Abstract
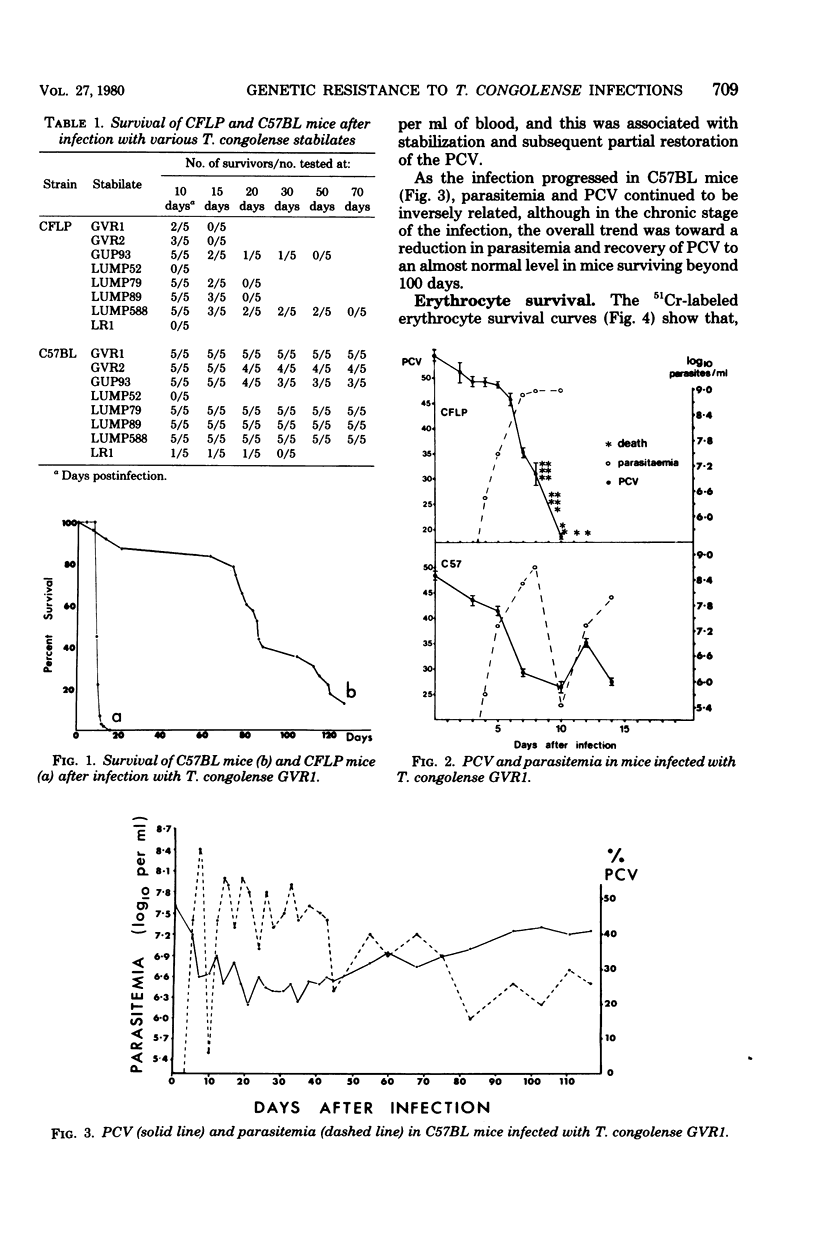
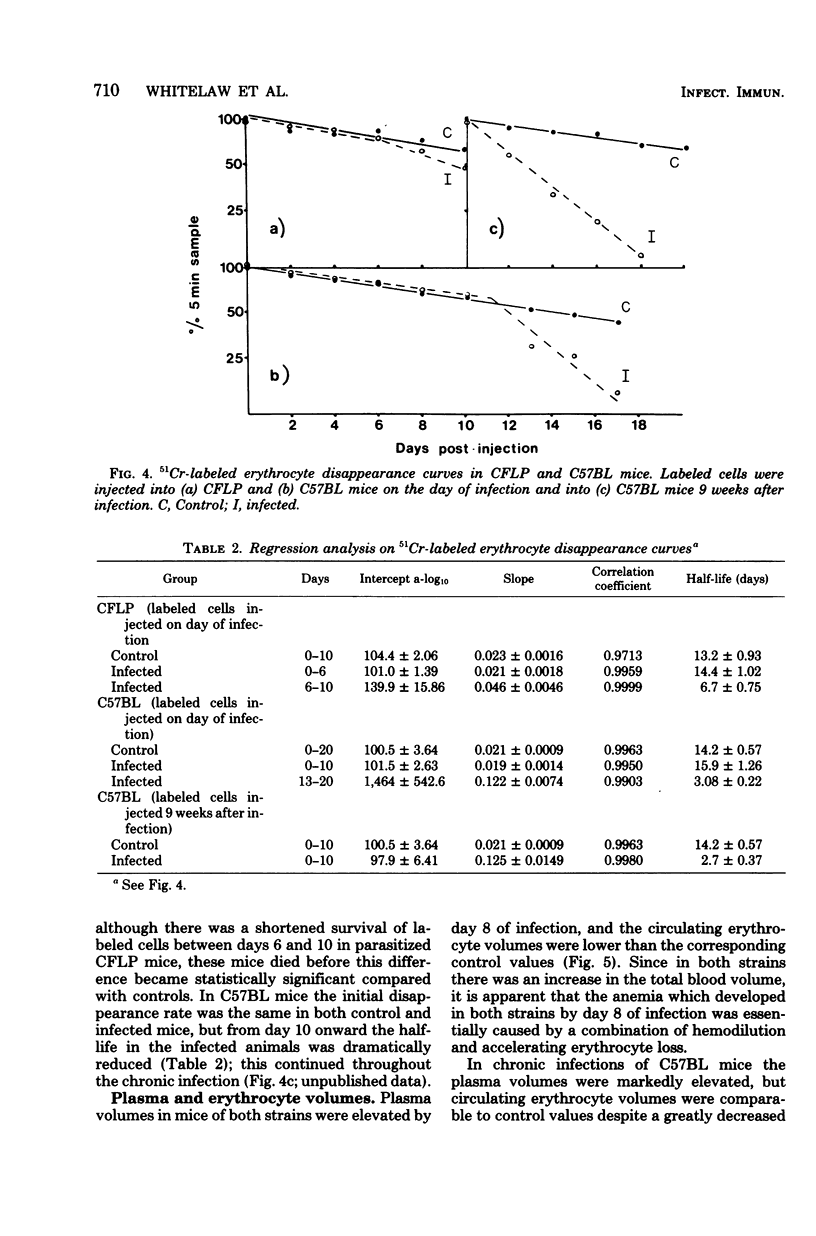
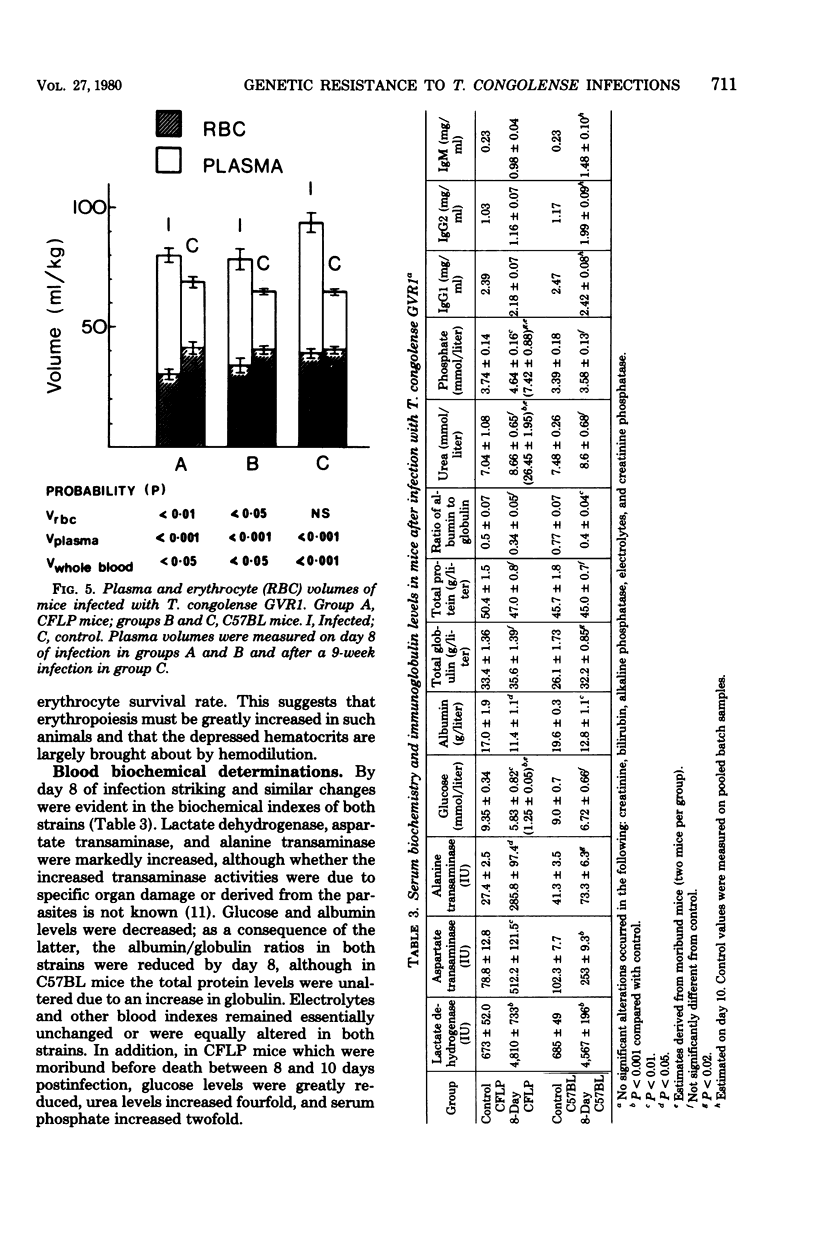
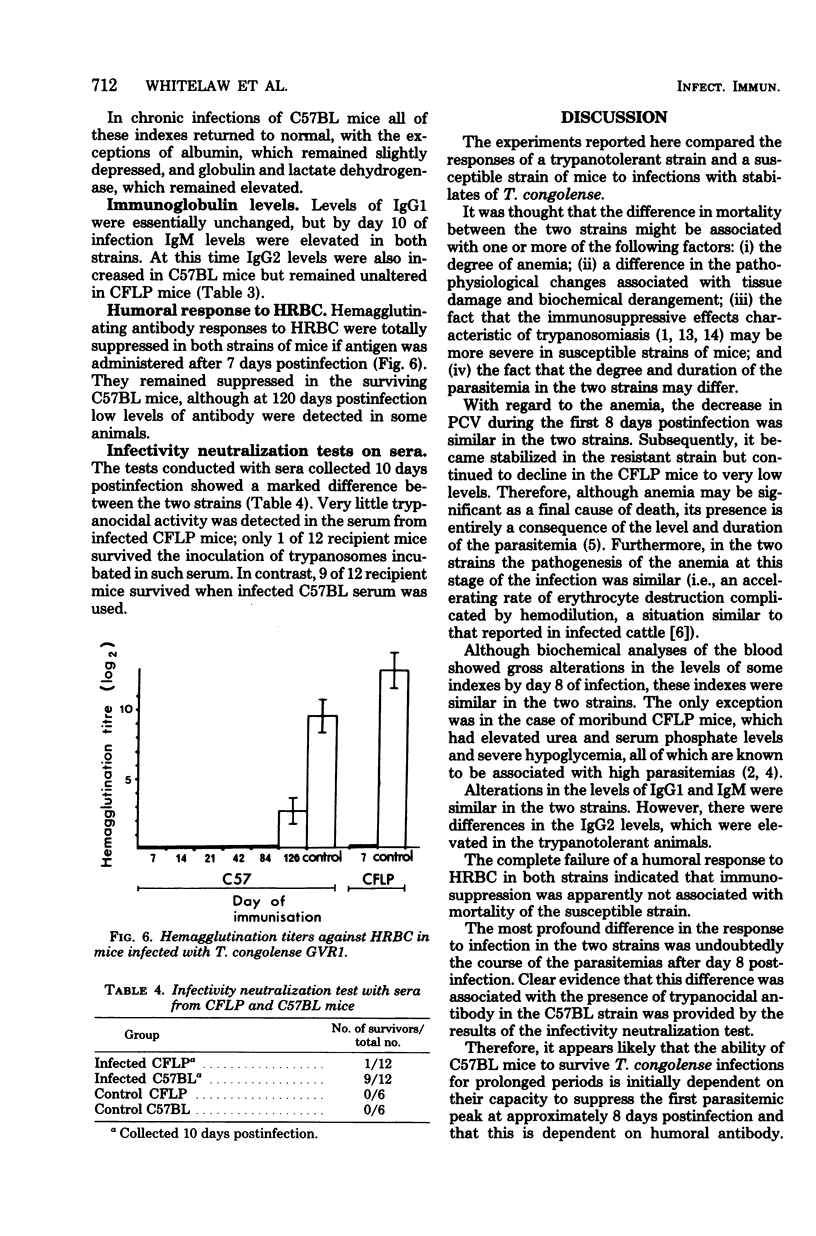
The mechanisms of genetic resistance or "trypanotolerance" to infection with Trypanosoma congolense were investigated in two strains of mice. One strain C57BL, is outstandingly resistant to most stabilates of T. congolense and can survive for over 80 days, whereas CFLP, in common with most other strains, generally succumbs in less than 20 days. Evaluation of several pathophysiological and immunological parameters showed that after infection both strains initially developed similar levels of parasitemia, anemia, biochemical derangement, and immunosuppression. The most outstanding difference was after