Abstract
Background
Cardiovascular magnetic resonance (CMR) T1 mapping characteristics are elevated in adult cancer survivors; however, it remains unknown whether these elevations are related to age or presence of coincident cardiovascular comorbidities.
Methods and Results
We performed blinded CMR analyses of left ventricular (LV) T1 and extracellular volume fraction (ECV) in 327 individuals (65% women, aged 64±12 years). Thirty-seven (37) individuals had breast cancer or a hematologic malignancy but had not yet initiated their treatment, and 54 cancer survivors that received either anthracycline-based (n=37) or non-anthracycline-based (n=17) chemotherapy 2.8±1.3 years earlier were compared to 236 cancer-free participants. Multivariable analyses were performed to determine the association between T1/ECV measures and variables associated with myocardial fibrosis. Age-adjusted native T1 was elevated pre- (1058±7 ms) and post- (1040±7 ms) receipt of anthracycline chemotherapy versus comparators (965±3 ms, p<0.0001 for both). Age-adjusted ECV, a marker of myocardial fibrosis, was elevated in anthracycline-treated cancer participants (30.4±0.7%) compared with either pre-treatment cancer (27.8±0.7%, p<0.01) or cancer-free comparators (26.9±0.2%, p<0.0001). T1 and ECV of non-anthracycline survivors was no different than pre-treatment survivors (p=0.17 and p=0.16, respectively). Native T1 and ECV remained elevated in cancer survivors after accounting for demographics (including age), myocardial fibrosis risk factors, and LV ejection fraction or myocardial mass index (p<0.0001 for all).
Conclusions
Three years after anthracycline-based chemotherapy, elevations in myocardial T1 and ECV occur independent of underlying cancer or cardiovascular comorbidities suggesting that imaging biomarkers of interstitial fibrosis in cancer survivors are related to prior receipt of a potentially cardiotoxic cancer treatment regimen.
Keywords: Cardiotoxicity, Cardiovascular magnetic resonance imaging, Extracellular volume, T1 mapping, Myocardial fibrosis
Anthracycline-based chemotherapy is associated with subclinical left ventricular (LV) dysfunction and future cardiovascular (CV) events.1–4 Previously, elevated cardiovascular magnetic resonance (CMR) measures of extracellular volume fraction (ECV), a marker of diffuse myocardial fibrosis associated with mortality and heart failure,5 have been observed among adult cancer survivors.6 Importantly, it is uncertain whether these elevations in ECV observed in cancer survivors were related to the increased prevalence of myocardial comorbidities in the individuals studied, the presence of cancer alone, or the prior receipt of treatment for their cancer. In addition, regional myocardial edema assessed by T2 mapping has been identified in acute cardiotoxicity7 but its possible presence in chronic elevations of ECV has not been studied.
Accordingly, we performed this study to determine how CMR-derived measures of ECV obtained from individuals who previously received anthracycline-based chemotherapy for breast cancer or hematologic malignancy were related to measures obtained from individuals with cancer or coincident CV co-morbidities. To accomplish this, we measured ECV (and its component measures including LV myocardial T1 with and without gadolinium contrast) using CMR among adult cancer survivors previously treated with anthracycline-based chemotherapy. Also, we assessed myocardial T2 mapping measures for myocardial edema. As comparators, we assessed newly diagnosed cancer patients prior to receipt of chemotherapy, cancer survivors treated with non-anthracycline chemotherapy, and cancer-free control participants with and without risk factors for CV fibrosis.
METHODS
Study population
A total of 327 participants were placed into one of three groups: 236 control participants without cancer, 37 cancer participants yet to receive scheduled cancer treatment, and 54 cancer survivors (of whom 37 received anthracycline-based chemotherapy and 17 received non-anthracycline chemotherapy). The 327 individuals were drawn from two NIH funded concurrent (years 2010–2012) cohort studies that underwent CMR scanning at Wake Forest Health Sciences on the same scanner and with the same imaging protocol. The first cohort included 224 participants from Exam 5 of the Multi-Ethnic Study of Atherosclerosis (MESA)8 with no history of cancer. The second cohort included 91 cancer participants consecutively recruited from the Comprehensive Cancer Center at Wake Forest School of Medicine (and 12 healthy community-dwelling comparators). Cancer participants were either scanned prior to receipt of chemotherapy or after completion and were uniquely classified into one study group (no serial scans included).
All participants were enrolled in research studies were approved by the local Institutional Review Board and all participants provided written, informed consent. From both cohorts, we excluded participants with evidence of myocardial infarction, hypertrophic cardiomyopathy, intra-cardiac masses, or other cardiovascular infiltrative disorders (e.g., amyloidosis or sarcoidosis) that could also alter ECV values.
CMR imaging acquisition
All study participants underwent their research CMR examination on a 1.5T Siemens Avanto scanner (Siemens Medical Systems, Erlangen, Germany) with a phased array chest coil. All participants underwent a non-contrasted CMR examination for evaluation of native T1, LV volumes, and LV mass. Participants with estimated glomerular filtration rates (assessed by serum creatinine levels in blood samples taken from venipuncture prior to CMR examination) of ≥ 45 ml/min received either 0.15 mmol/kg of Magnevist (Bayer Healthcare Pharmaceuticals, Montville, New Jersey; n=184) if in the first cohort (MESA)8 or 0.2 mmol/kg of ProHance (Bracco Diagnostics, Princeton, New Jersey, n=74) gadolinium contrast if in the second cohort (participants recruited from the Comprehensive Cancer Center). Matching imaging protocols (sequences, parameters, and timing with contrast) on a single scanner were used in both cohorts.
