Abstract
Background
Cutaneous plasmacytosis (CP) is a syndrome of multiple cutaneous plasma cell tumors, in the absence of multiple myeloma. Although rare in both humans and dogs, treatment recommendations are usually extrapolated from multiple myeloma protocols. To date, no case series of CP have been described in the veterinary literature.
Hypothesis/Objectives
To describe clinical presentation, determine treatment response rates and duration, and report overall survival of dogs with CP.
Animals
Twenty‐one client‐owned dogs with CP.
Methods
Medical records of 21 dogs with CP were reviewed. Diagnosis was based on histopathologic evaluation of at least 1 representative cutaneous or subcutaneous lesion in dogs with ≥3 lesions. Dogs with suspicion of multiple myeloma were excluded.
Results
The most commonly affected breeds were the golden (5/21) and Labrador retriever (3/21). Fourteen of 21 dogs had >10 lesions, with some having >100. Lesions commonly were described as round, raised, pink‐to‐red, and variably alopecic or ulcerated. The most commonly used drug protocol was combined melphalan and prednisone, with an overall response rate (ORR) of 73.7% (14/19 dogs). Single‐agent lomustine was associated with a similar ORR of 71.4% (5/7 dogs). For all treatments combined, the median progression‐free interval after the first treatment was 153 days. The median survival time from the first treatment was 542 days.
Conclusions and Clinical Importance
Alkylating agents were effective in inducing remission of CP; corticosteroids, melphalan, and lomustine were the most commonly used drugs. Survival times were similar to those reported in dogs with multiple myeloma treated with alkylating agents.
Keywords: Multiple myeloma, Plasma cell
Abbreviations
- CP
cutaneous plasmacytosis
- CR
complete response
- HCT
hematocrit
- HHV‐8
human herpesvirus 8
- HPF
high‐power fields
- IFE
immunofixation electrophoresis
- IL‐6
interleukin‐6
- ITP
immune‐mediated thrombocytopenia
- MUM‐1
multiple myeloma oncogene 1
- ORR
overall response rate
- PD
progressive disease
- PFI
progression‐free interval
- PR
partial response
- SD
stable disease
- VCOG‐CTCAE
veterinary cooperative oncology group‐common terminology criteria for adverse events
Plasma cell tumors are uncommon in dogs. Of soft tissue plasma cell tumors, termed extramedullary plasmacytomas, 86% are cutaneous, 9% appear in the oral cavity or lips, and 4% in the rectum or colon.1 The majority (>95%) of cutaneous plasmacytomas are solitary, and <1% are associated with multiple myeloma. They classically appear as alopecic, pink, raised, round, and well‐circumscribed growths, typically 1–2 cm in diameter. The head and limbs are the most common locations, but they can appear on any body surface.1, 2, 3, 4 Breed predisposition is suspected in American cocker spaniels, English cocker spaniels, and West Highland white terriers, and the median age at diagnosis is 9–10 years.1
Solitary cutaneous and nonosseous oral plasmacytomas are considered benign, with a good long‐term prognosis and low metastatic potential. Surgical excision for local control is often curative.3, 5 In a synopsis of reports, the local recurrence rate was 5%, and nodal or distant metastatic rate was 2%.6 As a result, adjuvant treatments are not routinely recommended for dogs with solitary cutaneous and nonosseous oral plasmacytomas.
A seemingly distinct clinical syndrome of multiple cutaneous plasma cell tumors that appear as red‐brown plaques or raised lesions has been reported in humans and is referred to as cutaneous plasmacytosis (CP). Definitive diagnosis is based on an expanded polyclonal population of mature plasma cells. Although very rare, there is significant predisposition among men of Asian and Pacific Islander descent.7, 8
Cutaneous plasmacytosis is distinguished from systemic plasmacytosis by the absence of disseminated disease in the bone marrow, liver, spleen, and lymph nodes. Polyclonal hypergammaglobulinemia is a characteristic feature of CP in people.9
Cutaneous plasmacytosis in humans typically is a benign disorder with indolent behavior, although a few reported cases had an aggressive course leading to death. Some patients have been reported to subsequently develop malignant lymphomas.10 Treatment is reserved for patients who develop clinical signs such as fever, lymphadenopathy, or compromised quality of life because of the cosmetic appearance of the lesions. Treatments have included topically and systemically administered corticosteroids, photodynamic therapy, or systemic chemotherapy such as cyclophosphamide, with limited reported efficacy.7
A syndrome similar to CP has been anecdotally reported to occur in dogs, but has not been described in detail in the veterinary literature. Within a case series of mucocutaneous plasmacytomas, 5/75 dogs had ≥3 lesions, although at least 1 of these dogs had characteristics of multiple myeloma.2 In a larger study, 2.2% of 406 dogs with cutaneous plasmacytomas had multiple lesions reported.11 We studied a group of dogs with characteristics of CP retrospectively to elucidate clinical features and treatment responses of this rare disorder.
