Abstract
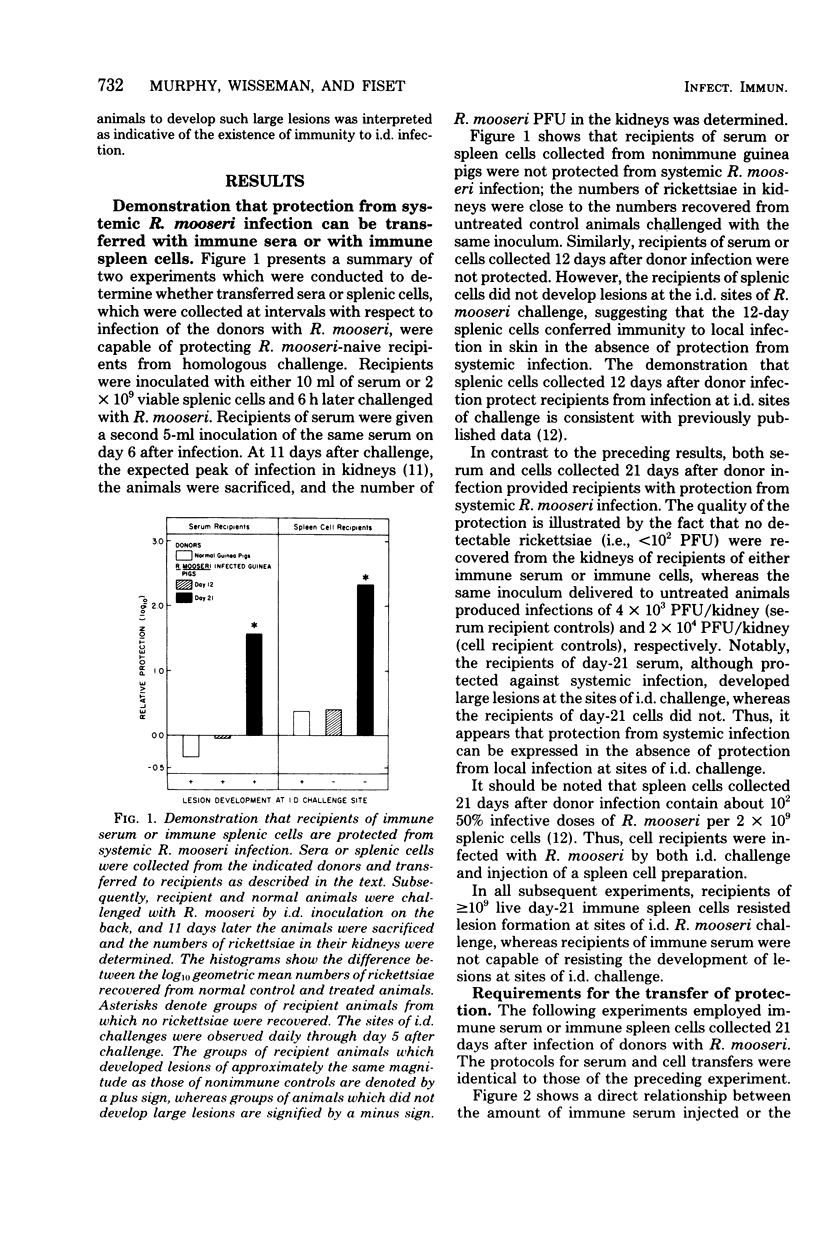
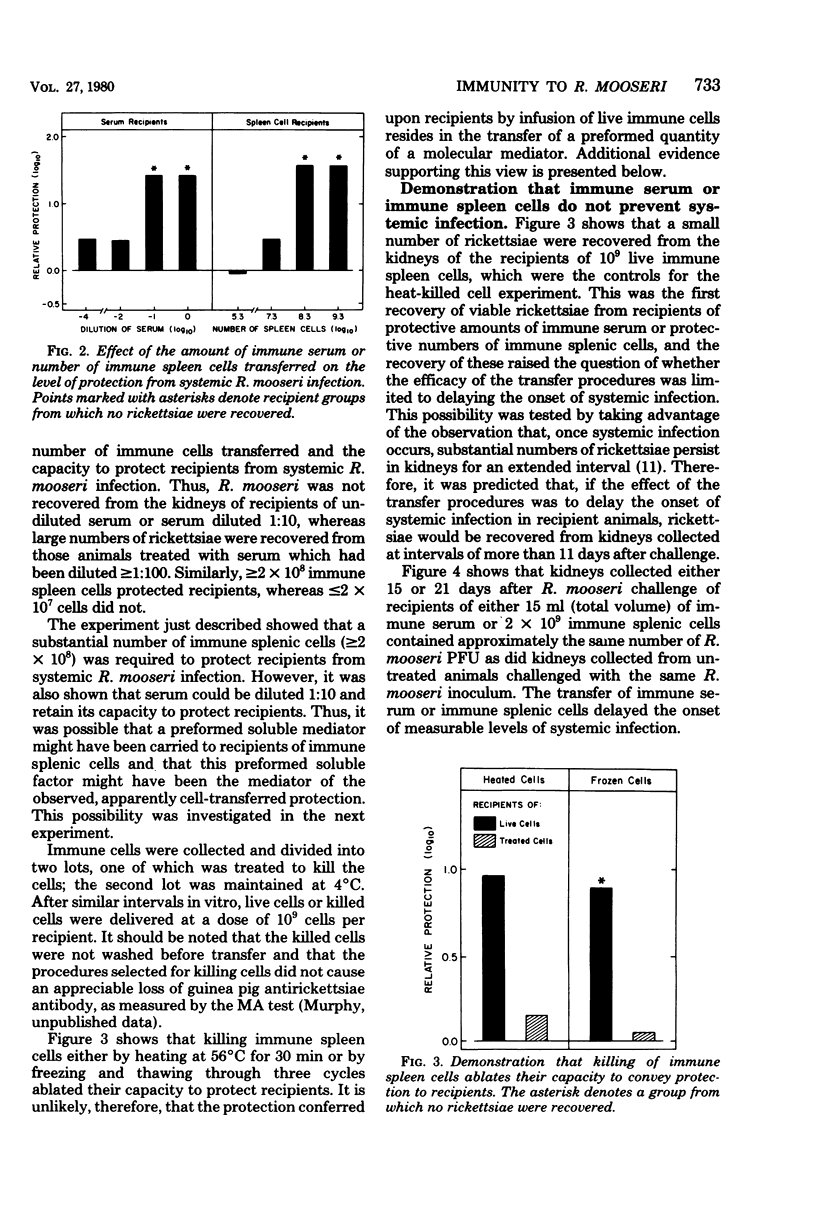
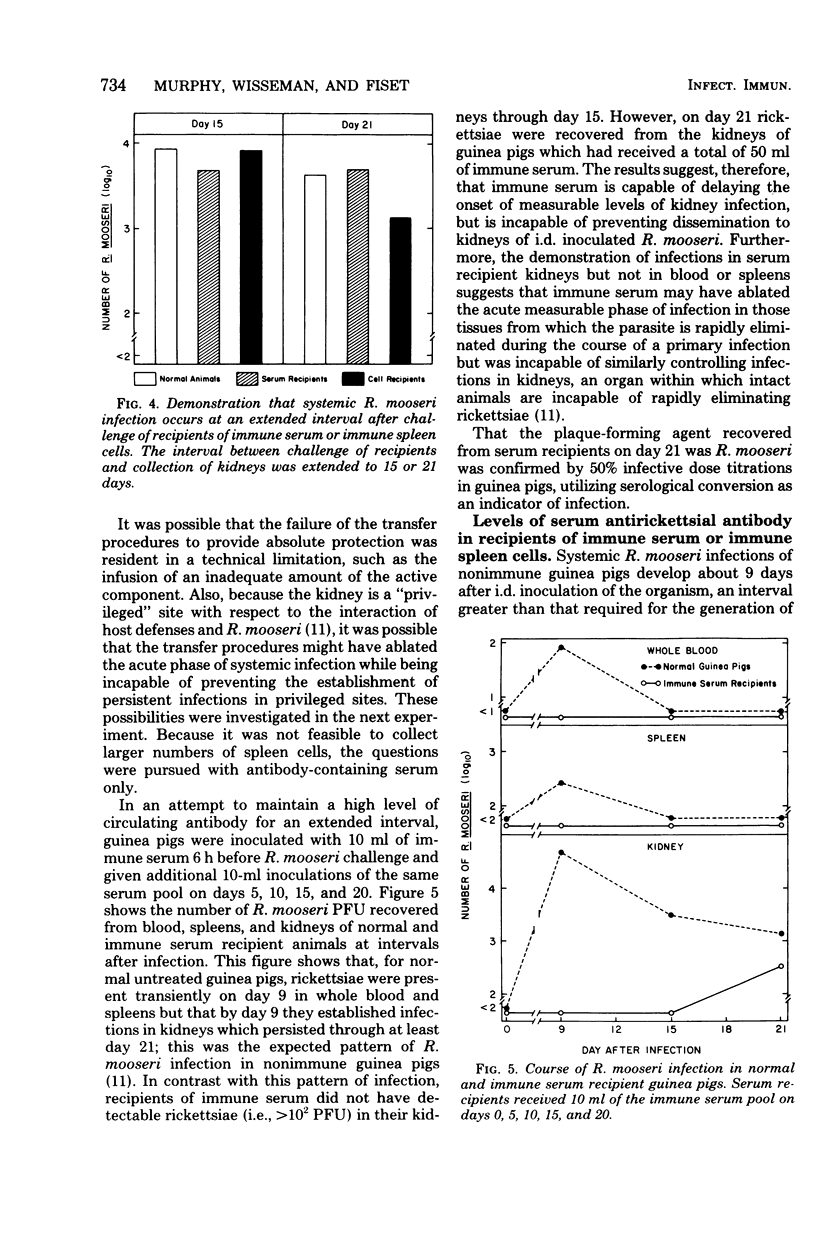
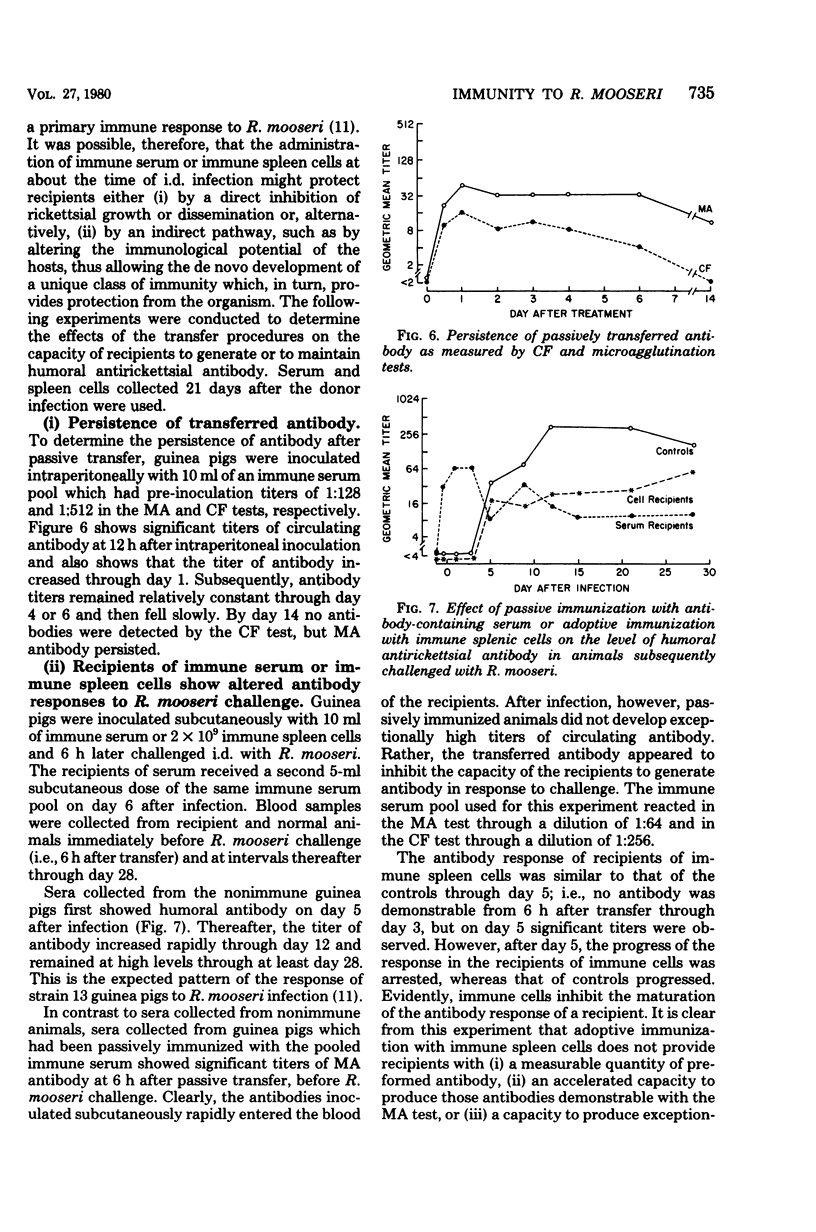
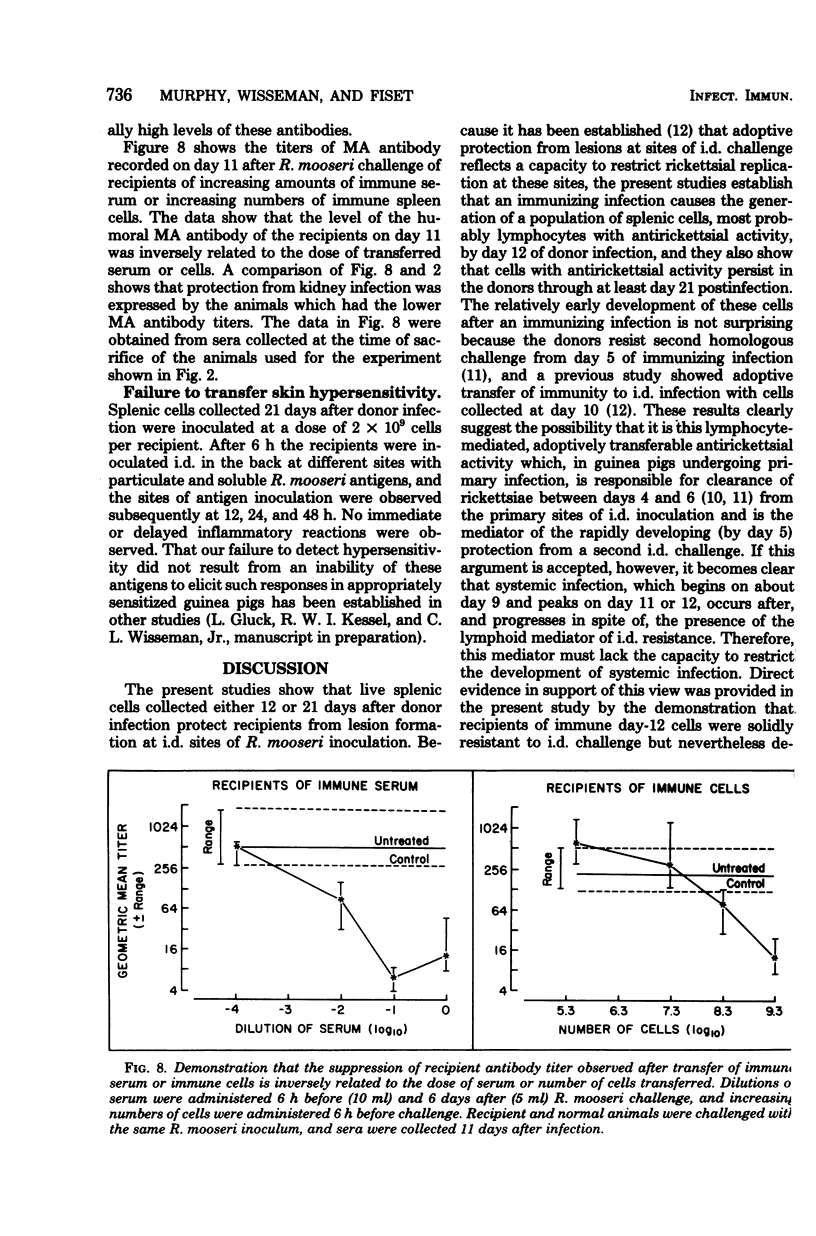
To study the mechanisms of immunity to Rickettsia mooseri (R. typhi) infection, sera and splenic cells collected from nonimmune and immune guinea pigs were inoculated separately into syngeneic nonimmune recipients which were subsequently challenged intradermally. Protection was measured by comparing the course of the challenge infections of recipients with infections initiated with the same rickettsial inocula in nonimmune animals. Recipients of splenic cells collected 21 days after donor infection were