Abstract
Background
Biochemical indicators for diagnosing liver disease are plasma alanine aminotransferase activity (ALT), alkaline phosphatase activity (ALP), and bile acid concentration (BA).
Objectives
To determine the sensitivity and specificity of ALT, ALP, and BA for detecting primary hepatitis (PH) in clinically healthy Labrador retrievers and investigate whether ALT and ALP can discriminate between dogs with PH and nonspecific reactive hepatitis (RH).
Animals
191 clinically healthy and 51 clinically ill Labrador retrievers with hepatic histopathology.
Methods
Retrospective study. Medical records were reviewed for ALT, ALP, preprandial BA, liver histopathology, and hepatic copper concentrations.
Results
In 64% (122/191) of the clinically healthy Labrador retrievers, hepatic histology revealed inflammatory infiltrates. This frequency might be biased because part of them was included as first‐line relatives of dogs with copper‐associated hepatitis. Sensitivity of ALT, ALP, and BA in this population for detecting acute hepatitis was 45, 15, and 15%, respectively. For chronic hepatitis, sensitivity was 71, 35, and 13%, respectively. Specificity of ALT, ALP, and BA was >90% for AH, CH, and RH. When increased liver enzymes were present, median ALT was significantly higher in PH cases (312 U/L, range 38–1,369) compared to RH cases (91 U/L, range 39–139) (P < .001). There was no difference in ALP between dogs with a PH and a RH (P = .361).
Conclusions and Clinical Importance
Histopathologic abnormalities in the liver were present in the majority of apparent clinically healthy Labrador retrievers. The sensitivity of ALT, ALP, and BA for detecting acute and chronic hepatitis in this population was low. More sensitive biomarkers are needed for early detection of liver disease in apparent clinically healthy dogs.
Keywords: Copper, Dog, Liver injury, ROC curve
Abbreviations
- AH
acute hepatitis
- ALP
plasma alkaline phosphatase activity
- ALT
plasma alanine aminotransferase activity
- AUC
area under the curve
- BA
preprandial plasma bile acid concentration
- CH
chronic hepatitis
- CI
confidence interval
- CuQ
quantitative copper
- dwl
dry weight liver
- NL
normal liver
- PH
primary hepatitis
- RH
nonspecific reactive hepatitis
- ROC
receiver operating characteristic
Primary hepatitis is one of the most frequently diagnosed parenchymal liver diseases in the dog.1 Examples of PH include acute hepatitis (AH), chronic hepatitis (CH), lobular dissecting hepatitis, and granulomatous hepatitis.2 CH is by far the most common observed form of PH.1, 3 In the majority of dogs with CH, the etiology remains undetermined and is considered to be of idiopathic origin.4 AH can be induced by a variety of stimuli, including toxins, adverse drug reactions, infectious disease (i.e, canine adenovirus‐1 infection, leptospirosis), or is considered idiopathic.1, 2, 5, 6, 7 In the last few decades, copper toxicosis is recognized with increased frequency as an etiologic factor in the development of both AH and CH in several dog breeds,8, 9, 10, 11, 12 including the Labrador retriever.13, 14, 15, 16
Clinical signs of liver disease are usually not very specific, except in end‐stage liver disease, where icteric mucous membranes and presence of ascites might indicate advanced liver disease.
Unfortunately, cases of CH are often only diagnosed in an advanced stage when there is extensive hepatocellular injury, loss of liver function, or both. At this stage, treatment is less likely to be effective and prognosis is usually very guarded.1 For successful management of the disease, early identification of (subclinical) dogs with PH is of great importance.
The most commonly used indicators of hepatobiliary disease are plasma liver enzyme activity and preprandial bile acid concentrations (BA).17, 18, 19, 20, 21, 22, 23 Alanine aminotransferase (ALT) is a liver specific cytosolic enzyme and a sensitive indicator for hepatocellular injury.17 Alkaline phosphatase (ALP) is believed to have a good sensitivity for liver diseases, with higher activities in cholestatic disease and chronic hepatitis/cirrhosis, although being less liver specific.17, 24, 25, 26 However, increases in plasma liver enzymes might be encountered in dogs wherein the liver parenchyma reacts to a variety of extra‐hepatic diseases (i.e, inflammatory bowel disease, pancreatitis)27, 28 leading to a nonspecific reactive hepatitis (RH).2 The concomitant increase in ALT, ALP, or both can make it difficult to distinguish RH from true primary liver disease. Whereas liver enzymes establish the presence of liver injury, bile acids are often used in the identification of hepatobiliary dysfunction due to decreased liver mass, intra‐ and extrahepatic cholestasis or portosystemic shunting.18, 29 Measuring BA is a sensitive method to establish liver dysfunction,26 but similar to liver enzymes, these measurements are not sufficient in specifying the underlying liver disease. Therefore, definitive diagnosis relies on an extensive clinical work‐up, usually including histopathologic evaluation of a liver biopsy specimen.
Although biochemical indicators with high sensitivity for liver disease (i.e, ALT and ALP) are essential to identify affected dogs in an early stage of disease, robust data considering sensitivity and specificity of ALT, ALP, and BA in dogs with subclinical hepatitis are currently missing. The main aims of the current study were (1) to calculate the sensitivity and specificity of ALT, ALP and BA for detecting hepatitis in clinically healthy Labrador retrievers and (2) to evaluate whether ALT and ALP aid in the discrimination between dogs with PH and RH. Further, correlation of ALT and ALP with grading and staging of the hepatitis was assessed and the influence of hepatic copper concentration on liver enzymes and BA was determined.
Material and Methods
Labrador Retrievers
All Labrador retrievers in this study were referred to the Department of Clinical Sciences of Companion Animals, Utrecht University, between 2003 and 2015. Dogs were referred because of possible liver‐related clinical signs or because of increased liver enzymes. In addition, client‐owned clinically healthy Labrador retrievers were used that participated in the ongoing research program into copper‐associated hepatitis lead by Fieten et al.30 These dogs were either first‐line relatives of dogs diagnosed with copper‐associated hepatitis or admitted for screening for copper‐associated hepatitis before breeding. Data of 299 Labrador retrievers, concerning signalment, medical history, clinical, laboratory, and histopathologic findings, were retrospectively identified from medical records. Only data from dogs that were admitted for the first time were included. At time of admission, liver biopsies and blood samples were collected from all dogs according to the Act on Veterinary Practice, as required under Dutch legislation and all procedures were known and approved by the Animal Welfare Body of the University of Utrecht.
Histopathology was considered as the gold standard for diagnosing dogs with parenchymal liver disease and was reviewed as described below. Dogs defined as normal (NL) in this study had no clinical signs of disease and absence of liver disease on histopathologic evaluation of liver biopsy specimen. Based on the localization and concentration of hepatic copper, these dogs were further subdivided in dogs with normal or high hepatic copper concentrations. Dogs were considered to be subclinically affected when they had no clinical signs of disease but histopathology revealed hepatitis, regardless of copper quantification or biochemical blood parameter results. Normal or subclinically affected dogs with nonsystemic concurrent disease were included in the study provided that they were not receiving any medication.
