Summary
Ever since its first application in clinical medicine, scientists have been urged to induce tolerance towards foreign allogeneic transplants and thus avoid rejection by the recipient's immune system. This would circumvent chronic use of immunosuppressive drugs (IS) and thus avoid development of IS‐induced side effects, which are contributing to the still unsatisfactory long‐term graft and patient survival after solid organ transplantation. Although manifold strategies of tolerance induction have been described in preclinical models, only three therapeutic approaches have been utilized successfully in a still small number of patients. These approaches are based on (i) IS withdrawal in spontaneous operational tolerant (SOT) patients, (ii) induction of a mixed chimerism and (iii) adoptive transfer of regulatory cells. Results of clinical trials utilizing these approaches show that tolerance induction does not work in all patients. Thus, there is a need for reliable biomarkers, which can be used for patient selection and post‐therapeutic immune monitoring of safety, success and failure. In this review, we summarize recent achievements in the identification and validation of such immunological assays and biomarkers, focusing mainly on kidney and liver transplantation. From the published findings so far, it has become clear that indicative biomarkers may vary between different therapeutic approaches applied and organs transplanted. Also, patient numbers studied so far are very small. This is the main reason why nearly all described parameters lack validation and reproducibility testing in large clinical trials, and are therefore not yet suitable for clinical practice.
Keywords: B cell, natural killer cells, regulatory T cells, tolerance/suppression/anergy, transplantation
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immune tolerance in transplantation. Clinical and Experimental Immunology 2017, 189: 133–4.
Transplantation tolerance: the big picture. Where do we stand, where should we go? Clinical and Experimental Immunology 2017, 189: 135–7.
Operational tolerance in kidney transplantation and associated biomarkers. Clinical and Experimental Immunology 2017, 189: 138–57.
Transplantation tolerance: don't forget about the B cells. Clinical and Experimental Immunology 2017, 189: 171–80.
Murine models of transplantation tolerance through mixed chimerism: advances and roadblocks. Clinical and Experimental Immunology 2017, 189: 181–9.
Chimerism‐based tolerance in organ transplantation: preclinical and clinical studies. Clinical and Experimental Immunology 2017, 189: 190–6.
Regulatory T cells: tolerance induction in solid organ transplantation. Clinical and Experimental Immunology 2017, 189: 197–210.
Moving away from chronic use of conventional immunosuppression
In the past two decades, considerable improvement in short‐term survival of transplant recipients and grafts has been achieved, mainly due to the contribution of immunosuppressive drugs (IS). However, at the same time, long‐term outcome of these patients has barely improved 1.
Commonly, IS regimens used in solid organ transplantation consist of triple therapy with a calcineurin inhibitor (CNI) or a mechanistic target of rapamycin (mTOR) antagonist, an anti‐metabolite, and corticosteroids 2, 3. Despite being effective in preventing acute rejection episodes, the chronic use of conventional IS is associated with development of severe side effects, such as increased rate of malignancies, infections, promotion of cardiovascular diseases and metabolic disorders such as diabetes 4, resulting from either reduced immune defence or direct toxicity of IS, respectively 5. Together, this contributes to the long‐term morbidity and mortality of transplant recipients 6.
Conversely, insufficient immunosuppression could result in rejection, with chronic rejection being one of the main reasons for late allograft loss 7, 8. Thus, it is essential to balance the therapy in order to protect recipients and the graft from side effects and/or inadequate immunosuppression 1.
To reduce or avoid undesirable effects of immunosuppression, there exist two options: (1) development of less toxic medications and treatment regimens, which are effective in preventing graft rejection without the adverse side effects of conventional IS or (2) induction of tolerance, the holy grail of transplant medicine, where the graft remains functional in the absence of IS, and at the same time the immune system is not altered in its ability to fight off malignancies and infections 9, 10.
Research on new immunomodulatory agents as alternatives to conventional IS drugs continues and some, such as the fusion protein cytotoxic T lymphocyte antigen 4‐immunoglobulin (CTLA4‐Ig), also known as belatacept, seem to be promising alternatives to CNIs 11. In addition, IS minimization over time in patients is an option already applied in clinical routine 12, 13.
Nevertheless, induction of a drug‐free tolerance would be the preferable option, as this would avoid chronic interference with the recipient's immune competence, thus increasing the risk for malignancies and infections. Although several different therapeutic approaches for tolerance induction have been tested in preclinical models, only three have been utilized successfully in a still small number of patients. These approaches are based on (i) IS withdrawal in spontaneous operational tolerant (SOT) patients, (ii) induction of a mixed chimerism and (iii) adoptive transfer of regulatory cells, e.g. regulatory T cells (Tregs) (Fig. 1), and are discussed in the other papers in this issue in more detail. Applying these strategies could not only reduce side effects and thus improve the long‐term graft outcome but also the quality of life of transplant recipients, and the costs of immunosuppressive therapy altogether 14, 15, 16.
Figure 1.
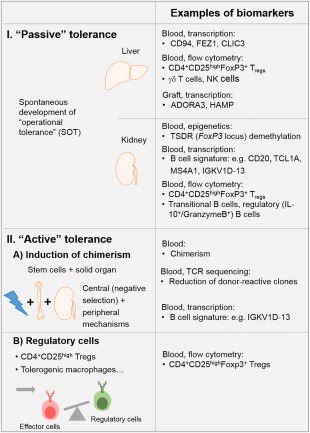
Examples for biomarkers identified upon (1) passive tolerance development in liver and kidney spontaneous operational tolerant (SOT) patients, (2) active tolerance achievement via (a) chimerism induction and (b) regulatory T cell transfer.
Interestingly, as also discussed elsewhere, tolerance occurs occasionally, and has been observed in some patients who had discontinued their IS due to various reasons, e.g. experiencing severe side effects or simply due to non‐compliance 10, 17, 18, 19. Importantly, these patients maintain graft function even long after discontinuation of IS 10, 20. This state is referred to as SOT.
Studies on SOT patients show that they do not have an increased risk for infections or malignancies, and their response to vaccination is similar to that of the healthy population 10, 18, 21, 22. Studying these patients could help further understanding of the mechanisms behind tolerance development and provide valuable knowledge for developing tolerance induction trials.
Cases of SOT in renal transplantation are rare, and it is estimated that approximately 7% of kidney transplant recipients could have developed tolerance 23, 24. In comparison, up to 20% of liver transplant recipients are estimated to have become tolerant, probably due to the immune privileged status of the liver 19, 25, 26. Moreover, in liver transplantation, the risk of graft loss upon withdrawal of medication is lower, as acute rejection episodes can be reversed upon reintroduction of IS 27.
It has been suggested that a fraction of patients with stable graft function who are still on IS have become tolerant 26, 28. These patients, if selected carefully, could profit from weaning/minimization trials. While there have been successful attempts of immunosuppression withdrawal in liver recipients, these trials have proved to be more difficult in renal transplantation 29, 30. In fact, two studies aimed to wean CNIs in small cohorts of stable kidney recipients concluded that CNIs, at present, cannot be withdrawn safely in these patients 31, 32. In addition, withdrawal and minimization trials in renal transplantation pose a greater risk due to the higher incidence of graft loss following rejection and reintroduction of IS 14, 30. This represents a major challenge in approaches aiming to modify IS therapy, and highlights the need for biomarkers as a proof of tolerance, especially as these weaning attempts have not been performed based on the measurement of potential tolerance biomarkers.
It has also to be considered that some IS inhibit the induction of tolerance through their interaction with the immune system as they could interfere with immune mechanisms contributing to the development of tolerance, such as development/expansion of alloantigen‐reactive Tregs 14, 33.
Another tolerance induction strategy, which has been tested successfully in standard preclinical models 34, 35, 36, 37, but also in humanized mouse models 38, 39, 40, involves adoptive transfer of cells with regulatory or tolerogenic function such as Tregs or tolerogenic macrophages.
The first safety and, in part, efficacy testing of such a cell therapy approach has been performed for Tregs in liver transplant recipients 41 and tolerogenic macrophages in patients receiving a kidney transplant 42. The safety and efficacy of adoptive transfer of different regulatory cell products given prior to or shortly after kidney transplantation is currently tested and compared in a multi‐centre study called ‘The ONE Study’.
However, to date, the only approach that has repeatedly shown efficacy in actively promoting drug‐free graft acceptance has been through induction of a chimerism by transferring haematopoietic donor stem cells together with the transplantation of the solid organ graft. This has been shown to result in tolerance induction in liver and kidney transplant recipients 43, 44, 45, 46.