Materials and Methods
Patient Selection
Our study was performed by a retrospective review of medical records of dogs with CP from 2005 to 2015 from 5 oncology referral clinics. The cases were reviewed for the inclusion criteria, including ≥3 simultaneous cutaneous plasma cell tumors with histopathologic confirmation of at least 1 lesion, and absence of evidence of multiple myeloma (the presence of ≥2 of the following criteria: bone marrow involvement, monoclonal gammopathy, lytic bone lesions, or light chain proteinuria)1 .
Data Collection
The following data were collected from the patients’ medical records: breed; sex; age and weight at diagnosis; date and method of diagnosis; clinical signs; number; size; location and description of plasma cell tumors; bone marrow and serum globulin diagnostic tests; other staging tests; all treatments including response and response duration; and date of death. In some cases, additional follow‐up information was obtained by phone or email contact with the pet owner or referring veterinarian. Treatment responses were recorded for each treatment protocol according to established response evaluation criteria for solid tumors in dogs (v1.0) criteria as progressive disease (PD), stable disease (SD), partial response (PR), or complete response (CR).12 Similarly, adverse events and pre‐existing clinicopathologic changes were characterized according to veterinary cooperative oncology group‐common terminology criteria for adverse events (VCOG‐CTCAE) v1.1.13
Statistics
For outcome calculations, dogs that died because of plasma cell disease or unknown cause were included. Dogs were censored at the time of last follow‐up if they were lost to follow‐up, died of a confirmed cause other than plasma cell disease, or were still alive at time of analysis. Progression‐free and overall survival estimates were calculated by the Kaplan‐Meier product limit method, and differences between groups were compared by log‐rank analysis. All statistical analysis was performed by commercial statistical software2. A P value < .05 was considered significant for all analyses.
Results
Patient Population
Twenty‐one dogs met the inclusion criteria. The median age of affected dogs was 8.5 years (range, 3–12 years), with a median weight of 26.5 kg (range, 5–47 kg). Thirteen of 21 dogs were males (11 were castrated). The most commonly affected breeds were the golden (5/21) and Labrador retriever (3/21).
Clinical Presentation
Ten or more lesions were noted in 14 of 21 dogs (66.7%), with 1 patient estimated to have >100 lesions, although a count was not provided (Fig 1). Three dogs had 3 lesions, 1 dog had 4 lesions, 2 dogs had 5 lesions, and 1 dog had 6 lesions. When descriptions were available (15 cases), lesions were most commonly described as round, raised, pink‐to‐red, and variably alopecic or ulcerated (3 cases), similar to solitary plasmacytomas. Patients were reported to be asymptomatic in 13 of 16 cases (81.3%) where this information was provided. Epistaxis was noted in 2 dogs and lethargy in 1 dog.
Figure 1.
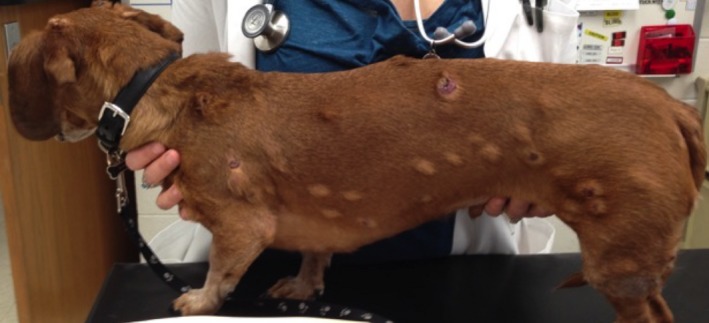
Miniature Dachshund patient with >100 cutaneous plasmacytomas.
