Abstract
Background
Chronic kidney disease (CKD) is an important cause of morbidity and mortality in dogs.
Objective
To evaluate the efficacy in prolonging survival and safety of benazepril administration to dogs with CKD.
Animals
Forty‐nine client‐owned dogs with CKD.
Methods
Dogs were randomized to benazepril (0.25 to <0.5 mg/kg) or placebo once daily for up to 2 years in a prospective, multicenter, blinded clinical trial. The primary endpoint variable was the renal survival time, defined as the time from inclusion in the study to the treatment failure endpoint of death or euthanasia or need for administration of parenteral fluids related to renal failure.
Results
No benefit of benazepril versus placebo was detected for renal survival time in all dogs; median (95% confidence interval (CI)) survival times were 305 (53–575) days in the benazepril group and 287 (152‐not available) in the placebo group (P = .53). Renal survival times were not significantly longer with benazepril compared to placebo for subgroups: hazard ratios (95% CI) were 0.50 (0.21–1.22) with P = .12 for initial urine protein‐to‐creatinine ratio (UPC) >0.5, and 0.38 (0.12–1.19) with P = .080 for initial UPC >0.5 plus plasma creatinine ≤440 μmol/L. Proteinuria, assessed from the UPC, was significantly (P = .0032) lower after treatment with benazepril compared to placebo. There were no significant differences between groups for clinical signs or frequencies of adverse events.
Conclusions and Clinical Relevance
Benazepril significantly reduced proteinuria in dogs with CKD. Insufficient numbers of dogs were recruited to allow conclusions on survival time.
Keywords: ACE inhibitor, Survival, Time‐to‐event analysis, Treatment failure
Abbreviations
- ACEI
angiotensin‐converting enzyme inhibitor
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- CHF
congestive heart failure
- CI
confidence interval
- CKD
chronic kidney disease
- CPH
Cox proportional hazards
- HR
hazard ratio
- IRIS
International Renal Interest Society
- NA
not available or applicable
- RBC
red blood cell
- RMANCOVA
repeated‐measures analysis of covariance
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
- WBC
white blood cell
Chronic kidney disease (CKD) is an important cause of morbidity and mortality in dogs, and the aims of therapy include improvement in quality of life and increased survival time.1 Angiotensin‐converting enzyme inhibitors (ACEIs) slow the progression of CKD in animal models and in humans.1, 2, 3 The beneficial actions of ACEIs in CKD appear to be mediated mainly by reduction in systemic and intraglomerular hypertension, reduction in proteinuria, and retardation of glomerulosclerosis and tubulointerstitial lesions.1, 4
In dogs, beneficial effects of the ACEI enalapril include reduction in proteinuria in cases of glomerulonephritis5 and proteinuric CKD6; reduction in proteinuria and slowed progression of hereditary nephritis in Samoyeds7; and reduction in glomerular and systemic hypertension, proteinuria, and glomerular and interstitial lesions in an experimental model of renal insufficiency.8 Beneficial actions of the ACEI benazepril include reductions in angiotensin II and aldosterone concentrations and systemic hypertension in dogs with experimental 7/8th renal ablation9; and improved clinical score, increased glomerular filtration rate, and reduced proteinuria in dogs with CKD.10
There remains a shortage of data from well‐controlled field studies with ACEIs in dogs with CKD. The objective of this study was to evaluate the effects of benazepril in comparison with placebo for the treatment of naturally occurring CKD in dogs.
Materials and Methods
The study was a prospective, multicenter, randomized, parallel‐group, blinded clinical trial involving 15 veterinary practices in France, Italy, Spain, and United Kingdom. The study was conducted in compliance with the Procedures and Principles of Good Clinical Practice1 and company internal review procedures and was approved by the respective regulatory authorities in each country taking into account animal welfare and ethical guidelines. All owners had to give their written informed consent before the start of the study.
The manuscript was prepared after consultation of the CONSORT statement for reporting of randomized clinical trials.11
Study Design
A standard case history was taken, and dogs were examined by investigators (veterinarians) at the selection visit on day −14 (range −18 to −14, and in addition on day −32 to −28 if needed) and at the inclusion visit on day 0 (range −4 to 0). Administration of the test items started on day 1, and the dogs were re‐examined on days 5 or 7, 30, 60, 120, 180, 240, 300 and 360, and thereafter for up to 2 years. At each visit, blood samples were taken for routine clinical chemistry and hematology. Also at each visit, urine samples were collected by free‐catch, catheter, or cystocentesis. Blood pressure measurements and ophthalmology examinations were not made routinely.
Animals
All dogs were client‐owned animals and were therefore fed, housed, and managed as pets. All dogs were fed, if they would accept it, a diet containing low amounts of phosphate, protein, and sodium from at least 14 days before the selection visit (hereafter referred to as “renal diet”). The renal diet consisted of either commercial diets,2 , 3 , 4 or (in France only) home‐made diets made according to instructions.5 Clients were requested not to change, as far as possible, the home management of their dogs during the study.
Dogs were recruited according to the following criteria. Inclusion criteria were dogs of all ages, breeds and both sexes; with body weight 2.5 to 80 kg (to permit accurate dosing with the test items); and with CKD with plasma creatinine concentration ≥142 μmol/L (1.6 mg/dL) and urine specific gravity (USG) ≤1.020 at both day −14 and day 0.
