Abstract
Background:
Rapid prototyping is an emerging technology that integrates common medical imaging with specialized production mechanisms to create detailed anatomic replicas. 3D-printed models of musculoskeletal anatomy have already proven useful in orthopedics and their applications continue to expand.
Case Description:
We present the case of a 10 year-old female with Down syndrome and left acetabular dysplasia and chronic hip instability who underwent periacetabular osteotomy. A rapid prototyping 3D model was created to better understand the anatomy, counsel the family about the problem and the surgical procedure, as well as guide surgical technique. The intricate detail and size match of the model with the patient’s anatomy offered unparalleled, hands-on experience with the patient’s anatomy pre-operatively and improved surgical precision.
Conclusions:
Our experience with rapid prototyping confirmed its ability to enhance orthopedic care by improving the surgeon’s ability to understand complex anatomy. Additionally, we report a new application utilizing intraoperative fluoroscopic comparison of the model and patient to ensure surgical precision and minimize the risk of complications. This technique could be used in other challenging cases. The increasing availability of rapid prototyping welcomes further use in all areas of orthopedics.
Introduction
Rapid prototyping is an emerging technology that creates detailed anatomic replicas from common medical imaging data. With roots in industrial product development, rapid prototyping was first applied to orthopedics in 1979 with the development of a custom pelvic implant.1 Initial widespread use was limited by cost and availability of production equipment. Recently, the technology has rapidly progressed and cost has greatly decreased. As a result, its use in medicine has increased in the last ten years.2,3
Current rapid prototyping hinges on the integration of medical imaging – computed tomography (CT) or magnetic resonance imaging (MRI) – with highly specialized production mechanisms. One such mechanism is three dimensional (3D) printing, where a life-sized replica is produced out of layered photopolymer-based resin.4 These replicas provide excellent anatomic detail with accuracy to 0.1 mm.2 High-resolution models of the skeletal system provide a unique tactile and visual experience useful in diagnosis, surgical planning, patient communication, and medical education.3 The models can also be used intraoperatively to guide technique and minimize surgical complications.
As rapid prototyping becomes more common in orthopedics, its use will continually evolve. Given the paucity of data on use of 3D models to help direct care in the orthopedic literature, it is important to disseminate helpful clinical experiences with these models. As with the implementation of any new technology, such cooperation can accelerate the learning curve to improve the standard of orthopedic care.
The purpose of this report is to describe our experience with and the benefits of rapid prototyping models in the treatment of orthopedic conditions in pediatric patients. We present a challenging case of a patient with hip instability and left acetabular dysplasia who underwent periacetabular osteotomy (PAO). The 3D model was utilized throughout the treatment process, which included anatomical demonstrations for the family, preoperative planning and simulation of the case, and intraoperative guidance of the surgery. Written informed consent was provided for print and electronic publication of this case report.
Case Report
A 10 year-old female with trisomy 21 presented with chronic bilateral hip instability and multiple prior left hip dislocations that failed non-operative treatment. A PAO and a varus derotational femoral intertrochanteric osteotomy of the left hip were planned for definitive correction.
Pre-operative Evaluation
Radiographs and CT imaging of the pelvis were obtained two weeks prior to surgery (Figure 1). Radiographs showed 80% subluxation of the left hip. The lateral center edge angle (LCEA) was -32 degrees, the anterior center edge angle (ACEA) was 0 degrees, and the acetabular index was 43 degrees. CT rotational evaluation showed that the left acetabulum was anteverted 2 degrees with significant posterior-lateral deficiency and that the left femoral neck was anteverted 30 degrees. A life-sized rapid prototyping 3D model of the patient’s pelvis and left proximal femur was created from the CT imaging data using Slicer 4.1.1 software and a Replicator 3D printer (Makerbot; Brooklyn, NY, USA) (Figure 2). It was composed of acrylonitrile butadiene styrene (ABS) filament. The model was utilized during the preoperative visit to demonstrate the abnormal anatomy to the patient’s family. The family was better able to understand the anatomy and where the osteotomies would be made. Prior to surgery, the osteotomies were templated on the model and the osteotomy fragment was rotated into a position which best stabilized the hip. During this surgical templating it was recognized that the posterior column was particularly narrow and angular (Figure 3) and that this cut may be difficult at surgery. The morning of surgery, the osteotomized and corrected models were shown to the patient’s family so that they would better understand the procedure (Figure 4).