Histopathology
The most common histopathologic description in patients of our series was dense sheets of anaplastic round cells with eccentric nuclei. Moderate‐to‐marked anisocytosis and anisokaryosis were described in 8 of 10 cases where this characteristic was noted. Multinucleated cells were noted in 5 of 7 cases where number of nuclei was available, but were most commonly described as scattered or occasional. When immunohistochemistry (IHC) for plasma cells was performed, neoplastic cells were consistently positive, including multiple myeloma oncogene‐1 (MUM‐1) in 4 cases (plasma cell), lambda light chain in 3 cases (plasma cell), and CD79a in 1 case (pan B cell). Immunohistochemistry was not performed in 13 cases. Additionally, CD3‐positive cells were noted at the periphery of 1 neoplasm, interpreted to be infiltrating T‐cells. Mitotic figures were variable, ranging from rare to 70 per 10 high‐power fields (hpf) (median, 15 per 10 hpf).
Clinical Pathology
Bone marrow cytology was performed for routine staging in 3 of 21 patients, none of which demonstrated malignant plasma cell involvement. Complete blood counts at diagnosis were available in 17 of 21 patients. These were normal in 13 cases. One dog had mild lymphocytosis of 4,800/μL and slight increase in band neutrophils of 200/μL. One dog had grade 1 anemia. One dog had grade 1 thrombocytopenia. One dog had severe grade 4 thrombocytopenia with 4 platelets/μL and moderate grade 2 anemia with a hematocrit (HCT) of 24%. This dog was clinically suspected to have immune‐mediated thrombocytopenia (ITP), and these results normalized with prednisone treatment (1 mg/kg/d).
Biochemistry profiles at diagnosis were available in 16 of 21 patients. Eleven patients had no clinically relevant changes on their biochemistry profiles. Grade 1 increases in liver enzyme activities were noted in 3 dogs, and abdominal ultrasound examination was performed in 1 of them, with no liver changes noted. One of these patients, additionally, had mildly increased serum cholesterol concentration at 9.6 mmol/L (reference range 3.9–7.8 mmol/L) and mildly increased ionized calcium concentration at 1.38 mmol/L (reference range 1.13–1.33 mmol/L).
Total globulin concentrations were increased in 3 of 20 patients, with concentrations of 3.7 and 4.3 g/dL (reference range 1.5–3.2 g/dL), and 1 reported as slightly increased, without laboratory results available for review. Further evaluation with serum protein electrophoresis was performed in 2 of these 3 dogs, and both had a polyclonal gammopathy. In 4 additional patients with normal total globulin concentrations, serum protein electrophoresis was performed as a screening test and was normal in all cases. Globulin quantification was normal in 1 additional patient with normal total globulin. No patient had confirmed monoclonal gammopathy.
Urine protein electrophoresis was performed as a screening test in 4 patients and was normal in all cases. One dog had 2+ proteinuria on routine urinalysis, but no further evaluation by electrophoresis.
Staging
A total of 6 dogs had suspected or confirmed lymph node involvement at diagnosis. Peripheral lymph node involvement was confirmed cytologically in 2 of 21 cases, and 1 additional dog had suspected lymph node involvement based on enlargement on rectal palpation, but had inconclusive cytology. Two additional dogs had cytologically confirmed involvement of enlarged iliac lymph nodes. Two dogs (1 included above with peripheral lymph node involvement) had suspected abdominal lymph node involvement on ultrasonography as described below.
Abdominal visceral involvement was suspected based on abdominal ultrasonography in 2 of 7 dogs in which it was performed at initial diagnosis. One dog had multifocal hepatic, splenic, and mesenteric nodules, and jejunal lymphadenopathy detected on ultrasonography, with no sample collected for cytology. One dog had gastric wall thickening and an enlarged regional lymph node. Although neither lesion was aspirated for cytology, both were suspected to be a result of plasma cell or another round cell neoplasm, based on measurable response to chemotherapy.
