Abstract
Background:
Men treated with androgen deprivation therapy (ADT) for prostate cancer are prone to multiple treatment-induced adverse effects, particularly with regard to a deterioration in bone health and altered body composition including decreased lean tissue mass and increased fat mass. These alterations may partially explain the marked increased risk in osteoporosis, falls, fracture and cardiometabolic risk that has been observed in this population.
Methods:
A review was conducted that assessed standard clinical guidelines for the management of ADT-induced adverse effects on bone health and body composition in men with prostate cancer.
Results:
Currently, standard clinical guidelines exist for the management of various bone and metabolic ADT-induced adverse effects in men with prostate cancer. However, an evaluation of the effectiveness of these guidelines into routine practice revealed that men continued to experience increased central adiposity, and, unless pharmacotherapy was instituted, accelerated bone loss and worsening glycaemia occurred.
Conclusions:
This review discusses the current guidelines and some of the limitations, and proposes new recommendations based on emerging evidence regarding the efficacy of lifestyle interventions, particularly with regard to exercise and nutritional factors, to manage ADT-related adverse effects on bone health and body composition in men with prostate cancer.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed male cancer in developed countries.1 Treatment for PCa includes surgery, radiotherapy, androgen deprivation therapy (ADT) and chemotherapy, which is influenced by the stage and aggressiveness of the cancer.2, 3 Although usually reserved for non-localised or more aggressive PCa, ADT is a standard systemic treatment that aims to reduce the activity and/or concentration of androgens, such as testosterone, to castration levels to prevent PCa growth and spread.4 Various modalities of ADT, including those administered neoadjuvant or adjuvant with other treatments are associated with improved survival in appropriately selected men with advanced PCa.5, 6, 7, 8, 9 In contrast, evidence that ADT prolongs survival in men with localised PCa remains limited and, therefore, debated.10, 11, 12 Despite this, ADT is commonly prescribed for both metastatic4 and non-metastatic PCa.13
Although ADT has been shown to improve survival outcomes, treatment-induced hypogonadism has been associated with multiple interconnected adverse effects such as decreased bone mineral density (BMD) and a loss in the structure and strength of bone,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 a loss in lean tissue mass and muscle cross-sectional area,17, 18, 20, 26, 27, 28, 29, 30, 31 an increase in fat mass and intermuscular adipose tissue17, 18, 20, 26, 27, 28, 31, 32 and an increased risk of falls33 and subsequent fractures in this clinical population group.34, 35, 36 Other commonly observed adverse effects of ADT include negatively altered blood lipid profiles, decreased insulin sensitivity, increased arterial stiffness, increased fatigue, increased depressive symptoms and sexual dysfunction.37, 38, 39 Collectively, these alterations may lead to increased cardiometabolic risk and decreased health-related quality of life in men treated with ADT.40, 41 The aim of this paper was to review the current guidelines available for management of various bone and metabolic ADT-induced adverse effects in men with PCa. A secondary aim was to propose new recommendations based on emerging evidence regarding the efficacy of lifestyle interventions, particularly with regard to exercise and nutritional factors, to manage ADT-related alterations to bone health and body composition in men with PCa.
Materials and methods
An electronic search of the National Institute of Health MEDLINE database was performed to identify all peer-reviewed articles published in English between January 2000 and June 2016. The following search terms were used: (‘androgen deprivation therapy’ or ‘hormone therapy’) and (‘guidelines’ or ‘strategies’ or ‘practice’) and (‘bone’ or ‘muscle’ or ‘fat’ or ‘body composition’). Overall, 276 articles were found, of which the titles and abstracts were evaluated by two authors (PJO and SFF). Additional articles were located via manual searches of relevant reference lists. Five articles discussing recently proposed and relevant lifestyle guidelines for the management of ADT-induced adverse effects on bone health and body composition were determined as the basis for this review.
Changes to bone health and body composition associated with androgen deprivation therapy
Bone mineral density, structure and strength
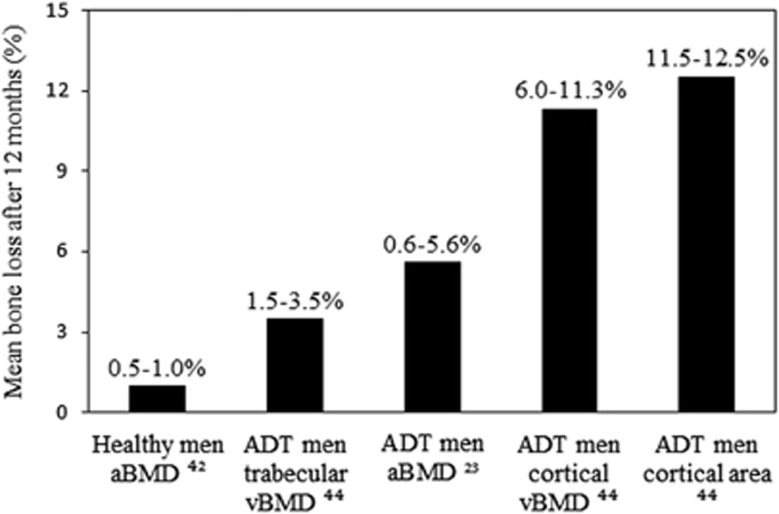
It is well established that there is a progressive age-related loss in areal BMD (aBMD) in healthy men, as commonly measured via dual-energy X-ray absorptiometry (DXA), which equates to ~0.5–1.0% per year at various skeletal sites including the femoral neck, total hip and lumbar spine42, 43 (Figure 1). In contrast, it has been reported that men treated with continuous pharmacological ADT experience aBMD annual losses of 0.6–3.9% at the femoral neck14, 15, 16, 21, 22, 23, 44 and 2.3–4.8% at the lumbar spine,15, 18, 21, 22, 23, 25, 44 particularly during the initial 6 months of treatment (Table 1 and Figure 1). Accelerated aBMD loss has also been observed at the hip,17, 18, 19, 20, 22, 23, 24, 25, 44, 45 radius18, 22, 24, 44 and whole body,17, 18 indicating that ADT has a systemic effect on bone (Table 1). The findings from a 12-month prospective study using high-resolution peripheral quantitative computed tomography, which quantifies changes in appendicular cortical and trabecular volumetric BMD and microarchitecture, reported that men treated with ADT (mean age; 71 years) experienced losses in distal radius and tibia cortical volumetric BMD of up to 11.3% and 6.0% per year, respectively44 (Figure 1). Losses in trabecular volumetric BMD of 3.5% and 1.5% were also observed at the distal radius and tibia, respectively44 (Figure 1). In addition, cortical area at the distal radius and tibia decreased by 5.1–5.2% within the first 6 months which increased to 11.5–12.5% after 12 months44 (Figure 1). This study indicates that both the density and structure of bone, which are both important determinants of whole-bone strength, are compromised in men treated with ADT. Furthermore, the study indicates that DXA-based measures of aBMD may underestimate the magnitude of bone loss associated with ADT.
