Abstract
Background
Investigations of antimicrobial use in companion animals are limited. With the growing recognition of the need for improved antimicrobial stewardship, there is urgent need for more detailed understanding of the patterns of antimicrobial use in this sector.
Objectives
To investigate antimicrobial use for medical and surgical conditions in dogs and cats by Australian veterinarians.
Methods
A cross‐sectional study was performed over 4 months in 2011. Respondents were asked about their choices of antimicrobials for empirical therapy of diseases in dogs and cats, duration of therapy, and selection based on culture and susceptibility testing, for common conditions framed as case scenarios: 11 medical, 2 surgical, and 8 dermatological.
Results
A total of 892 of the 1,029 members of the Australian veterinary profession that completed the survey satisfied the selection criteria. Empirical antimicrobial therapy was more common for acute conditions (76%) than chronic conditions (24%). Overall, the most common antimicrobial classes were potentiated aminopenicillins (36%), fluoroquinolones (15%), first‐ and second‐generation cephalosporins (14%), and tetracyclines (11%). Third‐generation cephalosporins were more frequently used in cats (16%) compared to dogs (2%). Agreement with Australasian Infectious Disease Advisory Panel (AIDAP) guidelines (generated subsequently) was variable ranging from 0 to 69% between conditions.
Conclusions and Clinical Importance
Choice of antimicrobials by Australian veterinary practitioners was generally appropriate, with relatively low use of drugs of high importance, except for the empirical use of fluoroquinolones in dogs, particularly for otitis externa and 3rd‐generation cephalosporins in cats. Future surveys will determine whether introduction of the 2013 AIDAP therapeutic guidelines has influenced prescribing habits.
Keywords: Antibiotic, Companion animals, Stewardship
Abbreviations
- AIDAP
Australasian Infectious Disease Advisory Panel
- C & S
culture and sensitivity
- IQR
interquartile range
- IRSAD
index for relative socioeconomic advantage‐disadvantage
- LUTI
lower urinary tract infection
Antimicrobial resistance develops in response to antimicrobial use1, 2, 3 regardless of the animal species being treated, with greater use likely to contribute to development of resistance to multiple drug classes. This is a growing threat in human hospitals, the community and in companion and production animals. Veterinary antimicrobial usage, has come under increasing scrutiny by medical, public health, and government officials, especially in food‐producing animals. In companion animals, an apparent increase, or increased reporting of multidrug‐resistant pathogens, especially coagulase‐positive staphylococcal species,4, 5, 6, 7 suggests that investigation of patterns of antimicrobial usage in companion animal practice is needed. Since the registration of fluoroquinolones (ie, enrofloxacin, marbofloxacin, difloxacin, orbifloxacin, and most recently pradofloxacin) starting in 1989 and an injectable long‐acting 3rd‐generation cephalosporin (cefovecin) in 2008, for specific use in dogs and cats, antimicrobial usage patterns in Australian companion animal practice have not been examined.
Data on antimicrobial use in companion animal practice in Australia are limited to a single cross‐sectional study carried out in 1997.8 In this survey, respondents were asked about patterns of use of various systemic antibacterial drugs and their approach to treatment of 9 specific medical scenarios. Penicillins and cephalosporins were the most commonly used drugs, with amoxicillin‐clavulanate the most frequently prescribed antimicrobial agent. Empiric antibiotic therapy was used in the vast majority of acute medical conditions (76–94% of cases) and was frequently used in chronic conditions (15–50% of cases).
The Australian Strategic and Technical Advisory Group on Antimicrobial Resistance have issued an importance rating and summary of antibacterials used in human health in Australia in 2015. Those given a high importance rating include piperacillin‐tazobactam, ticarcillin‐clavulanate (now no longer manufactured but available at the time of the survey), the 3rd‐ and 4th‐generation cephalosporins, aztreonam, tigecycline, vancomycin, teicoplanin, amikacin, the streptogramins (eg, pristinamycin), fluoroquinolones, and rifampicin.9 These antimicrobials should be treated as third‐line therapies and should only be used where culture and susceptibility (C & S) testing or other compelling clinical evidence indicates their use. Of the antimicrobials with a high importance rating, only the 3rd‐generation cephalosporins and fluoroquinolones are registered for use in dogs and cats in Australia.
The Australasian Infectious Disease Advisory Panel (AIDAP) was convened with a view to developing antimicrobial and therapeutic guidelines for common medical, surgical and dermatological conditions seen in general veterinary practice in Australia. These guidelines were released in 2013 and include evidence‐based recommendations, where possible, and specialist veterinary opinion where there was a limited evidence base.