protected from lesion development at sites of intradermal challenge and showed fewer rickettsiae in their kidneys. Cells obtained from nonimmune donors did not protect against either skin lesion development at sites of challenge or kidney infection. Antibody-containing sera collected 21 days after donor infection, but not normal sera, reduced levels of kidney infection, but immune sera did not protect against the development of lesions at sites of intradermal challenge. It was concluded that both immune sera and immune splenic cells possess capacities to effect a partial control of the systemic phase of R. mooseri infection in guinea pigs, but that immune splenic cells possess a capacity not shared by immune sera, i.e., the capacity to protect from infection at local sites of intradermal inoculation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect Immun. 1976 Oct;14(4):1071–1076. doi: 10.1128/iai.14.4.1071-1076.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese J. L., Wisseman C. L., Jr, Walsh W. T., Fiset P. Antibody and antibiotic action on Rickettsia prowazeki in body lice across the host-vector interface, with observations on strain virulence and retrieval mechanisms. Am J Epidemiol. 1973 Oct;98(4):262–282. doi: 10.1093/oxfordjournals.aje.a121556. [DOI] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect Immun. 1973 Oct;8(4):631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. G., Jr, Fiset P. Mechanisms of immunity in typhus infection: adoptive transfer of immunity to Rickettsia mooseri. Infect Immun. 1979 May;24(2):387–393. doi: 10.1128/iai.24.2.387-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Fiset P. Mechanisms of immunity in typhus infection: some characteristics of Rickettsia mooseri infection of guinea pigs. Infect Immun. 1978 Aug;21(2):417–424. doi: 10.1128/iai.21.2.417-424.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Fiset P. Mechanisms of immunity in typhus infection: some characteristics of intradermal Rickettsia mooseri infection in normal and immune guinea pigs. Infect Immun. 1978 Dec;22(3):810–820. doi: 10.1128/iai.22.3.810-820.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Snyder L. B. Plaque assay for Rickettsia mooseri in tissue samples. Proc Soc Exp Biol Med. 1976 Oct;153(1):151–155. doi: 10.3181/00379727-153-39499. [DOI] [PubMed] [Google Scholar]
- Wike D. A., Ormsbee R. A., Tallent G., Peacock M. G. Effects of various suspending media on plaque formation by rickettsiae in tissue culture. Infect Immun. 1972 Oct;6(4):550–556. doi: 10.1128/iai.6.4.550-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. Mechanisms of immunity in typhus infections. IV. Failure of chicken embryo cells in culture to restrict growth of antibody-sensitized Rickettsia prowazeki. Infect Immun. 1974 Mar;9(3):571–575. doi: 10.1128/iai.9.3.571-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



