Abstract
Background
N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) concentrations may be increased in cats with various cardiac disorders. The point‐of‐care (POC) ELISA assay uses the same biologic reagents as the quantitative NT‐proBNP ELISA. Previous studies have evaluated the sensitivity and specificity of the POC ELISA in cats with cardiac disease.
Objectives
To prospectively evaluate the diagnostic utility of the POC ELISA in a select population of cats.
Animals
Thirty‐eight client‐owned cats presented to the University of Florida Cardiology Service for cardiac evaluation. Fifteen apparently healthy cats recruited as part of another study.
Methods
Physical examination and echocardiography were performed in all cats. The POC ELISA was assessed visually as either positive or negative by a reader blinded to the echocardiographic findings, and results were analyzed relative to quantitative assay results.
Results
Twenty‐six cats were diagnosed with underlying cardiac disease, and 27 cats were considered free of cardiac disease. Cats with cardiac disease included: 21 with hypertrophic cardiomyopathy, 2 with unclassified cardiomyopathy, 2 with restrictive cardiomyopathy, and 1 with 3rd degree atrioventricular (AV) block. The POC ELISA differentiated cats with cardiac disease with a sensitivity of 65.4% and specificity of 100%.
Conclusions and Clinical Importance
The POC NT‐proBNP ELISA performed moderately well in a selected population of cats. A negative test result cannot exclude the presence of underlying cardiac disease, and a positive test result indicates that cardiac disease likely is present, but further diagnostic investigation would be indicated for a definitive diagnosis.
Keywords: Biomarker, Feline, Cardiac disease, NT‐proBNP
Abbreviations
- CHF
congestive heart failure
- HCM
hypertrophic cardiomyopathy
- NPV
negative predictive value
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- POC
point‐of‐care
- PPV
positive predictive value
- R/UCM
restrictive/unclassified cardiomyopathy
Echocardiography is currently the clinical gold standard test to diagnose cardiac disease in cats. Use of serum or plasma biomarkers as a means of providing further guidance in detecting underlying heart disease is attractive because of its minimally invasive nature, ease of sampling, widespread availability, quantitative nature, and cost‐effectiveness when compared to echocardiography. Amino‐terminal N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) concentrations are increased in humans, dogs, and cats with various cardiac disorders, and can be detected in circulation using standard laboratory techniques, such as ELISA.1, 2, 3, 4
A quantitative sandwich ELISA1 has been designed to measure the concentration of NT‐proBNP in feline plasma. Several studies have investigated the ability of this assay to distinguish echocardiographically normal cats from those with subclinical cardiomyopathy. These studies have shown that NT‐proBNP concentration can distinguish healthy cats from those with subclinical disease (sensitivity, 70.9–92.4%; specificity, 93.9–100%).1, 5, 6, 7, 8 Plasma NT‐proBNP concentrations >100 pmol/L indicate that morphologic cardiac changes may be present and further cardiac evaluation is warranted. Furthermore, studies evaluating measurement of NT‐proBNP to distinguish cardiogenic from noncardiogenic causes of respiratory clinical signs in cats indicated that NT‐proBNP concentrations >270 pmol/L support congestive heart failure (CHF) as the cause of these clinical signs (sensitivity, 90.2–93.9%; specificity, 87.8–87.9%).9, 10, 11
The quantitative sandwich ELISA assay is performed at a reference laboratory2 and may take 24–48 hours for test results to become available, limiting its usefulness during emergency situations. The NT‐proBNP point‐of‐care (POC) ELISA assay3 was designed to provide results in 10 minutes. The POC assay is a colorimetric ELISA providing results based on the color of the patient sample spot analyzed according to the reference spot. The POC assay results are either normal or abnormal based on a cutoff concentration of approximately 150–200 pmol/L based on manufacturer design.3 In 1 study, the POC assay had a sensitivity and specificity of 83.8 and 82.6%, respectively, in differentiating cats with moderate to severe occult heart disease from adult cats with either no, equivocal or mild cardiac disease based on echocardiography with an overall accuracy of 82.9%.12 The aim of our study was to evaluate the diagnostic utility of the NT‐proBNP POC ELISA in a selected population of cats (healthy and diseased) referred to a tertiary referral hospital. In addition, the performance of the POC ELISA was related to the quantitative NT‐proBNP ELISA.