Figure 1.
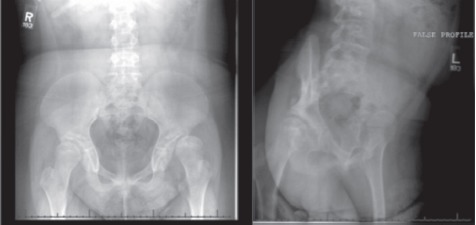
AP pelvis (left) and false profile left hip (right) radiographs demonstrating left hip dysplasia and subluxation.
Figure 2.
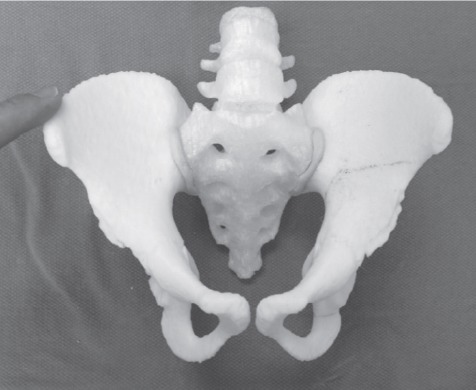
Rapid prototyping 3D model of the patient’s pelvis.
Figure 3.
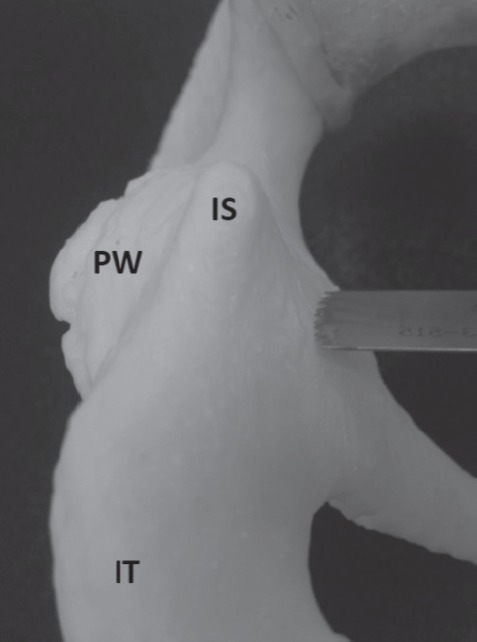
Posterior view of the 3D model of the patient’s pelvis demonstrating the very narrow and triangular shaped posterior column. IS=Ischial spine, PW=Posterior wall of the acetabulum, IT=Ischial tuberosity.
Figure 4.
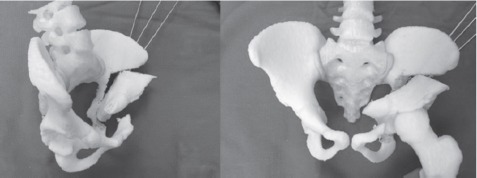
Corrected fragment position after simulation of the PAO on the 3D model.
Procedure
In the operating room prior to surgery, the osteotomized model was examined under fluoroscopy, both independently and while held over the patient’s pelvis to confirm a relative size match (Figure 5). A false profile image was taken of the osteotomized model and a Steinman pin was placed against the posteromedial wall of the acetabulum. This was done to demonstrate that a posterior column cut using that specific angle for the false profile view would ensure that neither the articular surface nor the sciatic notch would be penetrated (Figure 6). The model was then used to guide the iliac and posterior column osteotomies. The angle used for the false profile image of the 3D model was duplicated for the patient’s pelvis by comparing anatomic landmarks, including the distance between the left ischial spine and right pubic eminence and the shape of the obturator foramen. These fluoroscopic landmarks at similar angles optimized the angle of the posterior column osteotomy (Figure 7).