Imaging parameters for all imaging sequences were performed according to previously published techniques.9–13 For measurement of LV volumes and mass, a short-axis steady-state free precession cine slices were acquired from the cardiac apex to its base. Cine imaging parameters included a 360–400 mm field of view collected with a 256×160 matrix, a 20° flip angle, a 6 mm slice thickness with 4 mm slice gap, a 3–5 ms echo time, and an 8–10 ms repetition time.14 In participants from the Comprehensive Cancer Center cohort, T2 mapping was collected with a 360×360 field of view, 192×60 matrix, 70° flip angle, 6 mm slice thickness, acceleration factor of 2, and T2 preparation pulses of 0, 24, and 55 ms.
Myocardial T1 mapping was acquired with a modified Look-Locker inversion recovery sequence (MOLLI) in a mid-cavity short-axis slice pre-contrast (native T1) and again at 12 and 25 minutes after contrast administration in contrast eligible participants.11–13 The MOLLI imaging acquired 11 images in the span of 17 heartbeats with a 360×360 mm field of view collected with a 192×183 matrix, 35° flip angle, 8 mm slice thickness, 1.1 ms echo time, 2.2 ms repetition time, and an acceleration factor of 2.13
CMR data analysis
In accordance with previously published techniques, a 3-parameter curve fit with the Levenberg-Marquardt algorithm was applied to the MOLLI source images to create a T1 map.13 Myocardial endocardial and epicardial borders were carefully drawn manually on each cine series (at end-diastole and end-systole for LV volumes and mass) and on T1 and T2 maps to ensure exclusion of the LV blood pool and epicardial fat. The average T1 and T2 were calculated from the segmented myocardium; univariate linear modeling was performed to determine T1-related heart rate dependency.13
For calculation of extracellular volume (ECV) fraction, a region of interest was placed in the LV blood pool to determine the T1 of blood. Using the three timepoints (pre-contrast, and 12 and 25 min post-contrast), the partition coefficient was determined from the slope of 1/T1myo versus 1/T1blood and used to calculate ECV as previously described.11,15
Statistical analysis
Statistical means and standard deviations were calculated for all continuous variables and frequencies and percentages were tabulated for all categorical variables. One-way analyses of variance tests were performed to examine between group differences and two-sided Student’s t-tests were performed to determine a treatment effect between the pre-treatment and anthracycline-treated survivor cancer groups. Outcome variables (T1, ECV) were age-adjusted for the mean age of cancer participants using least-squares means. Finally, a series of multivariate model analyses were performed to determine the dependency outcome variables on covariates of participant demographics, CV profile, and LV remodeling parameters. All analyses were performed in SAS version 9.3 (SAS Institute, Cary, North Carolina) with p<0.05 considered statistically significant.
RESULTS
Participant baseline characteristics are summarized in Table 1. Participants were predominately white women with an admixture of CV disease risk factors. Cancer diagnoses included breast cancer, leukemia, lymphoma, and sarcoma. Cancer survivors underwent CMR examinations 2.8±1.3 after receipt of their treatment (3.0±1.5 years for anthracycline-treated survivors [n=37] and 2.6±0.0 years for non-anthracycline-treated survivors [n=17]). The median total doxorubicin equivalent dose was 238 mg/m2 (interquartile range 169–245 mg/m2). Due to the small size of the non-anthracycline survivors subset, we focus our modeling of outcomes on two cancer groups (anthracycline-treated survivors [“post-treatment” group] and newly diagnosed cancer patients [“pre-treatment” group]) and healthy comparators in order to investigate the association of cancer and Anth-bC on imaging markers of myocardial fibrosis. Exploratory analyses comparing the anthracycline-treated to non-anthracycline-treated breast cancer survivors showed significantly reduced LVEFs and trends to elevated ECV (p=0.02 and p=0.11, respectively; Supplemental Table and Supplemental Figure).
Table 1.
Study participant descriptive characteristics of demographics, cancer diagnosis, and cardiovascular risk factors.