Materials and Methods
Animals
Client‐owned cats presented to the University of Florida Cardiology Service for cardiac evaluation were prospectively recruited from April 2014 to February 2016. Exclusion criteria included cats <1 year of age and insufficient blood sample volume for both ELISA assays to be performed. Cats with severe concurrent systemic disease based on history, physical examination, and diagnostic testing done at the discretion of the attending clinician also were excluded. Fifteen of the apparently healthy cat samples were obtained as part of another study.13 The study was approved by the University of Florida Institutional Animal Care and Use Committee, and written consent to participate in the study was obtained from owners.
Cardiac Evaluation
A board‐certified cardiologist or a cardiology resident in training under the direct supervision of a board‐certified cardiologist performed all echocardiographic studies with an ultrasound unit equipped with a 5.5–7.5 MHz phased‐array transducer with continuous monitoring of the electrocardiogram.4 Echocardiographic examinations were performed without sedation on cats gently restrained in lateral recumbency to obtain short‐ and long‐axis views. All standard 2D and M‐mode variables were measured according to recommendations set by the American Society of Echocardiography and published methodology in the veterinary literature.14, 15 Cats were considered free of cardiac disease (normal group) if they did not have any color flow or spectral Doppler abnormalities, had normal left ventricular internal diameter in diastole and left ventricular internal diameter in systole, the left ventricular end diastolic caudal wall dimension and interventricular septal thickness at diastole both were <6.0 mm, the left atrium: aorta ratio in short axis (linear dimension) was <1.5, and there was no evidence of arrhythmias on a 6‐lead ECG or auscultation. Cats were diagnosed with various forms of cardiomyopathy (abnormal group) based on criteria described in a previous study13 with the attending cardiologist being responsible for the final classification of disease presence and severity. Cats with cardiac disease were classified as hypertrophic cardiomyopathy (HCM) or restrictive/unclassified cardiomyopathy (R/UCM), or as having noncardiomyopathic forms of cardiac disease (valvular disease, arrhythmias).
Measurement of Plasma NT‐proBNP
Approximately 3 mL of blood was collected from each cat by jugular or medial saphenous venipuncture. Each blood sample was placed in an EDTA tube and centrifuged within 1 hour of collection at 4°C for 10 minute at 604 g. All samples were stored at −80°C until shipped to an external commercial laboratory2 for batch measurement of plasma quantitative and POC NT‐proBNP. The POC ELISA was performed by trained individuals blinded to the echocardiography results. Three drops of EDTA plasma were mixed with 5 drops of assay conjugate in a sample tube and mixed by inversion 3–5 times. The sample was poured onto the POC ELISA sample well and flowed across the device until it reached an indicator window. At this time, the operator activated the device, initiating the wash and color development steps of the ELISA process. After a 10‐minute incubation period, the relative color densities of the patient and reference spots were evaluated by visual inspection. The assay result was based on the color of the sample spot compared to the color of the positive reference spot. Results were recorded as abnormal when the color of the sample spot was equal to or darker than the reference spot or normal when the color of the sample spot was lighter than the reference spot (Fig 1). The remaining plasma sample was used to determine the quantitative NT‐proBNP concentration by a second‐generation commercially available horseradish peroxidase, colorimetric end‐point assay,1 which utilizes the same second‐generation anti‐NT‐proBNP antibodies as does the POC ELISA test. The assay had been previously validated in cats.16
Figure 1.