Blood Analyses
Immediately before the biopsy procedure, plasma ALT, ALP, and fasting BA concentrations were measured from heparinized plasma and determined using a DXC‐600 Beckman analyzer1 at the Veterinary Diagnostic Laboratory of Utrecht University (UVDL). Reference ranges for ALT, ALP, and fasting BA were established by the UVDL and are <70 U/L, <89 U/L, and <10 μmol/L, respectively. Results of ALT, ALP, BA, and quantitative copper (CuQ) measurements were available for 236, 238, 233, and 222 dogs, respectively. In addition, citrate plasma was taken for analysis of the coagulation profile, including prothrombin time, activated partial thromboplastin time, and fibrinogen concentration. Platelet count was determined in an EDTA blood sample. When coagulation parameters were not within reference range, the liver biopsy procedure was postponed and dogs were treated with 1 mg/kg prednisone daily during 1 week to normalize coagulation parameters.31
Histopathology of Liver Specimens
At least 3 liver biopsies from the left lateral liver lobe were collected with a 14‐G needle using a Tru‐cut device under ultrasound guidance as described previously.32 Two biopsy specimens were fixed in 4% neutral buffered formalin for 3 hours and transferred to 70% ethanol and embedded in paraffin. Paraffin sections of biopsies were stained with hematoxylin and eosin and reticulin according to Gordon and Sweet.33 After histopathologic evaluation, liver biopsy results were categorized into PH or RH. PH included dogs with AH or CH (including dogs with cirrhosis). Dogs with normal liver histology on hematoxylin and eosin and reticulin were included in the normal liver (NL) group. A rubeanic acid stain was used to assess the presence and distribution of copper within the liver lobule. All samples were evaluated by a board certified pathologist (TSGAMvdI, diplomat ECVP) according to the standards of the WSAVA.2 Grading and staging of biopsy samples was performed according to the guidelines described by the WSAVA.2 A third biopsy specimen was collected in a copper free container and freeze‐dried before quantitative copper determination by Instrumental Neutron Activation Analysis.34 Quantitative copper (CuQ) concentrations >400 mg/kg dry weight liver (dwl) were considered to be abnormally elevated.35
Statistical Analysis
A Wilcoxon rank sum test was used to compare ALT, ALP, and BA between dogs with low and high hepatic copper concentrations. Associations between ALT and ALP and histologic grade and stage were analyzed using the Spearman's rank correlation. ALT, ALP, BA, and CuQ concentrations were analyzed by linear regression, where histologic diagnosis, age at time of biopsy, and sex were entered as fixed variables. The best fitting model for the data was determined with a stepwise forward model using Akaike's information criterion. The validity of all models was checked by studying the residuals on normality and constant variance. To ensure validity of the model, ALT, ALP, and CuQ were ln‐transformed. For BA, the transformation ln(BA+0.1) was used, as some dogs had BA concentrations of 0. A logit model was used to calculate the odds ratio for PH, with ALT as co‐factor, corrected for sex and age. NL and RH were used as reference category. Receiver operating characteristic (ROC) curve analyses were used to determine the sensitivity and specificity of ALT, ALP, and BA for detecting the presence of hepatitis in clinically healthy dogs. Normally distributed data were presented as mean ± standard deviation and non‐normally distributed data as median and range. P‐values were adjusted for multiple testing using the Bonferroni correction. Hereto, reported P‐values were multiplied by the number of tests performed. All data were analyzed using R statistics2. ROC curves were generated using the R package “pROC.”36
Results
Animal Characteristics
Records of 299 Labrador retrievers were evaluated. Five dogs had malignant lymphoma, 9 dogs had steroid induced hepatopathy, 1 dog had granulomatous hepatitis, and 1 dog had lobular dissecting hepatitis on liver histology and were all excluded from the study. Twenty‐six normal or subclinical dogs were excluded from the study because they were receiving medications for extra‐hepatic disease. Another 5 dogs were excluded due to concurrent extra‐hepatic cancer. Nine clinically ill dogs and 1 clinically healthy dog were excluded because they received corticosteroids for more than 3 weeks or for unknown duration or in an unknown dose. In total, 242 Labrador retrievers were included in the study.
Of the 242 Labrador retrievers, 155 dogs were female and 87 were male. The mean age at time of liver biopsy was 6.1 ± 2.9 years. Of all dogs, 81 dogs were single admitted cases and 161 dogs were family members of at least 1 other dog included in the study, representing 22 different families. Of the 51 clinically ill dogs, 12 dogs received 1 mg/kg body weight prednisolone to normalize coagulation. AF, ALT, and BA values were used before start of prednisolone and liver biopsies were taken within 1 week from initial presentation. These twelve dogs had no histopathologic signs of steroid induced hepatopathy. Of the 191 clinically healthy dogs, 17 dogs had concurrent nonsystemic disease that did not require therapy. Twelve dogs had primary epilepsy, with seizure frequency too low to require therapy (less than 1 seizure per 2 months) and no seizures in the previous months. The other dogs were diagnosed with ectopic ureters (n = 2), deafness (n = 1), arthrosis (n = 1), and partial cranial cruciate rupture (n = 1). Beside these diseases, there were no indications for other diseases in participating dogs. Fifty‐one Labradors were clinically ill and showed one or more of the following clinical signs: vomiting (n = 36), lethargy (n = 32), anorexia (n = 31), icterus (n = 27), polyuria and polydipsia (n = 18), weight loss (n = 15), diarrhea (n = 7), fever (n = 5), ascites (n = 6), and exercise intolerance (n = 3). Forty‐nine dogs had more than 1 clinical sign of disease.
Hepatic Copper Assessment
Hepatic copper granules in Labrador retrievers with increased hepatic copper concentrations were located in the centrilobular region of the liver lobule on rubeanic acid stain, indicating primary copper accumulating disease. Compared to normal liver (NL), biopsies with acute hepatitis (AH) and chronic hepatitis (CH) had increased hepatic copper concentrations (Fig 1). The highest copper concentrations were identified in dogs with AH (median 964 mg/kg dwl, range 243–3,030). In dogs with AH, copper concentrations were 1.7 times higher compared to dogs with normal liver histology (95% CI: 1.2–2.5; P = .002; Fig 1). Dogs with CH had a median copper concentration of 685 mg/kg dwl (range 85–5,084), which was also significantly increased compared to the NL group (estimate, 1.3; 95% CI: 1.0‐1.8; P = .035; Fig 1). There were no statistically significant differences in ALT, ALP, and BA concentrations in dogs (n = 222) with normal (<400 mg/kg dwl) or increased (>400 mg/kg dwl) hepatic copper concentrations (Fig S1). There were no statistically significant differences in ALT, ALP, and BA concentrations in dogs (n = 69) with normal (<400 mg/kg dwl) or increased (>400 mg/kg dwl) hepatic copper concentrations measured in dogs with normal liver histology (Fig S2). Because there was no difference in ALT, ALP, and BA level between dogs with high and normal copper concentrations, no differentiation between normal and high copper groups was made for the rest of the study.