| Control (No Cancer) Participants (n=236) | Cancer Pre-Treatment (n=37) | Cancer Survivors: Non-anthracycline Treatment (n=17) | Cancer Survivors: Anthracycline Treatment (n=37) | Group Difference p-value | |
|---|---|---|---|---|---|
| Age at exam, years | 67±9 | 56±15 | 57±12 | 53±13* | <0.0001 |
| Race/Ethnicity | 0.04 | ||||
| White | 149 (63) | 29 (78) | 15 (88) | 29 (78) | |
| Black | 86 (36) | 7 (19) | 2 (12) | 8 (22) | |
| Hispanic | 1 (<1) | 1 (3) | - | - | |
| Gender | <0.01 | ||||
| Female | 140 (59) | 25 (68) | 17 (100) | 29 (78)*,§ | |
| Male | 96 (41) | 12 (32) | - | 8 (22) | |
| Body mass index, kg/m2 | 28.6±5.2 | 28.3±5.5 | 28.8±7.6 | 29.2±6.0 | 0.92 |
| Cancer Type | - | ||||
| Breast | - | 13 (35) | 17 (100) | 21 (57) § | |
| Hematologic | - | 21 (57) | - | 16 (43) | |
| Sarcoma | - | 3 (8) | - | - | |
| CVD Risk Factors | |||||
| Known CAD | - | 2 (5) | 1 (6) | 2 (5)* | - |
| Diabetes | 38 (16) | 5 (14) | 2 (12) | 5 (14) | 0.93 |
| Hyperlipidemia | 19 (8) | 9 (24) | 4 (24) | 8 (22)* | <0.01 |
| Hypertension | 49 (21) | 13 (35) | 6 (35) | 13 (35) | 0.06 |
Measurements reported as frequency (percentage) or mean±standard deviation. CVD=cardiovascular disease; CAD=coronary artery disease. p<0.05 for anthracycline-treated survivors compared to*controls, †pre-treatment, and §non-anthracycline survivors.
The body habitus was similar across all groups (Table 1). The cancer groups were similar in age but younger than those in the control group without cancer (p<0.0001, Table 1). Compared to pre-treatment cancer participants, anthracycline-treated cancer participants exhibited a reduced LV ejection fraction and trended to have a smaller myocardial mass index (Table 2, p<0.0001 and p=0.06, respectively). The heart rate during T1 mapping was similar in all groups after adjustment for age and gender (p>0.30 for all comparisons). Additionally, among those who received gadolinium, hematocrit values were no different across groups (p=0.10). The published concordance correlation coefficient (CCC) of T1 and ECV in the MESA study showed excellent agreement among analysts and low inter-observer variability (CCC=0.90–0.96).16,17 Analysis of images from the second cohort was performed by one analyst (JHJ) with over-read by a secondary analyst (GCM) resulting in a mean ECV difference of 0.56±1.6% with a CCC= 0.84, also showing excellent agreement between analysts.
Table 2.
Cardiovascular magnetic resonance imaging measures.
| Control (No Cancer) Participants (n=236) | Cancer Pre-Treatment (n=37) | Cancer Survivors: Non-Anthracycline Treatment (n=17) | Cancer Survivors: Anthracycline Treatment (n=37) | Group Difference p-value | |
|---|---|---|---|---|---|
| Received gadolinium | 196 (83) | 27 (73) | 11 (79) | 24 (65) | 0.05 |
| Hematocrit, % | 39±4 | 37±5 | 39±4 | 38±4 | 0.10 |
| LV EDV index, ml/m2 | 63±13 | 65±13 | 57±13 | 59±16 | 0.06 |
| LV ESV index, ml/m2 | 24±7 | 24±7 | 23±7 | 28±10*,†,§ | 0.06 |
| LV stroke volume index, ml/m2 | 39±8 | 40±9 | 34±8 | 31±10*,† | <0.0001 |
| LV EF, % | 61±7 | 63±8 | 60±7 | 53±9*,†,§ | <0.0001 |
| LV mass index, g/m2 | 63±13 | 54±11 | 45±9 | 49±11* | <0.0001 |
| Native T1, ms | 965±3 | 1058±7 | 1041±10 | 1041±7* | <0.0001 |
| 15 minute post-contrast T1, ms | 442±3 | 436±8 | 384±712 | 410±8*,§ | <0.0001 |
| ECV, % | 26.9±0.2 | 27.8±0.7 | 29.5±1.0 | 30.4±0.7* | <0.0001 |
Values reported as frequency (percentage) or mean±standard deviation unless otherwise noted. Least squares estimates reported for age-adjusted T1 and ECV values. LV=left ventricular; EDV=end diastolic volume; ESV=end systolic volume; EF=ejection fraction; ECV=extracellular volume. p<0.05 for anthracycline-treated survivors compared to*controls, †pre-treatment, and §non-anthracycline survivors.
Myocardial native T1 was elevated in pre-treatment and post-treatment cancer participants (1058±7 and 1040±7 ms, respectively) compared to controls without cancer (965±3 ms, p<0.0001 for both; Figure 1). T2 was within normal ranges for both pre-treatment cancer participants (51.7±2ms) and anthracycline-treated cancer survivors (52.5±4ms) and was not correlated with native T1 (r=−0.01, p=0.94).
Figure 1. CMR Assessments of Myocardial T1.
Myocardial native T1 of control participants without cancer (965±3 ms) and cancer pre-treatment participants (1058±7 ms) compared with cancer survivors treated with anthracycline chemotherapy (1040±7 ms). Myocardial T1 is elevated in both cancer groups, reflecting potential myocardial fibrosis compared with cancer-free control participants (p<0.0001 for both).