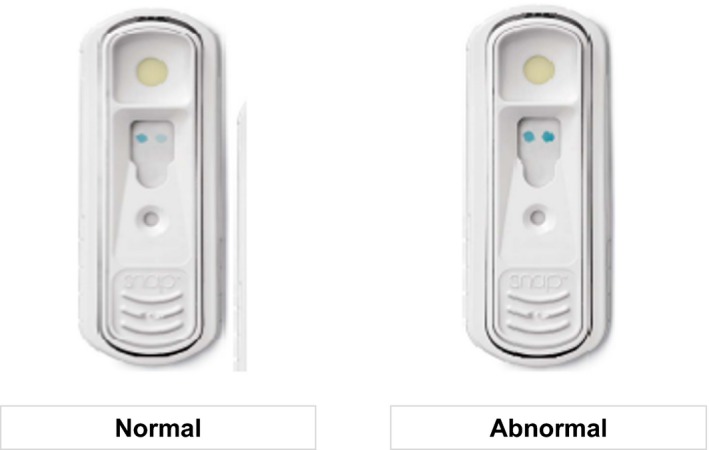
Image of the N‐terminal prohormone of brain natriuretic peptide Point‐of‐Care SNAP ELISA showing normal and abnormal results. Image is courtesy of IDEXX Laboratories, Inc.2
Statistical Analysis
Commercial software was used for statistical analysis5 . A Shapiro‐Wilk test was performed to evaluate normality. Nonparametric data are expressed as median and range. Amino‐terminal proBNP concentrations, age, body weight, and breed were compared between the normal and abnormal groups based on echocardiography results by the Mann‐Whitney rank sum test. In addition, the study population was grouped as normal and abnormal based on the POC ELISA, and NT‐proBNP concentrations were analyzed between the groups using the Mann–Whitney rank sum test. The clinical utility of the POC ELISA and quantitative second‐generation ELISA in differentiating normal from abnormal cats was assessed by calculating the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and negative likelihood ratio. Statistical significance was defined as P < 0.05.
Results
Study Population
The initial study population consisted of 54 cats. One cat was excluded because of insufficient sample quantity to perform all assays. The remaining 53 cats were used for analysis. Thirty‐eight were client‐owned cats that presented to the University of Florida Cardiology Service for cardiac evaluation, and 15 were apparently healthy cats recruited as part of another study.13 There were 3 intact females, 16 spayed females, 2 intact males, and 32 neutered males. The median age was 6 years (range, 1–17 years). The median weight was 5.17 kg (range, 2.8–9.0 kg). Breeds in the study population included 37 domestic shorthairs (DSH), 5 Sphynx, 4 Maine Coon, 2 Bambino, 1 American Bobtail, 1 Bengal, 1 Devon Rex, 1 Persian, and 1 Siamese. Twenty‐six cats (49%) were diagnosed with underlying cardiac disease, and 27 cats (51%) were considered free of cardiac disease. Of the cats with cardiac disease, 21 (80.7%) were diagnosed with HCM, 2 (7.7%) with UCM, 2 (7.7%) with RCM, and 1 (3.9%) with 3rd degree AV block. Three of the cats with cardiac disease (11.5%) were considered to be in CHF at the time of evaluation for the study. There was no significant difference in body weight or breed between the cats with cardiac disease and those without cardiac disease. Cats that were free of cardiac disease (median, 4.5 years; range, 1–16 years) were significantly younger than the cats with cardiac disease (median, 11 years; range, 1.5–17 years; P < 0.001).
Assay Evaluation
The median NT‐proBNP concentration in the abnormal cats (561.5 pmol/L [32–2,077 pmol/L]) was significantly different from normal cats (23 pmol/L [4–94 pmol/L]; P < 0.001; Fig 2). Thirty‐six (67.9%) POC ELISAs were assessed to be normal and 17 (32.1%) as abnormal. An abnormal POC ELISA result was associated with a median NT‐proBNP concentration of 854 pmol/L (204–2,077 pmol/L). A normal POC ELISA result was associated with a median NT‐proBNP concentration of 29 pmol/L (4–173 pmol/L), which was significantly less than positive POC ELISA NT‐proBNP concentrations (P < 0.001; Fig 3).
Figure 2.
