Abstract
Background
The renin‐angiotensin‐aldosterone system (RAAS) regulates blood pressure, electrolyte homeostasis, and renal function. Blood pressure, serum sodium concentrations, and urinary albumin excretion are higher in Greyhounds than other purebred and mixed‐breed dogs.
Hypothesis
Alterations in the RAAS in Greyhounds are associated with hemodynamic and clinicopathologic differences observed in the breed.
Animals
Clinically healthy Greyhound and non‐Greyhound dogs consecutively enrolled as blood donors (n = 20/group).
Methods
Prospective study. Standard chemical analysis was performed on serum and urine. Serum angiotensin‐converting enzyme (ACE) activity was determined by fluorometric assay. All other RAAS hormones were determined by radioimmunoassay. Symmetric dimethylarginine (SDMA) was measured by immunoassay. Measurements were compared to blood pressure and urine albumin concentration. Data are presented as mean ± SD or median, range.
Results
Serum creatinine (1.5 ± 0.2 vs 1.0 ± 0.1 mg/dL, P < .001), sodium (149, 147–152 vs 148, 146–150 mEq/L, P = .017), and SDMA (16.1 ± 2.9 vs 12.2 ± 1.8 μg/dL, P < .001) were significantly higher in Greyhounds versus non‐Greyhounds, respectively. Plasma renin activity (0.69, 0.10–1.93 vs 0.65, 0.27–2.93 ng/mL/h, P = .60) and ACE activity (4.5, 2.1–8.5 vs 4.6, 2.1–11.4 activity/mL; P = .77) were similar between groups and did not correlate with higher systolic pressures and albuminuria in Greyhounds. Plasma aldosterone concentration was significantly lower in Greyhounds versus non‐Greyhounds (11, 11–52 vs 15, 11–56 pg/mL, respectively, P = .002).
Conclusions and clinical importance
Basal RAAS activation did not differ between healthy Greyhounds and non‐Greyhounds. Lower aldosterone concentration in Greyhounds is an appropriate physiologic response to higher serum sodium concentration and blood pressure, suggesting that angiotensin II effects in the renal tubule predominate over those of aldosterone.
Keywords: Albuminuria, Atrial natriuretic peptide, Hypertension, Symmetric dimethylarginine
Abbreviations
- ACE
angiotensin‐converting enzyme
- ANP
atrial natriuretic peptide
- AT1
angiotensin receptor 1
- FE
fractional excretion
- PRA
plasma renin activity
- RAAS
renin‐angiotensin‐aldosterone system
- SDMA
symmetric dimethylarginine
- SP
systolic blood pressure
The prevalence of death from renal disease in retired racing Greyhounds is approximately 8%.1 Greyhounds exhibit certain physiologic traits, including higher blood pressure, than non‐Greyhound dogs and a tendency to develop albuminuria2, 3, 4, 5, 6, 7 that have been implicated in the development of renal disease.8 Persistent albuminuria is a marker of early renal disease in dogs and is associated with progressive glomerular and tubulointerstitial damage, ultimately resulting in the progressive loss of renal function.8 Likewise, hypertension has been implicated both as a consequence and cause of kidney disease in dogs.9, 10
Activation of the renin‐angiotensin‐aldosterone system (RAAS) is implicated in the development of both hypertension and proteinuria.11 In hypertensive states, the presence of either hyperreninemia or primary total body sodium excess is inappropriate and provides the basis for elevated blood pressure. In humans, hypertension is further characterized by high, low, or normal plasma renin activity (PRA).12 High‐renin hypertension is characterized by a disproportionate and persistent increase in peripheral vascular resistance, whereas low‐renin hypertension may be secondary to an increase in total body sodium concentration. In normal renin hypertension, renin and sodium remain within a normal range and fail to compensate appropriately.12 In addition to exhibiting tendencies toward hypertension and proteinuria, Greyhounds have also been documented to have disparities in the concentration of serum electrolytes regulated by the RAAS. Concentrations of serum sodium, chloride, and bicarbonate tend to be higher in Greyhounds, whereas serum potassium tends to be lower compared to other dog breeds and mixed breeds.13
Atrial natriuretic peptide (ANP) production by atrial myocytes is linked to the RAAS by incompletely understood mechanisms. ANP secretion is stimulated by oral salt or intravenous fluid infusion‐associated increases in extracellular fluid volume. ANP results in natriuresis and vasorelaxation and suppresses juxtaglomerular apparatus production of renin.14, 15, 16
The classification of hypertension using blood renin and aldosterone concentrations is helpful in not only understanding the physiologic basis for elevations in blood pressure, but also when formulating a treatment plan for patients with hypertension. RAAS blockade slows progression of renal injury in both people and dogs,11 suggesting that upregulation in RAAS activity precipitates or contributes to continued renal damage. Specifically in dogs, inhibition of angiotensin‐converting enzyme (ACE) is effective in slowing progression of renal injury and is associated with a concurrent decrease in glomerular and systemic hypertension and a decrease in albuminuria.17
The purpose of this study was to determine whether Greyhounds have alterations in their RAAS which may contribute to the hemodynamic and clinicopathologic differences observed in the breed. We hypothesized that RAAS hormones associated with sodium and water retention would significantly differ between Greyhounds and non‐Greyhounds and would be associated with differences in systolic blood pressure and with serum electrolyte, symmetric dimethylarginine (SDMA), and urine albumin concentrations.