Thoracic radiography was performed in 10 of 21 dogs, with no intrathoracic abnormalities reported. Suspected bone involvement was detected in 2 patients. Heterogeneous polyostotic lytic rib lesions were identified in 1 dog and a proximal humeral lytic lesion in the other. Neither lesion was investigated cytologically or histologically, and an independent disease such as osteosarcoma is possible. An additional dog had no bone lesions identified on survey skeletal radiographs.
Treatments and Responses
Nine patients were still alive at last contact, with a median follow‐up time of 519 days. Surgical excision of all plasma cell tumors was performed as a first‐line treatment in 3 dogs with 5, 4, and 3 lesions, with progression‐free intervals (PFI) of 43, 128, and 567 days respectively. Overall survival times in this group were 195, 128, and 567 days, but the latter 2 patients were lost to follow‐up (Table 1). An additional dog had palliative resection of its largest plasma cell tumor in the rescue setting, with a PFI of 242 days after surgery.
Table 1.
Objective response rates, complete response (CR), and progression‐free interval (PFI) in days for each treatment group among dogs with cutaneous plasmacytosis
| Primary Treatmenta | Number Treated | Number Treated First Line | Overall Response Rate, % | CR, % | PFI (Days) |
|---|---|---|---|---|---|
| Melphalan | 19 | 15 | 74 | 37 | 143 (29–823) |
| Lomustine | 7 | 1 | 71 | 57 | 84 (28–84) |
| Cyclophosphamide | 5 | 0 | 40 | 20 | 46, 70 |
| Combination protocolsb | 3 | 0 | 67 | 67 | 181, 229 |
| Radiation therapy | 3 | 1 | 67 | 33 | 43, 253 |
| Doxorubicin | 2 | 0 | 50 | 0 | 110 |
| Prednisone | 2 | 0 | 0 | 0 | 275 |
| Cannabis oil | 1 | 0 | 100 | 100 | 311 |
| Surgery | 4 | 3 | 100 | 75 | 43, >128, >242, >567 |
The majority of dogs treated with melphalan received concurrent prednisone (18 of 19).
Combination protocols included 1 dog treated with chlorambucil, cyclophosphamide, and prednisone, and 2 dogs treated with melphalan, lomustine, and cyclophosphamide. All other protocols did not include prednisone.
The most commonly used drugs were melphalan3 and prednisone, with an overall response rate (ORR) of 72.2% (13/18 dogs), 6 CR, and 7 PR. One patient treated with melphalan alone had a CR. Treatment with lomustine4, usually combined with prednisone, resulted in a similar ORR of 71.4% (5/7 dogs), 4 CR, and 1 PR. Single‐agent cyclophosphamide was associated with an ORR of 40% (2/5 dogs), 1 CR, and 1 PR. For all treatments combined, the median PFI after first treatment was 153 days. The median overall survival time from first treatment was 542 days (Fig 2).
Figure 2.
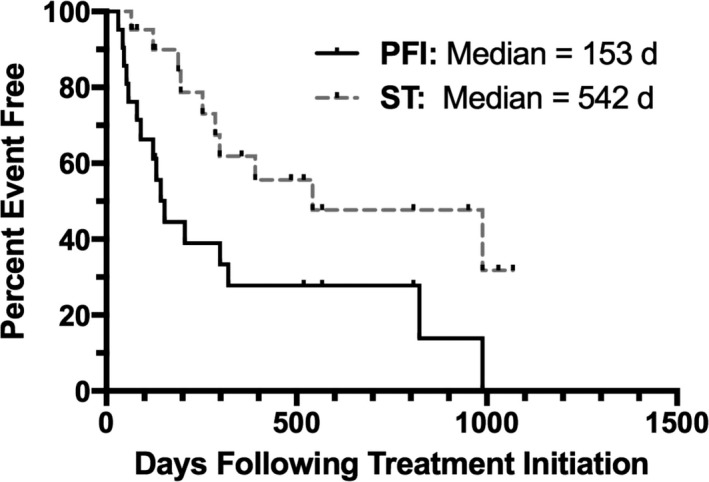
Kaplan‐Meier depicting progression‐free interval and overall survival times for all 21 dogs with cutaneous plasmacytosis.