However, with all tested therapeutic strategies, whether it be IS weaning, active tolerance induction by cell therapy or chimerism, it has become clear that it does not work for all patients. Thus, patients have to be selected carefully to avoid unnecessary risks and graft loss. This requires analysis of biomarkers, which predict success or failure of tolerance induction in patients. Most probably such biomarkers will reflect the global and/or donor‐reactive immune status, predisposing them to either high or low likelihood of accepting the foreign graft. For future clinical trials, we thus need to establish accompanying immune monitoring, which with a high specificity and reproducibility identifies patients in whom the therapy will be likely to succeed or fail. In addition, we need biomarkers which help in diagnosing the tolerant state itself.
Within this paper, we will describe what requirements such an immune monitoring would need to fulfil and which parameters have been described that could identify either tolerant patients or those who are likely to develop tolerance.
Principles of immune monitoring
Immune monitoring can be defined as a methodology which assesses immune reactivity by measuring phenotypical, molecular and functional correlates of the immune system, which together serve as a guide for clinical decisions.
Upon transplantation, cell types of the innate and adaptive immune system contribute to the development of tolerance or rejection of the foreign graft. Evidence suggests that the patient's alloresponse depends upon the relative proportion and interaction of inflammatory and anti‐inflammatory subpopulations of these cells 47, 48, 49, 50, 51, 52, 53. This means that, for both the innate immune cell compartment as well as the adaptive immune system, pro‐ and anti‐inflammatory subsets have been described and that the balance between them will most probably determine the outcome after transplantation. Such rejection is promoted by, e.g. donor‐reactive T helper type 1 (Th1) cells, whereas their activity is regulated by, e.g. interleukin (IL)‐10 and transforming growth factor (TGF)‐β‐producing Tregs 54, 55, 56. Similarly, both pathogenic and regulatory functions have been ascribed to B cells, and even plasma cells, but also macrophages 57, 58.
Thus, it will be not sufficient to determine only number, products or function of one cell subset, as is often performed; rather, simultaneous analysis of several immune cell compartments will be required. Furthermore, as composition and function of immune cells are influenced by internal and environmental challenges which might blur immune monitoring results, the creation of repositories from healthy individuals balanced for age and gender are needed 59. This will allow corrections at least for age and gender in the diagnosis of success or failure in tolerance induction.
What makes a biomarker a real biomarker?
The Biomarkers Definitions Working Group defines biomarkers as: ‘A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention’ 60.
In the context of transplantation medicine, biomarkers indicating a wide range of pathogenic processes are imaginable and have been identified. Prior to transplantation, biomarkers assessing organ quality and identifying presensitized patients who have a higher risk for developing acute rejection episodes in the post‐transplant course have been described 61, 62, 63, 64, 65, 66, 67, 68, 69. As already mentioned, there is a need for biomarkers which help us to identify the most suitable patients for enrolment into tolerance induction trials. Such patients may overlap but are not identical with low‐risk patients who are unlikely to develop acute rejection episodes.
In the post‐transplant course, we need biomarkers to monitor the success or failure of conventional IS and tolerance‐inducing therapies, respectively, for IS weaning approaches. Additionally, they are necessary for the surveillance of the general immune competence and monitoring of therapeutic IS levels.
In summary, with the use of biomarkers, IS protocols could be adapted to the patient's individual needs, allowing personalized medicine rather than empirical IS therapy.
As per definition, biomarkers in general and also in transplantation medicine should meet specific criteria (Box 1).
Box 1. Criteria which biomarkers in general and also in transplantation medicine should fulfil in order to be useful and applied in clinical routine.
Highly specific and sensitive for the clinical question addressed
Reproducible and standardized across different centres and laboratories
Measurable in easily accessible sample sources, preferably obtained using non‐invasive methods
Allow repetitive assessment often needed to appraise, e.g. development of tolerance
Measurement should be time‐effective, with clinical decision‐making requiring fast diagnosis
Cost‐effective and thus affordable
There are some challenges in finding biomarkers that would match all these criteria, starting with the identification of the right sampling compartment. To date, acute rejection is diagnosed by performing a histological analysis of a graft biopsy. Although being considered a ‘gold standard’ of rejection diagnosis, this method has some disadvantages, as it is invasive, relatively expensive and ethically questionable to perform, for example, on stable or especially SOT patients 70, 71, 72.
Peripheral blood is an appealing alternative to graft biopsy, as it is less invasive and obtained easily, even for repetitive analyses. Conversely, biomarkers in blood might not always reflect local immune processes controlling graft acceptance or rejection 73. Additionally, in case of renal transplantation, studying the urine has provided valuable information, as it is in direct contact with the graft. Collecting urine also has the advantage of being non‐invasive and less expensive. However, collecting and preparing urine samples requires specific caution and carefully tested procedures, as contained proteins, and especially RNAs, are degraded swiftly.
Examples of already described biomarkers and potential assays
Different methods and techniques have been developed for immune monitoring. As mentioned previously, many cells play a role in alloresponse to a graft, including B and T lymphocytes, dendritic cells, macrophages, natural killer (NK) cells or even granulocytes, all of which occur as inflammatory or anti‐inflammatory subpopulations 74, 75, 76, 77, 78, 79. Assessing the balance between these subpopulations might help to detect tolerance or rejection in patients.
This can be achieved by (1) simply determining the presence of immune cell subsets by, e.g. flow cytometry, genetic or sequencing‐based approaches 80, 81, (2) measuring immune cell products such as cytokines, chemokines or other effector molecules 82, 83, 84, 85, 86, 87 or (3) analysing their functionality and direct measuring of their proliferation, up‐regulation of activation markers or production of cytokines upon donor‐specific restimulation 67, 88, 89, 90, 91.
We will briefly summarize examples for all three possibilities, as follows.
(1) T cells play an important role in response against allogenic tissue through direct or indirect recognition of donor major histocompatibility complex (MHC) I and II alleles 92. It is generally accepted that the balance between donor‐reactive conventional effector and anti‐inflammatory Tregs determines the outcome of the anti‐donor immune response. Indeed, increased frequencies and absolute cell numbers of differentiated effector/memory subsets [e.g. CD45RA+C‐C chemokine receptor type 7 (CCR7)+terminally differentiated effector memory (TEMRA), CD57+] of CD4+ and CD8+ T cells in peripheral blood of kidney and liver transplant patients is associated with development of cellular or even humoral acute rejections 93, 94. In contrast, in some situations higher levels of CD4+CD25highCD127lowforkhead box protein 3 (FoxP3)+ Tregs seem to predispose for tolerance induction 48, 95. Similar observations have been made for other immune cells, such as the B cell lineage or myeloid cells 49, 96, 97, 98.
In addition to flow cytometry‐based assessment of immune cells, other technologies have been applied to follow immune cell subsets. In particular, the recent introduction of next‐generation sequencing (NGS) has been used to trace donor‐ or virus‐reactive conventional effector T cells in biopsies or urine samples 99. Determination of sequences within the complementarity determining region 3 (CDR3) region of T cell receptor (TCR)‐β chains alone or of both α and β chains with subsequent pairing allows identification of individual T cell clones, which can be tracked at any time post‐transplant and within almost any compartment. However, due to the immense T cell repertoire size, with the exception of Treg therapy, this will only be meaningful when combined with assays identifying antigen‐specific T cells (see also below). Thus, one could analyse simultaneously changes in the repertoire of donor‐reactive conventional and Treg cells, which would be an attractive immune monitoring option for tolerance‐inducing strategies based on Treg cell therapy or a mixed chimerism approach (see further below).
(2) Apart from direct measurement of immune cell subsets, quantifying their products such as chemokines, cytokines or antibodies has become a valuable diagnostic opportunity 89, 100, 101, 102. Analysis of donor (MHC)‐specific antibodies (DSA) has been used for decades to guide donor–recipient‐matching in the pretransplant phase 103, 104 or to diagnose humoral rejection episodes, especially after kidney transplantation 105, 106, 107, 108, 109, 110, 111. However, during the last 10 years it has become evident that detection of non‐human leucocyte antigen (HLA) antibodies such as anti‐MHC class I chain‐related protein A (MICA), anti‐angiotensin receptor or anti‐endothelin receptor antibodies have also been associated with the occurrence of humoral rejections and poor outcome 110, 112, 113, 114, 115, 116. Furthermore, associations of elevated concentrations of T and NK cell‐derived effector molecules, such as perforin or granzyme B, at mRNA expression level or shedding of activation markers such as soluble CD30 (sCD30) with cellular rejections have been described 63, 85, 117. In addition, interferon (IFN)‐γ‐induced chemokines such as chemokine (C‐X‐C motif) ligand (CXCL)9 or CXCL10 in serum or urine samples seem to help in predicting or diagnosing cellular rejections 83, 118, 119. Interestingly, measurement of products from anti‐inflammatory immune cell subsets has not been used for pre‐ and post‐transplant immune monitoring.
