatinib or nilotinib, or with the T315I mutation: longer-term follow up of the PACE trial. J Clin Oncol. 2014;32:5s. [Google Scholar]
- 11.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH S7A Safety Pharmacology Studies for Human Pharmaceuticals. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7A/Step4/S7A_Guideline.pdf 2001. Accessed February 7, 2016.
- 12.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH S7B Non-Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7B/Step4/S7B_Guideline.pdf. 2005. Accessed February 7, 2016.
- 13.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH S9 Nonclinical Evaluation for Anticancer Pharmaceuticals. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S9/Step4/S9_Step4_Guideline.pdf. 2009. Accessed February 7, 2016.
- 14.Janssen P.M.L., Lehnart S.E., Prestle J., Hasenfuss G. Preservation of contractile characteristics of human myocardium in multi-day cell culture. J Mol Cell Cardiol. 1999;31:1419–1427. doi: 10.1006/jmcc.1999.0978. [DOI] [PubMed] [Google Scholar]
- 15.Bird S.D., Doevendans P.A., Van Rooijen M.A. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58:423–434. doi: 10.1016/s0008-6363(03)00253-0. [DOI] [PubMed] [Google Scholar]
- 16.Bistola V., Nikolopoulou M., Derventzi A. Long-term primary cultures of human adult atrial cardiac myocytes: cell viability, structural properties and BNP secretion in vitro. Int J Cardiol. 2008;131:113–122. doi: 10.1016/j.ijcard.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 17.Brandenburger M., Wenzel J., Bogdan R. Organotypic slice culture from human adult ventricular myocardium. Cardiovasc Res. 2012;93:50–59. doi: 10.1093/cvr/cvr259. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa Y., Ghanefar M., Bayeva M. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H., Kari G., Dicker A.P., Rodeck U., Koch W.J., Force T. A novel preclinical strategy for identifying cardiotoxic kinase inhibitors and mechanisms of cardiotoxicity. Circ Res. 2011;109:1401–1409. doi: 10.1161/CIRCRESAHA.111.255695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasinoff B.B. The cardiotoxicity and myocyte damage caused by small molecule anticancer tyrosine kinase inhibitors is correlated with lack of target specificity. Toxicol Appl Pharmacol. 2010;244:190–195. doi: 10.1016/j.taap.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Tytgat J. How to isolate cardiac myocytes. Cardiovasc Res. 1994;28:280–283. doi: 10.1093/cvr/28.2.280. [DOI] [PubMed] [Google Scholar]
- 22.Merten K.E., Jiang Y., Feng W., Kang Y.J. Calcineurin activation is not necessary for doxorubicin-induced hypertrophy in H9c2 embryonic rat cardiac cells: involvement of the phosphoinositide 3-kinase-Akt pathway. J Pharmacol Exp Ther. 2006;319:934–940. doi: 10.1124/jpet.106.108845. [DOI] [PubMed] [Google Scholar]
- 23.Sardão V.A., Oliveira P.J., Holy J., Oliveira C.R., Wallace K.B. Morphological alterations induced by doxorubicin on H9c2 myoblasts: nuclear, mitochondrial, and cytoskeletal targets. Cell Biol Toxicol. 2009;25:227–243. doi: 10.1007/s10565-008-9070-1. [DOI] [PubMed] [Google Scholar]
- 24.Karagiannis T.C., Lin A.J.E., Ververis K. Trichostatin A accentuates doxorubicin-induced hypertrophy in cardiac myocytes. Aging (Albany NY) 2010;2:659. doi: 10.18632/aging.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X.-Y., Yang C.-T., Zheng D.-D. Hydrogen sulfide protects H9c2 cells against doxorubicin-induced cardiotoxicity through inhibition of endoplasmic reticulum stress. Mol Cell Biochem. 2012;363:419–426. doi: 10.1007/s11010-011-1194-6. [DOI] [PubMed] [Google Scholar]
- 26.Will Y., Dykens J.A., Nadanaciva S. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol Sci. 2008;106:153–161. doi: 10.1093/toxsci/kfn157. [DOI] [PubMed] [Google Scholar]
- 27.Hescheler J., Meyer R., Plant S., Krautwurst D., Rosenthal W., Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 28.Roy M.-L., Dumaine R., Brown A.M. HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation. 1996;94:817–823. doi: 10.1161/01.cir.94.4.817. [DOI] [PubMed] [Google Scholar]
- 29.Mohammad S., Zhou Z., Gong Q., January C.T. Blockage of the HERG human cardiac K+ channel by the gastrointestinal prokinetic agent cisapride. Am J Physiol Heart Circ Physiol. 1997;273:H2534–H2538. doi: 10.1152/ajpheart.1997.273.5.H2534. [DOI] [PubMed] [Google Scholar]
- 30.Redfern W.S., Carlsson L., Davis A.S. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann P., Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53:87–105. doi: 10.1016/j.vascn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Lacerda A.E., Kuryshev Y.A., Chen Y. Alfuzosin delays cardiac repolarization by a novel mechanism. J Pharmacol Exp Ther. 2008;324:427–433. doi: 10.1124/jpet.107.128405. [DOI] [PubMed] [Google Scholar]