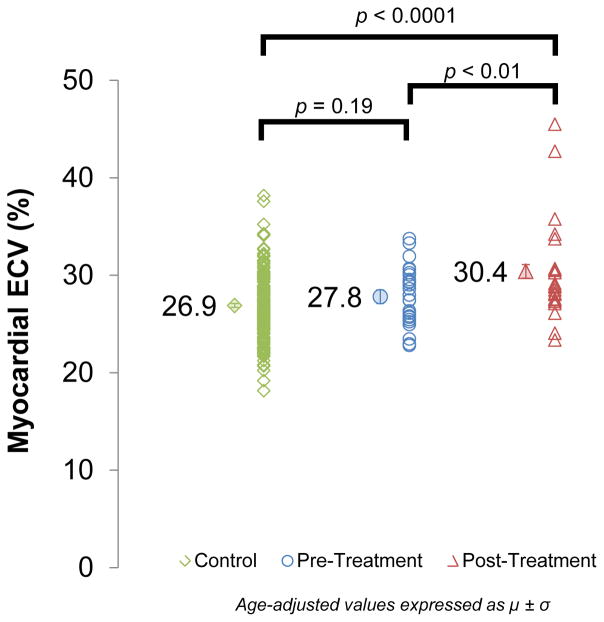
As shown in Figure 2, ECV was similar among cancer pre-treatment and controls (27.8±0.7 vs 26.9±0.2, respectively; p=0.19). However, ECV was elevated in cancer post-treatment participants (30.4±0.7%) compared with similarly-aged cancer pre-treatment and controls participants (p<0.01 for both). Figure 3 shows a representative image from a participant in each study group. There was no measured effect of the contrast agent type or dose on ECV after adjustment for age and gender among comparators (p=0.22). ECV was calculated with 2 post-contrast T1 values, however, no difference was found when compared to ECV calculated with 1 post-contrast T1 value (27.39 vs 27.36%, p=0.40). Additionally, there was no difference in ECV for breast cancer survivors treated with or without radiation therapy (33.2±6.4% vs 33.7%, respectively; p=0.94). No significant ECV were observed in the non-anthracycline breast cancer participants compared to similarly-aged pre-treatment breast cancer comparators, however, ECV trended higher in anthracycline-treated vs non-anthracycline-treated breast cancer survivors (Supplemental Figure). Table 3 displays the multivariable models used to determine whether covariates known to influence T1 indices were responsible for the statistical difference between the three study groups. The baseline models (Model 1) examined native T1 or ECV with the study group as a covariate and were found to be significantly different across the groups (p<0.0001 for both). Model 2 added age, race, gender, and an interaction for age*gender to Model 1 and again found that native T1 and ECV remained different across the groups (p<0.0001 for both). Model 3 added CV health and risk factors for CV events and myocardial fibrosis to Model 2 including: weight, heart rate, systolic blood pressure, and presence of known coronary artery disease, diabetes mellitus, hyperlipidemia, and hypertension. After accounting for participants’ demographics and CV comorbidities, native T1 and ECV were significantly different across the groups (p<0.0001 for both; Model 3). Finally, Model 4 added LV ejection fraction (LVEF) and LV mass index to Model 2. Again, CMR findings consistent with elevated myocardial fibrosis (T1 and ECV) remained elevated even after the adjustments in Model 4 (p<0.0001 for both).
Figure 2. CMR Assessments of Myocardial ECV.
Myocardial extracellular volume (ECV) measured by cardiovascular magnetic resonance in controls (26.9±0.2%) and cancer pre-treatment participants (27.8±0.7%) compared with cancer survivors treated with anthracycline chemotherapy (30.4±0.7%). ECV incrementally increases across the groups (p<0.0001) with a significant increase in post-treatment ECV values compared with pre-treatment ECV values (p<0.01).
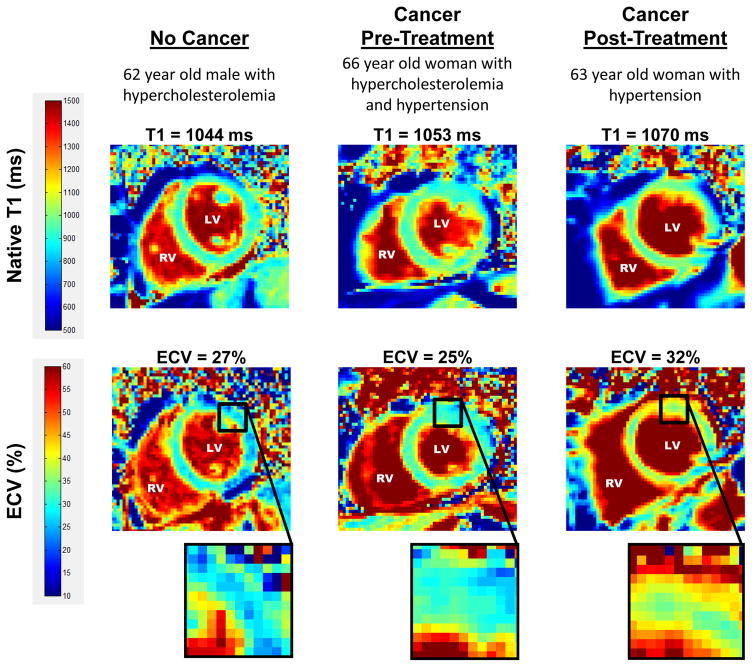
Figure 3. T1 and ECV map images.