day 8 postinfection, when the susceptible strain (CFLP) sustained high levels of parasitemia (10(9) trypanosomes per ml) until death 2 to 4 days later, whereas the resistant strain (C57BL) showed a marked decrease to less than 10(6) trypanosomes per ml by day 10 postinfection. Clear evidence that this was associated with the presence of trypanocidal antibody in the resistant mice was provided by the results of an infectivity neutralization test on serum collected from each strain at 10 days postinfection. Chronically infected C57BL mice showed declining waves of parasitemia and a slow restoration of most hematological and biochemical indexes to near normal levels by 80 days postinfection, although at this stage they remained immunosuppressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodwin L. G., Guy M. W. Tissue fluid in rabbits infected with Trypanosoma (Trypanozoon) brucei. Parasitology. 1973 Jun;66(3):499–513. doi: 10.1017/s0031182000046059. [DOI] [PubMed] [Google Scholar]
- Herbert W. J., Lumsden W. H. Trypanosoma brucei: a rapid "matching" method for estimating the host's parasitemia. Exp Parasitol. 1976 Dec;40(3):427–431. doi: 10.1016/0014-4894(76)90110-7. [DOI] [PubMed] [Google Scholar]
- Herbert W. J., Mucklow M. G., Lennox B. The cause of death in acute murine trypanosomiasis. Trans R Soc Trop Med Hyg. 1975;69(1):4–4. [PubMed] [Google Scholar]
- Jennings F. W., Whitelaw D. D., Holmes P. H., Urquhart G. M. The susceptibility of strains of mice to infection with Trypanosoma congolense. Res Vet Sci. 1978 Nov;25(3):399–400. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Moon A. P., Williams J. S., Witherspoon C. Serum biochemical changes in mice infected with Trypanosoma rhodesiense and Trypanosoma duttoni. Exp Parasitol. 1968 Feb;22(1):112–121. doi: 10.1016/0014-4894(68)90084-2. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. I. The role of the macrophage. Immunology. 1974 Nov;27(5):815–824. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Gray A. R. Studies on trypanosome-resistant cattle. II. The effect of trypanosomiasis on N'dama, Muturu and Zebu cattle. Trop Anim Health Prod. 1973 Nov;5(4):220–233. doi: 10.1007/BF02240423. [DOI] [PubMed] [Google Scholar]
- Taylor B. J. Cryostorage in polythene tubing. Trans R Soc Trop Med Hyg. 1972;66(4):544–544. [PubMed] [Google Scholar]