Figure 1.
Effects of ADT on bone mass, structure and strength. aBMD, areal bone mineral density; ADT, androgen deprivation therapy; vBMD, volumetric bone mineral density.
Table 1. Non-randomised controlled trials examining the effects of continual ADT in men with prostate cancer on aBMD.
| Authors | N | Average age (years) | Change in aBMD |
|---|---|---|---|
| GnRH agonist only | |||
| 6 months | |||
| Diamond et al.45 | 18 | 78 | FN−6.5% WT−7.5% Troch−6.2% |
| Maillefert et al.21 | 12 | 70 | LS−3.0% FN−2.7% |
| Hamilton et al.44 | 26 | 71 | LS−1.7% FN−1.5% Hip−1.5% 1/3Rad +9.1% |
| 12 months | |||
| Maillefert et al.21 | 12 | 70 | LS−4.6% FN−3.9% |
| Berruti et al.15 | 35 | 75 | LS−2.3% FN−0.6% |
| Mittan et al.22 | 15 | 75 | Hip−3.3% DRad−5.3% MRad−2.7% 1/3Rad−1.6% LS−2.8% FN−2.3% |
| Bergstrom et al.14 | 10 | 73 | FN−3.2% |
| Smith et al.25 | 26 | 65 | LS−2.5% Hip−1.4% |
| Lee et al.20 | 65 | 66 | Hip−1.9% |
| Morote et al.23 | 31 | 70 | LS−4.8% FN−3.0% WT−5.6% Troch−3.6% Hip−3.8% |
| Hamilton et al.44 | 26 | 71 | LS−3.9% FN−3.0% Hip−2.6% 1/3Rad +9.2% |
| 18 months | |||
| Maillefert et al.21 | 12 | 70 | LS−6.6% FN−7.1% |
| Anti-androgen only | |||
| 12 months | |||
| Smith et al.25 | 25 | 63 | LS +2.5% Hip +1.1% |
| CAB | |||
| 9 months | |||
| Higano et al.19 | 17 | 69 | LS−4.5% Hip−2.5% |
| Galvão et al.17 | 69 | 74 | Hip−1.5% LS−3.9% WB−2.4% |
| Pharmacological (any modality) | |||
| 12 months | |||
| Daniell et al.16 | 16 | 72 | FN−3.4% |
| Greenspan et al.18 | 80 | 69–71 | Acute ADT: Hip−2.5% Troch−2.4% TRad−2.6% WB−3.3% LS−4.0% Chronic ADT: TRad−2.0% |
| 24 months | |||
| Daniell et al.16 | 16 | 72 | FN−6.5% |
| Preston et al.24 | 23 | 73 | FN−1.9% Hip −1.5% Troch−2.0% LS−0.2% DRad +9.4% |
| Orchiectomy only | |||
| 12 months | |||
| Daniell et al.16 | 10 | 77 | FN−2.4% |
| Bergstrom et al.14 | 12 | 79 | FN−4.5% |
| 24 months | |||
| Daniell et al.16 | 10 | 77 | FN−10.0% |
Abbreviations: 1/3Rad, one-third radius; aBMD, areal bone mineral density; ADT, androgen deprivation therapy; CAB, combined androgen blockade; DRad, distal radius; FN, femoral neck; GnRH, gonadotropin-releasing hormone; Hip, total hip; LS, lumbar spine; MRad, mid radius; N, number; TRad, total radius; Troch, trochanter; WB, whole body; WT, Ward’s triangle.
There is also evidence that the rate of bone loss in men with PCa may be dependent on the modality of treatment. For instance, orchiectomy appears to lead to greater aBMD loss when compared with pharmacological ADT,14, 16 whereas anti-androgen monotherapy has been associated with reduced bone loss compared with gonadotropin-releasing hormone agonists.25 Duration of ADT may also influence the rate of bone loss, with the greatest losses occurring within the first 6 months of treatment.18 From a clinical perspective, accelerated loss of bone density and structure during ADT is important because it likely contributes to the marked increase in fracture risk (34–65%) in this susceptible population group, which has been associated with twice the rate of mortality when compared with men on ADT who did not sustain a fracture.34, 46 Importantly, there is also evidence of a significant dose–response relationship between the number of ADT doses received during the 12 months post diagnosis and the subsequent risk of fracture.35, 47 In addition to ADT, residual effects of radiotherapy have been postulated to influence androgen concentration,48 which supports the findings from a meta-analysis indicating that men who had not received ADT for PCa had lower aBMD than healthy older males, but not as low as men treated with ADT.49
Lean tissue mass
In healthy adult men, age-related losses in lean tissue mass range from 1.0-2.0% per year after the age of 40–45 years, with a more accelerated loss after the fifth decade of life.50, 51 However, lean tissue mass losses of 2.0–3.6% have been reported after a year of ADT18, 20, 28, 44 (Table 2). In addition, findings from a study of 25 men (mean age; 68 years) who had commenced ADT reported that lean tissue mass decreased by 1.4% in the first 12 weeks of treatment.29 Although the mediating factors contributing to muscle loss with ADT are multifaceted, duration of treatment and patient age appears to be important. For instance, men treated with ADT demonstrated the greatest losses in lean tissue mass (2.6–3.2%) during the initial 6–9 months of treatment.18, 44, 52 Furthermore, men on ADT aged >70 years have been shown to lose three times the amount of lean tissue mass when compared with men aged <70 years.52 These changes to lean tissue mass may adversely affect functional performance as observed during a follow-up of 50 men (median age; 78 years) treated with ADT in which 24% of the men demonstrated impairment during activities of daily living.33 Moreover, 22% of these men reported a recent fall,33 which may further increase the risk of fracture. Notably, after cessation of ADT and recovery of eugonadal testosterone level, lean tissue mass losses were not reversed over a 2-year follow-up in a cohort of 49 men (mean age; 73 years).32 This suggests that the associated complications of ADT may be prolonged after cessation of treatment.