The nature of disease progression could be defined in 11 cases: new cutaneous lesions occurred in 5 dogs, progressive growth of existing lesions in 3 dogs, lymph node metastasis in 1 dog, and new bone involvement in 2 dogs. No patients developed criteria for multiple myeloma diagnosis during follow‐up.
Three dogs were euthanized for causes likely unrelated to CP and were censored from survival analysis at the time of death; 1 each with congestive heart failure, T‐zone lymphoma, and metastatic pulmonary carcinoma. Only the patient with pulmonary carcinoma had a necropsy performed.
Adverse Events
Adverse events were reported in 4 of 8 dogs that received lomustine, leading to discontinued treatment in 2 dogs and dose reduction in 1 dog. Adverse events included grade 3 increases in liver enzyme activity (2 dogs), grade 2 increases in liver enzyme activity (1 dog), and grade 3 neutropenia (1 dog).
Adverse events were reported in 2 of 2 dogs that received doxorubicin‐based treatment, leading to discontinued treatment in both cases. One patient treated with doxorubicin had grade 3 diarrhea and hyporexia, requiring 2 days of hospitalization before recovery. Another dog developed palmar plantar erythrodysesthesia and cerebellar neurologic signs after having received pegylated liposomal doxorubicin. Magnetic resonance imaging and cerebrospinal fluid analysis were unremarkable in this patient, treatment was stopped, and these clinical signs resolved within 2 weeks.
Adverse events associated with prednisone were reported in 1 patient. This patient had clinically relevant panting, polyuria, and polydypsia during rescue treatment with prednisone alone, which led to an accelerated dose taper but continued treatment.
Adverse events were reported in 6 of 19 dogs treated with melphalan, leading to dose reduction in 2 cases; both were treated with pulsed melphalan. The most common adverse event was thrombocytopenia (5 dogs), with 2 grade 1, 2 grade 2 and 1 grade 4 events. There were 2 reported neutropenic episodes (1 grade 1 and 1 grade 2). Grade 1 anemia was noted in 2 dogs.
The only patient treated with a combination of cyclophosphamide, chlorambucil and prednisone experienced vomiting and cystitis, although grades or description were not provided. This adverse event resolved with supportive medications, and treatment was continued for an additional 6 months without additional adverse events reported.
One of 5 patients treated with cyclophosphamide alone experienced grade 2 neutropenia.
No adverse events were reported in association with surgery (7 dogs), radiation therapy (4 dogs), cannabis oil (1 dog), or the combination protocol of melphalan, lomustine, and cyclophosphamide (2 dogs).
Discussion
Cutaneous plasmacytosis may represent a multifocal neoplasm on the continuum between solitary plasmacytomas and multiple myeloma. The clinical features of CP resemble those of multiple myeloma, including cytologic appearance, median survival time (approximately 18 months), and responsiveness to alkylating agents.6 In this study, dogs with diagnostic criteria for multiple myeloma were excluded, making it difficult to ascertain the relationship between CP and multiple myeloma. To understand how often CP and multiple myeloma coincide, dogs with multiple myeloma could be reviewed for multiple cutaneous plasma cell lesions. In our case series, no patients went on to develop multiple myeloma during follow‐up.
The most commonly reported breeds in our study were golden retrievers (5/21) and Labrador retrievers (3/21). Given the regional differences among case submissions, including multiple clinics in the United States and Australia, a control population was not established for comparison of breed prevalence, and so, this finding could be due to the popularity of these breeds rather than a clear breed predisposition. American cocker spaniels, English cocker spaniels, and West Highland white terriers previously have been reported as potentially predisposed to solitary plasma cell tumors.1 None of these breeds was represented in our study population.
Although diagnosis of cutaneous plasma cell tumors often is accomplished by fine needle aspiration cytology, histopathologic confirmation is recommended. In cases of poorly differentiated round cell tumors, plasma cell‐specific immunohistochemical markers can be used for specific diagnosis, including immunoglobulin, light and heavy chains, MUM1, and thioflavin T.14 Immunohistochemical confirmation of plasma cell origin in our series included MUM‐1 (plasma cell), lambda light chain (plasma cell), and CD79a (pan B cell) markers. The majority of dogs (13 of 21) did not have IHC confirmation, leaving open the possibility of cutaneous lymphoma lesions with plasma cell differentiation. Histopathologic descriptions included nuclear atypia, and substantial anisocytosis or anisokaryosis or both in the majority of cases. These findings are not typical of CP in humans, who typically described have an expansion of mature plasma cells. A previous study showed no correlation between outcome and a grading scheme for canine extramedullary plasma cell tumors based on morphologic subtype and proliferation index.15 Correlation of histopathologic findings with outcome data was not attempted because of small numbers of patients, lack of available histopathology samples for review, variable treatment, and variable description of mitotic count.