Materials and Methods
The study was conducted in accordance with the guidelines of the Animal Care and Use Committee of The Ohio State University and with informed consent of the owners. Dogs consecutively enrolled in The Ohio State University Veterinary Medical Center Animal Blood Bank donor program over a 2‐month period were eligible for inclusion. Eligibility criteria for the blood donor program included dogs weighing >25.0 kg, which had never received a blood transfusion, and which tested negative for blood‐borne infectious diseases. Diet was not controlled. Dogs were excluded if any clinically relevant abnormalities were detected on physical examination, CBC, serum biochemistry profile, or routine urinalysis; in Greyhounds, we used specific breed‐related reference intervals.13 Dogs were also excluded if insufficient volume of blood or urine was collected for all required tests.
Blood Pressure Measurement
Feed was withheld from the dogs by their owners for 12 hours before presentation. Blood pressure was measured after a minimum of 5‐minute acclimation to the clinic environment and prior to physical examination or sample collection. Dogs were gently restrained in right lateral recumbency. Measurements were obtained using an oscillometric blood pressure monitor1 with a cuff size approximately 40% of limb circumference on the left pelvic limb, as previously described.18 Blood pressure values were determined by averaging 5 systolic (SP), diastolic, and mean arterial blood pressure readings.
Sample Collection
Midstream voided urine samples were collected following blood pressure measurement. Blood was then collected by jugular venipuncture using a butterfly catheter. Serum was collected for measurement of a routine biochemistry profile, SDMA concentration, serum ACE activity, and aldosterone concentration. Samples were collected in separate tubes containing EDTA for CBC and prechilled ETDA tubes for measurement of PRA; PRA samples were kept on ice until being frozen at −80°C. For measurement of ANP, blood was collected in chilled tubes containing EDTA and aprotinin (200 KIU/mL)2 and kept on ice until frozen at −80°C. Serum and plasma were separated within 1 hour of collection. Centrifugation of samples for PRA and ANP was carried out in a refrigerated centrifuge. Samples were stored at −80°C until time of analysis for SDMA, ACE activity, aldosterone, PRA, and ANP.
Sample Analysis
Routine urinalysis was performed within 1 hour of collection. Dogs were excluded from study enrollment if >3 leukocytes or >3 red blood cells per high power field were found on sediment examination. Urine creatinine, sodium, potassium, chloride, and protein were measured by standard biochemical analysis.3 Urine was centrifuged to remove sediment and frozen at −80°C for later determination of quantitative urine albumin concentration.4
Routine CBC5 with hand differential white blood cell count and biochemical analyses3 were performed within 1 hour of sample collection. Fractional excretion (FE) of electrolytes and albumin were calculated as [(Soluteurine × Creatinineserum)/(Soluteserum × Creatinineurine)] × 100%. Serum SDMA, an indicator of glomerular filtration rate, was measured by a commercial laboratory.6 Serum ACE activity was determined by fluorometric assay that measured formation of hippuric acid from hippuryl‐L‐histidyl‐L‐leucine substrate2 by ACE with a coefficient of variation (CV) of 11.8% as previously described.19, 20 Serum aldosterone was measured using a radioimmunoassay7 previously validated for dogs21 with an analytical sensitivity of 11 pg/mL and CV of 2.1%. PRA was measured by radioimmunoassay of angiotensin I generation in plasma8 as previously described in dogs22 and with an analytical sensitivity of 0.018 ng/tube and CV of 1.8%. Plasma ANP was measured using a radioimmunoassay9 as previously validated in dogs23 and with an analytical sensitivity of 0.04 ng/mL and CV of 10.3%. All hormone assays were run in duplicate. Curve fitting and determination of unknowns were calculated for the radioimmunoassays using commercial software.10
Statistical Analysis
Statistical analyses were performed using commercial software.10 Normality for each analyte was evaluated using the Shapiro–Wilk test. Groups were compared by t‐test and data expressed as mean ± SD for normally distributed data. Nonparametric data were compared by Mann‐Whitney U‐test and data expressed as median and range. Statistical significance was set at P < .05. Spearman rank correlations (r s) were used to examine associations between analytes.