Figure 1.
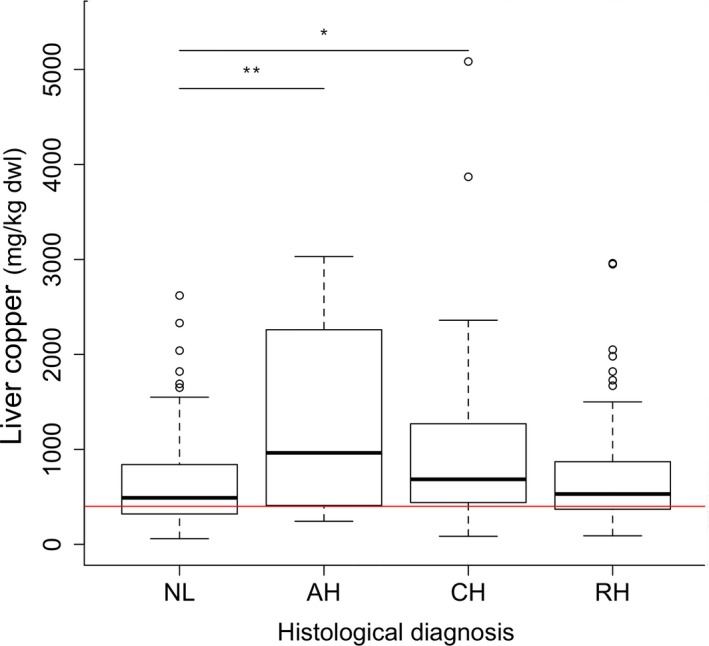
Quantitative copper concentrations in 222 Labrador retrievers with different histologic diagnosis. Horizontal red line indicates upper reference limit of normal hepatic copper concentration (400 mg/kg dwl). AH, acute hepatitis; CH, chronic hepatitis; CuQ, quantitative copper concentration; dwl, dry weight liver; NL, normal liver; RH, nonspecific reactive hepatitis. The thick black line represents the median (50th percentile), and also the first quartile and third quartile (25th and 75th percentile, respectively) are displayed. Outliers are depicted with an open dot, representing values higher than 1.5 times the interquartile range. *P < .05, **P < .01.
ALT, ALP and BA in Normal Liver, Acute‐ and Chronic Hepatitis and Nonspecific Reactive Hepatitis
In Table 1, the results of ALT, ALP, and BA measurements in 242 Labrador retrievers with different clinical presentations and liver histopathology are summarized. Of the 242 dogs, 28% (69/242) dogs had NL, 11% (27/242) AH, 23% (55/242) CH, and 38% (91/242) RH. In these 242 dogs, ALT, ALP, and BA concentrations were increased in dogs with AH and CH compared to dogs with NL or RH (P < .001; Fig 2). Dogs with CH had higher ALT and ALP concentrations compared to dogs with AH (P < .001; Fig 2A,B).
Table 1.
ALT, ALP, and BA in 242 Labrador retrievers
| Clinically healthy | Clinically ill | ||
|---|---|---|---|
| Subclinically affected n = 122 | n = 51 | ||
| Normal n = 69Normal histology NL, n = 69 | Abnormal histology PH, n = 37 (AH, n = 20, CH, n = 17) RH, n = 85 | Abnormal histology PH, n = 45 (AH, n = 7, CH, n = 38) RH, n = 6 | |
| ALT (U/L, median and range) |
33 (17–197) n = 68 |
40 (5–575) n = 122 |
376 (14–1,369) n = 46 |
| ALP (U/L, median and range) |
23 (8–109) n = 68 |
27 (12–478) n = 121 |
381 (20–1,500) n = 49 |
| BA (μmol/L, median and range) |
2 (0–16) n = 69 |
2 (0–146) n = 120 |
36 (1–325) n = 44 |
AH, acute hepatitis; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BA, bile acids; CH, chronic hepatitis; NL, normal liver; PH, primary hepatitis; RH, nonspecific reactive hepatitis.
Figure 2.
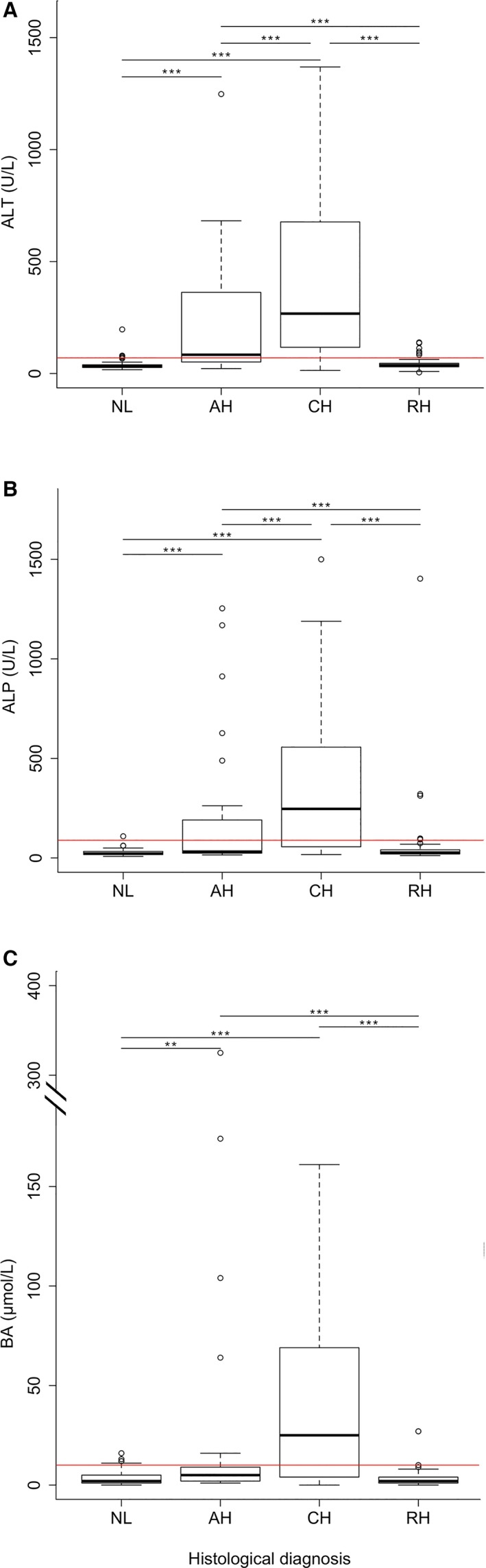
Concentrations of ALT, ALP and BA in Labrador retrievers. Concentrations of ALT (A), ALP (B), BA (C) in 242 Labrador retrievers used in the study. AH, acute hepatitis; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BA, bile acids; CH, chronic hepatitis; NL, normal liver; RH, nonspecific reactive hepatitis. Horizontal red lines indicate upper reference limits for ALT (<70 U/L), ALP (<89 U/L), and BA (<10 μmol/L). The thick black line represents the median (50th percentile), and also the first quartile and third quartile (25th and 75th percentile, respectively) are displayed. Outliers are depicted with an open dot, representing values higher than 1.5 times the interquartile range. **P < .01, ***P < .001.