Figure 5.
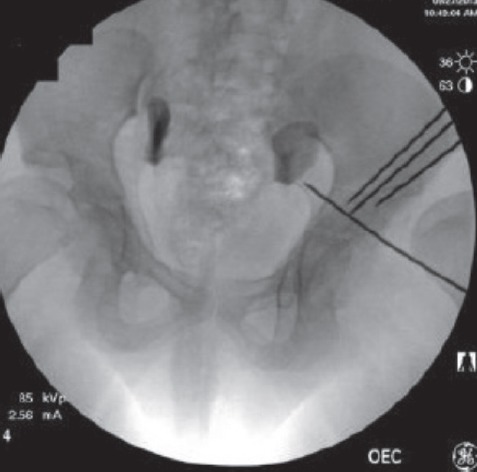
Fluoroscopic image of the model pelvis held over the patient’s pelvis to demonstrate size match. The Steinman pins are in the model.
Figure 6.
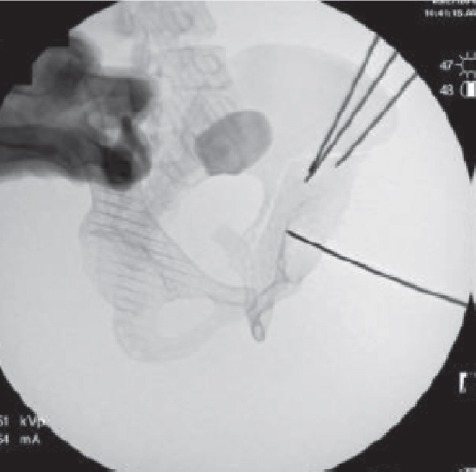
Fluoroscopic false profile image of the model pelvis with Steinman pin held against the posterior-medial wall of the acetabulum. The posterior column cut can be seen between the articular surface and the sciatic notch.
Figure 7.
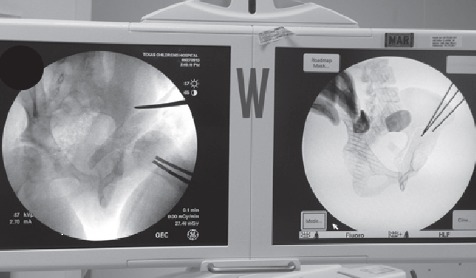
Intraoperative comparison of false profile views of the model (right) and patient’s pelvis (left) used to obtain the correct fluoroscopic view with which to safely make the posterior column osteotomy.
During surgery, the left varus derotational femoral intertrochanteric osteotomy was performed first using standard technique without complication. Following this, the PAO was performed utilizing the direct anterior abductor-sparing approach as described by Murphy et al.5 Comparing fluoroscopic images of the patient’s pelvis with the osteotomized and corrected model pelvis, the fragment was oriented similarly to the position found to allow for maximal hip stability in the 3D model. After internal fixation of the fragment, the hip was stable to full range of motion in all directions. All osteotomies were performed successfully without violation of either the sciatic notch or articular surface.
Post-operative Course
Post-operatively, the patient was placed in a singleleg spica cast due to concerns about adherence to weight bearing restrictions and discharged home on post-operative day four. At three weeks follow-up, radiographs in the cast showed a periprosthetic fracture at the inferior aspect of the femoral fixation plate. She was subsequently brought back to the operating room for fixation of this fracture with a locking proximal humeral plate placed anteriorly as the osteotomy plate had remained stably fixed to the proximal fragment (Figure 8). The hip remained stable in all directions. The rest of her post-operative course was unremarkable. She ambulated independently without pain at six months and demonstrated complete radiographic healing by 10 months (Figure 9). The post-operative LCEA, ACEA and acetabular index measured 25, 25, and 2 degrees respectively at that time. 21 months after her first surgery, the patient underwent the same procedure on the right hip. At 33 months follow-up from the original procedure, her hips remained clinically and radiographically stable with no complications (Figures 10A and B).