Representative left ventricular (LV) short axis native T1 (top row) and extracellular volume (ECV, bottom row) maps are shown in similarly-aged participants. The LV and right ventricular (RV) blood pool cavities are noted. On each image, the color of pixels in the images (color scales on left) identifies the native T1 (milliseconds) and ECV (%). Insets on the ECV maps demonstrate the change in color intensity within the anterolateral wall of each ventricle. As shown, ECV is elevated in the cancer survivor previously treated with anthracycline-based chemotherapy.
Table 3.
Multivariate models of myocardial T1 and ECV adjusting for participant demographics, cardiovascular profile, and parameters associated with LV remodeling.
| Myocardial Native T1 | Myocardial ECV | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Model | Covariates | Beta Estimate | (95% CI) | p-value | Beta Estimate | (95% CI) | p-value |
| 1 | Group | <0.0001 | <0.0001 | ||||
| Pre-treatment | 92.7 | (78.3,107.2) | <0.0001 | 0.92 | (−0.44,2.3) | <0.0001 | |
| Post-treatment | 75.3 | (60.6,89.9) | 3.5 | (2.1,5.0) | |||
| 2 | Group | ||||||
| Pre-treatment | 89.9 | (74.6,105.2) | 0.91 | 1.2 | (−0.08,2.6) | <0.01 | |
| Post-treatment | 71.1 | (55.0,87.2) | 0.60 | 3.8 | (2.4,5.2) | 0.77 | |
| Age | 0.41 | (−0.26,1.1) | 0.02 | 0.09 | (0.031,0.14) | <0.01 | |
| Non-white | −2.7 | (−12.5,7.2) | 0.047 | 0.12 | (−0.69,0.93) | 0.08 | |
| Female | 64.7 | (11.0,118.5) | <0.0001 | 6.8 | (2.4,11.3) | <0.0001 | |
| Age*Female | −0.77 | (−1.6,0.05) | −0.06 | (−0.13,0.008) | |||
| 3 | Group | ||||||
| Pre-treatment | 88.9 | (72.5,105.4) | 0.71 | 1.6 | (0.21,3.0) | <0.01 | |
| Post-treatment | 70.0 | (52.3,87.6) | 0.59 | 3.9 | (2.4,5.4) | 0.69 | |
| Age | 0.22 | (−0.49,0.93) | 0.06 | 0.08 | (0.02,0.14) | 0.01 | |
| Non-white | −2.9 | (−13.2,7.5) | 0.15 | 0.17 | (−0.67,1.0) | 0.19 | |
| Female | 54.2 | (−1.8,110.2) | 0.15 | 6.0 | (1.4,10.6) | 0.27 | |
| Age*Female | −0.63 | (−1.5,0.23) | 0.62 | −0.05 | (−0.12,0.02) | 0.22 | |
| Weight | −0.11 | (−0.26,0.04) | 0.77 | −0.01 | (−0.02,0.01) | 0.41 | |
| Systolic BP | −0.08 | (−0.42,0.25) | 0.83 | −0.02 | (−0.05,0.01) | 0.91 | |
| Known CAD | 6.5 | (−37.7,50.7) | 0.46 | −1.6 | (−5.3,2.2) | 0.16 | |
| Diabetes | 1.5 | (−12.0,15.0) | 0.80 | −0.06 | (−1.1,1.0) | 0.67 | |
| Hyperlipidemia | −6.0 | (−21.9,9.9) | <0.0001 | −1.1 | (−2.5,0.41) | <0.0001 | |
| Hypertension | 1.9 | (−13.0,16.9) | 0.28 | (−1.0,1.6) | |||
| 4 | Group | ||||||
| Pre-treatment | 90.0 | (74.5,105.4) | 0.82 | 1.3 | (−0.04,2.6) | <0.01 | |
| Post-treatment | 63.0 | (45.5,80.5) | 0.55 | 4.0 | (2.5,5.5) | 0.70 | |
| Age | 0.40 | (−0.28,1.1) | 0.03 | 0.07 | (0.02,0.13) | <0.01 | |
| Non-white | −3.1 | (−13.1,6.9) | 0.10 | 0.16 | (−0.66,0.98) | 0.16 | |
| Female | 60.8 | (6.0,115.5) | 0.04 | 6.4 | (1.8,11.0) | 0.81 | |
| Age*Female | −0.70 | (−1.5,0.13) | 0.44 | −0.05 | (−0.12,0.02) | 0.46 | |
| LVEF | −0.73 | (−1.4, −0.02) | 0.01 | (−0.05,0.06) | |||
| LV mass index | −0.17 | (−0.60,0.26) | 0.01 | (−0.02,0.05) | |||
Categorical reference variables include no cancer controls (group), white (race), and men (gender). BP=blood pressure; CAD=coronary artery disease; LV=left ventricular; EF=ejection fraction. Post-treatment=anthracycline-treated survivors.
DISCUSSION
The results of this study demonstrate several important findings. First, extracellular volume fraction measured with CMR is abnormally increased three years after receipt of anthracycline-based chemotherapy above and beyond that which occurs in the presence of cancer prior to treatment (Figure 2). Second, elevated ECV persists even after accounting for pre-existing CV comorbidities associated with subclinical fibrosis development (Table 3). Third, myocardial native T1 is elevated 3 years after cancer treatment and prior to the time individuals with cancer initiate their treatment for cancer. This elevation in T1 occurs with a small but not marked increase in ECV in untreated cancer patients and cancer survivors treated with non-anthracycline chemotherapy. Fourth, our study demonstrates that increased subclinical myocardial fibrosis, as manifest by ECV elevations, coincides with decreases in left ventricular ejection fraction and myocardial mass in adult cancer survivors three years after receiving anthracycline-based chemotherapy.