The preadmission exclusion criteria were dogs with acute kidney injury in the previous 28 days (including nephropathies of infectious or toxic origin); azotemia of prerenal or postrenal origin in the previous 28 days (including urinary tract obstruction); chronic heart failure (New York Heart Association class II, III, or IV); edema that required diuretic therapy; diabetes mellitus with uncontrolled hyperglycemia; clinical evidence of hepatic disease; malignant neoplasia; chronic gastrointestinal tract disease judged likely to interfere with the absorption of the test items; female animals that were pregnant or were planned to become pregnant in the next 12 months; and animals with uncooperative or noncompliant owners.
The following previous or concomitant treatments were forbidden: antibiotics with nephrotoxic properties (e.g., aminoglycosides); antihypertensive treatments (including ACEIs) other than benazepril as the test item; corticosteroids; diuretics; nonsteroidal anti‐inflammatory drugs; oral phosphate binders; and vitamin D and its derivatives. Dogs receiving any of these treatments could have the therapy withdrawn and return for the selection visit after a minimum washout period of 7 days.
Randomization
After inclusion in the study at day 0, dogs were allocated in sequence by permuted block randomization by the investigators to 1 of the 2 treatment groups in a 1:1 ratio. Separate randomization lists were generated by computer by the statistician for each investigator, with a block size of 4.
Test items
The investigational veterinary product, benazepril, was administered as the hydrochloride salt at a target minimum dose of 0.25 mg/kg (range 0.25 to <0.5 mg/kg) once daily in the form of divisible film‐coated tablets containing 5 or 20 mg benazepril hydrochloride.6 The negative control, placebo tablets, had the same appearance as the respective benazepril tablets and contained the same excipients, except that benazepril hydrochloride was replaced by lactose. Benazepril and placebo tablets were packed into identical bottles that were labeled A‐H, with 2 codes for each of the 4 test tablets. The blinding code was not broken in the study.
Owners were instructed to administer the test items, as far as possible, at the same time each day with or without food. The total duration of treatment was for up to 2 years.
The target dose of benazepril is the same as the starting dose registered in the EU for the treatment of congestive heart failure (CHF) in dogs, and shown to improve clinical signs and increase survival time in a field study in dogs with CHF.12 The dose was not adjusted according to the extent of renal impairment.13 No doubling of the dose (to 0.5 to <1.0 mg/kg as is allowed for the treatment of CHF) was permitted.
Evaluation of Efficacy
The primary endpoint was “treatment failure”, defined as a composite of the occurrence of death or euthanasia or the need for administration of parenteral fluids related to renal failure. The primary endpoint variable was the “renal survival time”, defined as the time from inclusion in the study to the occurrence of the treatment failure endpoint.
Secondary efficacy endpoints defined in the protocol were the progression of the following variables: urine protein‐to‐creatinine ratio (UPC); plasma creatinine and protein concentrations; body weight; and subjective assessments made by the investigator during clinical examinations and after questioning the owner. Clinical signs assessed as present or absent were as follows: bad breath, buccal cavity lesions, coat condition, diarrhea, neurological signs, retinal changes, and vomiting. The following variables were assessed by 4‐ or 5‐point numerical rating scales: appetite, general state (including dullness and weakness), polydipsia, and polyuria.
Evaluation of Safety
The tolerability of the test items was assessed from the frequency of reported adverse events, results of investigator clinical examinations, and clinical chemistry, hematology, and urine variables.
Statistical Analyses
All statistical tests were performed by computerized software.7 Reported P values are two‐tailed with P < .05 defined as significant.
Demographic and baseline data were compared between groups by the Mann‐Whitney U, Kruskal‐Wallis, or Fisher's exact tests.
The primary endpoint variable of the study was the renal survival time, defined as the time from inclusion in the study to the occurrence of treatment failure. Cases withdrawn from the study for a reason not defined as treatment failure, or lost to follow‐up or still alive and following the protocol at the end of the study, were included in the analysis up until the last time point at which they were known to be alive and following the protocol, and were thereafter censored in the analysis.
In the time‐to‐event analyses, the log‐rank test with right censoring was used to compare renal survival times between groups. The Kaplan‐Meier method was used to estimate the median and 95% confidence interval (CI) time to the primary endpoint in each group and to generate time‐to‐event plots. Univariate Cox proportional hazards (CPH) model analysis with right censoring was performed to determine the association between baseline variables and treatment and the risk of reaching the primary endpoint; the hazard ratio (HR) and 95% CI were calculated. Multivariate CPH model analysis was performed by the stepwise selection procedure.
The study was planned to include 80 dogs (40 in each group) in order to provide 80% power for a difference of 30% or more in renal survival time between groups.
Investigator subjective assessment, clinical chemistry, hematology, and urine variables were analyzed by repeated‐measures analysis of covariance (RMANCOVA). The covariates in the model included baseline, time, treatment (benazepril or placebo), and time × treatment interaction. Data were log‐transformed if that improved the normality of distributions. P values for deviation of distributions from normality were calculated by the Shapiro‐Wilk test.
The incidence of adverse events in the 2 groups was compared by Fisher's exact test.