Figure 8.
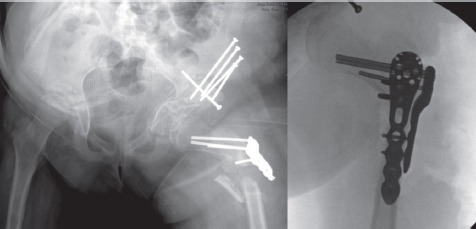
Three week post-operative radiograph showing periprosthetic femur fracture (left). Intra-operative fluoroscopic view of anterior plating of the periprosthetic fracture (right).
Figure 9.
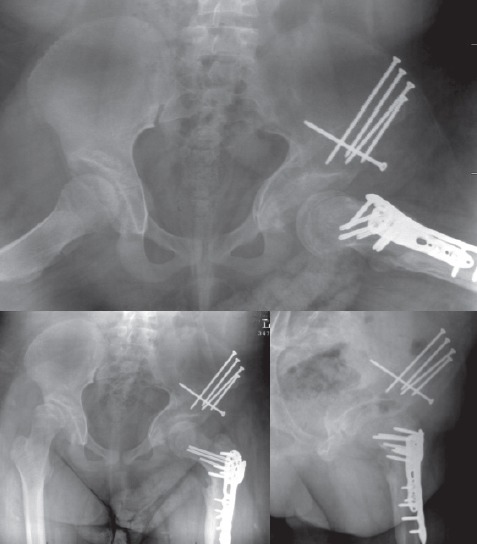
Frog-leg (top), AP (bottom left), and false profile (bottom right) views of the hip 10 months after the index surgery.
Figure 10.
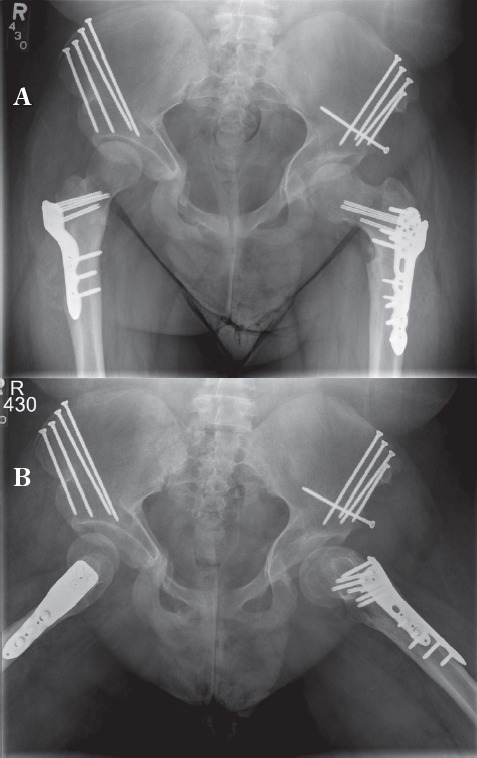
A) AP view of the pelvis 33 months after the index surgery. B) Frog lateral view of the hip 33 months after the index surgery.
Discussion
The use of 3D models in orthopedics has quickly expanded in recent years, likely due to decreased cost and improved quality of the models. Much of the interest and benefits are in patient-specific customization (unique surgical guides, implants, and fracture characterization), improved understanding of complex anatomy, patient communication, and the potential for increased surgical safety. Other benefits include the added advantage of “hands-on” evaluation.6 Reductions in operative time and amount of intra-operative fluoroscopy have also been noted, though we could not assess these findings in our case.7,8
Our case report confirmed many of the above findings. However, this case is unique in that it demonstrates the benefits of 3D printing at each stage of orthopedic surgical care, including its utility in performing a challenging PAO for a patient with global hip instability and unusual anatomy. Rapid prototyping models have been used in orthopedics for peri-operative guidance, but no previous studies have mentioned intra-operative fluoroscopic comparison of the model to the patient’s actual anatomy. This technique, as illustrated in this case report is simple and effective. It requires no additional equipment or training, but provides additional operative guidance above standard imaging. Such a technique may prove useful in cases with three dimensional complexity and limited operative visibility.