Table 2. Non-randomised controlled trials examining the effects of continual ADT in men with prostate cancer on lean tissue mass and fat mass.
| Authors | N | Average age (years) | Change in lean tissue mass (total body) | Change in fat mass |
|---|---|---|---|---|
| GnRH agonist only | ||||
| 26 weeks | ||||
| Boxer et al.31 | 30 | 72 | −2.1% | +9.5% |
| Hamilton et al.44 | 26 | 71 | −3.2% | +12.0% |
| 48 weeks | ||||
| Smith et al.27 | 32 | 66 | −2.7% | +9.4% |
| 52 weeks | ||||
| Lee et al.20 | 65 | 66 | −2.0% | +6.6% |
| Hamilton et al.44 | 26 | 71 | −3.6% | +14.1% |
| CAB | ||||
| 12 weeks | ||||
| Smith et al.29 | 25 | 68 | −1.4% | NA |
| 36 weeks | ||||
| Galvão et al.17 | 72 | 74 | −2.4% | +13.8% |
| 52 weeks | ||||
| Smith et al.28 | 26 | 65 | −3.6% | +11.2% |
| Pharmacological (any modality) | ||||
| 24 weeks | ||||
| Smith et al.30 | 22 | 67 | −2.7% | NA |
| 48 weeks | ||||
| Smith26 | 79 | 71 | −3.8% | NA |
| 52 weeks | ||||
| Greenspan et al.18 | 80 | 69–71 | Acute ADT: −3.5% Chronic ADT: NC | Acute ADT: +10.4% Chronic ADT: NC |
Abbreviations: ADT, androgen deprivation therapy; CAB, combined androgen blockade; GnRH, gonadotropin-releasing hormone; N, number; NA, not applicable; NC, no change.
Fat mass
During the ageing process, fat mass and percentage body fat commonly increases in men until the seventh decade, after which it typically remains stable or decreases slightly throughout the remainder of life.53 Conversely, men on ADT have been shown to experience a continuous increase in fat mass beyond this age-associated plateau (Table 2) with an average annual gain exceeding 11%.54 Furthermore, following a 2-year period after cessation of ADT, negative alterations to fat mass remained.32 To date, limited studies have quantified if there are region-specific gains in fat mass during ADT,27, 55, 56 but of the limited research available, ADT has been associated with an 11–13% increase in subcutaneous abdominal fat mass27, 55 and either an increase (22%)55 or no change27 in visceral abdominal fat mass after 48–52 weeks. In addition, findings from a study of 39 men (median age; 73 years) commencing ADT reported increased fat infiltration of lower limb muscles (anterior compartment of thigh), as assessed via computed tomography, after 14.6–20 weeks of treatment.56 Clinically, there are multiple adverse consequences associated with these ADT-related gains in total and regional fat mass and intramuscular fat. For instance, potential increases in subcutaneous and visceral fat may contribute to the marked increase in cardiometabolic disorders in men treated with ADT.28, 40, 57 In addition, increased fat infiltration within muscle has been shown to impair muscular function in non-ADT-treated older adults.58 As obesity is associated with an increase in inflammatory cytokines (or adipokines),59 and chronic systemic inflammation has been shown to contribute to age-related muscle wasting,60 it is possible that ADT-induced gains in fat mass may exacerbate losses in muscle in men with PCa.
Potential treatment options for addressing bone health and body composition changes associated with androgen deprivation therapy
Current guidelines for managing ADT-induced adverse effects on bone, muscle and fat generally apply a similar approach, that is, monitoring known risk factors, pharmacotherapy and/or lifestyle interventions, but these management guidelines appear to vary by country, organisation and expert opinion.37, 40, 61, 62, 63 Although these guidelines are often based on numerous evidence-based studies, it remains uncertain as to whether these guidelines are routinely implemented and followed over time and if they are effective in ameliorating or attenuating many of the adverse effects of ADT. To date, only one set of guidelines,40 focusing on bone and metabolic outcomes of ADT, has been implemented and evaluated.64 In a 2-year prospective study, 236 men with PCa commencing ADT attended a baseline clinic visit and received diet and lifestyle advice, with overweight and obese men offered referral to a dietician.64 If required, men also received treatment for hypertension, hypercholesterolaemia and osteoporosis as per standard management guidelines.40 Men attending this clinic were assessed at 3–6 monthly intervals.64 A summary of the assessment and management strategies used in this study is shown in Table 3. At the initial assessment, 87% of the men were overweight/obese, 61% had hypertension, 56% had hypercholesterolaemia, 27% had prior cardiovascular disease, 11% had osteoporosis and 40% had osteopenia.64 For the prospective study, 153 men had data available after 2 years of continuous ADT use.64 The main findings from this study were a mean loss of 3.4% and 2.5% in lumbar spine (L1-4) and hip aBMD, respectively, over 2 years, unless the men were treated with antiresorptive therapy (n=14), in which case aBMD was maintained at both the sites.64 No measures of muscle or fat mass or functional performance were assessed in this study, however, an increase in waist circumference of 2.7 cm was indicative of an increase in abdominal fat mass.64 In contrast, reductions were observed in blood pressure, total cholesterol and low-density lipoprotein cholesterol with treatment.64 Although it is important to acknowledge that this was an observational study, which precludes inferences about causality, these findings provide some evidence that adhering to the current management guidelines for men with PCa treated with ADT may not mitigate some of the metabolic and bone adverse effects associated with this treatment.