Additionally, the low incidence of polyclonal gammopathy (2/5 cases with globulins specifically evaluated) stands in contrast to a high incidence among humans with CP.9, 16 One additional patient with mild polyclonal gammopathy did not have further characterization of the gammopathy or bone marrow sampling performed. Although no other criteria of multiple myeloma were present in this patient, the diagnosis cannot definitively be ruled out. No patients in our study had monoclonal gammopathy, consistent with normal serum electrophoresis in 2 dogs with multiple plasma cell tumors in a previous report.2 However, none of the included patients were evaluated with serum immunofixation electrophoresis (IFE), a more accurate diagnostic test for increases in immunoglobulins. Immunofixation electrophoresis involves traditional protein electrophoresis followed by immunoglobulin‐specific antibody application and has been used to identify monoclonal gammopathies in patients without hyperglobulinemia or with polyclonal bands.17 As a result, IFE may be superior in evaluating patients with CP for multiple myeloma or other secretory B‐cell disorders, even in the absence of hyperglobulinemia.
No clear etiology for cutaneous or systemic plasmacytosis has been identified in people or dogs. Theories proposed have included interleukin‐6 (IL‐6) activation of B cells, underlying infectious disease such as Borrelia burgdorferi or Human Herpesvirus 8 (HHV‐8), collagen disorders, or chronic inflammatory disorders.9, 18 In several cases in humans, high concentrations of circulating IL‐6 have been identified, whereas underlying infectious diseases consistently have been ruled out. One hypothesis for CP pathogenesis is that the local production of IL‐6 creates a survival niche for plasma cells within the skin.
For dogs with relatively low numbers of lesions or palliation of symptomatic lesions, localized treatment with surgery or radiation therapy was fairly effective in delaying disease progression. Among the 4 dogs treated with surgery alone, the number of lesions (3, 4, 5 and >10) did not appear to correlate with PFI (567, 128, 43, and 242 days respectively). Due to small subgroup size and confounding factors such as number of lesions, direct comparison of outcomes between the surgery and chemotherapy groups was not attempted. Most dogs in our series had >10 lesions, making systemic treatment more appropriate. While acknowledging circumstantial differences among cases, localized treatment such as surgery or radiation therapy may be appropriate for dogs with ≤5 CP lesions.
Alkylating agents appeared to be effective in inducing clinical responses in the majority of dogs, although durable remissions were uncommon. The most common first‐line treatment was melphalan, with an ORR of 74% and median PFI of 143 days. Lomustine, cyclophosphamide, doxorubicin5, and combination protocols also caused objective responses. The median PFI for dogs treated with melphalan was longer than that of lomustine (median, 84 days) and cyclophosphamide (46 and 70 days). Melphalan predominantly was used as first‐line treatment, whereas the latter drugs were used in the rescue setting for all but 1 dog. Similarly, the relatively lower ORR for cyclophosphamide (40%) and doxorubicin (20%) may be due to the use of these drugs exclusively in the rescue setting in our study. Some dogs may have spontaneous remissions or prolonged PFI even in the absence of cytotoxic therapy. One patient received second‐line treatment with topical and oral cannabis oil alone had a CR and PFI of 311 days, and 1 patient treated with prednisone alone had SD with a PFI of 275 days.
Adverse events were noted frequently in dogs treated with doxorubicin or pegylated liposomal doxorubicin (2 of 2) and lomustine (4 of 8 dogs). These caused cessation of doxorubicin in both cases and of lomustine in 2 cases. An additional dog had dose reduction in lomustine as a result. The high proportion of adverse events and early treatment discontinuation with doxorubicin and lomustine may have contributed to early treatment failure.