The aims of this study were to investigate empirical antimicrobial use (ie, drug choices), the frequency of use of C & S testing as a tool for selecting antimicrobials, and the proportion of “high importance” rating antimicrobials, through case scenario presentations to identify likely practitioner prescribing behavior. A secondary aim was to determine the frequency of agreement of antimicrobial use with the AIDAP therapeutic guidelines which were generated after the survey had been conducted.
Methods
The source population for the survey was clinicians practicing veterinary medicine in Australia in 2011. At that time, there were an estimated 7,300 registered veterinarians in Australia. To be 95% certain that this estimate of the prevalence of veterinarians using a given class of antimicrobial was within 5% of a true prevalence of 50%, a total of 365 completed surveys were required. Sample size calculations were carried out assuming a 50% prevalence because this provided the largest sample size estimate for a constant margin of error. Respondents were self‐selected and were encouraged to participate through a variety of electronic and print media sources over a 4‐month period in 2011.
The survey was created online by web‐based designers in coordination with the AIDAP (questionnaire available as Supporting Information). There were 3 sections. The first section asked for the respondent's veterinary board registration number, year of graduation, and an estimate of the proportion of clinical work they performed on cats, dogs, horses, production animal, or other species. In the second section, respondents were asked to indicate their usual (>50% of the time) approach to the treatment of cats and dogs for each of 11 specific medical disorders when clinical evidence suggested the presumptive diagnosis. They were also asked about their approaches to 2 surgical conditions; routine desexing and dental scaling and polishing, with tooth extractions. The specified disorders included abscess/cellulitis, chronic gingivostomatitis/“faucitis,” acute febrile illness, peritonitis, chronic rhinosinusitis, pyothorax, acute upper and lower respiratory tract infections, acute and recurrent lower urinary tract infections (LUTI)/cystitis and LUTI with concurrent chronic kidney disease. The third section asked about management of selected dermatological conditions and otitis externa, including surface, superficial and deep pyodermas, dermatophytosis, and superficial yeast infections of the skin, as well as uncomplicated and refractory otitis externa. Both open and closed questions were used. Drop‐down menus provided lists of commercially available antimicrobials from which respondents could select their favored therapy.
Data were downloaded from the Website to spreadsheets (Microsoft Office Access, Microsoft Office Excel). Any questions not completed by a respondent were excluded from the analysis of that question. Simple descriptive statistics were computed with percentages being reported as a proportion of the total number of respondents answering a particular question. Given that this study did not use a simple random sampling design, data were analyzed to account for overrepresentation by state of practice.10 Sampling weights provided an estimate of the inverse probability of a veterinarian's involvement in the survey, W Hi, and were quantified as follows:
where N i is the number of registered veterinarians in the state in 2011, and n is the number of veterinarians from that state who completed the survey. Throughout this article, all profession level data are described using adjusted values based on survey design, sampling weights, and finite correction factors. Proportions of questionnaire responses are reported as unadjusted counts.
The index for relative socioeconomic advantage‐disadvantage (IRSAD) and usual resident population of each postcode for participants in the survey was accessed from the Australian Bureau of Statistics.11
Regression models were used to quantify the association between individual respondent‐level variables (year of graduation, percentage of small versus large animal practice, IRSAD) and the probability of a veterinarian prescribing in such a way that agreed with the AIDAP guidelines. For continuously distributed explanatory variables, a Shapiro‐Wilk test for normality was used. Differences in independent medians were assessed using the Mann‐Whitney tests. A binary logistic regression model was developed with year of graduation expressed as a 2‐level categorical variable: <5 years since graduation and 5 or greater years since graduation. The proportion of time spent on small animal practice was expressed as a 2‐level categorical variable: those that spent more than 70% of their time working with companion animals (“companion animal practitioners”) and those that spent 70% or less of their time working with companion animals (“mixed animal practitioners”). The binary outcome variable for this analysis was whether or not the reported antimicrobial usage patterns reported by the respondent were consistent with AIDAP guidelines or not. Descriptive analysis and the logistic regression analysis were carried out using Stata version 131 .
This study was organized and sponsored by a veterinary pharmaceutical company, and ethics clearance was not required by the University of Melbourne as no identifying information was used in the analysis.
Results
A total of 1,029 Australian veterinary practitioners completed the survey. Of these, 892 satisfied the selection criteria for inclusion, representing more than 12% of the total number of registered veterinarians in 2011. All states and territories were represented, as were recent and older graduates. More than 70% of respondents were companion animal practitioners. Only 4.5% of respondents had a caseload in which <50% of patients were dogs and cats.