Table 3. Clinical assessment and management guidelines for ADT-associated cardiometabolic and skeletal adverse effects.
| Cardiometabolic health |
| Metabolic risk assessment prior to ADT commencement: body mass index, waist circumference, blood pressure, fasting blood glucose, oral glucose tolerance test (if fasting glucose between 5.5 and 6.9 mmol l−1) and fasting lipid profile. |
| Six-monthly to yearly metabolic assessment during the first 24 months of ADT. |
| Lifestyle intervention and/or dietician to prevent weight gain and worsening of insulin resistance. |
| Smoking cessation. |
| Blood pressure <130/80 mm Hg. |
| Lipid targets according to NCEP ATP III treatment guidelines. |
| In men with diabetes, intensification of management as necessary to main HbA1c target. |
| Skeletal health |
| At commencement of ADT: assessment for history of minimal trauma fractures and risk factors for osteoporosis, aBMD measurement by DXA and, in men with osteopenia, postero-anterior as well as lateral thoracolumbar spine X-rays. |
| Yearly aBMD measurement during the first 24 months of ADT. |
| Advice regarding regular physical exercise, smoking cessation and alcohol consumption of ⩽2 standard drinks per day at each visit. |
| Total daily calcium intake of 1200–1500 mg through diet, supplements, or both, unless there is a history of renal calculi. |
| Vitamin D supplementation as necessary to achieve a target serum 25-hydroxyvitamin D level ⩾75 nmol l−1. |
| Commencement of treatment with a bisphosphonate in men with a minimal trauma fracture, an aBMD T-score of ⩽−2.5, or if 10-year absolute risk of a major osteoporotic fracture is >20%. |
Abbreviations: aBMD, areal bone mineral density; ADT, androgen deprivation therapy; DXA, dual-energy X-ray absorptiometry; HbA1c, glycated haemoglobin; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III.
Adapted from Cheung et al.64 on behalf of the Endocrine Society of Australia, the Australian and New Zealand Bone and Mineral Society and the Urological Society of Australia and New Zealand.
Bone mineral density, structure and strength
Current guidelines for managing bone health during ADT include several key recommendations.37, 40, 61, 62, 63
First, aBMD and fracture risk assessment should be performed prior to commencing ADT, after the first 2 years of treatment, and beyond this point pending individualised circumstances37, 40, 61 (Table 4). The use of DXA aBMD assessment within this population group has previously been deemed a cost-effective strategy to evaluate fracture risk.65 Despite these recommendations, it was reported that only 10.2% of a cohort of 28 960 men were referred to a DXA aBMD assessment prior to commencing ADT or within the first year of treatment.66
Table 4. Recommendations for managing adverse effects of ADT in men with prostate cancer on bone health, lean tissue mass and fat mass.
| Densitometry |
| Prior to commencing ADT, men should undergo a DXA scan for the assessment of hip and spine bone mineral density and a total body scan for the assessment of total and appendicular lean tissue mass and fat mass. These measures should be repeated yearly until the cessation of ADT. |
| Antiresorptive therapy |
| Clinicians should consider antiresorptive therapy if men: (1) experience a minimal trauma fracture, (2) have a hip and/or lumbar spine DXA-assessed bone mineral density T-score of <2.0 or (3) have a 10-year absolute risk of major osteoporotic fracture of >20% as determined by FRAX (http://www.shef.ac.uk/FRAX). |
| Exercise training |
| It is recommended that clinicians discuss and refer men to an individualised, multi-component exercise program incorporating the elements below: |
| Progressive resistance training: at least twice per week, 8–10 exercises (targeting major muscle groups, specifically the muscle attached to or near the hip and spine), 2–3 sets of 8–10 repetitions at moderate-to-high-intensity (70–85% of 1-RM or 5–8 ‘hard to very hard’ on the 10-point Borg RPE scale). |
| Weight-bearing impact exercises (jumping, bounding, hopping, skipping and bench stepping): at least 4 days per week, 2–4 impact exercises varying in magnitude and direction, progress to 50–100 jumps per session divided into 2–3 sets of 10–20 repetitions. *PRT is recommended first for those with low muscle strength and/or poor muscle function prior to commencing some impact activities. |
| Aerobic exercises: 5–7 days per week, 30 min of continuous, 55–75% of predicted heart rate maximum, modalities including cycling, walking, rowing or sports such as tennis. Aerobic training can be divided into shorter bouts if required (three by 10 min sessions). |
| The concept of specificity and progressive overload should be applied to all exercises and when possible, programs should initially be performed under the supervision of a tertiary-trained exercise professional (for example, an Accredited Exercise Physiologist in Australia). |
| Vitamin D |
| Prior to commencing ADT, men should have their serum 25(OH)D assessed. Men with 25(OH)D levels >50 nmol l−1 (>20 ng ml−1) should consider a daily supplement of 800 IU. Men with <50 nmol l−1 should supplement with 3000–5000 IU per day for at least 6–12 weeks under the guidance of a clinician. |
| Calcium |
| Include 3–4 serves of dairy food each day and if daily calcium intake is below the recommended dietary intake of 1000–1300 mg per day, supplement with 600 mg per day. |
| Protein |
| Daily protein intake of at least 1.2 g kg−1 body weight per day. |
| Consume 25–30 g of high-quality protein with each meal and on exercise training days, within the first few hours post exercise. |
| Tobacco and alcohol |
| Smoking cessation should be considered. |
| Alcohol consumption should be limited to <2 standard drinks per day. |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; ADT, androgen deprivation therapy; DXA, dual-energy X-ray absorptiometry; PRT, progressive resistance training; RM, repetition maximum.
Second, dietary and supplemental calcium and vitamin D are recommended, with recommended intakes varying from 1000–1500 mg per day and 800–2000 IU per day, respectively.37, 40, 62, 63 For men who are vitamin D deficient (serum 25-hydroxyvitamin D (25OHD) <50 nmol l−1), it is recommended that they supplement with 3000–5000 IU per day of vitamin D (pending level of deficiency) for at least 6–12 weeks under the guidance of a clinician.67 These recommendations are in line with a recent review, which concluded that 500–1000 mg calcium and 200–500 IU vitamin D per day was not adequate to prevent bone loss in men treated with ADT.68 In addition, Level 1 evidence from healthy older adults indicates that daily calcium plus vitamin D supplementation of >1000 mg and >800 IU can reduce fracture risk.67 Although it has been reported that an increased calcium intake (or dairy consumption) may be associated with an increased risk of PCa,69 calcium supplementation at doses of <1500 mg per day were not found to be associated with PCa progression.70 Similarly, vitamin D supplementation of 4000 IU per day for 1 year was associated with no adverse effects or PCa progression in men with PCa.71 Although further studies are needed to evaluate the efficacy of calcium and vitamin D on bone health in men with PCa treated with ADT, based on the available evidence it is advised that these men consider supplementation with calcium and vitamin D under the guidance of their urologist and/or general practitioner (Table 4).