(3) The earliest approaches to study the functional properties of donor‐reactive immune cells were utilizing mixed lymphocyte reactions (MLR) and assessment of T cell proliferation by [3H]‐thymidine incorporation or labelling with cell proliferation dyes 120, 121, 122. These analyses were performed without distinguishing between the contribution of conventional T cells versus Tregs. Furthermore, they were not designed to assess the functionality of formed memory T cells, which reflects the in vivo situation much more accurately. In contrast, whether donor‐reactive conventional memory T cells are present at elevated numbers, and thus contribute to inflammatory anti‐graft immune responses culminating in acute rejections, can be detected by measuring their cytokine production upon short‐term donor restimulation 67, 123, 124. In contrast to naive T cells, as indicated by the name, memory T cells (due to epigenetic imprinting 125) respond faster and produce cytokines within 24 h of stimulation. Cytokine measurement can be performed using an enzyme‐linked immunospot (ELISPOT) assay or intracellular cytokine staining and flow cytometry. ELISPOT seems to be more sensitive, and has thus been applied frequently by different laboratories 65, 88, 126. In addition to cytokine production‐based quantification of formed donor‐reactive memory T cells, assessment based on up‐regulation of activation marker expression on CD4+ and CD8+ T cells such as CD40L or CD137, respectively, has been described 127, 128.
In analogy, functionality of donor‐reactive Tregs can be determined by performing ex vivo suppression assays 129. Usually, they are designed to measure their ability to block conventional T cell proliferation in MLRs and thus do not reflect the in vivo situation 130. Suppression assays based on prevention of up‐regulation of activation markers such as CD40L appear to be much more promising 128. Instead of measuring their ability to suppress the function of conventional T cells, determining the balance of donor‐reactive Tregs up‐regulating CD137 expression and conventional T cells up‐regulating CD40L is also conceivable 128. This is also attractive when combined with TCR repertoire analysis, as mentioned above.
All these donor‐specific assays require the availability of intact donor cell material, which is logistically easier for live donation but may represent a challenge in case of cadaver donation. Thus, antigen non‐specific functional assays may also need to be incorporated. The only such assay tested frequently in transplantation is the ImmuKnow 131. In this assay, the adenosine triphosphate (ATP) levels produced by CD4+ T cells upon stimulation with a polyclonal mitogen are measured, which reflects their reactivity and, to a degree, immune competence. Although this assay is easy to perform, it is still non‐specific, and results from different clinical studies often contradict its value in predicting rejection 132, 133.
Biomarkers of tolerance and rejection in kidney and liver transplantation
As mentioned earlier, active tolerance induction has been achieved in transplant patients. Immune monitoring on SOT patients was performed in order to define their immune characteristics and to identify biomarkers for prospective IS weaning trials in stable patients (Fig. 1) 134. Due to the rarity of operational tolerance, especially in kidney recipients, this has proved to be difficult. Also, it should be borne in mind that it is ethically challenging to collect biopsies from clinically proven SOT patients so that, in some cases, subclinical rejections or inflammatory responses in a seemingly tolerant patient cannot be excluded. This, in turn, impedes the search for molecular markers of tolerance. Also, it should be noted that tolerance in SOT patients might collapse at a later time‐point 135. Thus, specific signatures of SOT patients should be treated with caution, especially when these signatures are supposed to be used for prospective IS withdrawal trials.
In SOT kidney recipients, tolerance signature seems to be dominated by B cells, as elevated levels of B cell‐related transcripts such as CD20, T cell leukaemia/lymphoma 1A (TCL1A), membrane spanning 4‐domains A1 (MS4A1), immunoglobulin kappa variable 1D‐13 (IGKV1D‐13) 24, 136, 137, 138 in peripheral blood and urine sediments and an overall shift towards naive and transitional B cells and fewer memory B cells 89 have been observed in several clinical trials. Interestingly, this B cell signature also allows differentiation between tolerance development and chronic rejection in preclinical transplant models 139 and is displayed by a proportion of rejection‐free stable patients at 12 months post‐transplant 140. Furthermore, it has also been reported that SOT patients have high numbers of regulatory B cells, which inhibit effector responses by CD4+CD25– effector T cells in a granzyme B‐dependent manner 49. In contrast, differentiation into ‘effector’ B cells and plasma cells secreting DSA is inhibited 89, most probably due to the observed defect of follicular helper T cells 141.
In addition to the shift towards more Breg subsets, SOT kidney transplant recipients have been shown to display higher frequencies of CD4+CD25high (FoxP3+) Tregs in peripheral blood compared to chronically rejecting and stable patients or even healthy controls 47, 48, 52, 142. Further investigations revealed that this elevation is caused by CD45RA‐FoxP3high memory Tregs, which also contribute to the increased demethylation of the Treg‐specific demethylation region (TSDR) within the FoxP3 locus in samples of these patients 75. Also, higher mRNA levels of FoxP3 were found in blood, graft and urine of tolerant patients 138, 143, 144. The immune cell profile from tolerant patients shares similarities to healthy volunteers. For instance, it was shown that SOT patients have CD4+CD25+ Treg, NK, CD8+ and B cell levels similar to those of healthy volunteers 48, 142, 145, 146, 147. Additionally, SOT patients also display conserved signalling pathways, such as signal transduction and activator of transcription (STAT)‐3, GATA binding protein 3 (GATA3), microRNA 142 (mir142)‐3p, TGF‐β and Toll‐like receptor (TLR)‐4/myeloid differentiation primary response gene 88 (MYD88) 52, 70, 138, 146, 147, 148, 149, all of which play a role in the maintenance of the immune response, suggesting that tolerance is a complicated and highly regulated procedure 70.
In contrast, maintenance of tolerance upon liver transplantation seems to involve other mechanisms compared to kidney transplantation, as SOT liver transplant patients were shown to be characterized by increased frequencies of NK cells or γδ T cells 150, 151, 152, 153, which was not found for SOT kidney patients (Fig. 1). Due to the complexity of the immune mechanisms involved, it is highly likely that a set of different biomarkers have to be used in detecting the immunological status of liver and kidney patients. Common biomarkers of rejection have been described for different solid organ transplantations 154, but a mutual ‘signature of tolerance’ in different organs has not been found 155. Thus, the role of cell types that contribute to tolerance seems to differ in liver and kidney transplant recipients. In liver SOT patients, however, increased numbers of CD4+CD25highFoxP3+ Tregs, either in the periphery or the graft, have also been described 156, 157.
With Tregs playing an important role for the induction and maintenance of transplant tolerance, it was only a matter of time until the transfer of induced/expanded Tregs was tested in patients. Upon first encouraging reports in the setting of allogeneic stem cell transplantation 158, trials to test their safety and also efficacy upon solid organ transplantation were initiated. Last year, Todo and colleagues reported on transfer of an ex‐vivo‐enriched Treg product, which was generated by a 2‐week culture of recipient lymphocytes with irradiated donor cells in the presence of anti‐CD80/86 monoclonal antibodies 41. In seven of 10 treated liver transplant patients IS could be withdrawn successfully until 18 months post‐transplant. Although this was not a controlled trial, these results indicate the power of adoptive Treg therapy. Unfortunately, the study was not accompanied with an extensive immune monitoring programme, but the authors detected a slight increase of peripheral Treg numbers upon adoptive therapy (Fig. 1). As described earlier, TCR sequencing should be incorporated into immune monitoring programmes especially upon Treg therapy, as this will allow tracking of transferred T cell clones in even difficult‐to‐access tissue compartments and regardless of ex vivo antigen restimulation. It is foreseeable that successful tolerance induction upon Treg therapy depends upon the longevity and stability of the transferred Tregs. The latter can be determined when combining TCR sequencing with transcriptomics analyses, as described recently 159.