In the 191 clinically healthy dogs (normal n = 69, subclinically affected n = 122), 146 dogs (76%) had ALT, ALP, and BA within reference range, and 1 dog had an increase of all 3 parameters (Fig 3A‐C). In all other dogs, at least 1 parameter was increased. Clinically healthy dogs with CH had a significantly increased ALP compared to dogs with NL (P < .001), AH (P = .029), and RH (P < .001) (Fig 3B). ALT concentrations were increased in dogs with AH and CH compared to dogs with NL or RH (P < .001; Fig 3A). There were no significant differences in BA concentrations between histology groups in the clinically healthy dogs (Fig 3C).
Figure 3.
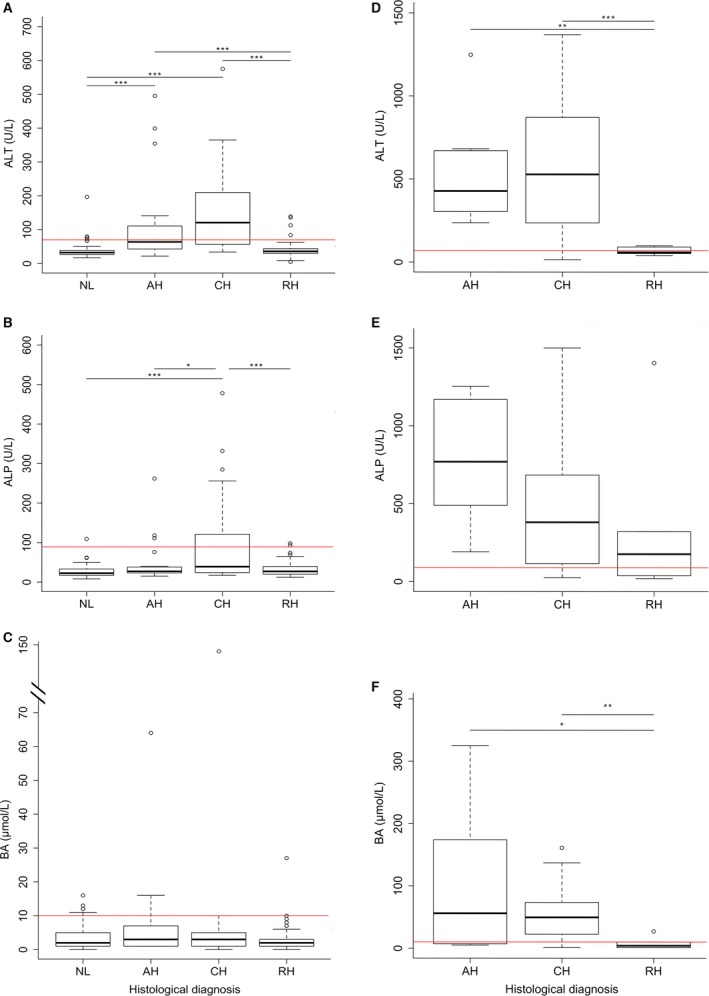
Concentrations of ALT, ALP, and BA in clinically healthy and clinically ill Labrador retrievers. Concentrations of ALT (A), ALP (B), and BA (C) in 191 clinically healthy Labrador retrievers. This includes 69 dogs with normal histology and 122 dogs with abnormal histology. Concentrations of ALT (D), ALP (E), and BA (F) in 51 clinically ill Labrador retrievers. AH, acute hepatitis; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BA, bile acids; CH, chronic hepatitis; NL, normal liver; RH, nonspecific reactive hepatitis. Horizontal red lines indicate upper reference limits for ALT (<70 U/L), ALP (<89 U/L), and BA (<10 μmol/L). The thick black line represents the median (50th percentile), and also the first quartile and third quartile (25th and 75th percentile, respectively) are displayed. Outliers are depicted with an open dot, representing values higher than 1.5 times the interquartile range. *P < .05, **P < .01, ***P < .001.
In clinically ill dogs, 88% (45/51) had a PH and 12% (6/51) a RH. Only 2 dogs had ALT, ALP, and BA within reference range (Fig 3D‐F). ALT and BA concentrations were significantly increased in dogs with AH and CH compared to dogs with RH, whereas ALP concentrations did not differ between groups (Fig 3D‐F).
When all dogs with hepatitis (n = 173), including subclinically affected (n = 122) and clinically ill dogs (n = 51), were considered, 98 dogs had ALT and ALP activity within reference range and 93 dogs had ALT, ALP, and BA within reference range. In the 69 dogs with normal liver histology, 13 dogs showed increase in either ALT, ALP, or BA.
ALT and ALP in Different Grades and Stages of Hepatitis
Grading of the necro‐inflammatory activity of hepatitis and staging of fibrosis were assessed in 95 liver biopsies (NL, n = 18; AH, n = 12; CH, n = 25; RH, n = 40) specimens of both clinically healthy and clinically ill dogs. Median grading scores (scores 0‐5) were 0 (NL), 1 (RH), 2 (AH), and 3 (CH). Median staging scores (scores 0‐4) were 0 (NL), 0 (RH), 0 (AH), and 3 (CH). A significant moderate positive correlation between ALT and grade of the hepatitis (r = 0.63, P < .001) was found (Fig 4A). A moderate association between ALT and hepatitis stage (r = 0.43, P < .001) was present (Fig 4B). For ALP, there was a moderate positive correlation with grade (r = 0.38, P < .001) and stage (r = 0.42, P < .001) of the hepatitis (4C,D).
Figure 4.
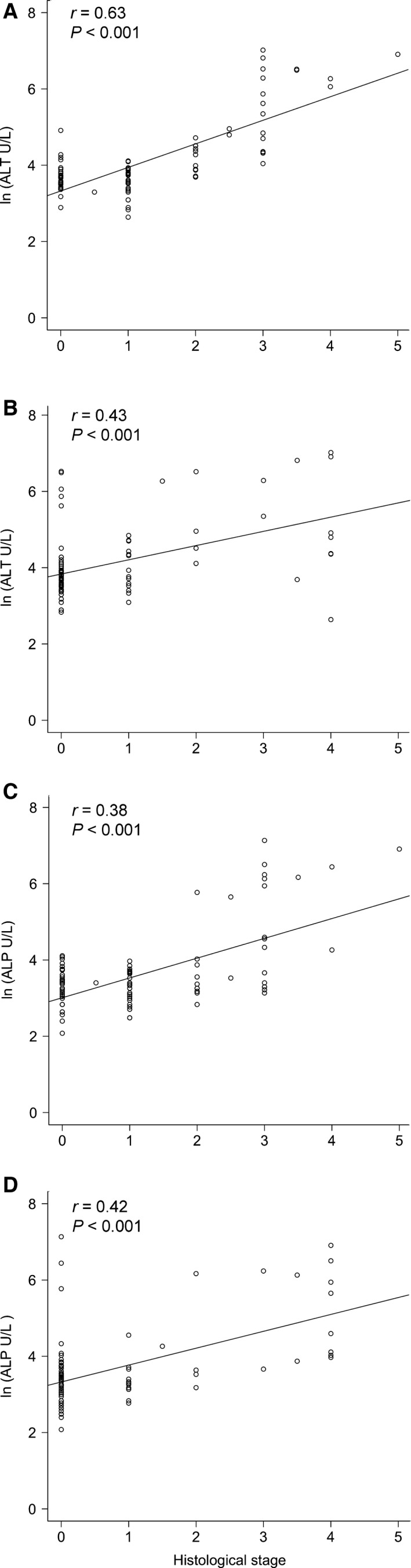
Histologic grade and stage. Correlation between histologic grade (A) and stage (B) with ALT. In the bottom row, the correlation between histologic grade (C) and stage (D) with ALP is depicted. ALT, alanine aminotransferase; ALP, alkaline phosphatase; r, Spearman rank correlation coefficient.