Results
The final study population consisted of 20 Greyhounds and 20 non‐Greyhound dogs. Of 44 dogs evaluated for possible inclusion in this study, 2 were excluded due to presence of leukocytes in the urine sediment and 2 for failure to collect urine. The 20 Greyhounds ranged from 3 to 8 years of age and included 10 spayed females and 10 neutered males. The 20 non‐Greyhound dogs ranged from 1 to 7 years of age and included 8 spayed females and 12 neutered males. There was no significant difference in ages between groups (P = .066). The non‐Greyhounds consisted of 15 mixed breeds, 2 Boxers, 1 Standard Poodle, 1 German Shepherd Dog, and 1 Great Dane.
Greyhounds had significantly higher serum creatinine (P < .001) and urea nitrogen (P = .033) concentrations than non‐Greyhounds (Table 1). Serum sodium (P = .017) and serum chloride (P = .028) concentrations were also significantly higher in Greyhounds than in non‐Greyhounds, but there was no difference in serum potassium concentration (P = .11) (Table 1). Urine specific gravity was not significantly different between groups (P = .44; Table 1). FE of sodium (P = .636), potassium (P = .62), and chloride (P = .12) were not different between groups (Table 2). However, urine albumin concentration (P = .006) and FE of albumin (P = .004) were significantly greater in Greyhounds compared to non‐Greyhound dogs (Table 2). One sample for SDMA from a Greyhound was lost. SDMA concentration was higher (P < .001) in Greyhounds compared to non‐Greyhounds (Table 2). Thirteen greyhounds (68%) had SDMA concentrations greater than the upper limit of the commercial laboratory's canine SDMA reference interval (0–14 μg/dL), while 6/19 (31%) had SDMA concentrations ≤14 μg/dL. In contrast, all but one of the non‐Greyhound dogs (95%) had SDMA ≤14 μg/dL. SDMA was significantly correlated with serum creatinine concentrations in the Greyhounds (r s = 0.657, P = .002) but not in the non‐Greyhounds (r s = 0.019, P = .94) (Fig 1). SDMA concentration was not correlated with BUN concentration in either group (r s = 0.348, P = .15 for Greyhound and r s = −0.148, P = .53 for non‐Greyhounds), nor with urine albumin concentration (r s = −0.264, P = .28 for Greyhounds and r s = −0.155, P = .52 for non‐Greyhounds).
Table 1.
Serum and urine variables in Greyhound and non‐Greyhound dogs (n = 20 per group)
| Greyhound | Non‐Greyhound | P value | |
|---|---|---|---|
| Serum Creatinine (mg/dL) | 1.5 ± 0.2 | 1.0 ± 0.1 | <.001 |
| BUN (mg/dL) | 20.0 (10.0–23.0) | 15.5 (10.0–29.0) | .033 |
| SDMA (μg/mL) | 16.1 ± 2.9 | 12.2 ± 1.8 | <.001 |
| Serum Sodium (mmol/L) | 149 (147–152) | 148 (146–150) | .017 |
| Serum Potassium (mmol/L) | 4.2 ± 0.3 | 4.3 ± 0.2 | .11 |
| Serum Chloride (mmol/L) | 113 ± 2 | 112 ± 2 | .028 |
| Serum Albumin (g/dL) | 3.7 ± 0.3 | 3.7 ± 0.2 | 1.0 |
| USG | 1.033 ± 0.011 | 1.036 ± 0.012 | .44 |
| FENa | 0.29 (0.08–1.39) | 0.41 (0.08–1.08) | .64 |
| FEK | 12.18 (4.78–18.30) | 13.09 (2.69–22.78) | .62 |
| FECl | 0.39 (0.05–1.78) | 0.65 (0.15–1.42) | .12 |
| FEAlbumin | 0.12 (0–1.07) | 0.02 (0–0.66) | .004 |
| Urine Albumin (mg/dL) | 1.0 (0–8.6) | 0.1 (0–2.1) | .006 |
BUN, blood urea nitrogen; SDMA, symmetric dimethylarginine; USG, urine specific gravity; FE, fractional excretion. Data are expressed as mean ± SD for normally distributed data and as median and range () for nonparametric data.