Other trials in liver and kidney transplant patients are ongoing such as, for example, the European Union (EU)‐funded ONE Study. It will be extremely interesting to determine whether Treg therapy can really fulfil its promise and result in similar changes in kidney and liver transplant patients.
The only active tolerance induction approach, which has repeatedly succeeded in IS withdrawal in liver and kidney transplant recipients, is the induction of chimerism by co‐transfer of donor stem cells together with transplantation of the solid organ graft 45, 160. This mechanism clearly aims at utilizing central tolerance mechanisms and hence elimination of donor‐reactive effector T cells. Thus, it is not surprising that utilizing TCR sequencing and tracking of donor‐reactive T cell clones identified prior to transplantation, Megan Sykes’ group could show that successful tolerance induction is accompanied by a deletion of donor‐reactive T cells 161 (Fig. 1). There is still a debate concerning whether persistent chimerism is a biomarker of successful tolerance induction in such patients 162, 163, 164. Interestingly, it has been reported that patients rendered tolerant via chimerism induction also show similar increases in B cell‐related transcripts as do SOT kidney recipients 53. Clearly, further investigations are needed to confirm this relationship.
Limitations of assays and biomarkers
Despite great advances that have been made in the past few years, there are still some challenges ahead before immune monitoring can become feasible for clinical practice. One major problem is that many biomarkers, and especially functional assays, are difficult to standardize, and standardization needs to be ensured before these assays can be used to achieve reliable and comparable results.
Another drawback of most assays is that their performance requires intense laboratory work and settings not available in all clinics. Additionally, many assays are time‐consuming and expensive to perform, and are therefore not applicable to clinical routine. Furthermore, as mentioned before in the case of antigen‐specific assays, donor cell material is required which is not always feasible, e.g. in cases of deceased donations.
It also has to be noted that some validated assays, such as the IFN‐γ ELISPOT, have failed to prove their prediction value in further studies 80, 165. However, this might also be due to differences in immunosuppression regimens among different centres. For instance, a treatment with anti‐thymocyte globulin (ATG) should result in depletion of donor‐reactive memory T cells, and thus prediction of acute rejection episodes is blurred in patients receiving ATG induction therapy. These differences in immunosuppression protocols and other methodical differences should be considered when results of different clinical trials are compared with each another.
Similarly, it has been shown that prior usage of different IS maintenance regimens affects peripheral gene expression in kidney SOT patients 166. This is very important when designing prospective IS weaning trials in stable transplant recipients.
This already indicates the next issue. Most, if not all, of the above‐described biomarkers have been identified by retrospective studies, and as yet only few have been validated in prospective studies. It also has to be noted that many biomarkers have been studied only in small cohorts, and still lack validation in larger cohorts and multi‐centre clinical trials.
Most probably, a single biomarker would not be sufficient to precisely diagnose, for example, successful tolerance induction. Thus, the evaluation of multiple biomarkers would be needed. This needs a greater effort in obtaining, measuring and interpreting the results correctly. Ultimately this will increase the costs and would also require collaboration with bioinformaticians.
Along the same lines, we know that immune cell composition and function changes with age and is different between men and women 59, 167, 168, 169, 170. However, many biomarkers lack validation according to age and gender specificity. This poses a problem, as the proper interpretation of the assays might be hampered if differences in age, gender and ethnicity are not taken into account. Thus, the lack of reliable and validated reference values impedes a correct analysis of the results.
There have already been a few studies which show changes in different immune cell subsets according to age and gender. For example, in a study published by Kverneland et al., an increased proportion of effector and memory CD4+ and CD8+ T cells in older patients and a higher CD4+ : CD8+ ratio in female patients was observed 59. Also, there has been evidence that females have higher peripheral levels of T cells with a demethylated TSDR (Niemann et al., unpublished).
Certainly, further investigations are required to specify age‐ and gender‐dependent variabilities in the immune system in general, and their impact on transplantation outcome in particular.
Conclusion
Despite considerable progress being made in developing tools and techniques for immune monitoring, there is still much to be done before it can become applied routinely in the care for transplant recipients, and especially applied in tolerance induction trials. Figure 2 shows a proposed roadmap for applying immune monitoring in transplant tolerance trials. As outlined above, some promising tolerance biomarker candidates have been identified which seem to vary within the tolerance induction approach. Although tempting, it is not clear whether the differences in identified biomarkers are related to mechanistic differences of the tolerance induction protocol, as not all promising biomarker candidates have been tested in all approaches. Thus, a comparative analysis of several promising candidates within different tolerance induction approaches is needed in future. Regardless of the test, there is an urgent need to obtain results from more patients and also to report on and correct for age‐, gender, IS‐ and environmental‐dependent differences. In particular, we need a better standardization and prospective validation of biomarkers in larger multi‐centre clinical trials, which requires closer collaboration. With more and more immune monitoring data acquired, a clearer understanding of the mechanisms involved during induction and maintenance of tolerance could help in guiding tolerance induction or IS weaning trials in transplant patients. This will help to adapt the therapy to each patient's individual needs.
Figure 2.
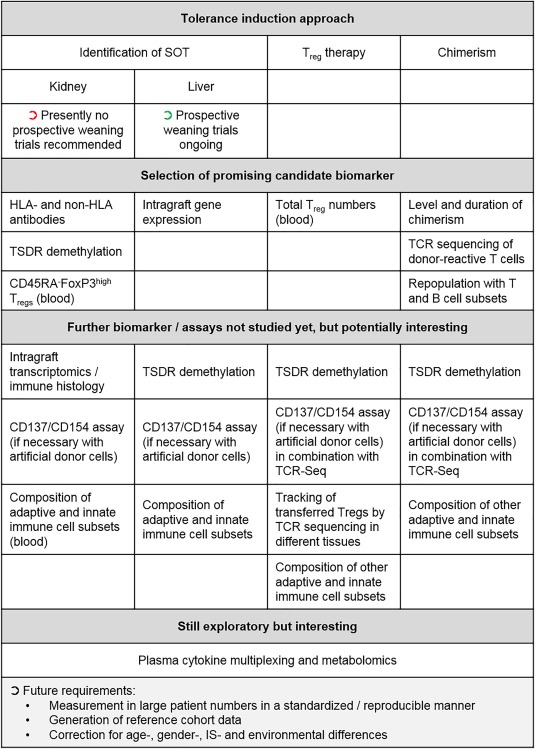
Roadmap for future immune monitoring in tolerance induction approaches based on identification of spontaneous operational tolerant (SOT) patients, regulatory cell transfer (here, focusing upon regulatory T cells) or chimerism. Existing promising biomarkers vary depending on the tolerance induction strategy. Also provided are examples of biomarkers which have not yet been tested rigorously with the indicated tolerance induction approach, but results from other approaches make it worthwhile for testing. For all tests, future requirements to achieve interpretable results are listed at the bottom.
Further investigations are needed to select the most suitable biomarkers for monitoring transplantation patients, and in the end, ease the burden of IS therapies.
Disclosure
The authors have no competing interests to declare.