Previously in a study of adults surviving beyond 7 years from their cancer treatment with clinically-indicated CMR examinations, myocardial ECV was elevated compared to healthy adults free of CV risk factors or cancer.6 In two studies of asymptomatic pediatric cancer survivors, myocardial T1 and ECV were within normal ranges for younger populations; however in these same studies, ECV was associated with cumulative anthracycline dose, reduced exercise capacity, myocardial wall thinning, increased end-systolic fiber stress, and reduced LV myocardial contractility.18,19 These prior studies did not address whether a) the ECV elevations were related to other CV comorbidities also associated with subclinical myocardial fibrosis or b) the presence of cancer in and of itself.
Our results indicate elevation of ECV 3±1 years (as opposed to the previously reported 7 years) after receipt of anthracycline-based chemotherapy (Figure 2). The magnitude of the elevation (30±1%) was greater than patients with CV co-morbidities without cancer (27±0.2%) and those yet to initiate their cancer treatment (28±1%) but lower than the previously reported 7-year survivors (36±3%).6 Differences in ECV between the cancer groups are not likely due to post-treatment anemia as the hematocrit levels in these groups was equivalent (Table 2, p=0.22). ECV was not different when calculated with one post-contrast time point, demonstrating the feasibility in using a shorter contrasted protocol that would improve clinical translation in future work. Chronically elevated ECV has been shown to represent either a) shift from intracellular to extracellular volume or b) myocardial fibrosis. The “intermediate” elevation in ECV between the comparator groups in this study and the previously reported higher values 7 years after treatment raises the possibility that there may be a transition in development of subclinical fibrosis after receipt of anthracycline-based chemotherapy. Recently, contrast-enhanced T1-weighted changes have been observed in cancer patients as early as 3 months after anthracycline-based chemotherapy initiation,20 however, it is unknown if ECV measures of fibrosis may be detected this early. A future longitudinal cohort study performed in patients before, during, and after cancer treatment could address these issues.
Our study design allowed us to compare ECV values in our survivor population with ECV values from those enrolled in the MESA cohort that possess risk factors for CV events and myocardial fibrosis.21 Selection of MESA as the comparator cohort was based on a) similar CMR protocol to the study cohort, and b) requirement of a comparator since no cancer-free comparator sub-group (with and without CV co-morbidities) with contemporary T1 and ECV data was available. Interstitial myocardial fibrosis, assessed by ECV, is known to increase with age, and in general is higher among women.11 In this study, we identified that ECV remained elevated in survivors after accounting for age, gender, and their interaction using multivariable models (Table 3); similar results were found in a sensitivity analysis with propensity score matching on factors that influence T1. These data suggest the ECV elevation we observed in survivors previously treated with anthracycline-based chemotherapy was not dependent on age or gender.
In addition, we performed other models that included CV comorbidities and LV remodeling measures such as LV ejection fraction or mass. As shown in Table 3, ECV remained elevated relative to those without cancer after accounting for weight, heart rate, systolic blood pressure, known coronary arterial disease, diabetes, hyperlipidemia, hypertension, LVEF, and LV mass index. These data confirm that elevations of ECV, a strong indicator of subclinical myocardial fibrosis, occurs 3 years after receipt of anthracycline-based chemotherapy independent of other CV co-morbidities previously associated with progressive LV myocardial fibrosis. Left ventricular myocardial ECV increases with age and the ECV of untreated cancer patients was similar to controls who were 10 years older.
To measure ECV, we calculated LV myocardial T1 before (“native T1”) and after the administration of gadolinium contrast (Figure 3). Overall, native T1 and ECV values of our comparator non-cancer group are similar to those previously reported in the literature.11,22 As expected, elevated native T1 coincided with elevated ECV among cancer survivors (Table 3). We included an exploratory analysis of non-anthracycline breast cancer survivors that showed an intermediate ECV between comparators and anthracycline-treated survivors; these preliminary findings should be further investigated in a larger study powered to determine the relationship of non-anthracycline chemotherapy with changes in myocardial fibrosis. Interestingly, we found that newly diagnosed cancer patients exhibited elevated native T1 but their ECV elevation was far less than to survivors who received anthracycline-based chemotherapy. T2 was within normal limits in anthracycline-treated cancer survivors (52.5ms) and thus indicates that their increased ECV is more likely related to myocardial fibrosis and not increased water content or myocardial edema.
It is important to note that ECV reflects interstitial processes that influence T1 whereas native T1 may reflect changes in myocellular composition (e.g., intra-myocellular edema, or deposition of protein, lipids, or iron), interstitial processes, and/or changes in myocardial oxygenation or perfusion.23,24 Previously, both skeletal and cardiac muscle have demonstrated increased intracellular edema related to inflammatory cytokines associated with cancer before treatment.25 Thus, our observation of T1-related changes in newly diagnosed cancer patients may be related to the presence of comorbidities, cancer-related processes, or a mixture of both.
