Abstract
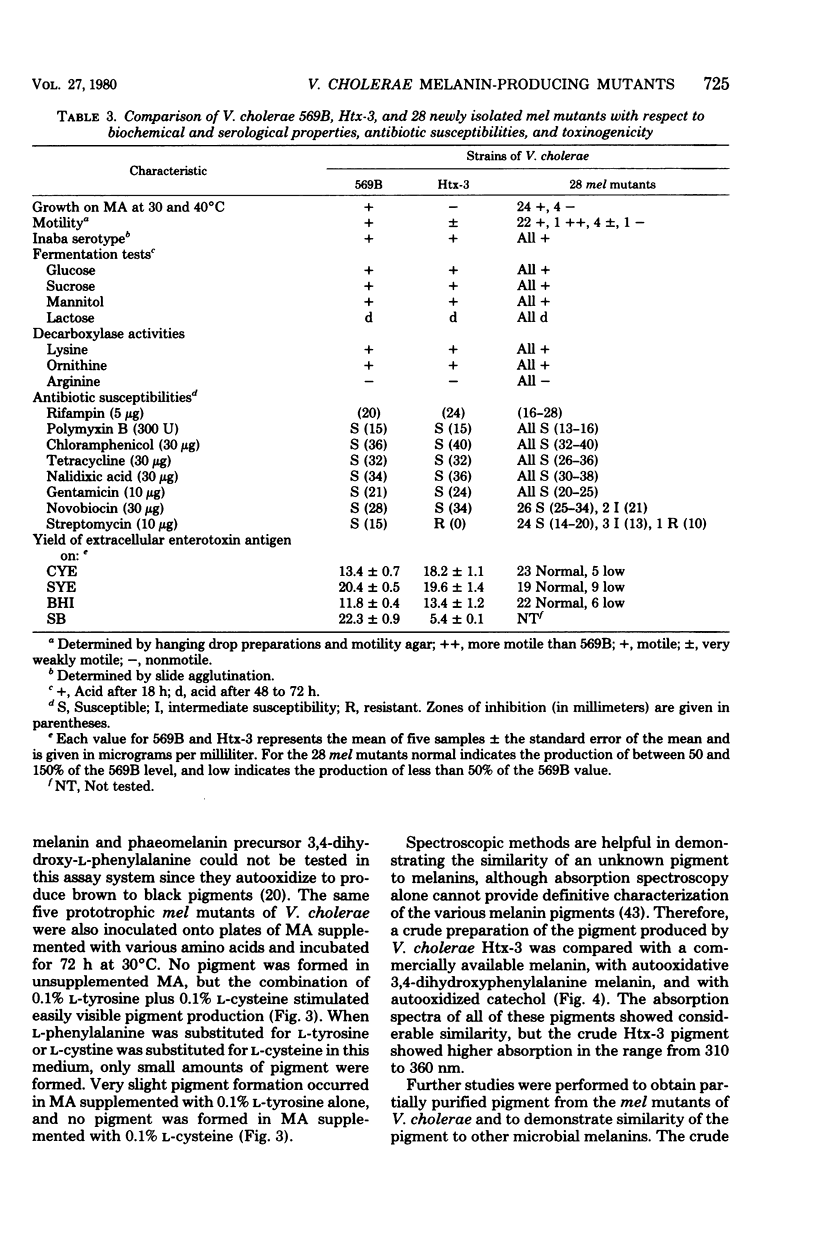
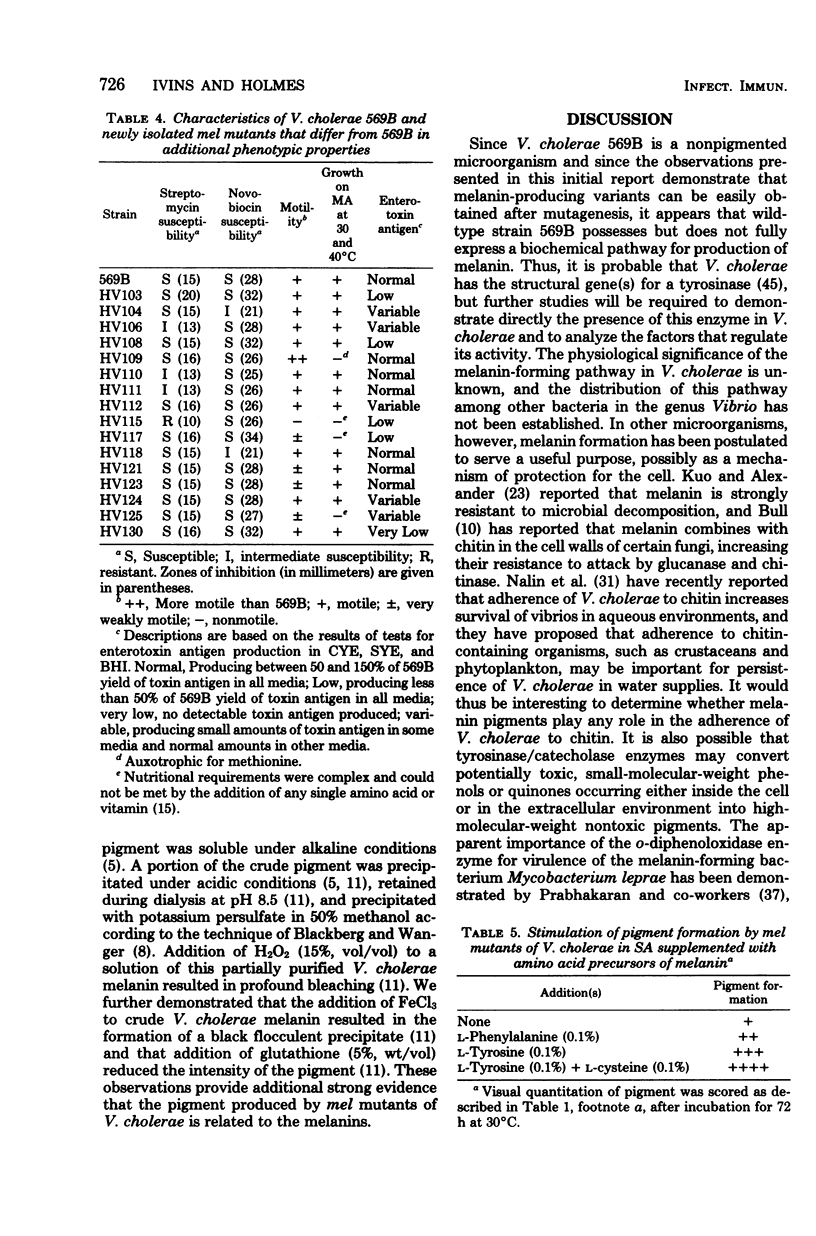
Vibrio cholerae strain Htx-3, a hypertoxinogenic mutant of V. cholerae 569B Inaba, produces a dark brown pigment under certain growth conditions, whereas strain 569B does not. We investigated the biochemical basis for this pigment production and the possible relationships between pigmentation and other phenotypic properties in V. cholerae. After mutagenesis of V. cholerae 569B with N-methyl-N′-nitro-N-nitrosoguanidine, 28 independently derived pigment-forming (mel) mutants were isolated and characterized. The mel mutants frequently differed from wild type in toxinogenicity or motility and occasionally differed in other phenotypic traits. Individual mel mutants differed from wild type both in the amount of toxin produced and in the growth conditions optimal for toxin production. It has not yet been established whether multiple phenotypic changes in individual mel mutants represent pleiotropic effects of single mutations or induction of multiple mutations by N-methyl-N′-nitro-N-nitrosoguanidine or both. Production of pigment by mel mutants occurred at temperatures from 22 to 40°C, was inhibited by anaerobiosis, and was stimulated by supplementation of growth media with the amino acid precursors of melanin (l-phenylalanine, l-tyrosine, or l-tyrosine plus l-cysteine). The pigment possessed several other properties reported for microbial melanins. We conclude that a biochemical pathway for melanin production is present in V. cholerae, that this pathway cannot be fully expressed in wild-type strain 569B, and that mutations in the gene(s) which we have designated mel can permit hyperproduction of melanin under appropriate conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Mikami Y. Chromogenicity of Streptomyces. Appl Microbiol. 1972 Feb;23(2):402–406. doi: 10.1128/am.23.2.402-406.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurstad K., Dahle H. K. The production and some properties of the brown pigment of Aeromonas liquefaciens. Acta Vet Scand. 1972;13(2):251–259. doi: 10.1186/BF03548579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baine W. B., Vasil M. L., Holmes R. K. Genetic mapping of mutations in independently isolated nontoxinogenic mutants of Vibrio cholerae. Infect Immun. 