Table 2.
RAAS variables and ANP in Greyhound and non‐Greyhound dogs (n = 20 per group)
| Greyhound | Non‐Greyhound | P value | |
|---|---|---|---|
| PRA (ng/mL/h) | 0.69 (0.10–1.93) | 0.65 (0.27–2.93) | .60 |
| ACE (activity/mL) | 4.5 (2.1–8.5) | 4.6 (2.1–11.4) | .77 |
| Aldosterone (pg/mL) | 11.0 (11–52) | 15 (11–56) | <.001 |
| Aldosterone/PRA ratio | 16.03 (5.70–107.60) | 27.95 (6.44–157.0) | .11 |
| ACE/PRA ratio | 6.02 (2.52–56.88) | 5.63 (1.70–28.25) | .27 |
| Aldosterone/ACE ratio | 2.47 (1.29–5.37) | 4.87 (0.96–14.79) | .049 |
| ANP (pg/mL) | 54 (15–399) | 45 (10–259) | .33 |
PRA, plasma renin activity; ACE, angiotensin‐converting enzyme; ANP, atrial natriuretic peptide. Data are expressed as median and range ().
Figure 1.
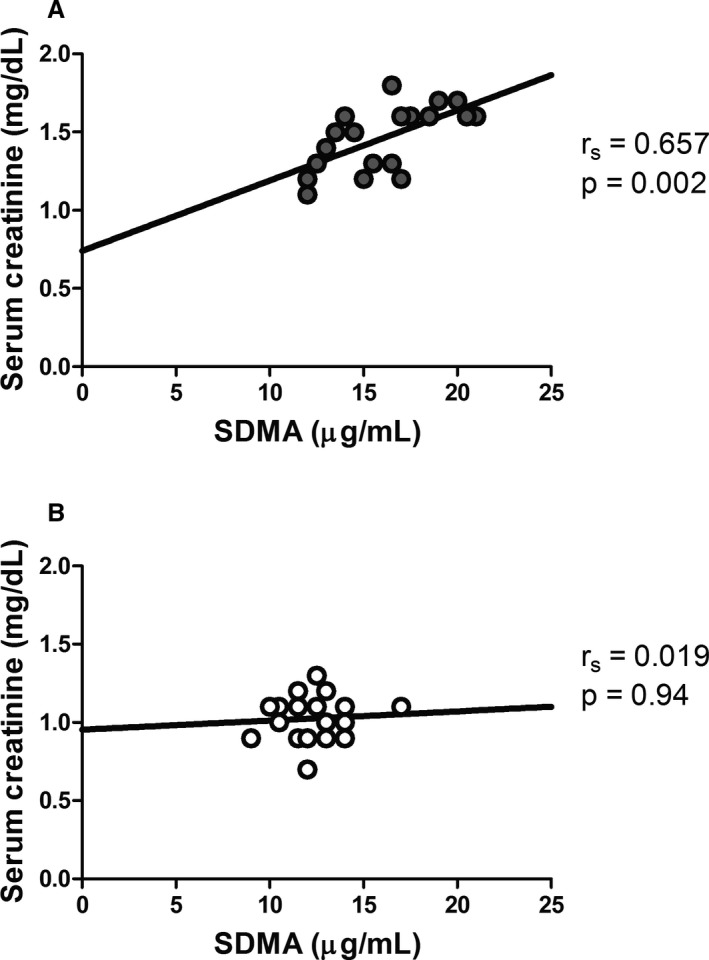
Regression analysis between serum creatinine and symmetric dimethylarginine (SDMA) in A. Greyhound and B. non‐Greyhound dogs. Serum creatinine and SDMA were significantly correlated only in the Greyhound dogs. Filled circles represent Greyhounds, and open circles represent non‐Greyhounds.
SP was significantly higher in Greyhounds than in non‐Greyhounds (P = .030) (Fig 2). There was no significant difference in diastolic blood pressure (P = .82) or mean arterial pressure (P = .66) between the 2 groups (Fig 2).