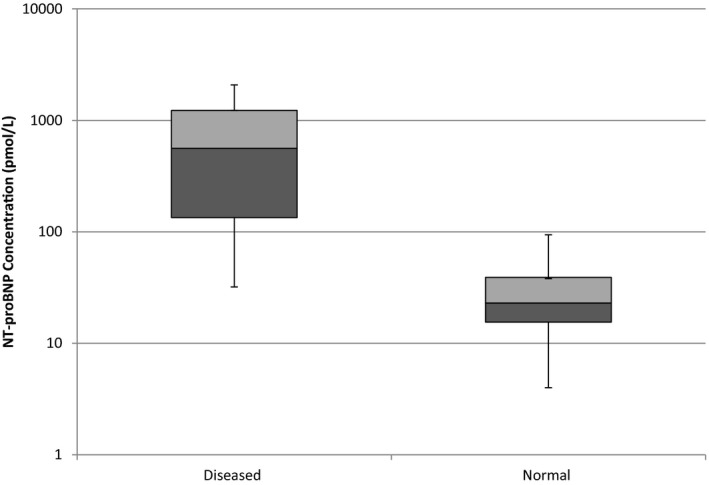
Box and Whisker plot of N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) concentration by a second‐generation NT‐proBNP quantitative ELISA assay in 26 adult cats with cardiac disease and 27 normal adult cats. The boxes encompass the interquartile range, and the line within the box denotes the median value. Note the use of a log scale on the y‐axis.
Figure 3.
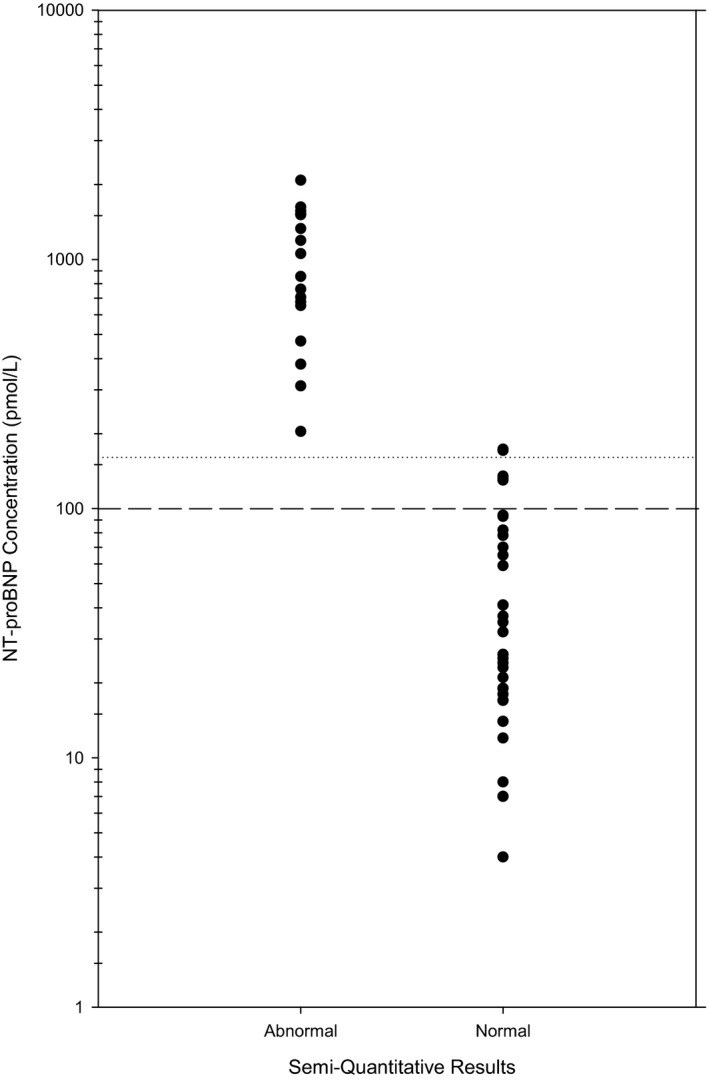
N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) concentration measured by a second‐generation NT‐proBNP plate assay from 36 cats with a negative visual assessment of a point‐of‐care NT‐proBNP SNAP ELISA and from 17 cats with a positive visual assessment. The dashed line represents the upper end of the reference interval for the NT‐proBNP plate assay in cats (100 pmol/L). The doted line represents the lower end of the range at which the SNAP ELISA is expected to turn abnormal (150 pmol/L). Note the use of a log scale on the y‐axis.