Differentiation between PH and RH using ALT and ALP
To investigate the possibility to discriminate PH from RH, based on the increase in ALT and ALP, Labrador retrievers in which ALT, ALP, or both were increased were selected. In 80 clinically healthy and clinically ill dogs, at least one of the liver enzymes was increased above reference range. Most of these dogs (65/80; 81%) had a PH, whereas 11% (9/80) had a RH, and 8% (6/80) had no abnormalities on histology. Median ALT activity of dogs with PH (312 U/L, range 38–1,369) was 4.3 times increased (95% CI 2.1–8.9; P < .001) compared to dogs with NL (median ALT of 75 U/L, range 17–197) and 3.6 times increased (95% CI 2.0–6.7; P < .001) compared to dogs with RH (median ALT of 91 U/L, range 39–139) (Fig 5A). There was no significant difference in ALT between dogs with NL and RH. Median ALP activity in dogs with PH (255 U/L, range 21–1,500) was significantly increased (estimate, 4.3; 95% CI: 1.5–12.4; P = .009) compared to dogs with NL (median ALP of 41 U/L, range 26–109) (Fig 5B). No difference was found in ALP activity between dogs with PH and RH (median ALP of 96 U/L, range 27–1,403) or between dogs with RH and NL.
Figure 5.
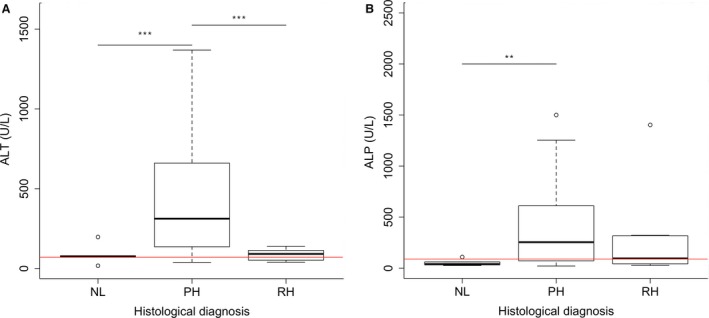
ALT and ALP activity in dogs with normal liver histology, primary hepatitis, or reactive hepatitis. This group included 80 normal, subclinically affected or clinically ill dogs of which at least one of the liver enzymes was increased above reference range. (A) ALT. Horizontal red line indicates upper reference limit of ALT (70 U/L). (B) ALP. Horizontal red line indicates upper reference limit of ALP (89 U/L). The thick black line represents the median (50th percentile), and also the first quartile and third quartile (25th and 75th percentile, respectively) are displayed. Outliers are depicted with an open dot, representing values higher than 1.5 times the interquartile range. Significant differences between groups are marked with stars (**P < .01, ***P < .001). ALT, alanine aminotransferase; ALP, alkaline phosphatase; NL, normal liver; PH, primary hepatitis; RH, nonspecific reactive hepatitis.
To measure the association between ALT and the presence of a PH, odds ratios were calculated. For every 1 U/L increase in ALT activity, a 2 percent increase in the odds of having a PH (versus having NL or a RH) is expected (95% CI: 1.01–1.04; P = .007).
Sensitivity and Specificity of ALT, ALP, and BA for Detection of Hepatitis in 191 Clinically Healthy Labrador Retrievers
ROC curves of ALT, ALP, and BA for discriminating normal dogs from dogs with AH, CH, or RH were made (Fig 6). Using our laboratory's threshold values, corresponding sensitivity and specificity of these parameters were determined (Table 2). Based on the area under the curves (AUC, Table 2), the power to discriminate dogs with AH from NL was significantly higher for ALT compared to ALP (P = .023) and BA (P = .016). For dogs with CH, ALT had a significant better ability to discriminate dogs with CH from dogs with NL than ALP (P = .005) and BA (P < .001). The discriminating ability of ALP was significantly better compared to BA (P = .040).
Figure 6.
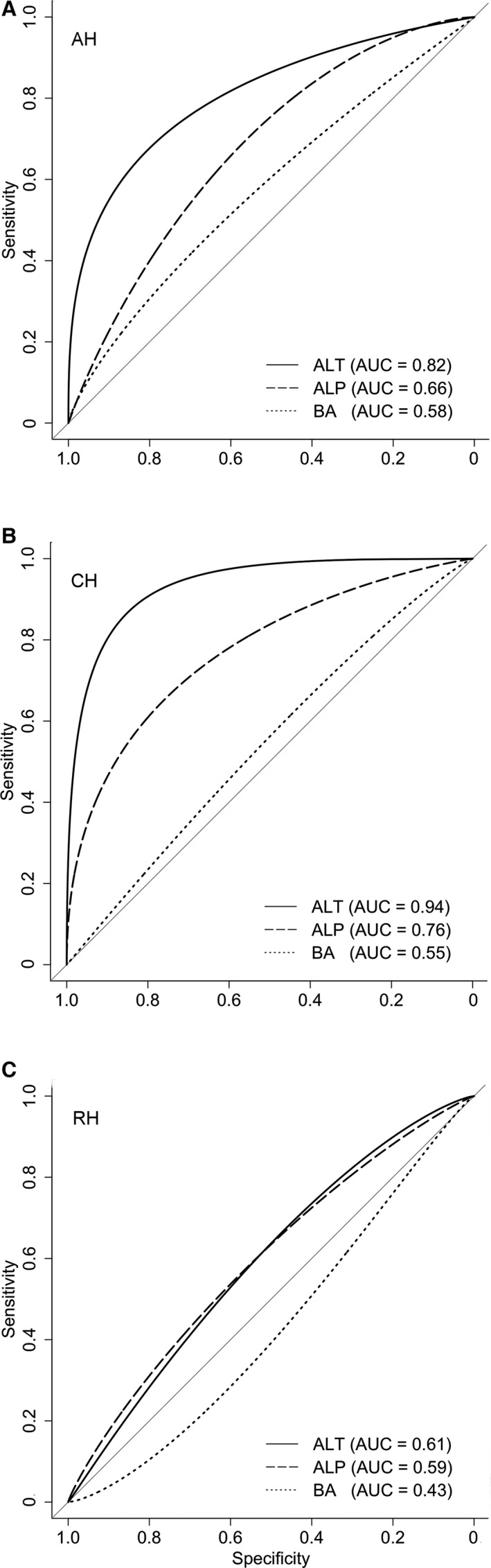
Receiver operator characteristic (ROC) curves for ALT, ALP, and BA in 191 clinically healthy Labrador retrievers. ROC curves for discriminating dogs with normal liver (n = 69) from dogs with acute hepatitis (n = 20) (A), dogs with chronic hepatitis (n = 17) (B), and dogs with nonspecific reactive hepatitis (n = 85) (C) ALT (solid line), ALP (dashed line), and BA (dotted line) ALT, alanine aminotransferase; ALP, alkaline phosphatase; AUC, area under curve; BA, bile acids.