As there was no difference in the frequency with which empirical antimicrobial therapy was used rather than antimicrobial therapy directed by the results of C & S testing between dogs and cats, the results were combined for each question. Overall, antimicrobial selections were empirical in 51% (7,290 of 14,414) of cases (range 8–79%), guided by C & S in 26% (3,694 of 14,414) of cases (range 0.1–70%), and empirical therapy was employed pending the results of C & S testing in 24% (3,430 of 14,414) (range 13–32%) of cases. There were 3 conditions in which C & S testing was used by >80% of respondents: pyothorax (80%, 95% CI 78–83%), recurrent LUTI (92%, 95% CI 91–94%) and LUTI with chronic kidney disease (80%, 95% CI 78–82%). Antimicrobial therapy guided by C & S was also commonly used for chronic rhinosinusitis (67% of responses, 95% CI 64–69%). Empirical antimicrobial therapy was more commonly used for acute conditions (median 63%, quartile 1 [Q1] 55 to quartile 3 [Q3] 77%) than chronic conditions (median 25%, Q1–Q3 17–44%, P = .01). Culture and susceptibility was used by at least 20% of respondents in all medical scenarios including abscesses. There was no difference between mixed and companion animal practitioners in the proportion of cases in which C & S was performed (4.7% higher for companion animal practitioners, 95% CI −2.5 to 12%, P = .199), although recent graduates (<5 years’ experience) used C & S guided antimicrobial therapy less commonly than older graduates (6.4% lower; 95% CI 2.4–11%, P = .002).
Routine prophylactic antimicrobial therapy was used by 25% (157 of 631) of respondents for routine desexing of cats (95% CI 22–28%) and 25% (154 of 618) of dogs (95% CI 22–28%). There was no difference in the choice of antimicrobial therapy between dogs and cats, with more than 90% of respondents indicating the use of aminopenicillins (51%), other β‐lactam drugs (25%) or potentiated aminopenicillins (17%). High importance rated antimicrobials were used by only 11 respondents for this indication (3.6%); 6 used 3rd‐generation cephalosporins (4 in cats, 2 in dogs), 3 reported using ticarcillin‐clavulanate (1 in cats, 2 in dogs), and 2 reported using enrofloxacin (1 in cats, 1 in dogs). Duration of therapy did not differ between dogs and cats with a median duration of therapy of 2 days (Q1–Q3 1–3 days).
Most respondents used antimicrobials for dental procedures, with extractions, in both cats (95% [639 of 675], 95% CI 93–96%) and dogs (94% [605 of 643], 95% CI 92–96%), and selection was empiric in 94% of cat cases (95% CI 92–96%) and 94% of dog cases (95% CI 92–96%). Antimicrobial therapy was initiated before dentistry by 64% (665 of 1,041, 95% CI 61–67%) of practitioners, and the median duration of therapy was 7 days (Q1–Q3 7–10 days). The choice of antimicrobial differed between dogs and cats with potentiated aminopenicillins (33%), clindamycin (30%), and 3rd‐generation cephalosporins (21%) used most frequently in cats, and potentiated aminopenicillins (46%) and clindamycin (35%) used most frequently in dogs. High importance rating antimicrobials were used by 13% of respondents, with the vast majority being a 3rd‐generation cephalosporin (190 of 203, 94%) which were predominately administered to cats (168 of 190, 88%).