Figure 2.
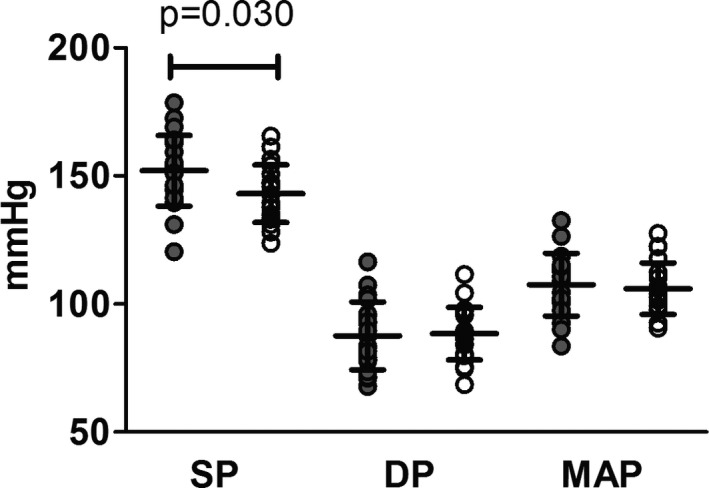
Systolic pressure (SP), diastolic pressure (DP), and mean arterial pressure (MAP) in Greyhound and non‐Greyhound dogs. Greyhounds had significantly higher SP compared to non‐Greyhounds (*P = .030). There was no significant difference between groups for DP or MAP. Filled circles represent Greyhounds, and open circles represent non‐Greyhounds.
PRA and ACE activity did not significantly differ between the 2 groups (P = .60 and P = .77, respectively, Table 2). However, serum aldosterone concentration was significantly lower in Greyhounds than in non‐Greyhounds (P = .002, Table 2), and the ratio of aldosterone to ACE activity was lower in Greyhounds compared to non‐Greyhound (P = .049, Table 2). No difference in ANP was observed between groups (P = .33, Table 2).
Discussion
The goal of this study was to evaluate whether Greyhounds have differences in their RAAS compared to non‐Greyhounds, and whether such differences contribute to higher blood pressure, albuminuria, and the prevalence of renal disease observed in the breed. Greyhounds in our study had higher SP and urinary albumin concentrations than non‐Greyhounds, which is consistent with previous reports.5, 11, 13, 24, 25
We measured serum SDMA as a noninvasive means to assess GFR. SDMA is a small molecule that is primarily eliminated by renal excretion and can serve as an endogenous marker of GFR.26 Increases in SDMA are used for early identification of decreased renal function in dogs and cats with kidney disease.26, 27, 28 Both SDMA and serum creatinine concentrations were significantly higher in Greyhounds compared to non‐Greyhounds. Sixty‐eight percent of the Greyhounds had SDMA concentrations above the recommended upper reference limit of 14 μg/dL26 compared to only 5% of the non‐Greyhound dogs. In addition, SDMA concentration was correlated with serum creatinine concentration in the Greyhounds but not in the non‐Greyhounds. This is similar to previous studies in which the correlation between SDMA and serum creatinine is stronger if dogs with “renal disease” are included, compared to groups that only include healthy animals.26 These findings could suggest some degree of decreased GFR in the Greyhounds compared with the non‐Greyhound dogs. However, in a previous study, we showed that Greyhounds have both higher GFR and serum creatinine concentrations than non‐Greyhounds and that the higher serum creatinine in Greyhounds could not be attributed to a lower GFR in this breed.29 Previous reports have suggested that breed does not influence SDMA concentration; however, Greyhounds were not included in those studies.30, 31 The reason for higher SDMA concentrations in our study Greyhound dogs remains unclear. Further studies are needed to determine the relationship between circulating SDMA concentrations and GFR in the Greyhound and whether breed‐specific reference intervals should be established for this analyte; a prospective study of SDMA concentration in Greyhounds is currently underway.