Results
Animals and Baseline Variables
Although a minimum of 80 dogs was planned in the protocol, recruitment was slow and inclusion was stopped by the sponsor after 49 dogs were recruited in the 29 months from March 1997 to July 2000.
The number of cases screened was not recorded, but 49 dogs enrolled into the study were included in the database; 24 received benazepril and 25 received placebo. All 49 cases were included in the statistical analyses, which can therefore be considered an “all‐randomized animal” or “intent‐to‐treat” analysis. No separate “per‐protocol” analysis was made.
Baseline data are shown in Table 1. There were no significant differences between groups for any variable. In the benazepril and placebo groups, respectively, 17 and 17 dogs had a reported recent history of any clinical sign, 8 and 16 had decreased appetite, 2 and 1 had diarrhea, and 4 and 4 had vomiting. A total of 12 dogs were in International Renal Interest Society (IRIS) stage 2 (plasma creatinine ≤180 μmol/L), 29 were in IRIS stage 3 (plasma creatinine >180 and ≤440 μmol/L), and 8 in IRIS stage 4 (plasma creatinine >440 μmol/L).14 Presence of Leishmania or other systemic parasite infections was not recorded, but only 1 dog was noted to have been treated previously for Leishmania infection.
Table 1.
Baseline data in all 49 dogs
| Variable (unit) | Benazepril (n = 24) | Placebo (n = 25) | P value |
|---|---|---|---|
| Median (range) or n | Median (range) or n | ||
| Age (year) | 10.2 (1.3–19.0) | 11.0 (0.58–18.0) | .89 |
| Body weight (kg) | 21.3 (4.3–38.0) | 18.0 (2.5–41.9) | .60 |
| Sexa | .38 | ||
| Entire female | 5 | 4 | |
| Neutered female | 7 | 4 | |
| Entire male | 2 | 1 | |
| Neutered male | 9 | 16 | |
| IRIS Stage | .68 | ||
| 2 | 6 | 6 | |
| 3 | 13 | 16 | |
| 4 | 5 | 3 | |
| Plasma chemistry | |||
| Calcium [mmol/L] | 2.4 (2.1–3.1) | 2.5 (2.1–2.8) | .25 |
| Creatinine [μmol/L] | 282.9 (88.4–671.8) | 229.8 (97.2–610.0) | .60 |
| Phosphate [mmol/L] | 1.6 (1.1–5.8) | 1.6 (0.84–3.0) | .88 |
| Potassium [mmol/L] | 4.7 (3.9–6.1) | 4.9 (3.4–6.6) | .67 |
| Sodium [mmol/L] | 141.5 (133.0–152.0) | 142.0 (133.0–152.0) | .88 |
| Total protein [g/L] | 69.0 (53.7–86.0) | 66.0 (57.5–110.0) | .21 |
| Urea [mmol/L] | 47.7 (10.7–164.2) | 50.0 (6.6–171.4) | .94 |
| ALP [IU/L] | 41.0 (18.0–123.0) | 47.0 (10.0–176.0) | .82 |
| ALT [IU/L] | 37.5 (13.0–222.3) | 35.0 (7.0–103.4) | .91 |
| Blood hematology | |||
| RBC [1012/L] | 5.2 (3.2–6.9) | 5.1 (2.5–8.0) | .92 |
| WBC [109/L] | 9.1 (5.3–21.1) | 9.5 (3.2–21.6) | .92 |
| Urine | |||
| UPC | 1.2 (0.21–17.5) | 1.3 (0.11–18.0) | .44 |
| USG | 1.013 (1.006–1.020) | 1.014 (1.009–1.022) | .78 |
The neutered status of 1 male dog in the benazepril group was not recorded.
P values were calculated with the Mann‐Whitney U, Kruskal‐Wallis, or Fisher's exact tests.
Some dogs included into the study did not fulfill the inclusion criteria. Although plasma creatinine and USG values from the investigators’ laboratories were in all cases in compliance with the defined criteria, the final values used in the data analysis were derived from a separate central laboratory. At day 0, a total of 8 dogs had plasma creatinine concentrations (range 88.4–141.4 μmol/L) lower than the value of 142 μmol/L defined as the inclusion criterion. One dog had a USG value of 1.022 at day 0, versus the inclusion criterion of ≤1.020. In accordance with the intention‐to‐treat approach, all cases were included in the analysis.
There were also no significant differences between the 2 groups at baseline for the subgroups of dogs with UPC >0.5 or UPC >0.5 and plasma creatinine ≤440 μmol/L (data not shown).
Test Items
Benazepril, as the hydrochloride salt, was administered at a mean/median dose of 0.37/0.36 mg/kg (range 0.26‐0.58 mg/kg) once daily. Treatment duration ranged from 7 to 714 days for benazepril and 9 to 725 days for placebo.
Concomitant Treatments
Concomitant treatments judged necessary to treat diseases other than CKD were permitted providing they had no known interactions with the test items. A total of 18/24 (75%) of dogs in the benazepril group received other treatments versus 18/25 (72%) in the placebo group. The commonest concomitant treatments were antibiotics, antiemetic drugs, and fluids. No dog received a drug with strong effects on the cardiovascular system, for example, amlodipine, or an oral phosphate binder.