The POC ELISA differentiated abnormal cats from normal cats with a sensitivity of 65.4% and specificity of 100% and a PPV and NPV of 100 and 75%, respectively (Table 1). The negative likelihood ratio was 0.35. The POC ELISA resulted in 9 false‐negative results with median NT‐proBNP concentration of 130 pmol/L (range, 32–173 pmol/L). The cats with false‐negative results were between 1.5 and 15 years of age and included 7 DSH, 1 Maine Coon, and 1 Siamese. Eight of the cats with false‐negative POC results were diagnosed with HCM, and one was diagnosed with RCM, consistent with the previous study.12 The quantitative plate ELISA assay differentiated abnormal cats from normal cats using a 100 pmol/L cutoff with a sensitivity of 84.6% and specificity of 100% and PPV and NPV of 100 and 87.1%, respectively (Table 1). The negative likelihood ratio was 0.15, indicating a moderate decrease in the likelihood of disease. The quantitative plate ELISA assay had 4 false‐negative results, which also had false‐negative results on the POC ELISA with NT‐proBNP concentrations of 32–82 pmol/L. Three of the cats with false‐negative results were diagnosed with HCM, and 1 was diagnosed with RCM.
Table 1.
Test performance data of visual point‐of‐care (POC) ELISA assay and quantitative ELISA plate assay
| POC ELISA | 95% Confidence Interval | Quantitative ELISA | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||
| Sensitivity (%) | 65.4 | 47% | 84% | 84.6 | 63% | 95% |
| Specificity (%) | 100 | 84% | 100% | 100 | 84% | 100% |
| Positive predicative value (%) | 1 | 77% | 100% | 100 | 81% | 100% |
| Negative predicative value (%) | 75 | 58% | 87% | 87.1 | 68% | 96% |
| Negative likelihood ratio | 0.35 | 0.18 | 0.57 | 0.15 | 0.07 | 0.39 |
Three (33%) of the 9 cats with false‐negative POC ELISA results were diagnosed with CHF at the time of evaluation for this study, and 1 also was diagnosed with an aortic thromboembolus. The diagnosis of CHF was determined by the attending veterinary cardiologist based on the results of diagnostic tests (echocardiogram, with or without thoracic radiographs), response to treatment or both. The NT‐proBNP concentration range for cats in CHF was 82–171 pmol/L. One of these cats with a false‐negative POC ELISA result also had a false‐negative result on the quantitative ELISA.
Discussion
The second‐generation quantitative plate ELISA used in our study had sensitivity and specificity (84.6 and 100%) similar to previously reported values (sensitivity, 71–92%; specificity, 78–100%) for both the first‐ and second‐generation quantitative assays.1, 5, 6, 7, 8 The POC ELISA had a lower sensitivity (65.4%) and higher specificity (100%) than previously reported (83.4 and 82.6%).12 The previous study focused on evaluating the ability of the POC ELISA to distinguish cats with moderate or severe cardiac disease from cats with mild or no cardiac disease. The study found that the percentage of cats with positive POC ELISA results increased as the severity of cardiac disease increased (normal [11.3%], equivocal [12.5%], mild [24%], moderate [83.9%], and severe [83.3%]).12 Several other studies have evaluated the ability of the quantitative NT‐proBNP to distinguish among different grades or severity of cardiomyopathy.5, 6 In these studies, cats with severe cardiomyopathy had significantly higher NT‐proBNP concentrations than did those with mild cardiomyopathy, and NT‐proBNP was found to be less accurate at identifying mild grades of disease, with more false‐negative results than false‐positive results.5, 6 However, each of these studies classified cats in different ways making direct comparisons between studies difficult. Currently, no consensus exists among veterinary cardiologists as to how to grade the severity of cardiomyopathy, but cats in CHF are considered to have severe cardiac disease. In our study, 3 (11.5%) of cats with cardiac disease were diagnosed with CHF at the time of evaluation and would be considered to have severe cardiac disease, but further grading of the severity of cardiac disease in the other cats with cardiac disease was not performed. Because grading of severity was not fully evaluated in all cats, it is unknown if a higher percentage of cats with mild cardiomyopathy and lower NT‐proBNP concentrations was present compared to previous studies. This may have resulted in the assay having lower sensitivity than in other studies. It is also possible that the differences in sensitivities are due to chance.