Melphalan led to adverse events in 6 of 19 dogs, but only 2 dogs had dose reductions, and no dog required a stop in treatment. All adverse events associated with melphalan were hematopoietic, with the most common being thrombocytopenia in 5 dogs. This finding is consistent with previous reports of common melphalan toxicities in dogs.6 Of note, the 2 dogs that required dose reductions due to grade 4 cytopenias both were treated with pulsed melphalan dosing.
Melphalan and prednisone are appropriate first‐line treatment for CP when lesions are not amenable to locoregional treatment. Although optimization of treatment protocols with additional or alternative agents is possible, those recommendations are beyond the scope of our study. Prednisone was used in all but 1 of the dogs treated primarily with melphalan. Prednisone use generally was not incorporated into other protocols, except where described in 1 dog that received a combination protocol along with cyclophosphamide and chlorambucil, and the cases palliated with prednisone alone. This could have influenced response rates and duration and limits the comparison of melphalan to other chemotherapy protocols. Although there were no OR reported with prednisone alone, 1 of 2 treated dogs had SD for 275 days. Considering previously identified corticosteroid responses of round cell tumors, and multiple myeloma in particular, inclusion of prednisone in first‐line treatment may be warranted.19
One limitation of our study was its retrospective design, evaluating a relatively small number of patients because of the comparative rarity of CP. The retrospective design led to substantial variation in diagnostic imaging and clinicopathologic evaluation of these patients. Degree of staging was limited in many cases, and necropsy was performed in only 2 patients. As a result, the number of dogs with systemic disease or metastasis, and the extent of disease likely were underestimated. Additionally, the identification of abdominal visceral plasma cell disease in at least 2 dogs raises the question of the origin of cutaneous plasma cell lesions. Some cases may originate primarily within the viscera, bone marrow, or elsewhere of patients that later present with secondary cutaneous lesions. As such, many of the CP cases in our series cannot be classified confidently as primary. Thorough staging for underlying round cell neoplasia should be performed after the identification of cutaneous plasma cell lesions.
Staging recommendations for dogs with CP should begin with standard multiple myeloma diagnostic evaluation including survey radiographs or more advanced imaging for lytic bone lesions, bone marrow cytology, serum electrophoresis including immunofixation when available, tissue sampling of lesions such as those of bone, and urine electrophoresis for Bence Jones proteinuria.6 A more standardized approach may allow future studies to evaluate patients for additional prognostic factors. Additionally, abdominal ultrasonography and fine needle aspiration of enlarged regional lymph nodes for cytology are recommended.
Another limitation to providing clear guidelines is that treatments varied widely due to both a lack of standard‐of‐care recommendations for CP and inclusion of cases from 5 different specialty practices. Assessment of treatment efficacy and overall survival time were further complicated by extensive but variable use of rescue treatments.
In conclusion, CP in dogs is a plasma cell neoplasm presenting with multiple cutaneous lesions and a low‐to‐moderate rate of metastasis to lymph nodes and abdominal viscera. Although the median PFI was 153 days, CP carries a fair to good prognosis with treatment, with a median survival time of 542 days in our patients. Treatment with a combination of alkylating agent and corticosteroid seems to be an appropriate first‐line systemic treatment for cases in which locoregional treatment is not adequate.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was primarily performed at Colorado State University Flint Animal Cancer Center. Case submissions were also received from the co‐authors’ clinics, along with significant contribution to writing and editing the manuscript. No grant funding was used to support of this study. These data were presented in abstract form at the Veterinary Cancer Society symposium in 2015.