Evaluation of the RAAS and ANP did not detect marked differences between Greyhounds and non‐Greyhound dogs to account for the increased SP and urine albumin excretion observed in the Greyhounds. If the RAAS was appropriately responsive, one would expect PRA to be lower and ANP increased in Greyhounds, given their higher SP and serum sodium concentrations. PRA was not significantly different in Greyhounds, suggesting that the higher SP in Greyhounds may be most consistent with normal renin hypertension. In people with normal‐renin essential hypertension, plasma aldosterone concentrations tend to have a unimodal distribution that parallels PRA and PRA falls within the normal reference interval.32, 33 The Greyhounds with normal PRA and low aldosterones do not completely fit with this condition. Alternatively, a variety of albuminuric conditions have been recognized in people to be associated with low aldosterone, variable ANP, salt sensitivity, and hypertension which appear to be related to alterations in renal tubular sodium channel activity.34 Further studies are warranted to evaluate renal sodium handling in the Greyhound breed.
ACE activity was used as a proxy for angiotensin II in this study. ACE is the enzyme that cleaves circulating angiotensin I to form angiotensin II. Angiotensin II is a key regulator of sodium homeostasis by stimulating aldosterone secretion and by direct effects on tubular sodium resorption mediated through the angiotensin receptor 1 (AT1). Additionally, angiotensin II has a direct effect on vasculature, resulting in vasoconstriction.35 There was no significant difference observed in ACE activity between Greyhounds and non‐Greyhounds, suggesting a similar production of angiotensin II. Interestingly, serum aldosterone concentrations were significantly lower in Greyhounds, as were aldosterone/ACE ratios. This is consistent with the higher serum sodium concentrations observed in Greyhounds, suggesting that aldosterone secretion is downregulated. If ACE activity reflects angiotensin II production, this would suggest that direct renal effects of angiotensin II on tubular sodium reabsorption could be more important than those of aldosterone in maintaining the higher serum sodium concentrations in the Greyhounds.14, 36 While chronic infusion of angiotensin II into the renal artery of Greyhounds results in increased systemic arterial pressure, baseline levels of angiotensin II have not been measured in this breed.37 It is possible that significant differences may exist in Greyhounds in actual angiotensin II levels, in response to angiotensin II at the cellular level, or in activation of alternative pathways of the renin‐angiotensin cascade. A study specifically evaluating angiotensin II and other components of the RAAS cascade would be needed to make this assessment.
Limitations of this study include failure to control for diet, which could have impacted concentrations of various electrolytes, most notably serum sodium. We attempted to minimize this effect by sampling in the morning after a 12‐hour fast. Additionally, secretion of aldosterone occurs throughout the day in a pulsatile manner, so single measurements may not be good predictors of average daily aldosterone levels. In people, 24‐hour urinary aldosterone measurements are used to screen patients for primary aldosteronism. More recently, urinary aldosterone‐to‐creatinine ratios have been used as a simpler means of ascertaining average daily aldosterone concentrations.38 Use of urinary aldosterone‐to‐creatinine ratio in this study may have been a better predictor of overall daily aldosterone levels. However, we were interested assessing in the interactions of renin, angiotensin II, and aldosterone. That the aldosterone to ACE ratio (with ACE as a surrogate for the effector molecule, angiotensin II) was lower in Greyhounds suggests altered regulation of aldosterone by angiotensin II or altered sensitivity of renal tubules to angiotensin II.
Based on the findings of this study, the increased SP, albuminuria, and SDMA concentration in Greyhounds is not associated with overt activation of the RAAS and, in fact, serum aldosterone concentrations are lower in this breed. Other considerations for why these differences are observed may be alterations in vascular reactivity, modulation of other vasoactive mediators, or differences in renal tubule sodium handling. Ultimately, further research is indicated to determine the mechanisms underlying the increased blood pressure and albuminuria as well as the possible decrease in GFR seen in this breed.
Acknowledgments
Dr. Martinez was funded in part through the Ohio State University Veterinary Scholar Summer Research Program. We thank Jane Robertson from IDEXX Laboratories for assistance with the SDMA measurements, which were performed at no cost to the investigators.
Conflict of Interest Declaration: Dr. Couto is a consultant for IDEXX Laboratories, Inc. Dr. Pressler is an employee of IDEXX Laboratories, Inc, which holds the SDMA immunoassay assay patent. The other authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was carried out at The Ohio State University College of Veterinary Medicine.
Support: Dr. Martinez was funded in part through the Ohio State University Veterinary Scholar Summer Research Program.
Data were presented in part at the American College of Veterinary Pathologists annual meeting, December 2016, in New Orleans, Louisiana.