Efficacy Endpoints
Primary Endpoint—Treatment Failure
There were no significant differences between groups in reasons for premature withdrawal from the study (Table 2). A total of 32 dogs reached the defined primary endpoint (treatment failure), of which 18 dogs were euthanized (benazepril 7, placebo 11), 14 required parenteral fluids (benazepril 8, placebo 6), and none died without euthanasia.
Table 2.
Reasons for premature withdrawal of dogs from the study
| Reason | Benazepril (n = 24) | Placebo (n = 25) | P value |
|---|---|---|---|
| N (%) | N (%) | ||
| Need for administration of parenteral fluids related to renal failurea | 8 (33.3) | 6 (24.0) | .54 |
| Euthanasia related to renal failurea | 7 (29.2) | 11 (44.0) | .38 |
| Death related to renal failurea | 0 (0.0) | 0 (0.0) | 1.0 |
| Serious adverse event | 14 (58.3) | 12 (48.0) | .57 |
| Increase in plasma creatinine concentration | 9 (37.5) | 4 (16.0) | .11 |
| Decision by investigator | 5 (20.8) | 4 (16.0) | .73 |
| Other reason | 5 (20.8) | 5 (20.0) | 1.0 |
| Failure of cooperation or compliance | 1 (4.2) | 3 (12.0) | .61 |
| Failure to administer test treatment | 0 (0.0) | 2 (8.0) | .49 |
| Decision to stop the trial by the sponsor | 0 (0.0) | 2 (8.0) | .49 |
| Withdrawal of owner's consent | 0 (0.0) | 0 (0.0) | 1.0 |
The primary endpoint, treatment failure, was a composite of these 3 variables.
Dogs could be classified in more than one category.
P values were calculated with Fisher's exact test.
For all dogs (n = 49), there was no statistically significant difference in the renal survival time between the 2 groups (P = .53, log‐rank test) (Fig 1, Table 3). The median (95% CI) renal survival time was 305 (53–575) days with benazepril and 287 (152‐not applicable (NA)) days with placebo.
Figure 1.
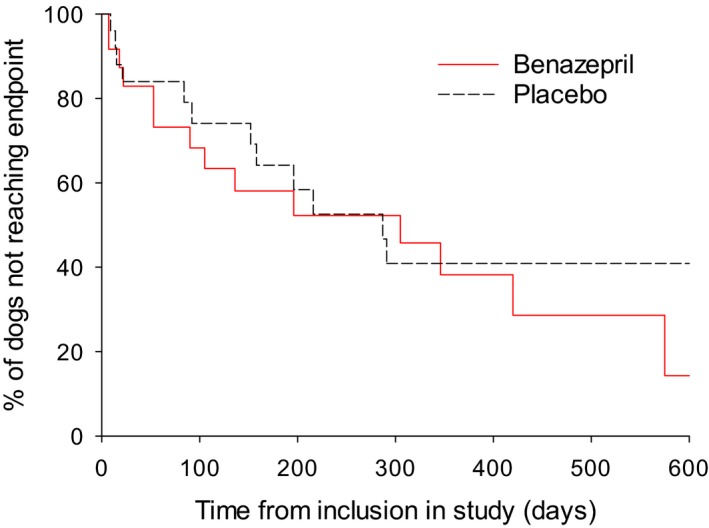
Kaplan‐Meier plot of time from inclusion to the primary endpoint (occurrence of death or euthanasia or the need for administration of parenteral fluids related to renal failure) in all dogs (n = 49). P = .53 (log‐rank test). Median (95% CI) time to the endpoint was 305 (53–575) days in the benazepril and 287 (152‐NA) days in the placebo group. The number of cases reaching the endpoint versus censored was 14 versus 10 for benazepril, and 12 versus 13 for placebo.
Table 3.
Summary of time‐to‐event analysis for the primary endpoint
| Group | Benazepril (n = 24) | Placebo (n = 25) | P Value | ||
|---|---|---|---|---|---|
| Number Reaching Endpoint/Censored | Renal Survival Time (days) | Number Reaching Endpoint/Censored | Renal Survival Time (days) | ||
| Median (95% CI) | Median (95% CI) | ||||
| All dogs | 14/10 | 305 (53–575) | 12/13 | 287 (152–NA) | .53 |
| UPC >0.5 | 13/7 | 196 (53–420) | 11/4 | 158 (15–287) | .12 |
| UPC ≤0.5 | 1/3 | NA (18–NA) | 0/8 | NA | .16 |
| Plasma creatinine >440 μmol/L | 5/0 | 90 (7–305) | 3/0 | 21 (9–291) | .83 |
| Plasma creatinine ≤440 μmol/L | 9/10 | 420 (53–NA) | 9/13 | 287 (152–NA) | .71 |
| 180< plasma creatinine ≤440 μmol/L | 8/5 | 346 (22–575) | 7/9 | 216 (92–NA) | .51 |
| Plasma creatinine ≤180 μmol/L | 0/2 | NA | 1/3 | NA (84–NA) | .48 |
| UPC >0.5 & plasma creatinine ≤440 μmol/L | 8/7 | 346 (53–575) | 8/4 | 158 (15–216) | .080 |
P values were calculated with the log‐rank test.