Diagnosing cardiac disease in cats can be challenging, and echocardiography currently is considered the clinical gold standard. This standard was used in our study to classify cats as having cardiac disease or being free of cardiac disease. Echocardiography has some limitations with reported inter‐ and intra‐observer variability of echocardiographic measurements in awake cats between 14.9 and 22.6%, depending on the specific measurement being evaluated.18 It is possible that some of the cats in our study population were misclassified as having cardiac disease, which also may have contributed to the lower sensitivity in our study.
The quantitative plate ELISA had 4 false‐negative results and the POC ELISA had 9 false‐negative results when compared with echocardiography assessment, resulting in differences between sensitivities of the ELISAs. The 5 false‐negative results of the POC ELISA that were considered abnormal on the quantitative ELISA had NT‐proBNP concentrations between 132 and 173 pmol/L with most of the concentrations falling within the range of transition between a negative and positive POC ELISA result (NT‐proBNP concentration of 150–200 pmol/L). This finding is similar to that of a previous study with 6 false‐negative POC results with NT‐proBNP concentrations between 66 and 165 pmol/L.12 The POC ELISA transition zone was designed with a higher cutoff value than the quantitative ELISA (<100 pmol/L) to increase the utility of a normal POC result to exclude moderate or severe occult cardiac disease and to better support differentiation of cardiac versus noncardiac causes of respiratory distress in cats based on previous reports that used a NT‐proBNP cutoff of <270 pmol/L (sensitivity, 90.2–93.9%; specificity, 87.8–87.9%)3.9, 10
Four cats had normal quantitative NT‐proBNP concentrations (<100 pmol/L) but were classified as having cardiac disease based on echocardiography, the current clinical gold standard. As previously discussed, echocardiography has some limitations and some of these cats may have been misclassified as having cardiac disease. It is also possible that some of these cats had mild cardiac disease that could not be detected by echocardiography before increased in NT‐proBNP occurred. If these cats had been followed over time, NT‐proBNP may have become increased with or without concurrent disease progression. No follow‐up was performed on these cats as part of our study, and thus, it is unknown if progression occurred. One of these cats was diagnosed with CHF based on the attending cardiologist's assessment of the patient and diagnostic tests (echocardiogram and thoracic radiographs) and thus was unlikely to be misclassified as having cardiac disease. The reason that this cat had a normal NT‐proBNP concentration (82 pmol/L) is unknown.
Sensitivity and specificity are values that describe test performance in a population and are not directly applicable to any 1 individual, whereas PPV and NPV also take into account prevalence of disease and indicate the probability of a true positive or negative in a single individual. In our study, the POC ELISA had a PPV of 100% and NPV of 75%. The study population included cats with a higher proportion of cardiac disease (49%) compared to what would be expected in the population of cats seen by general veterinary practitioners (14.7%) and may differ from that of other cardiology services.19 The study population consisted of cats that were referred to a cardiologist for further cardiac evaluation based on the presence of heart murmur, suspected cardiomegaly on thoracic radiographs or screening of predisposed breeds for cardiac disease. Prevalence of disease may not be the same from geographic region to region or hospital to hospital making extrapolation of this information to a clinical setting for an individual patient challenging. Using the sensitivity and specificity for the POC ELISA and estimating the prevalence in different populations (10, 20, 30, and 40%), the PPV would be 100% for all and the NPV would be 96, 92, 87, and 81%, respectively. Clinically, a normal POC test result cannot exclude the presence of underlying cardiac disease, and if there is suspicion of cardiac disease based on other findings, further diagnostic tests such as echocardiography may be needed to determine whether cardiac disease is truly present. An abnormal POC test indicates the presence of cardiac disease, but further diagnostic tests such as echocardiography, with or without thoracic radiographs, would be needed to further characterize the type and severity of cardiac disease and if treatment is needed.