Table 2.
Sensitivity and specificity for detecting acute hepatitis, chronic hepatitis, and nonspecific reactive hepatitis in clinically healthy Labrador retrievers
| Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | |
|---|---|---|---|
| Acute hepatitis | |||
| ALT 70 U/L | 0.45 (0.25–0.65) | 0.93 (0.85–0.99) | 0.82 (0.69–0.94) |
| ALP 89 U/L | 0.15 (0.00–0.35) | 0.99 (0.96–1.00) | 0.66 (0.53–0.79) |
| BA 10 μmol/L | 0.15 (0.00–0.30) | 0.90 (0.83–0.97) | 0.58 (0.43–0.72) |
| Chronic hepatitis | |||
| ALT 70 U/L | 0.71 (0.47–0.94) | 0.93 (0.85–0.99) | 0.94 (0.87–1.00) |
| ALP 89 U/L | 0.35 (0.12–0.59) | 0.99 (0.96–1.00) | 0.76 (0.63–0.89) |
| BA 10 μmol/L | 0.13 (0.00–0.31) | 0.90 (0.81–0.96) | 0.55 (0.39–0.71) |
| Nonspecific reactive hepatitis | |||
| ALT 70 U/L | 0.05 (0.01–0.09) | 0.93 (0.85–0.99) | 0.61 (0.52–0.70) |
| ALP 89 U/L | 0.02 (0.00–0.06) | 0.99 (0.96–1.00) | 0.59 (0.50–0.68) |
| BA 10 μmol/L | 0.04 (0–0.08) | 0.90 (0.83–0.97) | 0.43 (0.34–0.52) |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AUC, area under the curve; BA, bile acids; CI, confidence interval.
Discussion
Biochemical variables including ALT, ALP, and BA are often useful to make a presumptive diagnosis of liver disease in dogs with clinical signs that might be related to liver dysfunction. However, especially in cases of CH, there is a long subclinical phase in which dogs do not show clinical signs whereas hepatitis is already present. The first aim of this study was to determine the sensitivity and specificity of ALT, ALP, and BA for detecting AH, CH, and RH in clinically healthy Labrador retrievers. In the present study, we showed that ALT has a reasonable sensitivity to identify dogs with CH (71%), but lacks sensitivity to detect dogs with AH (45%). The sensitivity of ALP and BA for AH (15% for both) and RH (35 and 13%, respectively) was low, and these parameters are therefore not appropriate to use as screening parameters to detect early primary hepatic disease. Our second aim was to investigate the role of ALT, ALP, and BA in discriminating between PH and RH in Labradors. Hereto, data were used from dogs in which ALT, ALP, or both were increased. Our results show that ALP and BA cannot differentiate between both, but the odds of having a PH increase with increasing ALT.
The sensitivity of ALT, ALP, and BA was determined in dogs with NL, RH, AH, and CH. ALT was better in detecting dogs with AH and CH compared to ALP and BA, but overall sensitivity of these biochemical indicators for detecting dogs with inflammatory liver lesions was low. Hepatic changes in Labrador retrievers in the present study were only mild (Fig 5), which might be a reason for the relatively poor sensitivity of biochemical indicators to detect the presence of CH or AH. Another reason for the poor sensitivity could be that the current reference values are too high, at least for this population. In our laboratory, the upper limit of the reference range is used as cutoff to discriminate healthy animals from diseased animals. Reference ranges are usually determined in a group of clinically healthy dogs, but in most cases, no histopathology results are present to confirm the health status of these dogs. In this study population, a considerable number of apparent clinically healthy Labrador retrievers had inflammatory lesions in the liver. This might be due to the fact that the majority of this population of clinically healthy dogs was related to a Labrador retriever with clinical copper‐associated hepatitis and would possibly be more prone to have (early) inflammatory lesions in the liver. In this population of dogs at risk, it might be an option to decrease current ALT threshold to facilitate early detection of inflammatory lesions. Further, results from this study might not be directly translatable to other populations of dogs, Labrador retrievers or other breeds, without a predisposition to (copper‐associated) hereditary liver disease.
Preprandial bile acids had a sensitivity of less than 15%. As all dogs were clinically healthy dogs, the degree of liver dysfunction was assumed to be minimal reflecting low preprandial bile acid concentrations. Postprandial bile acids could potentially perform better than basal bile acids in some liver diseases. However, it has been reported that neither of both tests consistently outperforms the other, but does suggest measuring both preprandial and postprandial bile acids or either bile acids together with liver enzyme activities.26 Evaluation of data on sensitivity and specificity of postprandial bile acids in clinically healthy dogs with possible hepatitis would have been an interesting addition to our study; however, bile acid stimulation tests are not routinely performed in our hospital.
In the present study, the specificity of ALT and ALP for hepatitis in clinically healthy Labrador retrievers was found to be 93 and 99%, respectively. However, Center et al.17 reported much lower specificities for both enzymes, especially ALP. This difference can be explained because our study did not include dogs with known extra‐hepatic disease or taking medications influencing enzyme levels, and therefore, specificity might be overestimated compared to the general population.
Another goal of our study was to evaluate the possibility to discriminate between a PH and a RH in dogs with increased ALT, ALP, or both. In both RH and PH, dogs might present with elevated ALT, ALP, or both. In dogs with RH, it is the underlying disease that requires attention and usually the hepatic changes resolve once the extra‐hepatic diseases or stimuli are treated or removed. Interestingly, we found that ALT was significantly higher in dogs with a PH compared to dogs with a RH. ALT is present in the hepatocyte cytosol and leaks upon altered hepatocyte membrane integrity and is reported to be the first and most sensitive parameter to increase in dogs with hepatocellular inflammation and necrosis.17, 37 In RH, hepatocellular damage is usually limited, which can explain lower ALT. Although there is still overlap in ALT levels between dogs with a PH and with a RH, the magnitude of increase of ALT might aid in distinguishing PH from RH.