The primary endpoint was “treatment failure” defined as “the occurrence of death or euthanasia or the need for administration of parenteral fluids related to renal failure”. The primary endpoint variable (renal survival time) was the time from inclusion to the occurrence of the primary endpoint.
Censored cases became no longer available for analysis before or without reaching the defined endpoint.
It was preplanned in the protocol to take baseline plasma creatinine and UPC values into account in the analyses, and as noted previously, the 2 groups were not optimally matched at baseline. Therefore, additional analyses were conducted with stratification for baseline plasma creatinine and UPC, and by CPH analysis.
The database contained a total of 53 variables from 49 cases. In order to reduce the risk of overparameterization, variables were selected for the CPH analyses as follows. First, correlations between clinical chemistry and hematology variables were assessed via Spearman ρ and P values, and only one of highly correlated variables was selected. Hematocrit, hemoglobin concentration, and red blood cell (RBC) count were highly correlated (ρ > 0.95); therefore, RBC count was used. Second, presence or absence of bad breath, buccal cavity lesions, decreased appetite, diarrhea, dullness, neurological signs, vomiting, and weakness were summarized into a single category, the presence or absence of CKD clinical signs. A total of 18 variables were therefore included in the CPH models: age, body weight, and sex; treatment (benazepril); UPC and USG; plasma creatinine, urea, alkaline phosphatase (ALP), alanine aminotransferase (ALT), sodium, potassium, protein, calcium, and phosphate; RBC and white blood cell (WBC) counts; CKD clinical signs.
Dogs Stratified for Baseline Plasma Creatinine
There were no differences in renal survival time between groups in the subgroups with initial plasma creatinine >440 μmol/L or ≤440 μmol/L (Table 3).
Dogs Stratified for Baseline UPC
In the subgroup of 35 dogs with initial UPC >0.5 (defined as dogs with proteinuria), the median renal survival time was 196 days with benazepril and 158 days with placebo (P = .12) (Fig 2, Table 3).
Figure 2.
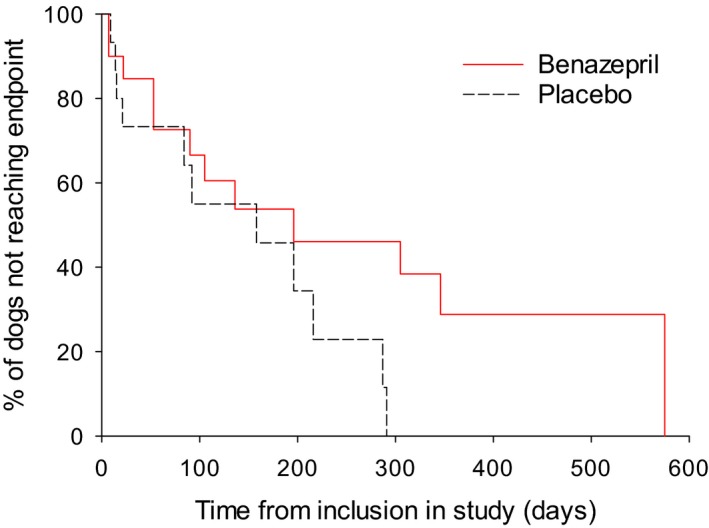
Kaplan‐Meier plot of time from inclusion to the primary endpoint (occurrence of death or euthanasia or the need for administration of parenteral fluids related to renal failure) in the subgroup of dogs with initial UPC >0.5 (n = 35). P = .12 (log‐rank test). Median (95% CI) time to the endpoint was 196 (53‐420) days in the benazepril and 158 (15–287) days in the placebo group. The number of cases reaching the endpoint versus censored was 13 versus 7 for benazepril, and 11 versus 4 for placebo.
Dogs Stratified for Baseline Plasma Creatinine and UPC
In the subgroup of 27 dogs with initial UPC >0.5 and plasma creatinine ≤440 μmol/L, the median renal survival time was 346 days with benazepril and 158 days with placebo (P = .080) (Fig 3, Table 3).
Figure 3.
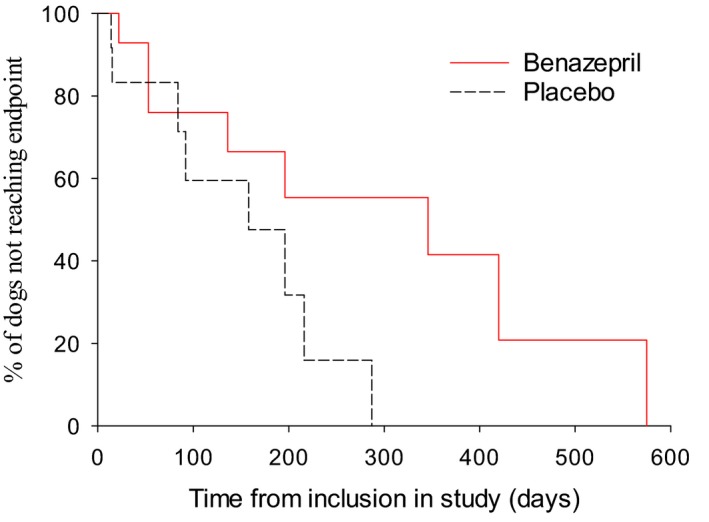
Kaplan‐Meier plot of time from inclusion to the primary endpoint (occurrence of death or euthanasia or the need for administration of parenteral fluids related to renal failure) in the subgroup of dogs with initial UPC >0.5 and plasma creatinine ≤440 μmol/L (n = 27). P = .080 (log‐rank test). Median (95% CI) time to the endpoint was 346 (53‐575) days in the benazepril and 158 (15–216) days in the placebo group. The number of cases reaching the endpoint versus censored was 8 versus 7 for benazepril, and 8 versus 4 for placebo.