Our study had some limitations. The samples were tested using both the quantitative ELISA and the POC ELISA as a batched testing event. A previous study of human plasma NT‐proBNP has shown up to 5 freeze–thaw cycles can be done before degradation of the sample occurs.20 This testing strategy minimized the effect of analytical variation but is unlikely to reflect use of the POC ELISA by veterinarians in the field where the assay would likely be performed shortly after sample collection from each individual cat. To address this limitation, further studies should involve on‐site recruitment and POC testing of cats to more closely reflect the likely clinical scenario when using a POC ELISA. In our study, the diagnosis of heart disease was based on the final opinion of a single‐blinded investigator. Further studies comparing diagnosis based on the opinion of a single‐blinded investigator compared to several investigators providing a consensus opinion diagnosis would be useful in better understanding the impact of a single investigator in the diagnosis of cardiac disease. Inter‐ and intra‐observer variability on echocardiographic measurements in awake cats has been reported to be between 14.9 and 22.6% depending on the specific measurement evaluated, and this variability may impact the diagnosis.18 The presence of hyperthyroidism and chronic kidney disease was not assessed for the cats in our study. These diseases are common comorbidities in geriatric cats that are known to affect measurement of NT‐proBNP.17, 21 The clinical utility of the POC ELISA in the context of these diseases currently is unknown, and further investigation is warranted.
In conclusion, the POC NT‐proBNP ELISA performed moderately well in a selected population of cats that presented to a cardiologist for evaluation. A negative test result cannot exclude the presence of underlying cardiac disease and should be assessed in the context of additional clinical findings. A quantitative test could be performed, which may increase identification of cats with underlying cardiac disease but, if still normal, could not exclude the presence of underlying cardiac disease. A positive test result indicates that cardiac disease is likely present and supports further diagnostic investigation to obtain a definitive diagnosis and assess if treatment is necessary. The value of measuring NT‐proBNP in response to treatment for cardiac disease or for monitoring cats for cardiac disease progression is unknown and potentially may be determined by future studies.
Acknowledgments
Grant support
This study was funded by IDEXX Laboratories, Inc., Westbrook, ME, USA, and an intramural resident research grant at the University of Florida, Gainesville, FL.
Conflict of Interest Declaration
Jancy Hanscom and Celine A. Mainville work for IDEXX Laboratories.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This study was performed at the College of Veterinary Medicine, University of Florida Gainesville, FL.
Footnotes
Cardiopet proBNP, IDEXX Laboratories, Inc., Westbrook, ME
IDEXX Laboratories, Inc.
SNAP Feline proBNP, IDEXX Laboratories Inc.