The early literature surrounding rapid prototyping in surgery is sparse and dominated by oral and maxillofacial surgery, where complex reconstructions benefit from having an accurate template for planning. Notable applications in this field include pre-bending plates based on 3D models and creating custom implants for craniofacial defects based on the model.9,10 The technology has also been used in neurosurgical education, where simulated surgery on 3D models allowed hands-on instruction with the opportunity to repeat procedures in a low risk setting until comfortable with the new skill.11 Custom-made guiding systems can now be produced from virtual models to optimize surgical approaches and implant positioning.12-15 Other computer-aided surgical techniques, such as navigational markers and patient-specific instrumentation, have enhanced outcomes and can include the use of rapid prototyping 3D models.13,15-17
Clinically, 3D prototyping was beneficial in our case in several ways. It was initially created with the intent to further define the patient’s exact anatomy and understand the area of greatest acetabular deficiency. The model confirmed a globally dysplastic left acetabulum with deficiency most severe posterior-laterally. We were able to perform the osteotomies on the model and determine the amount and direction of correction necessary to result in improved stability of the hip.
Hands-on evaluation of the model demonstrated a uniquely narrow and angular posterior column that could result in increased risk of penetration into the articular surface or the sciatic notch during the posterior column cut of the PAO. The replica’s intricate detail allowed the surgeon to better understand this anatomy and recognize that the standard intra-operative imaging technique might not be sufficient. Utilizing intra-operative fluoroscopy of the model we were able to identify the angle for the false profile image that would provide a view in the plane of the posterior column osteotomy in order to ensure protection of the sciatic notch and the acetabular articular surface.
In addition to clinical implications, 3D prototyping was beneficial in patient/caregiver communication. During the preoperative clinic visit, the model was used to physically demonstrate the instability of the dysplastic hip and explain to the patient and family the cuts that would be made in the bone. This enhanced their understanding of the problem and proposed solution.
In conclusion, we report our positive experience utilizing a 3D printed model in clinical practice to treat a patient with uniquely challenging anatomy. In doing so, we confirm previously reported positive experiences with 3D models in orthopedics. Similarly challenging or atypical cases could benefit from rapid prototyping models in the future. The increasing availability and diverse clinical, educational, and surgical applications of rapid prototyping make it a practical tool for the modern orthopedic surgeon.
Acknowledgment
The authors would like to thank Ifeoma A. Inneh (Director of Research, Baylor College of Medicine/Texas Children’s Hospital Division of Orthopedic Surgery) and Lee S. Haruno (Research Assistant, Texas Children’s Hospital Division of Orthopedic Surgery) for their support and input in the preparation of this manuscript.