Cox Proportional Hazards Analysis
All dogs (n = 49)
In the univariate analysis, the following variables were significant (P < .05) and associated with HRs >1: UPC and USG; plasma concentrations of creatinine, phosphate, protein, and urea (Table 4). In the multivariate analysis, plasma phosphate and protein were significant (P < .05) and were associated with HRs >1 (Table 5).
Table 4.
Results of univariate Cox proportional hazard analysis for the association between baseline variables and treatment and the risk of reaching the primary endpoint in all dogs (n = 49)
| Variable | Hazard Ratio | P Value |
|---|---|---|
| Estimate (95% Confidence Interval) | ||
| Age | 1.037 (0.961–1.118) | .35 |
| Body weight | 0.983 (0.952–1.015) | .29 |
| Sex (male versus female) | 1.790 (0.774–4.136) | .17 |
| Treatment (benazepril versus placebo) | 1.280 (0.591–2.775) | .53 |
| Clinical signs (present versus absent) | 2.294 (0.916–5.743) | .076 |
| Plasma calcium | 1.357 (0.146–12.60) | .79 |
| Plasma creatinine | 1.006 (1.003–1.009) | <.0001 |
| Plasma phosphate | 3.431 (1.943–6.059) | <.0001 |
| Plasma potassium | 1.305 (0.734–2.318) | .36 |
| Plasma total protein | 1.062 (1.019–1.107) | .0041 |
| Plasma sodium | 1.056 (0.974–1.145) | .18 |
| Plasma urea | 1.021 (1.011–1.031) | <.0001 |
| Plasma ALP | 0.992 (0.978–1.005) | .23 |
| Plasma ALT | 1.003 (0.995–1.011) | .43 |
| Blood RBC | 0.747 (0.525–1.062) | .10 |
| Blood WBC | 0.999 (0.890–1.122) | .99 |
| UPC | 1.124 (1.024–1.235) | .014 |
| USG | 1.021 (1.011–1.031) | <.0001 |
All variables were analyzed as continuous unless noted.
The primary endpoint was “treatment failure” defined as “death or euthanasia or need for administration of parenteral fluids related to renal failure”.
P values < .05 are shown in bold.
Table 5.
Results of multivariate Cox proportional hazard analyses for the association between baseline variables and the risk of reaching the primary endpoint
| Group and Variable | Hazard Ratio | P Value |
|---|---|---|
| Estimate (95% Confidence Interval) | ||
| All dogs (n = 49) | ||
| Plasma phosphate | 4.018 (2.086–7.741) | <.0001 |
| Plasma total protein | 1.072 (1.028–1.117) | .0011 |
| Baseline UPC >0.5 | ||
| Plasma phosphate | 3.733 (1.898–7.342) | .0001 |
| Baseline UPC >0.5 and plasma creatinine ≤440 μmol/L | ||
| Plasma phosphate | 27.58 (3.724–204.3) | .0012 |
| Plasma total protein | 1.164 (1.050–1.291) | .0039 |
Only results of variables with P < .1 are shown. Both variables shown were analyzed as continuous.
P values < .05 are shown in bold.
Dogs with UPC >0.5 (n = 35)
In the univariate analysis, the following variables were significant (P < .05) and associated with HRs >1: plasma concentrations of calcium, creatinine, phosphate, and urea (Table 6). For the treatment effect (benazepril versus placebo), the HR (95% CI) was 0.50 (0.21–1.22) with P = .12 in the log‐rank test.
Table 6.
Results of univariate Cox proportional hazard analysis for the association between baseline variables and the risk of reaching the primary endpoint in dogs with baseline UPC >0.5
| Variable | Hazard Ratio | P Value |
|---|---|---|
| Estimate (95% Confidence Interval) | ||
| Clinical signs (present versus absent) | 2.241 (0.861–5.830) | .098 |
| Plasma calcium | 10.29 (1.181–89.64) | .035 |
| Plasma creatinine | 1.005 (1.002–1.008) | .0026 |
| Plasma phosphate | 3.948 (1.979–7.876) | <.0001 |
| Plasma urea | 1.016 (1.005–1.028) | .0064 |
Only results of variables with P < .1 are shown.
Quoted variables except clinical signs were analyzed as continuous.
P values < .05 are shown in bold.
In the multivariate analysis, only plasma phosphate was significant (P < .05) and was associated with an HR >1 (Table 5).
Dogs with UPC >0.5 and plasma creatinine ≤440 μmol/L (n = 27)
In the univariate analysis, only plasma phosphate concentration was significant (P < .05) and was associated with an HR >1 (Table 7). For the treatment effect (benazepril versus placebo), the HR (95% CI) was 0.38 (0.12–1.19) with P = .080 in the log‐rank test. In the multivariate analysis, plasma phosphate and plasma protein concentration were significant (P < .05) and were associated with HRs >1 (Table 5).