Overall there were 22,748 antimicrobial therapies reported across the scenarios. The most commonly used antimicrobials were aminopenicillins (41% of dog therapies and 41% of cat therapies), followed by fluoroquinolones (18% of dog therapies and 11% of cat therapies), 1st‐ or 2nd‐generation cephalosporins (22% of dog therapies and 3% of cat therapies), and tetracyclines (7% of dog therapies and 17% of cat therapies) (Table 1). Use of antimicrobials with a high importance rating ranged from 12 to 47% (median 17%) for cats and 4 to 42% (median 15%) for dogs among the medical scenarios. Overall, 3rd‐generation cephalosporin use was more frequent in cats than dogs (16 versus 1.8%, P < .001) whereas fluoroquinolone use was more frequent in dogs (18 versus 11%, P < .001) (Table 1). In dogs, fluoroquinolones were also more frequently prescribed for chronic conditions than for acute conditions (18 and 15% respectively, P < .001). In cats, 3rd‐generation cephalosporins were more frequently prescribed for chronic than for acute conditions (18 and 14% respectively, P = .001). The amount of fluoroquinolone use was similar in dermatological conditions to medical conditions (11%, 95% CI 10–12%), but more frequent in otitis externa (41%, 95% CI 39–43%). In otitis externa, where bacterial rods were seen in cytological preparations, systemic fluoroquinolone use was reported by 61% (95% CI 58–64%) of respondents. For medical conditions in dogs, fluoroquinolones were used most frequently to treat pneumonia (29%, 95% CI 27–32%), pyothorax (31%, 95% CI 27–36%), and recurrent LUTI disorders (32%, 95% CI 27–37%). In cats, 3rd‐generation cephalosporins were used by more than 25% of respondents in 4 scenarios; cellulitis/abscesses (26%, 95% CI 24–28%), acute LUTI disease (33%, 95% CI 29–36%), recurrent LUTI disease (25%, 95% CI 21–31%), and LUTI with concurrent chronic kidney disease (26%, 95% CI 22–31%). In contrast, 3rd‐generation cephalosporins were much less frequently used for severe conditions in cats such as pneumonia (7.8%, 95% CI 6.3–9.6%), pyothorax (4.2%, 95% CI 2.8–6.3%), and peritonitis (3.5%, 95% CI 2.4–5.0%). The use of 3rd‐generation cephalosporins for dermatological cases was rare (1.9% overall, 95% CI 1.5–2.3%). Use of other antimicrobials with a high importance rating was rare and did not differ between dogs and cats (0.6 and 0.5%, respectively). The duration of therapy used by respondents choosing antimicrobials with a high importance rating did not differ from those choosing antimicrobials with low or medium importance rating. The distribution of the number of prescriptions of antimicrobials of high importance rating for each participant was positively skewed with lowest 50% of respondents prescribing 12% of these antimicrobials and higher 50% prescribing the remaining 88%. The low users of antimicrobials of high importance rating also used less therapy guided by C & S (38%) than high users (62%, P < .001). There was no difference in population, IRSAD, year of graduation or percentage time in companion animal practice, between low and high users of antimicrobials of high importance rating.
Table 1.
Overall frequency of antibiotic use across medical, surgical and dermatological scenarios posed in this survey
| Drug Class | Subclass or Drug | Frequency (%) | |
|---|---|---|---|
| Cats | Dogs | ||
| 1st‐ and 2nd‐generation cephalosporins | 327 (3.4) | 2,908 (22) | |
| 3rd‐generation cephalosporins | 1,548 (16) | 240 (1.8) | |
| Aminoglycosides | Gentamicin | 20 (0.2) | 54 (0.4) |
| Amikacin | 3 (<0.1) | 7 (<0.1) | |
| Total | 23 (0.2) | 61 (0.5) | |
| β‐Lactams | Unpotentiated | 487 (5.1) | 492 (3.7) |
| Potentiated | 3,330 (35) | 4,901 (37) | |
| High importance rating | 46 (0.5) | 69 (0.5) | |
| Total | 3,863 (41) | 5,462 (41) | |
| Macrolides | 706 (7.4) | 617 (4.7) | |
| Chloramphenicol | 0 (0) | 2 (<0.1) | |
| Tetracyclines | 1,579 (17) | 953 (7.2) | |
| Fluoroquinolones | 1,065 (11) | 2,389 (18) | |
| Metronidazole | 327 (3.4) | 374 (2.8) | |
| Rifampicin | 0 (0) | 29 (0.2) | |
| Trimethoprim/sulfonamides | 47 (0.5) | 161 (1.2) | |
| Other | 27 (0.3) | 40 (0.3) | |
| Total | 9,512 | 13,236 | |
Agreement with AIDAP therapeutic guidelines (posthoc) was evaluated for use of empirical therapy, use of antimicrobial therapy guided by C & S or treatment without the use of antimicrobials, as well as drug choice, duration of therapy, and overall agreement. The data were not normally distributed. Overall agreement was variable, ranging from 0 to 69% between conditions. There was no difference between medical and dermatological conditions in the extent of agreement with the guidelines. The median overall agreement was higher for dogs (38%, Q1–Q3 25–46%) than for cats (25%, Q1–Q3 16–31%, P < .001). The overall agreement with the guidelines was less than 33% for 4 conditions; gingivostomatitis (0% agreement for cats, 6.9% agreement for dogs), pyothorax (3.2% agreement for cats, 0.1% agreement for dogs), peritonitis (0.3% agreement for cats, 1.0% agreement for dogs), and acute LUTI disease/cystitis (8.8% agreement for cats, 16% agreement for dogs). For gingivitis and pyothorax, the decision to use empirical antimicrobials or C & S testing had much higher agreement with AIDAP guidelines, and the poor overall agreement was due to poor alignment with the recommendations for drug selection and duration of therapy recommendations (Fig 1A,B). For acute cystitis, the poor agreement was due to the common use of empirical antimicrobial therapy and failure to culture samples from these cases, whereas drug selection and duration of therapy were in better agreement (Fig 1C). Finally, for peritonitis, there was poor agreement in terms of both empirical choice of drug, use of C & S testing, and with the selection of drug (Fig 1D), with drugs with a limited spectrum of activity being chosen for most cases rather than the extended spectrum (usually via combination therapy) advocated in the AIDAP guidelines (96%, 95% CI 95–97%). Overall, the choice of empirical or therapy guided by C & S or treatment without the use of antimicrobials showed the best agreement with the guidelines, with a median of 83% (Q1–Q3 42–95%). There was no difference between responses about treatment of dogs or cats. The agreement with the guidelines with respect to choice of drug, where indicated, did not differ between dogs and cats, with an overall median 43% (Q1–Q3 5–57%). Similarly, agreement with the guidelines on duration of therapy and drug selection, where indicated, had the same level of agreement between dogs and cats (overall median 36%, Q1–Q3 25–67%). There was no significant difference in agreement with AIDAP guidelines for practitioners who were recent graduates (past 5 years) compared to older graduates, nor between practitioners who were predominately small animal veterinarians compared to veterinarians working in “mixed practices.”