Our findings of reduced LVEF and LV mass loss in adult survivors treated with anthracyclines are consistent with prior reports.26–28 The higher LV mass among cancer-free comparators is likely age-related. Neilan, et al., reported reduced LV mass and LVEF in anthracycline-treated survivors with elevated ECV6 and lower LV mass was related to increased risk for CV events in survivors with anthracycline-induced cardiomyopathy.26 Importantly, LV mass is determined during CMR by measuring the myocardial volume residing between the epi- and endocardial contours from three-dimensional image acquisitions spanning the cardiac apex to its base planned in the LV short axis plane.9 This tissue volume is multiplied by a widely accepted specific density constant for normal myocardial tissue. In the setting of abnormal myocardial tissue –as in anthracycline cardiotoxicity – the true LV “myocellular” mass may be underestimated when replaced by fibrous tissue. Thus, these study results indicate that not only do 3-year cancer anthracycline-treated survivors have a reduced LV mass, but also 30% of the tissue categorized as mass is actually “extra-myocellular volume” that does not include functioning cardiac myocytes.
These study results have important clinical ramifications. Myocardial extracellular remodeling with increased collagen and fibrosis stiffens the left ventricle, and in other populations, contributes to LV dysfunction, fatigue, and reduced exercise capacity.29–31 To date, most anthracycline cardiotoxicity research has focused on myocellular injury. The 3-year post-treatment ECV elevation warrants additional research to investigate the time-course of onset and the clinical importance of myocardial fibrosis and whether it relates to the CV mortality and exercise intolerance observed in cancer survivors.18,32 Current surveillance strategies focused only on LVEF after symptoms could miss the onset of subclinical fibrosis,3,33 and therapies for fibrosis differ from those that prevent myocellular injury.34
Our results are interpreted in the setting of the following limitations. First, our cross-sectional analysis does not allow us to determine the time-course of ECV elevation among individuals treated for cancer. A prospective longitudinal study with matched control participants would aid in understanding the time-course of myocardial fibrosis development, inflammatory processes, LV functional deterioration and associations with CV events. Second, only 69% of cancer patients exhibited estimated GFR values ≥45 ml/min and consented to receipt of contrast; thus, we are uncertain of the assessment of ECV in those with reduced GFR values. Third, two different contrast agents were administered in the study with a mild decrease in dose for the cancer participants. ECV assessments comparing other types of gadolinium contrast agents observed a mean ECV difference of only 0.01%;35 a similar effect in this study would not alter our observation of elevated ECV in anthracycline-treated survivors. Furthermore, we found no significant effect of the contrast agent and dose on ECV measurements among control participants. Future prospective studies could use a single contrast agent and dose in order to eliminate this potential confounder.
In conclusion, in adult cancer survivors treated with anthracycline-based chemotherapy, myocardial ECV – a marker of diffuse fibrosis – is abnormally elevated three years after treatment. This elevation occurs after accounting for other risk factors such as age, the presence of cancer, or co-existent cardiovascular co-morbidities thereby suggesting receipt of anthracycline-based chemotherapy is associated with the adverse accumulation of left ventricular interstitial myocardial fibrosis in cancer survivors.
Supplementary Material
Clinical Perspective.
This study found that in adult cancer survivors treated with anthracycline-based chemotherapy, myocardial extracellular volume (ECV)—a marker of diffuse fibrosis—is abnormally elevated three years after cancer treatment compared with untreated cancer patients and comparators without cancer. This elevation occurred independent of other risk factors and CV co-morbidities previously associated with LV myocardial fibrosis. T2 was within normal limits in anthracycline-treated cancer survivors and thus indicates that their increased ECV is more likely related to myocardial fibrosis. These study results have important clinical ramifications. Myocardial extracellular remodeling with increased collagen and fibrosis stiffens the left ventricle, and contributes to LV dysfunction, fatigue, and reduced exercise capacity. To date, most anthracycline cardiotoxicity research has focused on myocellular injury. Current surveillance strategies focused only on LVEF after symptoms could miss the onset of subclinical fibrosis, and therapies for fibrosis differ from those that prevent myocellular injury. This study could serve as a foundation for additional research to investigate the onset of myocardial fibrosis and its relation to CV mortality and exercise intolerance observed in cancer survivors so that new clinical pathways can be created that best account for this occurrence and improve the cardiovascular health of cancer survivors.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. ProHance contrast agent was provided for the study by Bracco Diagnostics (Princeton, NJ).
SOURCES OF FUNDING
This research was supported by contracts N01-HC-95165 and N01-HC-95168 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. Additionally, this work was supported in part by NIH grants R33CA12196, R01HL118740, R01CA167821, and P30CA012197 and the Susan G. Komen Foundation (BCTR07007769).
Footnotes
DISCLOSURES
None.