Third, for patients who have experienced a minimal trauma fracture have an initial aBMD hip or lumbar spine T-scores of <2.0 or have a 10-year absolute risk of major osteoporotic fracture of >20% as determined by FRAX (http://www.shef.ac.uk/FRAX), antiresorptive therapy is recommended.37, 40, 61, 62, 63 The efficacy of antiresorptive therapy, such as bisphosphonates, has been extensively reviewed and shown to prevent bone loss and fragility fractures in men treated with ADT.72 However, in countries such as Australia, the Pharmaceutical Benefits Scheme only subsidises antiresorptive therapy for secondary prevention for those who have suffered a minimal trauma fracture and primary prevention for those aged over 70 years with T-scores ⩽−2.5 or ⩽−3.0, pending therapy already prescribed. There may be a potential lack of accessibility to these therapies in men treated with ADT in some countries. Furthermore, there are concerns surrounding the overall safety of antiresorptive therapy with adverse effects such as flu-like symptoms, hypercalcaemia and hepatic failure reported in men treated with ADT.73 Finally, as antiresorptive therapy has no effect on other key fracture risk factors, such as muscle strength, muscle mass, balance and gait, all of which are associated with an increased risk of falls and fracture, independent of BMD, alternative or adjunct interventions should be made available to patients currently undergoing ADT (Table 4).
Lifestyle measures such as exercise training, smoking cessation and limited alcohol consumption (<2 standard drinks per day) are an alternative to pharmacological intervention and are commonly recommended in men treated with ADT.40, 62 Suggested exercise training modalities include weight-bearing aerobic training (AT; for example, walking), progressive resistance training (PRT) and balance training.40, 62 However, these guidelines provide limited information on the precise exercise prescription variables such as the training intensity, frequency and duration. Despite initial concerns, regular exercise training has been shown to have no effect on PSA or testosterone levels.74 Currently only four studies have examined the role of exercise on skeletal adaptations in men treated with ADT.75, 76, 77, 78 Galvao et al.75 reported that a 20-week PRT program, including two training sessions per week utilising exercises that progressed from two sets at 12-repetition maximum (RM) to four sets at 6-RM, did not improve femoral neck, trochanter or Ward’s triangle aBMD in 10 men (mean age; 70 years) currently treated with ADT (mean duration; 3.1 years). Similarly, the findings from a 16-week PRT program in 28 men (mean age; 66 years) currently treated with ADT (mean duration; 9 months) demonstrated no significant differences in femoral neck, trochanter, total hip and lumbar spine (L1-4) aBMD when compared with men undergoing usual care (n=30).78 In this study, the training program included three sessions per week, with each session including 9 exercises that progressed from two sets of 10 repetitions at 40–50% 1-RM to 1–3 sets at 6–10-RM in a daily undulating periodisation model.78 Consistent with these results, Cormie et al.77 observed no significant change in femoral neck or lumbar spine (L2-4) aBMD, as well as tibia volumetric BMD following a 12-week combined PRT and AT program in 32 men (mean age; 70 years) initiating ADT when compared with usual care (n=31). The PRT component of the exercise program was administered during two sessions per week and included eight exercises progressing from 1–4 sets of 6–12RM.77 In all these studies, the sample sizes were relatively modest and the lack of any beneficial skeletal effects was not unexpected given that the typical bone remodelling cycle takes 6–8 months to complete, and to demonstrate clinically relevant and observable physiological changes, longer-term trials with appropriate sample sizes are needed.
To date, only one trial of adequate duration has assessed the role of exercise training on bone health in men treated with ADT.76 Winters-Stone et al.76 reported that a 12-month targeted PRT and weight-bearing impact loading program did not significantly improve femoral neck, total hip, greater trochanter or lumbar spine (L1-4) aBMD in 29 men (mean age; 70 years) currently treated with ADT (mean duration; 39 months) when compared with an exercise placebo group consisting of stretching (n=22). It was suggested that due to concerns with treatment-induced muscle weakness, frailty and incontinence, a cautious approach was taken towards exercise prescription and this may have not provided an adequate stimulus to elicit improvements in aBMD.76 For example, the program only included one impact exercise (two-footed jumps), performed three times per week, which progressed from 10–50 repetitions and 0–10% body weight (via weighted vests).76 Previous research in 180 healthy and osteopenic older men (age range; 50–79 years) showed that a combination of moderate-to-high-intensity PRT (60–85% 1-RM) with weight-bearing impact exercises (90–180 impact loads) performed three times per week that varied in the type, magnitude and direction of loads was effective at improving femoral neck (1.7%) and lumbar spine (L1-4; 2.1%) aBMD over 12 months compared with non-exercising controls.79 Similarly, a meta-analysis of randomised controlled trials in older adults also found that multi-component exercise programs incorporating moderate-to-high-intensity PRT and weight-bearing impact exercises were most effective for maintaining (or improving) hip and spine aBMD.80 Although further studies are warranted to evaluate the safety and efficacy of targeted, multi-component exercise program on bone health in this susceptible population group, based on the available evidence in healthy and osteopenic older men, it is advised that men treated with ADT be prescribed a similar exercise program under the supervision of an appropriately qualified exercise physiologist or trainer (Table 4).