Footnotes
Cardell 9402 BP/SpO2, Sharn Veterinary INC, Tampa, FL
Sigma Aldrich, St. Louis, MO
Cobas 6000 c501; Roche, Indianapolis, IN
Canine urine microalbumin, Antech Diagnostics, Southhaven, MS
Advia 2120, Siemens Medical Solutions, Malvern, PA
IDEXX SDMA™ test, IDEXX Laboratories, Westbrook, ME
Siemens Coat‐A‐Count Aldosterone, Malvern, PA
Gamma Coat Plasma Renin Acivity RIA, Diasorin, Stillwater, MN
Atrial Natriuretic Factor RIA Kit, Bachem Peninsula, San Carlos, CA
GraphPad Prism, version 5.04, GraphPad Software, La Jolla CA
References
- 1. Lord LK, Yaissle JE, Marin L, et al. Results of a web‐based health survey of retired racing Greyhounds. J Vet Intern Med 2007;21:1243–1250. [DOI] [PubMed] [Google Scholar]
- 2. Schneider HP, Truex RC, Knowles JO. Comparative observations of the hearts of mongrel and Greyhound dogs. Anat Rec 1964;149:173–179. [DOI] [PubMed] [Google Scholar]
- 3. Cox RH, Peterson LH, Detweiler DK. Comparison of arterial hemodynamics in the mongrel dog and the racing greyhound. Am J Physiol 1976;230:211–218. [DOI] [PubMed] [Google Scholar]
- 4. Pape LA, Price JM, Alpert JS, et al. Hemodynamics and left ventricular function: A comparison between adult racing greyhounds and greyhounds completely untrained from birth. Basic Res Cardiol 1986;81:417–424. [DOI] [PubMed] [Google Scholar]
- 5. Bodey AR, Rampling MW. Comparison of haemorrheological parameters and blood pressure in various breeds of dog. J Small Anim Pract 1999;40:3–6. [DOI] [PubMed] [Google Scholar]
- 6. Surman S, Couto CG, Dibartola SP, et al. Arterial blood pressure, proteinuria, and renal histopathology in clinically healthy retired racing greyhounds. J Vet Intern Med 2012;26:1320–1329. [DOI] [PubMed] [Google Scholar]
- 7. Martinez JT, Rogers LK, Kellogg C, et al. Plasma vasoprotective eicosanoid concentrations in healthy greyhounds and non‐greyhound dogs. J Vet Intern Med 2016;30:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grauer GF. Canine glomerulonephritis: New thoughts on proteinuria and treatment. J Small Anim Pract 2005;46:469–478. [DOI] [PubMed] [Google Scholar]
- 9. Wehner A, Hartmann K, Hirschberger J. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non‐renal diseases. Vet Rec 2008;162:141–147. [DOI] [PubMed] [Google Scholar]
- 10. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003;222:322–329. [DOI] [PubMed] [Google Scholar]
- 11. Lattanzio MR, Weir MR. Does blockade of the Renin‐Angiotensin‐aldosterone system slow progression of all forms of kidney disease? Curr Hypertens Rep 2010;12:369–377. [DOI] [PubMed] [Google Scholar]
- 12. Viola A, Monticone S, Burrello J, et al. Renin and aldosterone measurements in the management of arterial hypertension. Horm Metab Res 2015;47:418–426. [DOI] [PubMed] [Google Scholar]
- 13. Zaldivar‐Lopez S, Marin LM, Iazbik MC, et al. Clinical pathology of Greyhounds and other sighthounds. Vet Clin Pathol 2011;40:414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schweda F. Salt feedback on the renin‐angiotensin‐aldosterone system. Pflugers Arch 2015;467:565–576. [DOI] [PubMed] [Google Scholar]
- 15. Doorenbos CJ, Iestra JA, Papapoulos SE, et al. Atrial natriuretic peptide and chronic renal effects of changes in dietary protein and sodium intake in man. Clin Sci (Lond) 1990;78:565–572. [DOI] [PubMed] [Google Scholar]
- 16. Overlack A, Ruppert M, Kolloch R, et al. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension 1993;22:331–338. [DOI] [PubMed] [Google Scholar]
- 17. Brown SA, Finco DR, Brown CA, et al. Evaluation of the effects of inhibition of angiotensin converting enzyme with enalapril in dogs with induced chronic renal insufficiency. Am J Vet Res 2003;64:321–327. [DOI] [PubMed] [Google Scholar]
- 18. Marino CL, Cober RE, Iazbik MC, et al. White‐coat effect on systemic blood pressure in retired racing Greyhounds. J Vet Intern Med 2011;25:861–865. [DOI] [PubMed] [Google Scholar]