SOURCE OF FUNDING
None
References
- 1.Frame M, Huntley JS. Rapid prototyping in orthopaedic surgery: a user’s guide. Scientific World Journal. 2012;2012:838575. doi: 10.1100/2012/838575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Eng. 1997;79:169–74. [PMC free article] [PubMed] [Google Scholar]
- 3.Starosolski ZA, Kan JH, Rosenfeld SD, Krishnamurthy R, Annapragada A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr Radiol. 2014;44(2):216–21. doi: 10.1007/s00247-013-2788-9. [DOI] [PubMed] [Google Scholar]
- 4.Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. Am J Roentgenol. 2011;196(6):W683–8. doi: 10.2214/AJR.10.5681. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SB, Millis MB. Periacetabular osteotomy without abductor dissection using direct anterior exposure. Clin Orthop Relat Res. 1999;364:92–8. doi: 10.1097/00003086-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Guarino J, Tennyson S, McCain G, Bond L, Shea K, King H. Rapid prototyping technology for surgeries of the pediatric spine and pelvis. J Pediatr Orthop. 2007;27(8):955–60. doi: 10.1097/bpo.0b013e3181594ced. [DOI] [PubMed] [Google Scholar]
- 7.Brown GA, Firoozbakhsh K, Decoster TA, Reyna JR, Jr, Moneim M. Rapid prototyping: the future of trauma surgery? J Bone Joint Surg. 2003;85:49–55. [PubMed] [Google Scholar]
- 8.Yang JC, Ma XY, Xia H, W ZH, Ai FZ, Zhang K, Yin QS. Clinical application of computer-aided design-rapid prototyping in C1-C2 operation techniques for complex atlantoaxial instability. J Spinal Disord Tech. 2014;27(4):E143–50. doi: 10.1097/01.bsd.0000450173.95940.ed. [DOI] [PubMed] [Google Scholar]
- 9.Azuma M, Yanagawa T, Ishibashi-Kanno N, Uchida F, Ito T, Yamagata K, Hasegawa S, Sasaki K, Adachi K, Tabuchi K, Sekido M, Bukawa H. Mandibular reconstruction using plates prebent to fit rapid prototyping 3 dimensional printing models ameliorates contour deformity. Head Face Med. 2014;10:45. doi: 10.1186/1746-160X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes da Silva AL, Borba AM, Simao NR, Pedro FL, Borges AH, Miloro M. Customized polymethyl methacrylate implants for the reconstruction of craniofacial osseous defects. Case Rep Surg. 2014. 2014:358569. doi: 10.1155/2014/358569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waran V, Narayanan V, Karuppiah R, Pancharatnam D, Chandran H, Raman R, Rahman ZA, Owen SL, Aziz TZ. Injecting realism in surgical training- initial simulation experience with custom 3D models. J Surg Educ. 2014;71(2):193–7. doi: 10.1016/j.jsurg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Chai W, Xu M, Zhang GQ, Zhang LH, Gou WL, Ni M, Chen JY. Computer-aided design and custom made guide in corrective osteotomy for complex femoral deformity. J Huazhong Univ Sci Technolog Med Sci. 2013;33(3):398–405. doi: 10.1007/s11596-013-1131-x. [DOI] [PubMed] [Google Scholar]
- 13.Iannotti J, Baker J, Rodriguez E, Brems J, Ricchetti E, Mesiha M, Bryan J. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Joint Surg Am. 2014;96(9):e71. doi: 10.2106/JBJS.L.01346. [DOI] [PubMed] [Google Scholar]
- 14.Otsuki B, Takemoto M, Kawanabe K, Awa Y, Akiyama H, Fujibayashi S, Nakamura T, Matsuda S. Developing a novel custom cutting guide for curved peri-acetabular osteotomy. Int Orthop. 2013;37(6):1033–8. doi: 10.1007/s00264-013-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YZ, Lu S, Chen B, Zhao JM, Liu R, Pei GX. Application of computer aided design osteotomy template for treatment of cubitus varus deformity in teenagers: a pilot study. J Shoulder Elbow Surg. 2011;20:51–6. doi: 10.1016/j.jse.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Araujo PH, Moloney G, Rincon G, Carey R, Zhang X, Harner C. Use of a fluoroscopic overlay to guide femoral tunnel placement during posterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(11):2673–9. doi: 10.1177/0363546514549007. [DOI] [PubMed] [Google Scholar]
- 17.Dobbe JGG, Kievit AJ, Schafroth MU, Blankevoort L, Streekstra GJ. Evaluation of a CT-based technique to measure the transfer accuracy of a virtually planned osteotomy. Med Eng Phys. 2014;36:1081–7. doi: 10.1016/j.medengphy.2014.05.012. [DOI] [PubMed] [Google Scholar]