- 33.Navarrete E.G., Liang P., Lan F. Screening drug-induced arrhythmia using human induced pluripotent stem cell–derived cardiomyocytes and low-impedance microelectrode arrays. Circulation. 2013;128(Suppl 1):S3–S13. doi: 10.1161/CIRCULATIONAHA.112.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kehat I., Kenyagin-Karsenti D., Snir M. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J., Klos M., Wilson G.F. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lian X., Hsiao C., Wilson G. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian X., Zhang J., Azarin S.M. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsa E., Burridge P.W., Wu J.C. Human stem cells for modeling heart disease and for drug discovery. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008921. 239ps6–9ps6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao F., Wagner R.A., Wilson K.D. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PloS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germanguz I., Sedan O., Zeevi-Levin N. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2011;15:38–51. doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J., Guo L., Fiene S.J. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang H.S., Kryshtal D.O., Feaster T.K. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. J Mol Cell Cardiol. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moretti A., Bellin M., Welling A. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 44.Spach M.S., Heidlage J.F., Barr R.C., Dolber P.C. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1:500–515. doi: 10.1016/j.hrthm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson M.K.B., Vos M.A., Mirams G.R. Application of human stem cell-derived cardiomyocytes in safety pharmacology requires caution beyond hERG. J Mol Cell Cardiol. 2012;52:998–1008. doi: 10.1016/j.yjmcc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Kamakura T., Makiyama T., Sasaki K. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77:1307–1314. doi: 10.1253/circj.cj-12-0987. [DOI] [PubMed] [Google Scholar]
- 47.Chen I.Y., Matsa E., Wu J.C. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol. 2016;13:333–349. doi: 10.1038/nrcardio.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krischer J.P., Epstein S., Cuthbertson D.D., Goorin A.M., Epstein M.L., Lipshultz S.E. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 49.Hershman D., McBride R., Jacobson J.S. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 50.Calvo E., Baselga J. Ethnic differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol. 2006;24:2158–2163. doi: 10.1200/JCO.2006.06.5961. [DOI] [PubMed] [Google Scholar]
- 51.Caspi O., Itzhaki I., Kehat I. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cell Dev. 2009;18:161–172. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 52.Braam S.R., Tertoolen L., van de Stolpe A., Meyer T., Passier R., Mummery C.L. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–116. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Liang P., Lan F., Lee A.S. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease specific patterns of cardiotoxicity. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.001883. 113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doherty K.R., Wappel R.L., Talbert D.R. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol Appl Pharmacol. 2013;272:245–255. doi: 10.1016/j.taap.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Guo L., Abrams R., Babiarz J.E. Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell derived cardiomyocytes. Toxicol Sci. 2011;123:281–289. doi: 10.1093/toxsci/kfr158. [DOI] [PubMed] [Google Scholar]
- 56.Guo L., Coyle L., Abrams R.M.C., Kemper R., Chiao E.T., Kolaja K.L. Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013;136:581–594. doi: 10.1093/toxsci/kft205. [DOI] [PubMed] [Google Scholar]
- 57.Burridge P.W., Li Y.F., Matsa E. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKernan R., Watt F.M. What is the point of large-scale collections of human induced pluripotent stem cells? Nat Biotechnol. 2013;31:875–877. doi: 10.1038/nbt.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cong L., Ran F.A., Cox D. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herman E.H., Ferrans V.J. Preclinical animal models of cardiac protection from anthracycline-induced cardiotoxicity. Semin Oncol. 1998;25:15–21. [PubMed] [Google Scholar]
- 61.Herman E.H., El-Hage A.N., Ferrans V.J., Ardalan B. Comparison of the severity of the chronic cardiotoxicity produced by doxorubicin in normotensive and hypertensive rats. Toxicol Appl Pharmacol. 1985;78:202–214. doi: 10.1016/0041-008x(85)90284-4. [DOI] [PubMed] [Google Scholar]
- 62.Moulin M., Piquereau J., Mateo P. Sexual dimorphism of doxorubicin-mediated cardiotoxicity potential role of energy metabolism remodeling. Circ Heart Fail. 2015;8:98–108. doi: 10.1161/CIRCHEARTFAILURE.114.001180. [DOI] [PubMed] [Google Scholar]