1978 Jul;21(1):194–200. doi: 10.1128/iai.21.1.194-200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramucci M. G., Holmes R. K. Radial passive immune hemolysis assay for detection of heat-labile enterotoxin produced by individual colonies of Escherichia coli or Vibrio cholerae. J Clin Microbiol. 1978 Aug;8(2):252–255. doi: 10.1128/jcm.8.2.252-255.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull A. T. Chemical composition of wild-type and mutant Aspergillus nidulans cell walls. The nature of polysaccharide and melanin constituents. J Gen Microbiol. 1970 Sep;63(1):75–94. doi: 10.1099/00221287-63-1-75. [DOI] [PubMed] [Google Scholar]
- Bull A. T. Inhibition of polysaccharases by melanin: enzyme inhibition in relation to mycolysis. Arch Biochem Biophys. 1970 Apr;137(2):345–356. doi: 10.1016/0003-9861(70)90448-0. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Ryder R. C., Richardson S. H. Biochemistry of vibrio cholerae virulence. II. Skin permeability factor-cholera enterotoxin production in a chemically defined medium. Infect Immun. 1971 Nov;4(5):611–618. doi: 10.1128/iai.4.5.611-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- GRIFFIN P. J., SNIESZKO S. F., FRIDDLE S. B. Pigment formation by Bacterium salmonicida. J Bacteriol. 1953 Jun;65(6):652–659. doi: 10.1128/jb.65.6.652-659.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Ito S., Nicol J. A. A new amino acid, 3-(2,5-SS-dicysteinyl-3,4-dihydroxyphenyl)alanine, from the tapetum lucidum of the gar (Lepisosteidae) and its enzymic synthesis. Biochem J. 1977 Mar 1;161(3):499–507. doi: 10.1042/bj1610499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. J., Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967 Sep;94(3):624–629. doi: 10.1128/jb.94.3.624-629.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONDER C., WILLIAMS J. N., Jr, WAISMAN H. A. Studies on the non-enzymic conversion of dopa to melanin. I. Studies on autoxidation. Arch Biochem Biophys. 1957 Dec;72(2):255–270. doi: 10.1016/0003-9861(57)90203-5. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Affinity filters, a new approach to the isolation of tox mutants of Vibrio cholerae. Proc Natl Acad Sci U S A. 1978 Feb;75(2):941–945. doi: 10.1073/pnas.75.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Purification of cholera toxin and its subunits: new methods of preparation and the use of hypertoxinogenic mutants. Infect Immun. 1978 May;20(2):552–558. doi: 10.1128/iai.20.2.552-558.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Sublett R. D., Romig W. R. Genetic mapping of toxin regulatory mutations in Vibrio cholerae. J Bacteriol. 