Table 7.
Results of univariate Cox proportional hazard analysis for the association between baseline variables and treatment and the risk of reaching the primary endpoint in dogs with baseline UPC >0.5 and plasma creatinine ≤440 μmol/L
| Variable | Hazard Ratio | P Value |
|---|---|---|
| Estimate (95% Confidence Interval) | ||
| Treatment (benazepril versus placebo) | 0.380 (0.122–1.186) | .080 |
| Plasma phosphate | 5.818 (1.791–18.91) | .0034 |
| Plasma total protein | 1.076 (0.999–1.159) | .054 |
| UPC | 1.125 (0.980–1.291) | .093 |
Only results of variables with P < .1 are shown.
Quoted variables except treatment were analyzed as continuous.
P values < .05 are shown in bold.
Secondary Efficacy Endpoints
Mean (SD) data for UPC and plasma creatinine are shown up to day 180 in Figs 4 and 5. Data became increasingly unreliable after day 180 due to the loss of cases from the study.
Figure 4.
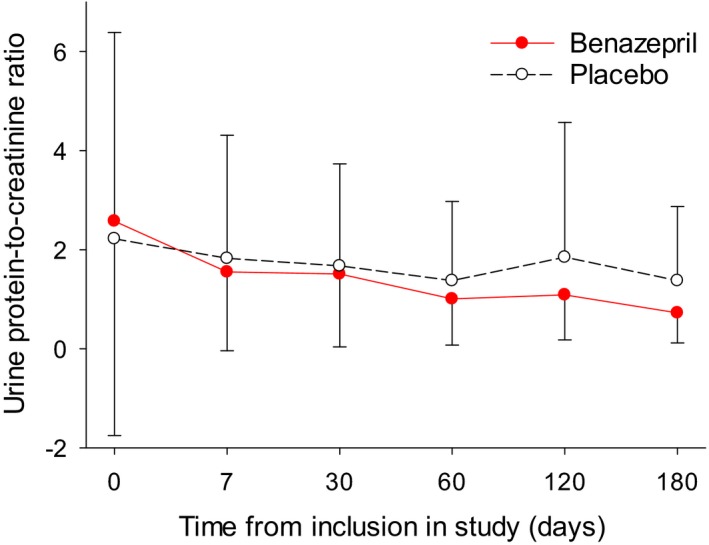
Mean (SD) urine protein‐to‐creatinine ratio (UPC) in all dogs.
Figure 5.
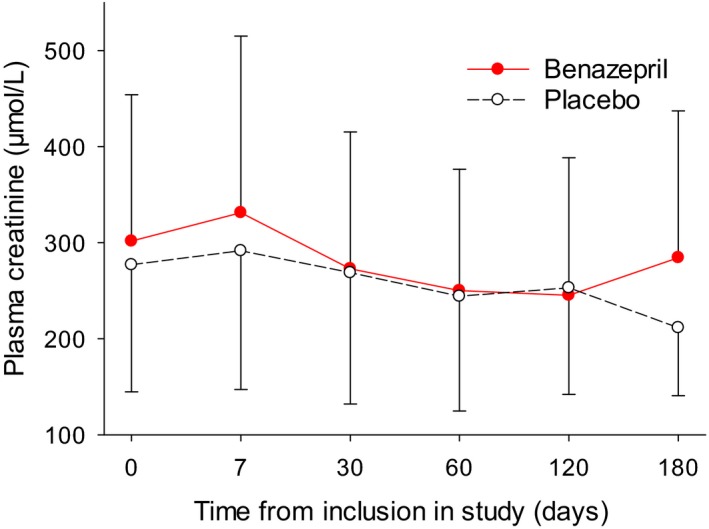
Mean (SD) plasma creatinine concentrations in all dogs.
Proteinuria was assessed from the UPC. Data were log‐transformed as this improved the normality distribution of the data, although in all dogs and the 2 subgroups the distributions remained significantly different (P < .05) from normal (Table 8). UPC values (Fig 4) were significantly lower during treatment with benazepril compared to placebo for all 3 groups tested (Table 8). Mean ± SD UPC values during treatment (i.e., after day 0) in the benazepril and placebo groups were, respectively, 1.51 ± 1.37 and 1.94 ± 2.22 in all dogs, 1.76 ± 1.40 and 2.98 ± 2.41 in the subgroup with baseline UPC>0.5, and 1.70 ± 1.06 and 3.23 ± 2.52 in the subgroup with baseline UPC > 0.5 and plasma creatinine ≤440 μmol/L.
Table 8.