Figure 1.
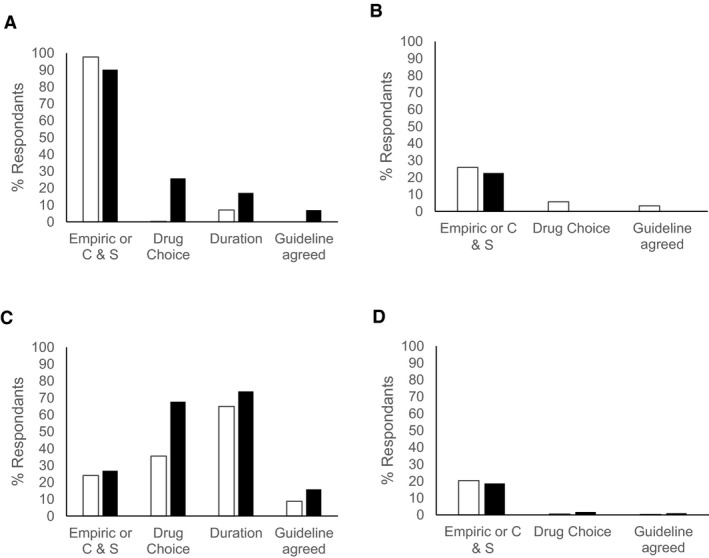
Agreement with Australasian Infectious Disease Advisory Panel guidelines for choice of empirical or antimicrobial therapy guided by culture and susceptibility (C & S), choice of drug and duration of therapy, and overall agreement with the guidelines for treatment of (A) gingivitis, (B) pyothorax, (C) acute cystitis, and (D) peritonitis. White columns indicate treatment choices for cats, and black columns indicate the treatment choices for dogs.
Discussion
This study has shown that empirical antimicrobial therapy is very common in Australian veterinary practice as is indicated for many conditions both in veterinary12 and medical practice.13 This is a similar outcome to that reported in a cross‐sectional study of practicing veterinarians in New Zealand where therapy, guided by C & S, was used in 19% of cases.14 Fluoroquinolones were used empirically at a high rate for specific conditions in dogs, as were 3rd‐generation cephalosporins in cats. The rate of empiric 3rd‐generation cephalosporin use in cats in this study is similar to findings by others.15, 16 Prophylactic antimicrobial use for routine desexing was less common. The most commonly used antimicrobial classes are aminopenicillins, particularly potentiated aminopenicillins, fluoroquinolones, early‐generation cephalosporins, and tetracyclines. This is consistent with findings in New Zealand (amoxicillin‐clavulanate 48%, cephalexin 31%),14 Canada (aminopenicillins 56%, cephalexin 33%),16 and the United Kingdom (aminopenicillins 59%, cephalexin 13%).15 Interestingly, there was very low use of older broad‐spectrum antimicrobials such as trimethoprim sulfonamide combinations (0.9%) and chloramphenicol (0.01%). There was also limited use of other drugs with a low importance rating, such as macrolides (6%), which have traditionally been mainstays of therapy, particularly in cats.