Lean tissue mass
The guidelines for managing metabolic health during ADT briefly discuss the link between lean tissue mass and insulin resistance, yet do not recommend any interventions specifically intended to preserve or improve muscle health.37, 40, 61, 63 Regular exercise training and/or physical activity are recommended, but greater emphasis is placed on weight management as an outcome, rather than lean tissue mass.37, 40, 61, 63 In healthy older men, there is consistent and compelling evidence demonstrating that PRT is safe and effective for improving muscle mass and strength, with the greatest benefits typically observed with high-intensity training.81 However, in men with PCa treated with ADT, a recent systematic review74 concluded that there was inadequate evidence to produce specific exercise prescription guidelines for this population group, and therefore suggested that these men adopt general guidelines for cancer patients.82, 83, 84, 85 For example, Exercise and Sport Science Australia83 recommend 1–3 PRT sessions per week that include 6–10 exercises targeting different muscle groups, completed at an intensity of 50–80% 1-RM or 6–12-RM in sets of 1–4. A growing body of evidence examining the role of exercise training in men treated with ADT has shown that interventions including at least two PRT sessions per week for 12 weeks or greater can maintain77, 78, 86 or improve87 (0.5–0.7 kg) total body and/or appendicular lean tissue mass. In contrast, no improvement in lean tissue mass was demonstrated following a 24-week AT program, including three sessions per week progressing from 15–45 min at an intensity of 50–75% VO2peak, in 25 men (mean age; 66 years) treated with ADT (mean duration; 106 days).86 Therefore, it is advised that all men treated with ADT be prescribed a moderate-to-high-intensity PRT program, with a specific focus on muscles attached to or near the hip, spine and forearm, as these are the most common fracture sites (Table 4).
Emerging evidence in older adults has also provided a rationale for the potential use of protein supplementation alongside PRT in clinical populations susceptible to muscle loss, such as those treated with ADT.88 Although yet to be examined within this population group, a recent meta-analysis of randomised controlled trials conducted in healthy older adults concluded that protein supplementation combined with PRT may elicit further gains in muscle mass than PRT alone.88 However, the responses appeared to vary according to the type, amount, spread and/or change in protein intake, timing, pattern or distribution of intake and the potential influence of co-ingestion with other nutrients.89 There is a growing body of evidence to suggest that high-quality, rapidly digested, leucine-rich protein sources (for example, whey protein), when compared with other types of protein supplementation (for example, casein and soy), can produce additive or synergistic benefits on post-exercise skeletal muscle protein synthesis and promote increases in muscle mass in older adults.90 Similarly, emerging evidence suggests that 20–40 g of high-quality protein may be required to elicit maximal gains in muscle mass in older adults.91 Moreover, the timing of protein ingestion in relation to the exercise session is also important with early (within 1–4 h) post-PRT consumption of protein reported to be most effective for optimising muscle protein synthesis.91, 92 Due to the lack of randomised controlled trials examining the role of protein supplementation in men treated with ADT, the potential disease-specific risks are unknown, however increased protein intake is commonly shown to be well tolerated and accompanied by no serious adverse events in older population groups.93, 94 Therefore, further evaluation is still required in men treated with ADT to elucidate both the risks and benefits of protein supplementation when used in combination with exercise training as a strategy to optimise muscle health (Table 4).
Fat mass
Most guidelines for managing adverse effects of ADT have placed greater emphasis on weight management, and do not specifically focus on monitoring and preventing the accumulation of fat mass via robust measures such as DXA.37, 40 Although not specifically stated within the current guidelines,37, 40 exercise training is one potential intervention to address the accumulation of fat mass during ADT. However, evidence regarding the efficacy of exercise training in reducing fat mass during ADT is currently mixed.77, 78, 87, 95, 96 In a sample of 32 men (mean age; 70 years) commencing ADT, fat mass was shown to decrease by 0.6 kg following a 12-week-combined PRT and AT program when compared with usual care (n=31).77 Exercise training was conducted twice weekly and involved 20–30 min AT at 70–85% of estimated heart rate maximum and PRT prescribed at 6–12-RM in sets of 1–4.77 Similarly, a 24-week PRT program performed three times per week (1–2 sets of 8–12 repetitions at 60–70% 1-RM) decreased the accumulation of body fat percentage when compared with usual care (+0.4% vs +3.2%), whereas a concurrent AT program also performed three times per week (progressing from 15–45 min at 50–75% of maximal heart rate) of the trial showed no change to fat mass when compared with usual care.86 No change in fat mass was also observed following interventions including PRT performed two or more times per week for 12–52 weeks.78, 87, 96 Despite these negative findings, the lack of change in fat mass may be viewed as beneficial due to the expected accumulation of fat mass during ADT. Although it is clear that further studies are needed to evaluate the effect of various types and dose of exercise training on total body and regional fat in men with PCa treated with ADT, it is recommended that AT be included as part of a multi-component exercise program for men with PCa treated with ADT (Table 4).
Conclusion
Despite ADT improving overall survival in appropriately selected patients, it is accompanied with a range of adverse effects, including negative alterations to BMD, structure and strength, lean tissue mass and fat mass, which may contribute to the increased risk of osteoporosis, falls, fractures and cardiometabolic-related events observed in this population group. Although various guidelines exist for men with PCa treated with ADT, there is a need to implement and evaluate the growing body of evidence supporting the use of lifestyle interventions to ameliorate and manage the adverse effects of ADT. Clinicians should consider referring suitable men to practitioners within the allied health profession (for example, exercise physiologists and dieticians) to ensure these men receive the greatest quality of evidence-based care. Our proposed recommendations are outlined in Table 4. Future trials are required to assess both the risk and benefits of adhering to these guidelines and only then may these be fully incorporated into standard practice for men treated with ADT.