References
- 1. Lodhi SA, Lamb KE, Meier‐Kriesche HU. Solid organ allograft survival improvement in the United States: the long‐term does not mirror the dramatic short‐term success. Am J Transplant 2011; 11:1226–35. [DOI] [PubMed] [Google Scholar]
- 2. EASL clinical practice guidelines: liver transplantation. J Hepatol 2016; 64:433–85. [DOI] [PubMed] [Google Scholar]
- 3. Kasiske BL, Zeier MG, Chapman JR et al KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 2010; 77:299–311. [DOI] [PubMed] [Google Scholar]
- 4. Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009; 69:2227–43. [DOI] [PubMed] [Google Scholar]
- 5. Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol 2013; 37:602–12. [DOI] [PubMed] [Google Scholar]
- 6. Womer KL, Kaplan B. Recent developments in kidney transplantation – a critical assessment. Am J Transplant 2009; 9:1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Girmanova E, Hruba P, Viklicky O. Circulating biomarkers of tolerance. Transplant Rev 2015; 29:68–72. [DOI] [PubMed] [Google Scholar]
- 8. Sellares J, de Freitas DG, Mengel M et al Understanding the causes of kidney transplant failure: the dominant role of antibody‐mediated rejection and nonadherence. Am J Transplant 2012; 12:388–99. [DOI] [PubMed] [Google Scholar]
- 9. Ashton‐Chess J, Giral M, Soulillou JP, Brouard S. Can immune monitoring help to minimize immunosuppression in kidney transplantation? Transpl Int 2009; 22:110–9. [DOI] [PubMed] [Google Scholar]
- 10. Brouard S, Pallier A, Renaudin K et al The natural history of clinical operational tolerance after kidney transplantation through twenty‐seven cases. Am J Transplant 2012; 12:3296–307. [DOI] [PubMed] [Google Scholar]
- 11. Vincenti F, Rostaing L, Grinyo J et al Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med 2016; 374:333–43. [DOI] [PubMed] [Google Scholar]
- 12. Lerut JP, Pinheiro RS, Lai Q et al Is minimal, [almost] steroid‐free immunosuppression a safe approach in adult liver transplantation? Long‐term outcome of a prospective, double blind, placebo‐controlled, randomized, investigator‐driven study. Ann Surg 2014; 260:886–9; discussion 891–2. [DOI] [PubMed] [Google Scholar]
- 13. Thierry A, Le Meur Y, Ecotiere L et al Minimization of maintenance immunosuppressive therapy after renal transplantation comparing cyclosporine A/azathioprine or cyclosporine A/mycophenolate mofetil bitherapy to cyclosporine A monotherapy: a 10‐year postrandomization follow‐up study. Transpl Int 2016; 29:23–33. [DOI] [PubMed] [Google Scholar]
- 14. Baron D, Giral M, Brouard S. Reconsidering the detection of tolerance to individualize immunosuppression minimization and to improve long‐term kidney graft outcomes. Transpl Int 2015; 28:938–59. [DOI] [PubMed] [Google Scholar]
- 15. Earnshaw SR, Graham CN, Irish WD, Sato R, Schnitzler MA. Lifetime cost‐effectiveness of calcineurin inhibitor withdrawal after de novo renal transplantation. J Am Soc Nephrol 2008; 19:1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karam VH, Gasquet I, Delvart V et al Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation 2003; 76:1699–704. [DOI] [PubMed] [Google Scholar]
- 17. Comerci GD Jr, Williams TM, Kellie S. Immune tolerance after total lymphoid irradiation for heart transplantation: immunosuppressant‐free survival for 8 years. J Heart Lung Transplant 2009; 28:743–5. [DOI] [PubMed] [Google Scholar]
- 18. Massart A, Pallier A, Pascual J et al The DESCARTES–Nantes survey of kidney transplant recipients displaying clinical operational tolerance identifies 35 new tolerant patients and 34 almost tolerant patients. Nephrol Dial Transplant 2016; 31:1002–13. [DOI] [PubMed] [Google Scholar]
- 19. Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatol 2009; 50:1247–57. [DOI] [PubMed] [Google Scholar]
- 20. Starzl TE, Murase N, Demetris AJ et al Lessons of organ‐induced tolerance learned from historical clinical experience. Transplantation 2004; 77:926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ballet C, Roussey‐Kesler G, Aubin JT et al Humoral and cellular responses to influenza vaccination in human recipients naturally tolerant to a kidney allograft. Am J Transplant 2006; 6:2796–801. [DOI] [PubMed] [Google Scholar]
- 22. Roussey‐Kesler G, Giral M, Moreau A et al Clinical operational tolerance after kidney transplantation. Am J Transplant 2006; 6:736–46. [DOI] [PubMed] [Google Scholar]
- 23. Dugast E, Chesneau M, Soulillou JP, Brouard S. Biomarkers and possible mechanisms of operational tolerance in kidney transplant patients. Immunological Reviews 2014; 258(1):208–17. [DOI] [PubMed] [Google Scholar]
- 24. Baron D, Ramstein G, Chesneau M et al A common gene signature across multiple studies relate biomarkers and functional regulation in tolerance to renal allograft. Kidney Int 2015; 87:984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev 2006; 213:101–18. [DOI] [PubMed] [Google Scholar]
- 26. Heidt S, Wood KJ. Biomarkers of operational tolerance in solid organ transplantation. Expert Opin Med Diagn 2012; 6:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alex Bishop G, Bertolino PD, Bowen DG, McCaughan GW. Tolerance in liver transplantation. Best Pract Res Clin Gastroenterol 2012; 26:73–84. [DOI] [PubMed] [Google Scholar]
- 28. Newell KA, Turka LA. Tolerance signatures in transplant recipients. Curr Opin Organ Transplant 2015; 20:400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benitez C, Londono MC, Miquel R et al Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013; 58:1824–35. [DOI] [PubMed] [Google Scholar]
- 30. Mastoridis S, Martinez‐Llordella M, Sanchez‐Fueyo A. Biomarkers and immunopathology of tolerance. Curr Opin Organ Transplant 2016; 21:81–7. [DOI] [PubMed] [Google Scholar]
- 31. Dugast E, Soulillou JP, Foucher Y et al Failure of calcineurin inhibitor (tacrolimus) weaning randomized trial in long‐term stable kidney transplant recipients. Am J Transplant 2016; 16:3255–61. [DOI] [PubMed] [Google Scholar]
- 32. Hricik DE, Formica RN, Nickerson P et al Adverse outcomes of tacrolimus withdrawal in immune‐quiescent kidney transplant recipients. J Am Soc Nephrol 2015; 26:3114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noris M, Casiraghi F, Todeschini M et al Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol 2007; 18:1007–18. [DOI] [PubMed] [Google Scholar]
- 34. Riquelme P, Tomiuk S, Kammler A et al IFN‐gamma‐induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol Ther 2013; 21:409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schliesser U, Chopra M, Beilhack A et al Generation of highly effective and stable murine alloreactive Treg cells by combined anti‐CD4 mAb, TGF‐beta, and RA treatment. Eur J Immunol 2013; 43:3291–305. [DOI] [PubMed] [Google Scholar]
- 36. Schliesser U, Streitz M, Sawitzki B. Tregs: application for solid‐organ transplantation. Curr Opin Organ Transplant 2012; 17:34–41. [DOI] [PubMed] [Google Scholar]
- 37. Siepert A, Ahrlich S, Vogt K et al Permanent CNI treatment for prevention of renal allograft rejection in sensitized hosts can be replaced by regulatory T cells. Am J Transplant 2012; 12:2384–94. [DOI] [PubMed] [Google Scholar]
- 38. Boardman DA, Philippeos C, Fruhwirth GO et al Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am J Transplant 2017; 17:931–43. [DOI] [PubMed] [Google Scholar]
- 39. MacDonald KG, Hoeppli RE, Huang Q et al Alloantigen‐specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest 2016; 126:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noyan F, Zimmermann K, Hardtke‐Wolenski M et al Prevention of allograft rejection by use of regulatory T cells with an MHC‐specific chimeric antigen receptor. Am J Transplant 2017; 17:917–30. [DOI] [PubMed] [Google Scholar]
- 41. Todo S, Yamashita K, Goto R et al A pilot study of operational tolerance with a regulatory T‐cell‐based cell therapy in living donor liver transplantation. Hepatology 2016; 64:632–43. [DOI] [PubMed] [Google Scholar]
- 42. Hutchinson JA, Riquelme P, Sawitzki B et al Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol 2011; 187:2072–8. [DOI] [PubMed] [Google Scholar]
- 43. Kawai T, Sachs DH, Sprangers B et al Long‐term results in recipients of combined HLA‐mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 2014; 14:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim SY, Kim DW, Choi JY et al Full donor chimerism using stem‐cell transplantation for tolerance induction in the human leukocyte antigen‐matched liver transplant setting. Transplantation 2009; 88:601–3. [DOI] [PubMed] [Google Scholar]