Copper‐associated hepatitis is one of the most common forms of primary hepatitis in dogs as shown by a study conducted in 101 purebred and crossbred dogs in the Netherlands, of which up to 36% of the CH and 24% of the AH cases were copper‐associated.1 The Labrador retriever is a dog breed known to be affected with hereditary copper‐associated hepatitis,38 influenced by environmental factors such as dietary copper intake.15, 30 In the current study, increased hepatic copper concentrations were present in Labrador retrievers with different histologic diagnosis, including histologically normal liver, but concentrations were highest in dogs with AH. In contrast to dogs with AH, dogs with CH have fibrosis with or without cirrhosis. Neither fibrotic nor regenerative nodules contain accumulated copper granules, diluting total copper content of the original hepatocyte population.39, 40, 41 Liver biopsies might not be representative due to the sampling of fibrotic tissue only, but this was likely compensated by taking 3 different 14G samples. However, biopsies were only taken from the left liver lobe, which might account for discordant results, as variation in the distribution of histopathologic abnormalities between liver lobes might be present.42 There was no difference between dogs with normal and high hepatic copper concentrations in ALT, ALP, and BA, which was measured in dogs with and without concurrent presence of inflammatory liver disease. Although this result was not surprising, the current data provide evidence that ALT, ALP, and BA are not sensitive enough to detect increased hepatic copper concentrations in liver biopsies without additional inflammatory changes. Liver histology, including copper staining and copper quantification remains necessary to obtain a diagnosis.
Although this study was performed in the Labrador retriever, results from this study might potentially be of value to dogs from other breeds. However, without availability of hepatic biopsies in clinically healthy dogs, incidence of inflammatory lesions and estimation of sensitivity and specificity of biochemical indicators in these dogs is impossible. Further, the prevalence of early hepatic lesions in other dog breeds remains to be determined.
A limitation of the study was the retrospective nature; however, all data were obtained using a consistent clinical protocol which is used in all dogs with parenchymal liver diseases. None of the dogs was fed a (combination of) low copper or high zinc diet, but no information was collected with respect to dietary copper content.
This study did not include dogs with extra‐hepatic disease influencing enzyme levels (e.g, hyperadrenocorticism or bone disease), which might be considered a limitation of this study. The lack of these samples is obviously due to the fact that there is no indication for liver histopathology in these dogs. If these dogs were included in the study and specificity was determined in all dogs, true specificity of biochemical indicators would likely be lower. In the present study, twelve dogs received 1 mg/kg prednisone treatment for 1 week to normalize coagulation parameters,1, 31 and liver biopsy was therefore precluded. It is known that prednisone treatment can lead to glycogen accumulation in the hepatocytes.43 In addition, prednisone has beneficial effects on inflammation and in some dogs might even reduce or limit the progression of fibrotic changes.31 In our study, no steroid induced hepatocellular changes (ballooning) were present on histopathology, but it cannot be excluded that short‐term prednisone treatment ameliorated necro‐inflammatory activity in these dogs.
In conclusion, in this study, the sensitivity of ALT for detecting dogs with CH was reasonable, whereas it was poor for detecting dogs with AH or RH. ALP and BA are not sensitive enough for the detection of AH, CH, or RH in clinically healthy dogs. Both ALT and ALP correlate with necro‐inflammatory grade of hepatitis and stage of fibrosis. The magnitude of increase in ALT might aid the clinician in determining whether PH rather than RH is present in Labrador retrievers in which ALT, ALP, or both are increased. Hepatic copper accumulation in itself is not correlated to ALT, ALP, or BA.
Supporting information
Figure S1. Concentrations of ALT (A), ALP (B) and BA (C) in all Labrador retrievers with known hepatic quantitative copper concentrations (n = 222).
Figure S2. Concentrations of ALT (A), ALP (B) and BA (C) in Labrador retrievers with normal liver histology (n = 69).
Acknowledgments
The authors thank biostatistician Hans Vernooij (Theoretical Epidemiology, Utrecht University) for providing statistical advice.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Faculty of Veterinary Medicine, Utrecht University, Utrecht, the Netherlands.
Footnotes
Beckman Coulter, Woerden, the Netherlands
R Foundation for Statistical Computing, Vienna, Austria 2014
References
- 1. Poldervaart JH, Favier RP, Penning LC, et al. Primary hepatitis in dogs: A retrospective review (2002–2006). J Vet Intern Med 2009;23:72–80. [DOI] [PubMed] [Google Scholar]
- 2. Van den Ingh TSGAM, Van Winkle TJ, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver In: Rothuizen J. and Cullen JM, eds. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases, 1st ed Philadelphia: Saunders Elsevier; 2006:85–101. [Google Scholar]
- 3. Watson P, Roulois A, Scase T, et al. Prevalence of hepatic lesions at post‐mortem examination in dogs and association with pancreatitis. J Small Anim Pract 2010;51:566–572. [DOI] [PubMed] [Google Scholar]
- 4. Watson PJ. Chronic hepatitis in dogs: A review of current understanding of the aetiology, progression, and treatment. Vet J 2004;167:228–241. [DOI] [PubMed] [Google Scholar]
- 5. Boomkens SY, Penning LC, Egberink HF, et al. Hepatitis with special reference to dogs. A review on the pathogenesis and infectious etiologies, including unpublished results of recent own studies. Vet Q 2004;26:107–114. [DOI] [PubMed] [Google Scholar]
- 6. Lester C, Cooper J, Peters RM, Webster CR. Retrospective evaluation of acute liver failure in dogs (1995–2012): 49 cases. J Vet Emergency Critical Care 2016;559–567. [DOI] [PubMed] [Google Scholar]
- 7. Weingarten MA, Sande AA. Acute liver failure in dogs and cats. J Vet Emergency Critical Care 2015;25:455–473. [DOI] [PubMed] [Google Scholar]
- 8. Johnson GF, Sternlieb I, Twedt DC, et al. Inheritance of copper toxicosis in bedlington terriers. Am J Vet Res 1980;41:1865–1866. [PubMed] [Google Scholar]
- 9. Haywood S, Rutgers HC, Christian MK. Hepatitis and copper accumulation in skye terriers. Vet Pathol 1988;25:408–414. [DOI] [PubMed] [Google Scholar]
- 10. Thornburg LP, Shaw D, Dolan M, et al. Hereditary copper toxicosis in west highland white terriers. Vet Pathol 1986;23:148–154. [DOI] [PubMed] [Google Scholar]