Footnotes
The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011; 59: 61–71. [DOI] [PubMed] [Google Scholar]
- Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol Esp 2011; 35: 565–579. [DOI] [PubMed] [Google Scholar]
- Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer 2010; 17: R305–R315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff R-O et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised rial. Lancet 2002; 360: 103–108. [DOI] [PubMed] [Google Scholar]
- Denham JW, Steigler A, Lamb DS, Joseph D, Mameghan H, Turner S et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol 2005; 6: 841–850. [DOI] [PubMed] [Google Scholar]
- Granfors T, Modig H, Damber J-E, Tomic R. Combined orchiectomy and external radiotherapy versus radiotherapy alone for nonmetastatic prostate cancer with or without pelvic lymph node involvement: a prospective randomized study. J Urol 1998; 159: 2030–2034. [DOI] [PubMed] [Google Scholar]
- Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7: 472–479. [DOI] [PubMed] [Google Scholar]
- Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys 2005; 61: 1285–1290. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004; 292: 821–827. [DOI] [PubMed] [Google Scholar]
- Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola R et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008; 300: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS et al. Fifteen-year survival outcomes following primary androgen-deprivation therapy for localized prostate cancer. JAMA Intern Med 2014; 174: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liede A, Hallett DC, Hope K, Graham A, Arellano J, Shahinian VB. International survey of androgen deprivation therapy (ADT) for non-metastatic prostate cancer in 19 countries. ESMO Open 2016; 1: e000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström I, Gustafsson H, Sjöberg K, Arver S. Changes in bone mineral density differ between gonadotrophin-releasing hormone analogue- and surgically castrated men with prostate cancer: a prospective, controlled, parallel-groupstudy. Scand J Urol Nephrol 2004; 38: 148–152. [DOI] [PubMed] [Google Scholar]
- Berruti A, Dogliotti L, Terrone C, Cerutti S, Isaia G, Tarabuzzi R et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol 2002; 167: 2361–2367. [PubMed] [Google Scholar]
- Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol 2000; 163: 181–186. [PubMed] [Google Scholar]
- Galvão DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 2008; 102: 44–47. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 2005; 90: 6410–6417. [DOI] [PubMed] [Google Scholar]
- Higano C, Shields A, Wood N, Brown J, Tangen C. Bone mineral density in patients with prostate cancer without bone metastases treated with intermittent androgen suppression. Urology 2004; 64: 1182–1186. [DOI] [PubMed] [Google Scholar]
- Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer 2005; 104: 1633–1637. [DOI] [PubMed] [Google Scholar]
- Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol 1999; 161: 1219–1222. [PubMed] [Google Scholar]
- Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GNRH analogs. J Clin Endocrinol Metab 2002; 87: 3656–3661. [DOI] [PubMed] [Google Scholar]
- Morote J, Orsola A, Abascal JM, Planas J, Trilla E, Raventos CX et al. Bone mineral density changes in patients with prostate cancer during the first 2 years of androgen suppression. J Urol 2006; 175: 1679–1683. [DOI] [PubMed] [Google Scholar]
- Preston DM, Torrens JI, Harding P, Howard RS, Duncan WE, McLeod DG. Androgen deprivation in men with prostate cancer is associated with an increased rate of bone loss. Prostate Cancer Prostatic Dis 2002; 5: 304. [DOI] [PubMed] [Google Scholar]
- Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol 2004; 22: 2546–2553. [DOI] [PubMed] [Google Scholar]
- Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology 2004; 63: 742–745. [DOI] [PubMed] [Google Scholar]
- Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002; 87: 599–603. [DOI] [PubMed] [Google Scholar]
- Smith MR, Lee H, McGovern F, Fallon MA, Goode M, Zietman AL et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostatecancer. Cancer 2008; 112: 2188–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006; 91: 1305–1308. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 2001; 86: 4261–4267. [DOI] [PubMed] [Google Scholar]
- Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male 2005; 8: 207–212. [DOI] [PubMed] [Google Scholar]
- Spry NA, Taaffe DR, England PJ, Judge JS, Stephens DA, Peddle-McIntyre C et al. Long-term effects of intermittent androgen suppression therapy on lean and fat mass: a 33-month prospective study. Prostate Cancer Prostatic Dis 2013; 16: 67–72. [DOI] [PubMed] [Google Scholar]
- Bylow K, Dale W, Mustian K, Stadler WM, Rodin M, Hall W et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology 2008; 72: 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai SMH, Duong-Hua M, Cheung AM, Sutradhar R, Warde P, Fleshner NE et al. Fracture types and risk factors in men with prostate cancer on androgen deprivation therapy: a matched cohort study of 19,079 men. J Urol 2010; 184: 918–924. [DOI] [PubMed] [Google Scholar]
- Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005; 352: 154–164. [DOI] [PubMed] [Google Scholar]
- Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 2005; 23: 7897–7903. [DOI] [PubMed] [Google Scholar]
- Saylor P, Keating N, Smith M. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med 2009; 24: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer 2009; 115: 2388–2399. [DOI] [PubMed] [Google Scholar]
- Lee M, Jim HS, Fishman M, Zachariah B, Heysek R, Biagioli M et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psychooncology 2015; 24: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann M, Hamilton EJ, Gilfillan C, Bolton D, Joon DL, Zajac JD. Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust 2011; 194: 301–306. [DOI] [PubMed] [Google Scholar]
- Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl 2012; 14: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PWF et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000; 15: 710–720. [DOI] [PubMed] [Google Scholar]
- Sheu Y, Cauley JA, Wheeler VW, Patrick AL, Bunker CH, Ensrud KE et al. Age-related decline in bone density among ethnically diverse older men. Osteoporosis Int 2011; 22: 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EJ, Ghasem-Zadeh A, Gianatti E, Lim-Joon D, Bolton D, Zebaze R et al. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. J Clin Endocrinol Metab 2010; 95: E456–E463. [DOI] [PubMed] [Google Scholar]
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer 1998; 83: 1561–1566. [PubMed] [Google Scholar]
- Beebe-Dimmer JL, Cetin K, Shahinian V, Morgenstern H, Yee C, Schwartz KL et al. Timing of androgen deprivation therapy use and fracture risk among elderly men with prostate cancer in the United States. Pharmacoepidemiol Drug Saf 2012; 21: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-T, Yang Y-H, Chen P-C, Chen M-F, Chen W-C. Androgen deprivation increases the risk of fracture in prostate cancer patients: a population-based study in Chinese patients. Osteoporosis Int 2015; 26: 2281–2290. [DOI] [PubMed] [Google Scholar]
- Fiorino C, Valdagni R, Rancati T, Sanguineti G. Dose–volume effects for normal tissues in external radiotherapy: pelvis. Radiother Oncol 2009; 93: 153–167. [DOI] [PubMed] [Google Scholar]