GE Vivid 7 Dimension, General Electric Company, Fairfield, CT
SigmaPlot Version 13, Systat Software Inc, San Jose, CA
References
- 1. Connolly DJ, Soares Magalhaes RJ, Syme HM, et al. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med 2008;22:96–105. [DOI] [PubMed] [Google Scholar]
- 2. Goetze JP. Biochemistry of pro‐B‐type natriuretic peptide‐derived peptides: The endocrine heart revisited. Clin Chem 2004;50:1503–1510. [DOI] [PubMed] [Google Scholar]
- 3. Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract 2004;34:1105–1126. [DOI] [PubMed] [Google Scholar]
- 4. Roland RJ, van Kimmednade RR, Januzzi JL. The evolution of the natriuretic peptides‐Current application in human and animal medicine. J Vet Cardiol 2009;11:s9–s21. [DOI] [PubMed] [Google Scholar]
- 5. Wess G, Daisenberger P, Mahling M, et al. Utility of measuring plasma N‐terminal pro‐brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol 2011;40:237–244. [DOI] [PubMed] [Google Scholar]
- 6. Fox PPR, Rush JE, Reyonolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐proBNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011;25:1010–1016. [DOI] [PubMed] [Google Scholar]
- 7. Tominaga Y, Miyagawa Y, Toda N, et al. The diagnostic significance of plasma N‐terminal pro‐B‐type natriuretic peptide concentration in asymptomatic cats with cardiac enlargement. J Vet Med Sci 2011;73:971–975. [DOI] [PubMed] [Google Scholar]
- 8. Singh MK, Cocchiaro MF, Kittleson MD. NT‐proBNP measurement fails to reliably identify subclinical hypertrophic cardiomyopathy in Maine Coon cats. J Feline Med Surg 2010;12:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connolly DJ, Soares Magalhaes RJ, Fuentes VL, et al. Assessment of the diagnostic accuracy of circulating natriuretic peptide concentrations to distinguish between cats with cardiac and non‐cardiac causes of respiratory distress. J Vet Cardiol 2009;11(Suppl 1):S41–S50. [DOI] [PubMed] [Google Scholar]
- 10. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009;11(Suppl 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 11. Hassdenteufel E, Henrich E, Hildebrandt N, et al. Assessment of circulating N‐terminal pro B‐type natriuretic peptide concentration to differentiate between cardiac from noncardiac causes of pleural effusion in cats. J Vet Emerg Crit Care 2013;23:416–422. [DOI] [PubMed] [Google Scholar]
- 12. Machen MC, Oyama MA, Gordon SG, et al. Multi‐centered investigation of a point‐of‐care NT‐proBNP ELISA assay to detect moderate to severe occult (pre‐clinical) feline heart disease in cats referred for cardiac evaluation. J Vet Cardiol 2014;16:245–255. [DOI] [PubMed] [Google Scholar]
- 13. Harris AN, Estrada AH, Gallagher AE, et al. Biological variability of N‐terminal pro‐brain natriuretic peptide in adult healthy cats. J Feline Med Surg 2016;19:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petric AD, Rishniw M, Thomas WP. Two‐dimensionally‐guided M‐mode and pulsed wave Doppler echocardiographic evaluation of the ventricles of apparently healthy cats. J Vet Cardiol 2012;14:423–430. [DOI] [PubMed] [Google Scholar]
- 15. Mar‐Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics ASE/EAE consensus statement on methodology and indications. J Am Soc Echocardiogr 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- 16. Mainville CA, Clark GH, Esty KJ, et al. Validation of immunoassay for the quantification of N‐Terminal Pro‐B‐Type natriuretic peptide in feline blood. J Vet Diagn Invest 2015;27:414–421. [DOI] [PubMed] [Google Scholar]
- 17. Menaut P, Connolly DJ, Volk A, et al. Circulating natriuretic peptide concentrations in hyperthyroid cats. J Small Anim Pract 2012;53:673–678. [DOI] [PubMed] [Google Scholar]
- 18. Chetboul V, Concordet D, Pouchelon JL, et al. Effect of inter—and intra‐ observer variability on echocardiographic measurements in awake cats. J Vet Med A Physiol Pathol Clin Med 2003;50:326–331. [DOI] [PubMed] [Google Scholar]
- 19. Payne JR, Brodbelt DC, Fuentes VL. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan Study). J Vet Cardiol 2015;17:S244–S257. [DOI] [PubMed] [Google Scholar]
- 20. Collinson PO, Barnes SC, Gaze DC, et al. Analytical performance of the N terminal pro B type natriuretic peptide (NT‐proBNP) assay on the Elecsys 1010 and 2010 analysers. Eur J Heart Fail 2004;6:365–368. [DOI] [PubMed] [Google Scholar]
- 21. Lalor SM, Connolly DJ, Elliott J, et al. Plasma concentrations of natriuretic peptides in normal cats and normotensive and hypertensive cats with chronic kidney disease. J Vet Cardiol 2009;11(Suppl 1):S71–S79. [DOI] [PubMed] [Google Scholar]