- 63.Newell D.R., Burtles S.S., Fox B.W., Jodrell D.I., Connors T.A. Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics. Br J Cancer. 1999;81:760. doi: 10.1038/sj.bjc.6690761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newell D.R., Silvester J., McDowell C., Burtles S.S. The cancer research UK experience of pre-clinical toxicology studies to support early clinical trials with novel cancer therapies. Eur J Cancer. 2004;40:899–906. doi: 10.1016/j.ejca.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Tomaszewski J.E. Multi-species toxicology approaches for oncology drugs: the US perspective. Eur J Cancer. 2004;40:907–913. doi: 10.1016/j.ejca.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 66.Myers C.E., McGuire W.P., Liss R.H., Ifrim I., Grotzinger K., Young R.C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- 67.Zhang S., Liu X., Bawa-Khalfe T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 68.Lee C.-L., Moding E.J., Cuneo K.C. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal. 2012;5:52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crone S.A., Zhao Y.-Y., Fan L. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 70.Richards C.J., Je Y., Schutz F.A.B. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29:3450–3456. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 71.Kerkela R., Woulfe K.C., Durand J.B. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci. 2009;2:15–25. doi: 10.1111/j.1752-8062.2008.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Driever W., Solnica-Krezel L., Schier A.F. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- 73.Haffter P., Granato M., Brand M. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- 74.White R.M., Sessa A., Burke C. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell stem cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker K., Warren K.S., Yellen G., Fishman M.C. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci U S A. 1997;94:4554–4559. doi: 10.1073/pnas.94.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milan D.J., Peterson T.A., Ruskin J.N., Peterson R.T., MacRae C.A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 77.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y., Asnani A., Zou L. Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3010189. 266ra170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aronov A.M. Predictive in silico modeling for hERG channel blockers. Drug Discov Today. 2005;10:149–155. doi: 10.1016/S1359-6446(04)03278-7. [DOI] [PubMed] [Google Scholar]
- 80.O'Hara T., Virág L., Varró A., Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PloS Comput Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramer J., Obejero-Paz C.A., Myatt G. MICE models: superior to the HERG model in predicting Torsade de Pointes. Sci Rep. 2013;3:2100. doi: 10.1038/srep02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirams G.R., Cui Y., Sher A. Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk. Cardiovasc Res. 2011;91:53–61. doi: 10.1093/cvr/cvr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sager P.T., Gintant G., Turner J.R., Pettit S., Stockbridge N. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am Heart J. 2014;167:292–300. doi: 10.1016/j.ahj.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Mertens A.C., Yasui Y., Neglia J.P. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 85.Barac A., Murtagh G., Carver J.R. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol. 2015;65:2739–2746. doi: 10.1016/j.jacc.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kremer L.C.M., Van der Pal H.J.H., Offringa M., Van Dalen E.C., Voute P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 87.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 88.Diller L., Chow E.J., Gurney J.G. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–2355. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith L.A., Cornelius V.R., Plummer C.J. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moja L., Tagliabue L., Balduzzi S. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Armstrong G.T., Kawashima T., Leisenring W. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014:1055. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thavendiranathan P., Poulin F., Lim K.-D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 93.Visscher H., Ross C.J.D., Rassekh S.R. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2011:3467. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 94.Visscher H., Ross C.J.D., Rassekh S.R. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatric Blood Cancer. 2013;60:1375–1381. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 95.Aminkeng F., Bhavsar A.P., Visscher H. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47:1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roden D.M., Denny J.C. Integrating electronic health record genotype and phenotype datasets to transform patient care. Clin Pharmacol Ther. 2016;99:298–305. doi: 10.1002/cpt.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Denny J.C., Bastarache L., Ritchie M.D. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31:1102–1111. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denny J.C., Bastarache L., Roden D.M. Phenome-wide association studies as a tool to advance precision medicine. Annu Rev Genomics Hum Genet. 2016;17:11.1–11.21. doi: 10.1146/annurev-genom-090314-024956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uraizee I., Cheng S., Moslehi J. Reversible cardiomyopathy associated with sunitinib and sorafenib. N Engl J Med. 2011;365:1649–1650. doi: 10.1056/NEJMc1108849. [DOI] [PubMed] [Google Scholar]
- 100.U.S. Food and Drug Administration. Drugs@FDA: Approved Drug Products. Available at: https://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed February 7, 2016.