References
- 1.Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: an overview of current uses and future directions of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:66. doi: 10.1186/1532-429X-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drafts BC, Twomley KM, D’Agostino R, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to Moderate Dose Anthracycline-Based Chemotherapy Is Associated With Early Noninvasive Imaging Evidence of Subclinical Cardiovascular Disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal PK, Iliskovic N. Doxorubicin-Induced Cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 4.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 5.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2013:eht193. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, Pierre-Mongeon F, Heydari B, Francis SA, Moslehi J, Kwong RY, Jerosch-Herold M. Myocardial Extracellular Volume by Cardiac Magnetic Resonance Imaging in Patients Treated With Anthracycline-Based Chemotherapy. Am J Cardiol. 2013;111:717–722. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thavendiranathan P, Amir E, Bedard P, Crean A, Paul N, Nguyen ET, Wintersperger BJ. Regional myocardial edema detected by T2 mapping is a feature of cardiotoxicity in breast cancer patients receiving sequential therapy with anthracyclines and trastuzumab. J Cardiovasc Magn Reson. 2014;16:P273. [Google Scholar]
- 8.Kramer H, Palmas W, Kestenbaum B, Cushman M, Allison M, Astor B, Shlipak M. Chronic Kidney Disease Prevalence Estimates among Racial/Ethnic Groups: The Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol. 2008;3:1391–1397. doi: 10.2215/CJN.04160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehr RB, Malloy CR, Filipchuk NG, Peshock RM. Left ventricular volumes measured by MR imaging. Radiology. 1985;156:717–719. doi: 10.1148/radiology.156.3.4023232. [DOI] [PubMed] [Google Scholar]
- 10.Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S, Simonetti OP, Raman SV. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. 2012;5:102–110. doi: 10.1161/CIRCIMAGING.111.967836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C-Y, Liu Y-C, Wu C, Armstrong A, Volpe GJ, Van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugander M, Oki AJ, Hsu L-Y, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012:ehr481. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 14.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 15.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpe GJ, Rizzi P, Nacif MS, Ricketts EP, Venkatesh BA, Liu C-Y, Gomes AS, Hundley WG, Prince MR, Carr JC, et al. Lessons on Quality Control in Large Scale Imaging Trials: the Multi-Ethnic Study of Atherosclerosis (MESA) Curr Cardiovasc Imaging Rep. 2015;8:1–13. [Google Scholar]
- 17.Liu YC, Liu C-Y, van der Geest RJ, Liu S, Nacif M, Lima JA, Bluemke DA. Myocardial T1 measurement: comparison of modified Look-Locker inversion recovery (MOLLI) and TI scout in the Multi-ethnic Study of Atherosclerosis (MESA) J Cardiovasc Magn Reson. 2012;14:P265. [Google Scholar]
- 18.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toro-Salazar OH, Gillan E, O’Loughlin M, Burke GS, Ferranti J, Stainsby J, Liang B, Mazur W, Raman S, Hor K. Occult Cardiotoxicity in Childhood Cancer Survivors Exposed to Anthracycline Therapy. Circ Cardiovasc Imaging. 2013;6:873–80. doi: 10.1161/CIRCIMAGING.113.000798. [DOI] [PubMed] [Google Scholar]
- 20.Jordan JH, D’Agostino RB, Hamilton CA, Vasu S, Hall ME, Kitzman DW, Thohan V, Lawrence JA, Ellis LR, Lash TL, Hundley WG. Longitudinal Assessment of Concurrent Changes in Left Ventricular Ejection Fraction and Left Ventricular Myocardial Tissue Characteristics After Administration of Cardiotoxic Chemotherapies Using T1-Weighted and T2-Weighted Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2014;7:872–879. doi: 10.1161/CIRCIMAGING.114.002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Dabir D, Child N, Kalra A, Rogers T, Gebker R, Jabbour A, Plein S, Yu C-Y, Otton J, Kidambi A, McDiarmid A, Broadbent D, Higgins DM, Schnackenburg B, Foote L, Cummins C, Nagel E, Puntmann VO. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:69. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wacker CM, Bock M, Hartlep AW, Beck G, van Kaick G, Ertl G, Bauer WR, Schad LR. Changes in myocardial oxygenation and perfusion under pharmacological stress with dipyridamole: assessment using T* 2 and T1 measurements. Magn Reson Med. 1999;41:686–695. doi: 10.1002/(sici)1522-2594(199904)41:4<686::aid-mrm6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Kazemi-Bajestani SMR, Becher H, Fassbender K, Chu Q, Baracos VE. Concurrent evolution of cancer cachexia and heart failure: bilateral effects exist. J Cachexia Sarcopenia Muscle. 2014;5:95–104. doi: 10.1007/s13539-014-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, Moslehi J, Kwong RY. Left Ventricular Mass in Patients With a Cardiomyopathy After Treatment With Anthracyclines. Am J Cardiol. 2012;110:1679–1686. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iarussi D, Galderisi M, Ratti G, Tedesco MA, Indolfi P, Casale F, Tullio MTD, De Divitiis O, Iacono A. Left ventricular systolic and diastolic function after anthracycline chemotherapy in childhood. Clin Cardiol. 2001;24:663–669. doi: 10.1002/clc.4960241006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Donovan FD, Metzger ML, Arevalo A, Durand J-B, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 30.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 33.Kongbundansuk S, Hundley WG. Noninvasive Imaging of Cardiovascular Injury Related to the Treatment of Cancer. JACC Cardiovasc Imaging. 2014;7:824–838. doi: 10.1016/j.jcmg.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6:107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 35.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA. T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012;14:26. doi: 10.1186/1532-429X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.