- 45. Scandling JD, Busque S, Shizuru JA et al Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant 2015; 15:695–704. [DOI] [PubMed] [Google Scholar]
- 46. Tryphonopoulos P, Tzakis AG, Weppler D et al The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant 2005; 5:608–13. [DOI] [PubMed] [Google Scholar]
- 47. Braudeau C, Racape M, Giral M et al Variation in numbers of CD4+CD25highFOXP3+ T cells with normal immuno‐regulatory properties in long‐term graft outcome. Transpl Int 2007; 20:845–55. [DOI] [PubMed] [Google Scholar]
- 48. Braza F, Dugast E, Panov I et al Central role of CD45RA– Foxp3hi memory regulatory T cells in clinical kidney transplantation tolerance. J Am Soc Nephrol 2015; 26:1795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chesneau M, Michel L, Dugast E et al Tolerant kidney transplant patients produce B cells with regulatory properties. J Am Soc Nephrol 2015; 26:2588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol 2011; 23:252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harmon C, Sanchez‐Fueyo A, O'Farrelly C, Houlihan DD. Natural killer cells and liver transplantation: orchestrators of rejection or tolerance? Am J Transplant 2016; 16:751–7. [DOI] [PubMed] [Google Scholar]
- 52. Moraes‐Vieira PM, Takenaka MC, Silva HM et al GATA3 and a dominant regulatory gene expression profile discriminate operational tolerance in human transplantation. Clin Immunol 2012; 142:117–26. [DOI] [PubMed] [Google Scholar]
- 53. Newell KA, Asare A, Sanz I et al Longitudinal studies of a B cell‐derived signature of tolerance in renal transplant recipients. Am J Transplant 2015; 15:2908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gorbacheva V, Fan R, Wang X, Baldwin WM 3rd, Fairchild RL, Valujskikh A. IFN‐gamma production by memory helper T cells is required for CD40‐independent alloantibody responses. J Immunol 2015; 194:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu F, Dai W, Li C et al Role of IL‐10‐producing regulatory B cells in modulating T‐helper cell immune responses during silica‐induced lung inflammation and fibrosis. Sci Rep 2016; 6:28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trinchieri G. Interleukin‐10 production by effector T cells: Th1 cells show self control. J Exp Med 2007; 204:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fillatreau S. Regulatory plasma cells. Curr Opin Pharmacol 2015; 23:1–5. [DOI] [PubMed] [Google Scholar]
- 58. Salehi S, Reed EF. The divergent roles of macrophages in solid organ transplantation. Curr Opin Organ Transplant 2015; 20:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kverneland AH, Streitz M, Geissler E et al Age and gender leucocytes variances and references values generated using the standardized ONE‐Study protocol. Cytometry A 2016; 89:543–64. [DOI] [PubMed] [Google Scholar]
- 60. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69:89–95. [DOI] [PubMed] [Google Scholar]
- 61. Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre‐transplant IFN‐gamma ELISPOTs are associated with post‐transplant renal function in African American renal transplant recipients. Am J Transplant 2005; 5:1971–5. [DOI] [PubMed] [Google Scholar]
- 62. Caro‐Oleas JL, Gonzalez‐Escribano MF, Gonzalez‐Roncero FM et al Clinical relevance of HLA donor‐specific antibodies detected by single antigen assay in kidney transplantation. Nephrol Dial Transplant 2012; 27:1231–8. [DOI] [PubMed] [Google Scholar]
- 63. Cinti P, Pretagostini R, Arpino A et al Evaluation of pretransplant immunologic status in kidney‐transplant recipients by panel reactive antibody and soluble CD30 determinations. Transplantation 2005; 79:1154–6. [PubMed] [Google Scholar]
- 64. Crespo E, Lucia M, Cruzado JM et al Pre‐transplant donor‐specific T‐cell alloreactivity is strongly associated with early acute cellular rejection in kidney transplant recipients not receiving T‐cell depleting induction therapy. PLOS ONE 2015; 10:e0117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hricik DE, Rodriguez V, Riley J et al Enzyme linked immunosorbent spot (ELISPOT) assay for interferon‐gamma independently predicts renal function in kidney transplant recipients. Am J Transplant 2003; 3:878–84. [DOI] [PubMed] [Google Scholar]
- 66. Lucia M, Luque S, Crespo E et al Preformed circulating HLA‐specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int 2015; 88:874–87. [DOI] [PubMed] [Google Scholar]
- 67. Nickel P, Presber F, Bold G et al Enzyme‐linked immunosorbent spot assay for donor‐reactive interferon‐gamma‐producing cells identifies T‐cell presensitization and correlates with graft function at 6 and 12 months in renal‐transplant recipients. Transplantation 2004; 78:1640–6. [DOI] [PubMed] [Google Scholar]
- 68. Pelzl S, Opelz G, Daniel V, Wiesel M, Susal C. Evaluation of posttransplantation soluble CD30 for diagnosis of acute renal allograft rejection. Transplantation 2003; 75:421–3. [DOI] [PubMed] [Google Scholar]
- 69. Terasaki PI, Ozawa M, Castro R. Four‐year follow‐up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant 2007; 7:408–15. [DOI] [PubMed] [Google Scholar]
- 70. Dugast E, Chesneau M, Soulillou JP, Brouard S. Biomarkers and possible mechanisms of operational tolerance in kidney transplant patients. Immunol Rev 2014; 258:208–17. [DOI] [PubMed] [Google Scholar]
- 71. Haas M. The revised (2013) Banff classification for antibody‐mediated rejection of renal allografts: update, difficulties, and future considerations. Am J Transplant 2016; 16:1352–7. [DOI] [PubMed] [Google Scholar]
- 72. Haas M, Sis B, Racusen LC et al Banff 2013 meeting report: inclusion of C4D‐negative antibody‐mediated rejection and antibody‐associated arterial lesions. Am J Transplant 2014; 14:272–83. [DOI] [PubMed] [Google Scholar]
- 73. Ashton‐Chess J, Dugast E, Colvin RB et al Regulatory, effector, and cytotoxic T cell profiles in long‐term kidney transplant patients. J Am Soc Nephrol 2009; 20:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Assadiasl S, Sepanjnia A, Aghili B et al Natural killer cell subsets and IL‐2, IL‐15, and IL‐18 genes expressions in chronic kidney allograft dysfunction and graft function in kidney allograft recipients. Int J Organ Transplant Med 2016; 7:212–7. [PMC free article] [PubMed] [Google Scholar]
- 75. Braza F, Durand M, Degauque N, Brouard S. Regulatory T cells in kidney transplantation: new directions?. Am J Transplant 2015; 15:2288–300. [DOI] [PubMed] [Google Scholar]
- 76. Kim JI, Rothstein DM, Markmann JF. Role of B cells in tolerance induction. Curr Opin Organ Transplant 2015; 20:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Podesta MA, Cucchiari D, Ponticelli C. The diverging roles of dendritic cells in kidney allotransplantation. Transplant Rev 2015; 29:114–20. [DOI] [PubMed] [Google Scholar]
- 78. Vasu S, Geyer S, Bingman A et al Granulocyte colony‐stimulating factor‐mobilized allografts contain activated immune cell subsets associated with risk of acute and chronic graft‐versus‐host disease. Biol Blood Marrow Transplant 2016; 22:658–68. [DOI] [PubMed] [Google Scholar]
- 79. Weisheit CK, Engel DR, Kurts C. Dendritic cells and macrophages: sentinels in the kidney. Clin J Am Soc Nephrol 2015; 10:1841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. DeWolf S, Shen Y, Sykes M. A new window into the human alloresponse. Transplantation 2016; 100:1639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dieterlen MT, Eberhardt K, Tarnok A, Bittner HB, Barten MJ. Flow cytometry‐based pharmacodynamic monitoring after organ transplantation. Methods Cell Biol 2011; 103:267–84. [DOI] [PubMed] [Google Scholar]
- 82. Ding R, Li B, Muthukumar T et al CD103 mRNA levels in urinary cells predict acute rejection of renal allografts. Transplantation 2003; 75:1307–12. [DOI] [PubMed] [Google Scholar]
- 83. Hricik DE, Nickerson P, Formica RN et al Multicenter validation of urinary CXCL9 as a risk‐stratifying biomarker for kidney transplant injury. Am J Transplant 2013; 13:2634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hu H, Kwun J, Aizenstein BD, Knechtle SJ. Noninvasive detection of acute and chronic injuries in human renal transplant by elevation of multiple cytokines/chemokines in urine. Transplantation 2009; 87:1814–20. [DOI] [PubMed] [Google Scholar]
- 85. Li B, Hartono C, Ding R et al Noninvasive diagnosis of renal‐allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med 2001; 344:947–54. [DOI] [PubMed] [Google Scholar]
- 86. Muthukumar T, Ding R, Dadhania D et al Serine proteinase inhibitor‐9, an endogenous blocker of granzyme B/perforin lytic pathway, is hyperexpressed during acute rejection of renal allografts. Transplantation 2003; 75:1565–70. [DOI] [PubMed] [Google Scholar]