Empirical antimicrobial therapy was less common in this survey than in the only other survey of antimicrobial usage in dogs and cats in Australia, which was performed in 1997. In that study, empirical use for acute conditions ranged from 76 to 94% of cases,8 whereas in this study, the range was 19–79%. The increased use of C & S testing as a tool for directing antimicrobial therapy that was detected in this survey is likely to reflect improved antimicrobial stewardship and might be expected to improve clinical outcomes in veterinary practices, although there are likely to be concerns about its cost‐effectiveness for many animal owners. In addition, this survey did not investigate the methods used for C & S testing by veterinarians or veterinary laboratories. Use of rigorous methodology and veterinary specific break points is critical for ensuring reliable results from C & S testing. Regardless, this promising trend may reflect a growing willingness of the public to invest in disease investigations, an increase in these services being offered to clients, increased awareness by the profession of the benefit of testing, and/or an increase in treatment failures necessitating further investigation. Interestingly, C & S testing was used relatively frequently in the treatment of abscess (21%). Further investigation is warranted to evaluate the reasoning behind the high level of C & S testing for this scenario. The results may indicate a degree of prevarication bias in the survey (ie, survey respondents altering their answers to survey questions in a way that matches the perceived expectations of those carrying out the survey) and should be validated.
The AIDAP therapeutic guidelines recommend the use of cefovecin, the only 3rd‐generation cephalosporin registered for use in companion animals in Australia, only for cases where there is likely to be poor compliance with oral antimicrobial therapy. As a reflection of this, 3rd‐generation cephalosporins were much more commonly used in cats compared to dogs by practitioners completing this survey. The most frequent scenarios were those in which infection could be effectively treated with orally administered antimicrobial agents with a lower importance rating (ie, cellulitis/bite‐wound abscess and LUTI). Further, survey findings highlight the need for LUTI in cats to be confirmed by in‐house microscopic evaluation of a urine sample before initiating antimicrobial therapy due to the high prevalence of noninfectious cystitis in cats.17, 18, 19 The reported high usage of 3rd‐generation cephalosporins in cats likely reflects poor compliance in administration of oral drugs to cats compared to dogs, as cats are less likely to ingest medications in food, as has been found in a recent study from the United Kingdom.20
Different factors may account for fluoroquinolone administration to dogs. The rate of fluoroquinolone administration for some of the scenarios included in this survey was higher than expected. In complicated canine otitis cases involving Gram‐negative pathogens, such as Pseudomonas aeruginosa with rupture of the tympanic membrane, there are limited therapeutic options and the use of topical fluoroquinolones in this scenario is often warranted. However, the high frequency of systemic use of fluoroquinolones for both complicated and uncomplicated otitis cases suggests a need for improved antimicrobial stewardship by veterinarians in treating this disease. Awareness by veterinarians of the high concentrations of fluoroquinolones that can be achieved with topically applied formulations, and hence low risk of resistance development,21 may be lacking. Efforts should be made to alert veterinary practitioners that combined topical and systemic antimicrobial therapy should only be necessary in complicated cases where there is middle ear involvement with vestibular or facial nerve dysfunction and especially when there is osteomyelitis of the tympanic bulla. The use of systemic therapy alone is less likely to achieve the concentrations at the site of infection required to eliminate the pathogen and prevent development of resistance.22 The introduction of the AIDAP therapeutic guidelines, after this survey, may have improved veterinary prescribing in this area and ongoing monitoring of prescribing practices is warranted.
The Australian Veterinary Association has also recently recommended that antimicrobials with a high importance rating such as 3rd‐generation cephalosporins and fluoroquinolones “should be used only when other options are unavailable and wherever possible only after susceptibility testing has been completed”.23 Several drugs with a high importance require authorization before administration in human medicine in Australia.9 Half of the population of veterinarians that participated in this survey accounted for 88% of the usage of antimicrobials of high importance rating. The factors that influence these prescribing habits could not be elucidated in this study. There was no difference in population or socioeconomic variables based on postcode, or in year of graduation of prescribers. The concurrent low use of directed antimicrobial therapy in low users of antimicrobials of high importance rating may suggest that these practitioners have a client base that is less willing to invest economically in their animals, as both C & S testing and antimicrobials of high importance rating tend to be expensive in Australian veterinary practices. However, it may also reflect a more proactive clientele that present cases earlier and therefore the need for antimicrobials of high importance rating, and directed therapy, is perceived to be less. Further investigation into the factors driving the high use of these antimicrobials by a selection of the veterinary population is warranted.