- 19. Lieberman J. Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis. Am J Med 1975;59:365–372. [DOI] [PubMed] [Google Scholar]
- 20. Moesgaard SG, Pedersen LG, Teerlink T, et al. Neurohormonal and circulatory effects of short‐term treatment with enalapril and quinapril in dogs with asymptomatic mitral regurgitation. J Vet Intern Med 2005;19:712–719. [PubMed] [Google Scholar]
- 21. Behrend EN, Weigand CM, Whitley EM, et al. Corticosterone‐ and aldosterone‐secreting adrenocortical tumor in a dog. J Am Vet Med Assoc 2005;226:1662–1666 1659. [DOI] [PubMed] [Google Scholar]
- 22. Walsh KP, Williams TD, Canepa‐Anson R, et al. Effects of endogenous atrial natriuretic peptide released by rapid atrial pacing in dogs. Am J Physiol 1987;253:R599–R604. [DOI] [PubMed] [Google Scholar]
- 23. Greco DS, Biller B, Van Liew CH. Measurement of plasma atrial natriuretic peptide as an indicator of prognosis in dogs with cardiac disease. Can Vet J 2003;44:293–297. [PMC free article] [PubMed] [Google Scholar]
- 24. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc 2005;226:393–400. [DOI] [PubMed] [Google Scholar]
- 25. Lien YH, Hsiang TY, Huang HP. Associations among systemic blood pressure, microalbuminuria and albuminuria in dogs affected with pituitary‐ and adrenal‐dependent hyperadrenocorticism. Acta Vet Scand 2010;52:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hokamp JA, Nabity MB. Renal biomarkers in domestic species. Vet Clin Pathol 2016;45:28–56. [DOI] [PubMed] [Google Scholar]
- 27. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med 2015;29:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall JA, Yerramilli M, Obare E, et al. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Intern Med 2016;30:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drost WT, Couto CG, Fischetti AJ, et al. Comparison of glomerular filtration rate between greyhounds and non‐Greyhound dogs. J Vet Intern Med 2006;20:544–546. [DOI] [PubMed] [Google Scholar]
- 30. Moesgaard SG, Holte AV, Mogensen T, et al. Effects of breed, gender, exercise and white‐coat effect on markers of endothelial function in dogs. Res Vet Sci 2007;82:409–415. [DOI] [PubMed] [Google Scholar]
- 31. Pedersen LG, Tarnow I, Olsen LH, et al. Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethylarginines in dogs. Res Vet Sci 2006;80:336–342. [DOI] [PubMed] [Google Scholar]
- 32. Buhler FR, Laragh JH, Sealey JE, et al. Plasma aldosterone‐renin interrelationships in various forms of essential hypertension. Studies using a rapid assay of plasma aldosterone. Am J Cardiol 1973;32:554–561. [DOI] [PubMed] [Google Scholar]
- 33. Adlin EV, Braitman LE, Vasan RS. Bimodal aldosterone distribution in low‐renin hypertension. Am J Hypertens 2013;26:1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Svenningsen P, Andersen H, Nielsen LH, Jensen BL. Urinary serine proteases and activation of ENaC in kidney—implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch Eur J Physiol 2015;467:531–542. [DOI] [PubMed] [Google Scholar]
- 35. Goodman HM. Basic Medical Endocrinology, 4th ed Amsterdam; Boston: Elsevier/Academic Press; 2009;xxxiii, 309 p. [Google Scholar]
- 36. Mochel JP, Danhof M. Chronobiology and pharmacologic modulation of the renin‐angiotensin‐aldosterone system in dogs: What have we learned? Rev Physiol Biochem Pharmacol 2015;169:43–69. [DOI] [PubMed] [Google Scholar]
- 37. Fitzgerald SM, Stevenson KM, Evans RG, et al. Systemic hemodynamic responses to chronic angiotensin II infusion into the renal artery of dogs. Am J Physiol 1997;273:R1980–R1989. [DOI] [PubMed] [Google Scholar]
- 38. Wu CH, Yang YW, Hu YH, et al. Comparison of 24‐h urinary aldosterone level and random urinary aldosterone‐to‐creatinine ratio in the diagnosis of primary aldosteronism. PLoS ONE 2013;8:e67417. [DOI] [PMC free article] [PubMed] [Google Scholar]