- 87. Tatapudi RR, Muthukumar T, Dadhania D et al Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP‐10 and CXCR3 in urine. Kidney Int 2004; 65:2390–7. [DOI] [PubMed] [Google Scholar]
- 88. Bestard O, Cruzado JM, Lucia M et al Prospective assessment of antidonor cellular alloreactivity is a tool for guidance of immunosuppression in kidney transplantation. Kidney Int 2013; 84:1226–36. [DOI] [PubMed] [Google Scholar]
- 89. Chesneau M, Pallier A, Braza F et al Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant 2014; 14:144–55. [DOI] [PubMed] [Google Scholar]
- 90. Schlickeiser S, Boes D, Streitz M, Sawitzki B. The use of novel diagnostics to individualize immunosuppression following transplantation. Transpl Int 2015; 28:911–20. [DOI] [PubMed] [Google Scholar]
- 91. Sindhi R, Ashokkumar C, Higgs BW et al Allospecific CD154 + T‐cytotoxic memory cells as potential surrogate for rejection risk in pediatric intestine transplantation. Pediatr Transplant 2012; 16:83–91. [DOI] [PubMed] [Google Scholar]
- 92. Sawitzki B, Schlickeiser S, Reinke P, Volk HD. Monitoring tolerance and rejection in organ transplant recipients. Biomarkers 2011; 16:S42–50. [DOI] [PubMed] [Google Scholar]
- 93. Gerlach UA, Vogt K, Schlickeiser S et al Elevation of CD4+ differentiated memory T cells is associated with acute cellular and antibody‐mediated rejection after liver transplantation. Transplantation 2013; 95:1512–20. [DOI] [PubMed] [Google Scholar]
- 94. Yap M, Boeffard F, Clave E et al Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8+ T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol 2014; 25:1856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bestard O, Cunetti L, Cruzado JM et al Intragraft regulatory T cells in protocol biopsies retain foxp3 demethylation and are protective biomarkers for kidney graft outcome. Am J Transplant 2011; 11:2162–72. [DOI] [PubMed] [Google Scholar]
- 96. Dilek N, Poirier N, Usal C, Martinet B, Blancho G, Vanhove B. Control of transplant tolerance and intragraft regulatory T cell localization by myeloid‐derived suppressor cells and CCL5. J Immunol 2012; 188:4209–16. [DOI] [PubMed] [Google Scholar]
- 97. Sumpter TL, Lunz JG III, Castellaneta A et al Dendritic cell immunobiology in relation to liver transplant outcome. Front Biosci 2009; 1:99–114. [DOI] [PubMed] [Google Scholar]
- 98. Tokita D, Mazariegos GV, Zahorchak AF et al High PD‐L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T‐regulatory cells in liver transplant tolerance. Transplantation 2008; 85:369–77. [DOI] [PubMed] [Google Scholar]
- 99. Dziubianau M, Hecht J, Kuchenbecker L et al TCR repertoire analysis by next generation sequencing allows complex differential diagnosis of T cell‐related pathology. Am J Transplant 2013; 13:2842–54. [DOI] [PubMed] [Google Scholar]
- 100. Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ. Elevation of CXCR3‐binding chemokines in urine indicates acute renal‐allograft dysfunction. Am J Transplant 2004; 4:432–7. [DOI] [PubMed] [Google Scholar]
- 101. Kalavrizioti D, Gerolymos M, Rodi M et al T helper (Th)‐cytokines in the urine of patients with primary glomerulonephritis treated with immunosuppressive drugs: can they predict outcome?. Cytokine 2015; 76:260–9. [DOI] [PubMed] [Google Scholar]
- 102. Rotondi M, Netti GS, Lazzeri E et al High pretransplant serum levels of CXCL9 are associated with increased risk of acute rejection and graft failure in kidney graft recipients. Transpl Int 2010; 23:465–75. [DOI] [PubMed] [Google Scholar]
- 103. Farmer DG, Venick RS, Colangelo J et al Pretransplant predictors of survival after intestinal transplantation: analysis of a single‐center experience of more than 100 transplants. Transplantation 2010; 90:1574–80. [DOI] [PubMed] [Google Scholar]
- 104. Gloor JM, Winters JL, Cornell LD et al Baseline donor‐specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant 2010; 10:582–9. [DOI] [PubMed] [Google Scholar]
- 105. de Souza PS, David‐Neto E, Panajotopolous N et al Dynamics of anti‐human leukocyte antigen antibodies after renal transplantation and their impact on graft outcome. Clin Transplant 2014; 28:1234–43. [DOI] [PubMed] [Google Scholar]
- 106. DeVos JM, Patel SJ, Burns K et al De novo donor specific antibodies and patient outcomes in renal transplantation. Clin Transpl 2011; 351–8. [PubMed] [Google Scholar]
- 107. Dieplinger G, Everly MJ, Rebellato LM et al Changes in successive measures of de novo donor‐specific anti‐human leukocyte antigen antibodies intensity and the development of allograft dysfunction. Transplantation 2014; 98:1097–104. [DOI] [PubMed] [Google Scholar]
- 108. Dragun D, Muller DN, Brasen JH et al Angiotensin II type 1‐receptor activating antibodies in renal‐allograft rejection. N Engl J Med 2005; 352:558–69. [DOI] [PubMed] [Google Scholar]
- 109. Everly MJ, Rebellato LM, Haisch CE et al Incidence and impact of de novo donor‐specific alloantibody in primary renal allografts. Transplantation 2013; 95:410–7. [DOI] [PubMed] [Google Scholar]
- 110. Gerlach UA, Lachmann N, Sawitzki B et al Clinical relevance of the de novo production of anti‐HLA antibodies following intestinal and multivisceral transplantation. Transpl Int 2014; 27:280–9. [DOI] [PubMed] [Google Scholar]
- 111. Taniguchi M, Rebellato LM, Cai J et al Higher risk of kidney graft failure in the presence of anti‐angiotensin II type‐1 receptor antibodies. Am J Transplant 2013; 13:2577–89. [DOI] [PubMed] [Google Scholar]
- 112. Filippone EJ, Farber JL. Humoral immune response and allograft function in kidney transplantation. Am J Kidney Dis 2015; 66:337–47. [DOI] [PubMed] [Google Scholar]
- 113. Jackson AM, Sigdel TK, Delville M et al Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol 2015; 26:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Reinsmoen NL, Lai CH, Heidecke H et al Anti‐angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation 2010; 90:1473–7. [DOI] [PubMed] [Google Scholar]
- 115. Yu R, Xu S, Wang Y, Cai H, Xu P. Role of MICA expression, anti‐MICA antibodies and serum MICA during acute rejection in a rat‐to‐mouse cardiac transplantation model. Int J Clin Exp Pathol 2015; 8:14514–20. [PMC free article] [PubMed] [Google Scholar]
- 116. Zou Y, Stastny P, Susal C, Dohler B, Opelz G. Antibodies against MICA antigens and kidney‐transplant rejection. N Engl J Med 2007; 357:1293–300. [DOI] [PubMed] [Google Scholar]
- 117. Susal C, Pelzl S, Dohler B, Opelz G. Identification of highly responsive kidney transplant recipients using pretransplant soluble CD30. J Am Soc Nephrol 2002; 13:1650–6. [DOI] [PubMed] [Google Scholar]
- 118. Jackson JA, Kim EJ, Begley B et al Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant 2011; 11:2228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Schaub S, Nickerson P, Rush D et al Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant 2009; 9:1347–53. [DOI] [PubMed] [Google Scholar]
- 120. Mehrotra A, Leventhal J, Purroy C, Cravedi P. Monitoring T cell alloreactivity. Transplant Rev 2015; 29:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sood S, Testro AG. Immune monitoring post liver transplant. World J Transplant 2014; 4:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Truong DQ, Bourdeaux C, Wieers G, Saussoy P, Latinne D, Reding R. The immunological monitoring of kidney and liver transplants in adult and pediatric recipients. Transpl Immunol 2009; 22:18–27. [DOI] [PubMed] [Google Scholar]
- 123. Bestard O, Nickel P, Cruzado JM et al Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol 2008; 19:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nather BJ, Nickel P, Bold G et al Modified ELISPOT technique – highly significant inverse correlation of post‐Tx donor‐reactive IFNgamma‐producing cell frequencies with 6 and 12 months graft function in kidney transplant recipients. Transpl Immunol 2006; 16:232–7. [DOI] [PubMed] [Google Scholar]
- 125. de Araujo‐Souza PS, Hanschke SC, Viola JP. Epigenetic control of interferon‐gamma expression in CD8 T cells. J Immunol Res 2015; 2015:849573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bestard O, Crespo E, Stein M et al Cross‐validation of IFN‐gamma Elispot assay for measuring alloreactive memory/effector T cell responses in renal transplant recipients. Am J Transplant 2013; 13:1880–90. [DOI] [PubMed] [Google Scholar]