- 11. Cooper VL, Carlson MP, Jacobson J, Schneider NR. Hepatitis and increased copper levels in a Dalmatian. J Vet Diagn Invest 1997;9:201–203. [DOI] [PubMed] [Google Scholar]
- 12. Mandigers PJ, van den Ingh TS, Bode P, et al. Association between liver copper concentration and subclinical hepatitis in Doberman pinschers. J Vet Intern Med 2004;18:647–650. [DOI] [PubMed] [Google Scholar]
- 13. Shih JL, Keating JH, Freeman LM, Webster CR. Chronic hepatitis in Labrador retrievers: Clinical presentation and prognostic factors. J Vet Intern Med 2007;21:33–39. [DOI] [PubMed] [Google Scholar]
- 14. Smedley R, Mullaney T, Rumbeiha W. Copper‐associated hepatitis in Labrador retrievers. Vet Pathol 2009;46:484–490. [DOI] [PubMed] [Google Scholar]
- 15. Johnston AN, Center SA, McDonough SP, et al. Hepatic copper concentrations in Labrador retrievers with and without chronic hepatitis: 72 Cases (1980–2010). J Am Vet Med Assoc 2013;242:372–380. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann G, van den Ingh TS, Bode P, Rothuizen J. Copper‐associated chronic hepatitis in Labrador retrievers. J Vet Intern Med 2006;20:856–861. [DOI] [PubMed] [Google Scholar]
- 17. Center SA. Interpretation of liver enzyms. Vet Clin North Am: Small Animal Practice 2007;37:297–333. [DOI] [PubMed] [Google Scholar]
- 18. Schlesinger DP, Rubin SI. Serum bile acids and the assessment of hepatic function in dogs and cats. Can Vet J 1993;34:215–220. [PMC free article] [PubMed] [Google Scholar]
- 19. van Straten G, Spee B, Rothuizen J, et al. Diagnostic value of the rectal ammonia tolerance test, fasting plasma ammonia and fasting plasma bile acids for canine portosystemic shunting. Vet J 2015;204:282–286. [DOI] [PubMed] [Google Scholar]
- 20. Bosje JT, Bunch SE, van den Brom W, Rothuizen J. Plasma 14C‐cholic acid clearance in healthy dogs and dogs with cholestasis or a congenital portosystemic shunt. Vet Rec 2005;157:109–112. [DOI] [PubMed] [Google Scholar]
- 21. Rothuizen J, de Vries‐Chalmers Hoynck van Papendrecht R, van den Brom WE. Post prandial and cholecystokinin‐induced emptying of the gall bladder in dogs. Vet Rec 1990;126:505–507. [PubMed] [Google Scholar]
- 22. Kerr MG, van Doorn T. Mass screening of Irish wolfhound puppies for portosystemic shunts by the dynamic bile acid test. Vet Rec 1999;144:693–696. [DOI] [PubMed] [Google Scholar]
- 23. Center SA. Serum bile acids in companion animal‐medicine. Vet Clin N Am: Small Anim Pract 1993;23:625–657. [DOI] [PubMed] [Google Scholar]
- 24. Center SA, Slater MR, Manwarren T, Prymak K. Diagnostic efficacy of serum alkaline phosphatase and gamma‐glutamyltransferase in dogs with histologically confirmed hepatobiliary disease: 270 cases (1980–1990). J Am Vet Med Assoc 1992;201:1258–1264. [PubMed] [Google Scholar]
- 25. Sevelius E. Diagnosis and prognosis of chronic hepatitis and cirrhosis in dogs. J Small Anim Pract 1995;36:521–528. [DOI] [PubMed] [Google Scholar]
- 26. Center SA, ManWarren T, Slater MR, Wilentz E. Evaluation of twelve‐hour preprandial and two‐hour postprandial serum bile acids concentrations for diagnosis of hepatobiliary disease in dogs. J Am Vet Med Assoc 1991;199:217–226. [PubMed] [Google Scholar]
- 27. Webster CRL. History, clinical signs and physical findings in hepatobiliary disease In: Ettinger S, Feldman E, eds. Textbook of Veterinary Internal Medicine, 6th ed St Louis: Elsevier Saunders; 2005:1422–1434. [Google Scholar]
- 28. Dirksen K, Verzijl T, van den Ingh TS, et al. Hepatocyte‐derived microRNAs as sensitive serum biomarkers of hepatocellular injury in Labrador retrievers. Vet J 2016;211:75–81. [DOI] [PubMed] [Google Scholar]
- 29. Gerritzen‐Bruning M, Ingh T, Rothuizen J. Diagnostic value of fasting plasma ammonia and bile acid concentrations in the identification of portosystemic shunting in dogs. J Vet Intern Med 2006;20:13–19. [DOI] [PubMed] [Google Scholar]
- 30. Fieten H, Hooijer‐Nouwens B, Biourge V, et al. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in labrador retrievers. J Vet Intern Med 2012;26:1274–1280. [DOI] [PubMed] [Google Scholar]
- 31. Favier RP, Poldervaart JH, van den Ingh TSGAM, et al. A retrospective study of oral prednisolone treatment in canine chronic hepatitis. Vet Q 2013;33:113–120. [DOI] [PubMed] [Google Scholar]
- 32. Rothuizen J, Desmet VJ, Van Den Ingh TSGAM, et al. Sampling and Handling of Liver Tissue. Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Philadelphia, PA: Saunders Elsevier; 2006:5–14. [Google Scholar]
- 33. Gordon H, Sweets HH. A simple method for the silver impregnation of reticulum. Am J Pathol 1936;12:1. [PMC free article] [PubMed] [Google Scholar]
- 34. Bode P, Bueno MI, Bortoleto GG, et al. Neutron activation analysis and X‐Ray Rayleigh and Raman scattering of hair and nail clippings as noninvasive bioindicators for Cu liver status in Labrador retrievers. Anal Bioanal Chem 2008;390:1653–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puls R. Mineral Levels in Animal Health: Diagnostic Data, 2nd ed BC, Canada: Sherpa International, Clearbrook; 1994. [Google Scholar]
- 36. Robin X, Turck N, Hainard A, et al. pROC: An open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 2011;12:77 2105‐12‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dixon MF, Fulker MJ, Walker BE, et al. Serum transaminase levels after experimental paracetamol‐induced hepatic necrosis. Gut 1975;16:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fieten H, Gill Y, Martin AJ, et al. The menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: A new canine model for copper‐metabolism disorders. Disease Models Mechan 2016;9:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thornburg LP. A perspective on copper and liver disease in the dog. J Vet Diagn Invest 2000;12:101–110. [DOI] [PubMed] [Google Scholar]
- 40. Goldfischer S, Popper H, Sternlieb I. The significance of variations in the distribution of copper in liver disease. Am J Pathol 1980;99:715–730. [PMC free article] [PubMed] [Google Scholar]
- 41. Thornburg LP, Rottinghaus G, Dennis G, Crawford S. The relationship between hepatic copper content and morphologic changes in the liver of west highland white terriers. Vet Pathol 1996;33:656–661. [DOI] [PubMed] [Google Scholar]
- 42. Kemp S, Zimmerman K, Panciera D, et al. Histopathologic variation between liver lobes in dogs. J Vet Intern Med 2015;29:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fittschen C, Bellamy JE. Prednisone‐induced morphologic and chemical changes in the liver of dogs. Vet Pathol 1984;21:399–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Concentrations of ALT (A), ALP (B) and BA (C) in all Labrador retrievers with known hepatic quantitative copper concentrations (n = 222).
Figure S2. Concentrations of ALT (A), ALP (B) and BA (C) in Labrador retrievers with normal liver histology (n = 69).