There have been no previous reports on the frequency of antimicrobial use for routine surgical procedures in companion animal practice in Australia. Antibiotics are considered unnecessary for routine short surgeries conducted under sterile conditions, such as routine desexing.12 Over 75% of respondents in this survey did not use antimicrobial prophylaxis for routine desexing. However, with almost one quarter of Australian veterinarians still routinely using antimicrobials for neutering, and the number of these procedures performed in general practice, this topic requires a specific education program. Antimicrobials were frequently used in patients undergoing dental procedures including extractions in this survey (90% of respondents). The AIDAP guidelines recommend prophylactic antimicrobials if there are extractions or likely to be bleeding.12 Interestingly, as the release of the AIDAP guidelines, the recommendations for use of antimicrobials in dentistry have changed in human medicine with antimicrobials now only recommended for dental procedures performed on patients at a high risk for cardiac disease, to mitigate against the risk of infective endocarditis.24 In addition, these recommendations are now not in line with current accepted veterinary practice, which does not recommend the use of prophylactic antimicrobial therapy for routine dental procedures.25 This suggests that further study of the need for antimicrobial therapy after dental procedures is warranted in veterinary medicine.
Agreement with AIDAP guidelines was used as an indicator of gold standard therapy in this survey. The guidelines were introduced in 2013, 2 years after the survey was conducted, so some changes in usual therapy may have occurred after the survey was conducted while guidelines were being generated. However, there were no significant introductions of new antimicrobial drugs into the Australian companion animal market over this period and use of an indicator of best practice will allow for further investigation of factors confounding prescribing habits. Disagreement with guidelines was mainly due to drug selection and duration of therapy. This was due to both overuse of therapy and lack of recognition and treatment of severe sepsis. There was no difference in the prescribing habits between recently graduated veterinarians compared to older veterinarians.
There are several features of this study that may have influenced the results. Nonrandom, self‐selection of survey respondents can result in selection bias; for example, veterinarians more aware or interested in antimicrobial stewardship may have been more likely to respond. Recall bias can occur with retrospective questionnaire‐based surveys. In order to minimize this, generic hypothetical scenarios were posed rather than asking clinicians to recall specific cases. Prevarication bias was also possible. Given that this was not an anonymous survey, respondents may have felt pressured to respond in a certain way resulting in under‐ or over‐reporting of prescribing practices and use of C & S testing. While bias may have affected responses to some questions, it is the authors’ opinion, given the consistency with other studies and our clinical experience, we can have a reasonable level of confidence in the external validity of these findings.
In conclusion, this survey has shown that generally the choice to use antimicrobials by Australian veterinarians is appropriate and that in the majority of scenarios, antimicrobials with a low or medium importance rating are used. The use of antimicrobials with a high importance rating, particularly fluoroquinolones in dogs and 3rd‐generation cephalosporins in cats, as an empirical therapeutic choice warrants further investigation now that the AIDAP guidelines have been introduced.
Supporting information
Appendix S1. Questionnaire.
Acknowledgments
Grant support: This project was funded by Zoetis Animal Health. S.A. Holloway, D.J. Trott, M. Shipstone, V. Barrs, R. Malik, and M. Burrows, and J. Morton received financial remuneration for their participation in AIDAP.
Conflict of Interest Declaration: S.A. Holloway, D.J. Trott, M. Shipstone, V. Barrs, R. Malik, M. Burrows, and J. Morton received financial remuneration for their participation in AIDAP. Given that this was a cross‐sectional survey, respondents were self‐selected, and the primary author and last author are not part of AIDAP, the risk of bias is low.
Off‐label Antimicrobial Declaration: Cefovecin and fluoroquinolones were used off‐label.
The survey was designed by the AIDAP panel and undertaken by Pfizer Animal Health Australia in 2011 (now Zoetis Animal Health Australia). Analysis of the survey data was performed at the University of Melbourne.
The article has not been presented at any meetings.