1979 Sep;139(3):859–865. doi: 10.1128/jb.139.3.859-865.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin D. R., Daya V., Reid A., Levine M. M., Cisneros L. Adsorption and growth of Vibrio cholerae on chitin. Infect Immun. 1979 Aug;25(2):768–770. doi: 10.1128/iai.25.2.768-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaus R. A., Piattelli M., Fattorusso E. The structure of melanins and melanogenesis. IV. On some natural melanins. Tetrahedron. 1964 May;20(5):1163–1172. doi: 10.1016/s0040-4020(01)98983-5. [DOI] [PubMed] [Google Scholar]
- Ogunnariwo J., Hamilton-Miller J. M. Brown- and red-pigmented Pseudomonas aeruginosa: differentiation between melanin and pyorubrin. J Med Microbiol. 1975 Feb;8(1):199–203. doi: 10.1099/00222615-8-1-199. [DOI] [PubMed] [Google Scholar]
- Parker C., Gauthier D., Tate A., Richardson K., Romig W. R. Expanded linkage map of Vibrio cholerae. Genetics. 1979 Feb;91(2):191–214. doi: 10.1093/genetics/91.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H., Murthy V. V. Purification and properties of tyrosinases from Vibrio tyrosinaticus. Arch Biochem Biophys. 1974 Jan;160(1):73–82. doi: 10.1016/s0003-9861(74)80010-x. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K., Harris E. B., Kirchheimer W. F. The nature of the phenolase enzyme in Mycobacterium leprae: structure-activity relationships of substrates and comparison with other copper proteins and enzymes. Microbios. 1972;5(20):273–281. [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F., Harris E. B. Oxidation of phenolic compounds by Mycobacterium leprae and inhibition of phenolase by substrate analogues and copper chelators. J Bacteriol. 1968 Jun;95(6):2051–2053. doi: 10.1128/jb.95.6.2051-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Sen M., Sen S. P. Interspecific transformation in Azotobacter. J Gen Microbiol. 1965 Oct;41(1):1–6. doi: 10.1099/00221287-41-1-1. [DOI] [PubMed] [Google Scholar]
- Swan G. A. Structure, chemistry, and biosynthesis of the melanins. Fortschr Chem Org Naturst. 1974;31(0):521–582. doi: 10.1007/978-3-7091-7094-6_9. [DOI] [PubMed] [Google Scholar]
- Tanaka T. The decomposition of L-tyrosine and its derivatives by Proteus vulgaris. 2. Production of p-hydroxyphenylacetic acid, p-hydroxybenzaldehyde and melanin from L-tyrosine. Bull Pharm Res Inst. 1965 Nov;59:1–10. [PubMed] [Google Scholar]