- Lassemillante AC, Doi SA, Hooper JD, Prins JB, Wright OR. Prevalence of osteoporosis in prostate cancer survivors II: a meta-analysis of men not on androgen deprivation therapy. Endocrine 2015; 50: 344–354. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001; 56: B209–B217. [DOI] [PubMed] [Google Scholar]
- Sehl ME, Yates FE. Kinetics of human aging: I. rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci 2001; 56: B198–B208. [DOI] [PubMed] [Google Scholar]
- Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TLJ, Ke C et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol 2012; 30: 3271–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudali S, Dobs AS. Effects of testosterone on body composition of the aging male. Mech Ageing Dev 2004; 125: 297–304. [DOI] [PubMed] [Google Scholar]
- Storer TW, Miciek R, Travison TG. Muscle function, physical performance and body composition changes in men with prostate cancer undergoing androgen deprivation therapy. Asian J Androl 2012; 14: 204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol 2011; 74: 377–383. [DOI] [PubMed] [Google Scholar]
- Chang D, Joseph DJ, Ebert MA, Galvão DA, Taaffe DR, Denham JW et al. Effect of androgen deprivation therapy on muscle attenuation in men with prostatecancer. J Med Imaging Radiat Oncol 2014; 58: 223–228. [DOI] [PubMed] [Google Scholar]
- Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 2006; 24: 3979–3983. [DOI] [PubMed] [Google Scholar]
- Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014; 2014: 309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology 2015; 96A: 124–134. [DOI] [PubMed] [Google Scholar]
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol 2015; 22: 100–106. [DOI] [PubMed] [Google Scholar]
- Graham J, Kirkbride P, Cann K, Hasler E, Prettyjohns M. Prostate cancer: summary of updated NICE guidance. BMJ 2014; 348: f7524. [DOI] [PubMed] [Google Scholar]
- Lee CE, Leslie WD, Czaykowski P, Gingerich J, Geirnaert M, Lau YKJ. A comprehensive bone-health management approach for men with prostate cancer receiving androgen deprivation therapy. Curr Oncol 2011; 18: E163–E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015; 67: 825–836. [DOI] [PubMed] [Google Scholar]
- Cheung AS, Pattison D, Bretherton I, Hoermann R, Lim Joon D, Ho E et al. Cardiovascular risk and bone loss in men undergoing androgen deprivation therapy for non-metastatic prostate cancer: implementation of standardized management guidelines. Andrology 2013; 1: 583–589. [DOI] [PubMed] [Google Scholar]
- Ito K, Elkin EB, Girotra M, Morris MJ. Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med 2010; 152: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans AK, Smith MR, O'Malley AJ, Keating NL. Bone density testing among prostate cancer survivors treated with androgen-deprivation therapy. Cancer 2013; 119: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust 2012; 196: 686–687. [DOI] [PubMed] [Google Scholar]
- Datta M, Schwartz GG. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical review. Oncologist 2012; 17: 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015; 100: 87–117. [DOI] [PubMed] [Google Scholar]
- Greenspan SL. Approach to the prostate cancer patient with bone disease. J Clin Endocrinol Metab 2008; 93: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DT, Savage SJ, Garrett-Mayer E, Keane TE, Hollis BW, Horst RL et al. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocr Metab 2012; 97: 2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpa Neto A, Tobias-Machado M, Esteves M, Senra M, Wroclawski M, Fonseca F et al. A systematic review and meta-analysis of bone metabolism in prostate adenocarcinoma. BMC Urol 2010; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpa Neto A, Tobias-Machado M, Esteves MAP, Senra MD, Wroclawski ML, Fonseca FLA et al. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2012; 15: 36–44. [DOI] [PubMed] [Google Scholar]
- Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol 2014; 32: 335–346. [DOI] [PubMed] [Google Scholar]
- Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sport Exerc 2006; 38: 2045–2052. [DOI] [PubMed] [Google Scholar]
- Winters-Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc 2014; 46: 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormie P, Galvão DA, Spry N, Joseph D, Chee R, Taaffe DR et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int 2015; 115: 256–266. [DOI] [PubMed] [Google Scholar]
- Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol 2015; 54: 1805–1813. [DOI] [PubMed] [Google Scholar]
- Kukuljan S, Nowson CA, Bass SL, Sanders K, Nicholson GC, Seibel MJ et al. Effects of a multi-component exercise program and calcium-vitamin-D3-fortified milk on bone mineral density in older men: a randomised controlled trial. Osteoporosis Int 2009; 20: 1241–1251. [DOI] [PubMed] [Google Scholar]
- Marques E, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age 2012; 34: 1493–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports MedicineACSM's Resource Manual for Guidelines for Exercise Testing and Prescription, 6th edn. Lippincott Williams & Wilkins: Philadelphia, USA, 2010. [Google Scholar]
- Campbell A, Stevinson C, Crank H. The BASES expert statement on exercise and cancer survivorship. J Sport Sci 2012; 30: 949–952. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Spence RR, Galvão DA, Newton RU. Australian association for exercise and sport science position stand: optimising cancer outcomes through exercise. J Sci Med Sport 2009; 12: 428–434. [DOI] [PubMed] [Google Scholar]
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012; 62: 242–274. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exerc 2010; 42: 1409–1426. [DOI] [PubMed] [Google Scholar]
- Alberga A, Segal R, Reid R, Scott C, Sigal R, Khandwala F et al. Age and androgen-deprivation therapy on exercise outcomes in men with prostate cancer. Support Care Cancer 2012; 20: 971–981. [DOI] [PubMed] [Google Scholar]
- Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010; 28: 340–347. [DOI] [PubMed] [Google Scholar]
- Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LHT, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med 2014; 45: 245–255. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Rasmussen B. Dietary protein recommendations and the prevention of sarcopenia: protein, amino acid metabolism and therapy. Curr Opin Clin Nutr Metab Care 2009; 12: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf S, Egert S, Heer M. Effects of whey protein supplements on metabolism: evidence from human intervention studies. Curr Opin Clin Nutr Metab Care 2011; 14: 569–580. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Holwerda AM, Phillips SM, van Loon LJC. What is the optimal amount of protein to support post-exercise skeletal muscle reconditioning in the older adult? Sports Med 2016; 46: 1205–1212. [DOI] [PubMed] [Google Scholar]
- Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011; 93: 322–331. [DOI] [PubMed] [Google Scholar]
- Daly RM, O'Connell SL, Mundell NL, Grimes CA, Dunstan DW, Nowson CA. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlledtrial. Am J Clin Nutr 2014; 99: 899–910. [DOI] [PubMed] [Google Scholar]
- Chale A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 2013; 68: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud'Homme DG et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 2009; 27: 344–351. [DOI] [PubMed] [Google Scholar]
- Winters-Stone KM, Dieckmann N, Maddalozzo GF, Bennett JA, Ryan CW, Beer TM. Resistance exercise reduces body fat and insulin during androgen-deprivation therapy for prostate cancer. Oncol Nurs Forum 2015; 42: 348–356. [DOI] [PubMed] [Google Scholar]