Footnotes
Cytoxan, Bristol‐Myers Squibb Company, Princeton, NJ
Prism v6.0b, GraphPad Software, La Jolla, CA
Alkeran, GlaxoSmithKline, Research Triangle Park, NC
CeeNU, Bristol‐Myers Squibb Company
Adriamycin, Pharmacia, Milan, Italy
References
- 1. Lucke VM. Primary cutaneous plasmacytomas in the dog and cat. J Small Anim Pract 1987;28:49–55. [Google Scholar]
- 2. Rakich PM, Latimer KS, Weiss R, et al. Mucocutaneous plasmacytomas in dogs: 75 cases (1980–1987). J Am Vet Med Assoc 1989;6:803–810. [PubMed] [Google Scholar]
- 3. Clark GN, Berg J, Engler SJ, et al. Extramedullary plasmacytomas in dogs: Results of surgical excision in 131 cases. J Am Anim Hosp Assoc 1992;28:105–111. [Google Scholar]
- 4. Cangul IT, Wijnen M, Van Garderen E, et al. Clinico‐pathological aspects of canine cutaneous and mucocutaneous plasmacytomas. J Vet Med A Physiol Pathol Clin Med. 2002;49:307–312. [DOI] [PubMed] [Google Scholar]
- 5. Wright ZM, Rogers KS, Mansell J. Survival data for canine oral extramedullary plasmacytoma: A retrospective analysis (1996–2006). J Am Anim Hosp Assoc 2008;44:75–81. [DOI] [PubMed] [Google Scholar]
- 6. Vail DM. Hematopoietic tumors In: Withrow SJ, Vail DM, Page RL, eds. Withrow and MacEwen's Small Animal Clinical Oncology, 5th ed Philadelphia, PA: W.B. Saunders; 2007:665–676. [Google Scholar]
- 7. Honda R, Cerroni L, Tanikawa A, et al. Cutaneous plasmacytosis: Report of 6 cases with or without systemic involvement. J Am Acad Dermatol 2013;68:978–985. [DOI] [PubMed] [Google Scholar]
- 8. Haque M, Hou JS, Hisamichi K, et al. Cutaneous and systemic plasmacytosis vs. cutaneous plasmacytic castleman disease: Review and speculations about pathogenesis. Clin Lymphoma Myeloma Leuk 2011;11:453–461. [DOI] [PubMed] [Google Scholar]
- 9. Kodama A, Tani M, Hori K, et al. Systemic and cutaneous plasmacytosis with multiple skin lesions and polyclonal hypergammaglobulinaemia: Significant serum interleukin‐6 levels. Br J Dermatol 1992;127:49–53. [DOI] [PubMed] [Google Scholar]
- 10. Nitta Y. Case of malignant lymphoma associated with primary systemic plasmacytosis with polyclonal hypergammaglobulinemia. Am J Dermatopathol 1997;19:289–293. [DOI] [PubMed] [Google Scholar]
- 11. Goldschmidt MH, Shofer FS. Skin Tumors of the Dog and Cat. Oxford: Pergamon Press; 1992:265–270. [Google Scholar]
- 12. Nguyen SM, Thamm DH, Vail DM, et al. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2015;13:176–183. [DOI] [PubMed] [Google Scholar]
- 13. Veterinary Cooperative Oncology Group – common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016;14:417–446. [DOI] [PubMed] [Google Scholar]
- 14. Kyriazidou A, Brown PJ, Lucke VM. An immunohistochemical study of canine extramedullary plasma cell tumors. J Comp Pathol 1989;100:259–266. [DOI] [PubMed] [Google Scholar]
- 15. Platz SJ, Breuer W, Pfleghaar S, et al. Prognostic value of histopathological grading in canine extramedullary plasmacytomas. Vet Pathol 1991;28:125–130. [DOI] [PubMed] [Google Scholar]
- 16. Wagner G, Rose C, Klapper W, et al. Cutaneous and systemic plasmocytosis. J Dtsch Dermatol Ges 2013;11:1161–1167. [DOI] [PubMed] [Google Scholar]
- 17. Seelig DM, Perry JA, Zaks K, et al. Monoclonal immunoglobulin protein production in two dogs with secretory B‐cell lymphoma with Mott cell differentiation. J Am Vet Med Assoc 2011;239:1477–1482. [DOI] [PubMed] [Google Scholar]
- 18. Kanbe N, Kurosawa M, Akimoto S, et al. Systemic plasmacytosis with deposition of interleukin (IL)‐6 and elevated expression of IL‐6 mRNA in the skin lesions. Br J Dermatol 1998;138:721–723. [DOI] [PubMed] [Google Scholar]
- 19. Cunningham D, Paz‐Ares L, Milan S, et al. High‐dose melphalan and autologous bone marrow transplantation as consolidation in previously untreated myeloma. J Clin Oncol 1994;12:759–763. [DOI] [PubMed] [Google Scholar]