Footnote
StataCorp, 2013, Stata Statistical Software: Release 13, StataCorp LP, College Station, TX
References
- 1. Jiang X, Yang H, Dettman B, et al. Analysis of fecal microbial flora for antibiotic resistance in ceftiofur‐treated calves. Foodborne Pathog Dis 2006;3:355–365. [DOI] [PubMed] [Google Scholar]
- 2. Rentala M, Lahti E, Kuhalampi J, et al. Antimicrobial resistance in Staphlococcus spp., Escherichia coli and Enterococcus spp. in dogs given antibiotics for chronic dermatological disorders compared with non‐treated control dogs. Acta Vet Scand 2004;45:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leite‐Martins LR, Mahu MI, Costa AL, et al. Prevalence of antimicrobial resistance in enteric Escherichia coli from domestic pets and assessment of associated risk markers using a generalized linear mixed model. Prev Vet Med 2014;117:28–39. [DOI] [PubMed] [Google Scholar]
- 4. Davis JA, Jackson CR, Fedorka‐Cray PJ, et al. Carriage of methicillin‐resistant Staphylococci by healthy companion animals in the US. Lett Appl Microbiol 2014;59:1–8. [DOI] [PubMed] [Google Scholar]
- 5. Loeffler A, Pfeiffer DU, Lindsay JA, et al. Prevalence of and risk factors for MRSA carriage in companion animals: A survey of dogs, cats and horses. Epidemiol Infect 2011;139:1019–1028. [DOI] [PubMed] [Google Scholar]
- 6. Boost MV, O'Donoghue MM, James A. Prevalence of Staphylococcus aureus carriage among dogs and their owners. Epidemiol Infect 2008;136:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grinberg A, Kingsbury DD, Gibson IR, et al. Clinically overt infections with methicillin‐resistant Staphylococcus aureus in animals in New Zealand: A pilot study. N Z Vet J 2008;56:237–242. [DOI] [PubMed] [Google Scholar]
- 8. Watson ADJ, Maddison JE. Systemic antibacterial drug use in dogs in Australia. Aust Vet J 2001;79:740–746. [DOI] [PubMed] [Google Scholar]
- 9. Australian Strategic and Technical Advisory Group on Antimicrobial Resistance . Importance rating and summary of antibacterials used in human health in Australia. Commonwealth of Australia; 2015. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-amr.htm. Accessed August 26, 2016. [Google Scholar]
- 10. Doohoo I, Martin W, Stryhn H. Veterinary Epidemiology Research, 2nd ed Charlottetown, CA: VER Inc; 2009. [Google Scholar]
- 11. Australian Bureau of Statistics . 2033.0.55.001 census of population and housing: Socio‐economic indexes for areas (SEIFA), Australia; 2011. Available at: http://www.abs.gov.au/ 2011. Accessed November 11, 2016.
- 12. Holloway S, Trott DJ, Shipstone M, et al. Antibiotic prescribing detailed guidelines. Australasian Infectious Diseases Advisory Panel; 2013. Available at: http://www.ava.com.au/sites/default/files/AVA_website/pdfs/AIDAP guidelines.pdf. Accessed August 12, 2016.
- 13. Therapeutic guidelines Limited . eTG complete; 2016. Available at: http://www.tgldcdp.tg.org.au/. Accessed October 14, 2016.
- 14. Pleydell EJ, Souphavanh K, Hill KE, et al. Descriptive epidemiological study of the use of antimicrobial drugs by companion animal veterinarians in New Zealand. N Z Vet J 2012;60:115–122. [DOI] [PubMed] [Google Scholar]
- 15. Mateus A, Brodbelt DC, Barber N, et al. Antimicrobial usage in dogs and cats in first opinion veterinary practices in the UK. J Small Anim Pract 2011;52:515–521. [DOI] [PubMed] [Google Scholar]
- 16. Murphy CP, Reid‐Smith R, Boerlin P, et al. Out‐patient antimicrobial use in dogs and cats for new disease events from community companion animal practices in Ontario. Can Vet J 2012;53:291–298. [PMC free article] [PubMed] [Google Scholar]
- 17. Defauw PA, Van de Maele I, Duchateau L, et al. Risk factors and clinical presentation of cats with feline idiopathic cystitis. J Feline Med Surg 2011;13:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kruger JM, Osborne CA, Goyal SM, et al. Clinical evaluation of cats with lower urinary tract disease. J Am Vet Med Assoc 1991;199:211–216. [PubMed] [Google Scholar]
- 19. Buffington CA, Chew DJ, Kendall MS, et al. Clinical evaluation of cats with nonobstructive urinary tract diseases. J Am Vet Med Assoc 1997;210:46–50. [PubMed] [Google Scholar]
- 20. Burke S, Black V, Sanchez‐Vizcaino F, et al. Use of cefovecin in a UK population of cats attending first‐opinion practices as recorded in electronic health records. J Feline Med Surg 2016;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wetzstein HG. Comparative mutant prevention concentrations of pradofloxacin and other veterinary fluoroquinolones indicate differing potentials in preventing selection of resistance. Antimicrob Agents Chemother 2005;49:4166–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blondeau JM. New concepts in antimicrobial susceptibility testing: The mutant prevention concentration and mutant selection window approach. Vet Dermatol 2009;20:383–396. [DOI] [PubMed] [Google Scholar]
- 23. Australian Veterinary Association . Veterinary use of antibiotics critical to human health; 2014. Available at: http://www.ava.com.au/. Accessed October 24, 2016.
- 24. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: Guidelines from the American Heart Association. J Am Dent Assoc 2008;139:S3–S24. [DOI] [PubMed] [Google Scholar]
- 25. British Small Animal Veterinary Association . PROTECT; 2016. Available at: http://www.bsava.com/Resources/PROTECT.aspx. Accessed August 22, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Questionnaire.