- 127. Litjens NH, de Wit EA, Baan CC, Betjes MG. Activation‐induced CD137 is a fast assay for identification and multi‐parameter flow cytometric analysis of alloreactive T cells. Clin Exp Immunol 2013; 174:179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schoenbrunn A, Frentsch M, Kohler S et al A converse 4–1BB and CD40 ligand expression pattern delineates activated regulatory T cells (Treg) and conventional T cells enabling direct isolation of alloantigen‐reactive natural Foxp3+ Treg. J Immunol 2012; 189:5985–94. [DOI] [PubMed] [Google Scholar]
- 129. McMurchy AN, Levings MK. Suppression assays with human T regulatory cells: a technical guide. Eur J Immunol 2012; 42:27–34. [DOI] [PubMed] [Google Scholar]
- 130. Collison LW, Vignali DA. In vitro Treg suppression assays. Methods Mol Biol 2011; 707:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kowalski R, Post D, Schneider MC et al Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant 2003; 17:77–88. [DOI] [PubMed] [Google Scholar]
- 132. Ling X, Xiong J, Liang W et al Can immune cell function assay identify patients at risk of infection or rejection? A meta‐analysis. Transplantation 2012; 93:737–43. [DOI] [PubMed] [Google Scholar]
- 133. Rodrigo E, Lopez‐Hoyos M, Corral M et al ImmuKnow as a diagnostic tool for predicting infection and acute rejection in adult liver transplant recipients: a systematic review and meta‐analysis. Liver Transpl 2012; 18:1245–53. [DOI] [PubMed] [Google Scholar]
- 134. Sarwal MM. Fingerprints of transplant tolerance suggest opportunities for immunosuppression minimization. Clin Biochem 2016; 49:404–10. [DOI] [PubMed] [Google Scholar]
- 135. Soulillou JP, Giral M, Brouard S. Operational tolerance in kidney transplantation‐improved terminology may enable more precise investigation. Transplantation 2013; 96:e36–8. [DOI] [PubMed] [Google Scholar]
- 136. Brouard S, Mansfield E, Braud C et al Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci USA 2007; 104:15448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Newell KA, Asare A, Kirk AD et al Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 2010; 120:1836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sagoo P, Perucha E, Sawitzki B et al Development of a cross‐platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 2010; 120:1848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Siepert A, Brosel S, Vogt K et al Mechanisms and rescue strategies of calcineurin inhibitor mediated tolerance abrogation induced by anti‐CD4 mAb treatment. Am J Transplant 2013; 13:2308–21. [DOI] [PubMed] [Google Scholar]
- 140. Viklicky O, Krystufkova E, Brabcova I et al B‐cell‐related biomarkers of tolerance are up‐regulated in rejection‐free kidney transplant recipients. Transplantation 2013; 95:148–54. [DOI] [PubMed] [Google Scholar]
- 141. Chenouard A, Chesneau M, Bui Nguyen L et al Renal operational tolerance is associated with a defect of blood Tfh cells that exhibit impaired B cell help. Am J Transplant 2017; 17: 1490–1501. [DOI] [PubMed] [Google Scholar]
- 142. Louis S, Braudeau C, Giral M et al Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug‐free tolerance. Transplantation 2006; 81:398–407. [DOI] [PubMed] [Google Scholar]
- 143. Muthukumar T, Dadhania D, Ding R et al Messenger RNA for FOXP3 in the urine of renal‐allograft recipients. N Engl J Med 2005; 353:2342–51. [DOI] [PubMed] [Google Scholar]
- 144. Zuber J, Grimbert P, Blancho G et al Prognostic significance of graft Foxp3 expression in renal transplant recipients: a critical review and attempt to reconcile discrepancies. Nephrol Dial Transplant 2013; 28:1100–11. [DOI] [PubMed] [Google Scholar]
- 145. Baeten D, Louis S, Braud C et al Phenotypically and functionally distinct CD8+ lymphocyte populations in long‐term drug‐free tolerance and chronic rejection in human kidney graft recipients. J Am Soc Nephrol 2006; 17:294–304. [DOI] [PubMed] [Google Scholar]
- 146. Moraes‐Vieira PM, Silva HM, Takenaka MC et al Differential monocyte STAT6 activation and CD4(+)CD25(+)Foxp3(+) T cells in kidney operational tolerance transplanted individuals. Hum Immunol 2010; 71:442–50. [DOI] [PubMed] [Google Scholar]
- 147. Silva HM, Takenaka MC, Moraes‐Vieira PM et al Preserving the B‐cell compartment favors operational tolerance in human renal transplantation. Mol Med 2012; 18:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Braudeau C, Ashton‐Chess J, Giral M et al Contrasted blood and intragraft toll‐like receptor 4 mRNA profiles in operational tolerance versus chronic rejection in kidney transplant recipients. Transplantation 2008; 86:130–6. [DOI] [PubMed] [Google Scholar]
- 149. Danger R, Pallier A, Giral M et al Upregulation of miR‐142–3p in peripheral blood mononuclear cells of operationally tolerant patients with a renal transplant. J Am Soc Nephrol 2012; 23:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bohne F, Martinez‐Llordella M, Lozano JJ et al Intra‐graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest 2012; 122:368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Martinez‐Llordella M, Lozano JJ, Puig‐Pey I et al Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest 2008; 118:2845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Martinez‐Llordella M, Puig‐Pey I, Orlando G et al Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant 2007; 7:309–19. [DOI] [PubMed] [Google Scholar]
- 153. Puig‐Pey I, Bohne F, Benitez C et al Characterization of gammadelta T cell subsets in organ transplantation. Transpl Int 2010; 23:1045–55. [DOI] [PubMed] [Google Scholar]
- 154. Khatri P, Roedder S, Kimura N et al A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J Exp Med 2013; 210:2205–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lozano JJ, Pallier A, Martinez‐Llordella M et al Comparison of transcriptional and blood cell‐phenotypic markers between operationally tolerant liver and kidney recipients. Am J Transplant 2011; 11:1916–26. [DOI] [PubMed] [Google Scholar]
- 156. Sawitzki B. Liver transplant patients with operational tolerance: what can the graft itself tell us? Am J Transplant 2016; 16:1049–50. [DOI] [PubMed] [Google Scholar]
- 157. Taubert R, Danger R, Londono MC et al Hepatic infiltrates in operational tolerant patients after liver transplantation show enrichment of regulatory T cells before proinflammatory genes are downregulated. Am J Transplant 2016; 16:1285–93. [DOI] [PubMed] [Google Scholar]
- 158. Sawitzki B, Brunstein C, Meisel C et al Prevention of graft‐versus‐host disease by adoptive T regulatory therapy is associated with active repression of peripheral blood Toll‐like receptor 5 mRNA expression. Biol Blood Marrow Transplant 2014; 20:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Stubbington MJ, Lonnberg T, Proserpio V et al T cell fate and clonality inference from single‐cell transcriptomes. Nat Methods 2016; 13:329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mineo D, Ricordi C. Chimerism and liver transplant tolerance. J Hepatol 2008; 49:478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Morris H, DeWolf S, Robins H et al Tracking donor‐reactive T cells: evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med 2015; 7:272ra210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kawai T, Cosimi AB, Spitzer TR et al HLA‐mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA‐mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2013; 368:1850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. LoCascio SA, Morokata T, Chittenden M et al Mixed chimerism, lymphocyte recovery, and evidence for early donor‐specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation 2010; 90:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hricik DE, Augustine J, Nickerson P et al Interferon gamma ELISPOT testing as a risk‐stratifying biomarker for kidney transplant injury: results from the CTOT‐01 multicenter study. Am J Transplant 2015; 15:3166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rebollo‐Mesa I, Nova‐Lamperti E, Mobillo P et al Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment?. Am J Transplant 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Castle SC. Clinical relevance of age‐related immune dysfunction. Clin Infect Dis 2000; 31:578–85. [DOI] [PubMed] [Google Scholar]
- 168. Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine 2000; 18:1717–20. [DOI] [PubMed] [Google Scholar]
- 169. Malaguarnera L, Ferlito L, Imbesi RM et al Immunosenescence: a review. Arch Gerontol Geriatr 2001; 32:1–14. [DOI] [PubMed] [Google Scholar]
- 170. Yan J, Greer JM, Hull R et al The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing 2010; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]