Results of the RMANCOVA models for secondary efficacy variables
| Group | N | P Values from RMANCOVA | P Value for Normality | |||
|---|---|---|---|---|---|---|
| Trt | Time | Trt × time | Baseline | |||
| All dogs | ||||||
| Body weight | 46 | .33 | .76 | .69 | <.0001 | .0025 |
| Plasma creatinine | 46 | .033 | .043 | .66 | <.0001 | .0016 |
| Plasma total protein | 39 | .61 | .073 | .94 | <.0001 | .041 |
| UPC | 44 | .0032 | .83 | .24 | <.0001 | .041 |
| Baseline UPC >0.5 | ||||||
| Body weight | 32 | .043 | .0027 | .0009 | <.0001 | .50 |
| Plasma creatinine | 32 | .41 | .0031 | .87 | <.0001 | .50 |
| Plasma total protein | 26 | .84 | .024 | .051 | <.0001 | .28 |
| UPC | 32 | .0008 | .12 | .047 | <.0001 | .0008 |
| Baseline UPC >0.5 and plasma creatinine ≤440 μmol/L | ||||||
| Body weight | 25 | .17 | .15 | .40 | <.0001 | .048 |
| Plasma creatinine | 25 | .72 | .046 | .87 | <.0001 | .15 |
| Plasma total protein | 22 | .27 | .027 | .13 | <.0001 | .17 |
| UPC | 25 | .0074 | .20 | .049 | <.0001 | .014 |
Trt, treatment. Data for body weight, plasma creatinine, and UPC were log‐transformed.
P values for deviation of distributions from normality were calculated by the Shapiro‐Wilk test.
P values < .05 are shown in bold.
There was a significant treatment effect with higher plasma creatinine concentrations during treatment in the benazepril (301.8 ± 152.2 μmol/L) compared to the placebo group (277.0 ± 134.9 μmol/L) for all dogs (Fig 5, Table 8), but no significant differences in the 2 subgroups. There were no significant changes from baseline in creatinine in either group for all dogs or the 2 subgroups.
Body weight was significantly higher in the benazepril group (16.4 ± 1.03 kg) versus the placebo group (16.11 ± 0.81) for the dogs with baseline UPC >0.5 (Table 8). There was no significant treatment effect for plasma total protein concentration. There were no significant differences between groups for frequencies or scores for clinical signs (P > .05, data not shown).
Data on arterial blood pressure and changes to the fundus of the eye were too sparse to be analyzed (data not shown).
Safety
Adverse Events
As many dogs were treated for a long period (up to 2 years) and had CKD, the frequency of adverse events was high, mostly anorexia, death, diarrhea, or vomiting (Table 9). There was no difference between groups in the frequency of all reported adverse events: benazepril 21 of 24 dogs (87.5%) versus placebo 23 of 25 dogs (92.0%) (P = .67); or serious adverse events, benazepril 5 of 24 dogs (20.8%) versus placebo 6 of 25 dogs (24.0%) (P = 1.0).
Table 9.
Number of dogs with reported adverse events
| Reason | Benazepril (n) | Placebo (n) | P value |
|---|---|---|---|
| Vomiting | 16 | 16 | 1.0 |
| Death | 11 | 14 | .57 |
| Anorexia | 10 | 10 | 1.0 |
| Diarrhea | 7 | 11 | .38 |
| Dehydration | 5 | 1 | .098 |
| Seizure | 2 | 1 | .61 |
| Dermatitis | 1 | 2 | 1.0 |
| Tremor | 1 | 2 | 1.0 |
| Fever | 0 | 2 | .49 |
| Urinary tract infection | 0 | 2 | .49 |
Adverse events reported only in 1 dog per group are not shown.
P values were calculated by Fisher's exact test.
A total of 11 dogs in the benazepril group and 14 in the placebo group died or were euthanized (P = .57). In addition to the cases euthanized for renal failure (benazepril n = 7, placebo n = 11), 7 dogs (benazepril n = 4, placebo n = 3) died for reasons unrelated to CKD or renal failure.
There were no differences between groups for the frequency of adverse events in the subgroups of dogs with baseline UPC > 0.5 (P = 1.0) or UPC > 0.5 and creatinine ≤440 μmol/L (P = 1.0) (data not shown).
Plasma Chemistry, Urinalysis, and Hematology
Plasma urea (P = .0001) and ALT (P = .029) were significantly lower in the benazepril compared to the placebo group in all dogs, with no significant differences in the subgroups of dogs with baseline UPC > 0.5.
There were no significant differences between groups for other plasma chemistry, hematology, or urinalysis variables including plasma calcium, phosphate, potassium, and sodium concentrations, ALP activity; hematocrit, hemoglobin concentration, RBC and WBC counts; and USG (data not shown).
Discussion
The main results of this study are that the ACEI benazepril significantly reduced proteinuria in dogs with CKD compared to placebo, but there were no differences in renal survival time.
The demonstrated reduction in proteinuria by benazepril in dogs in this study confirms previous results with this ACEI in cats15 and humans.2 This action is attributed to reduction in glomerular hypertension and improved function of the glomerular basement membrane. In humans, reduction in proteinuria is a therapeutic goal and proteinuria is an independent risk factor for progression of renal disease.16 The ACEI enalapril has been reported to reduce proteinuria in dogs with clinical or experimental CKD,5, 6, 7, 8 and proteinuria has been demonstrated to be a risk factor for disease progression in dogs with CKD.17, 18
In humans with CKD, ACEIs prolong survival by inhibiting the progression of disease, with greatest effect in patients with proteinuria.2, 3 The benefit of benazepril on prolonging survival in humans with CKD was shown in the AIPRI study.2 Some but nonconclusive evidence also exists that the ACEI enalapril inhibits the progression of CKD in dogs.5 The present study with benazepril in dogs also was nonconclusive regarding survival time, with no significant effects.
The main limitations of the study are discussed below. First, the number of dogs was lower (n = 49) than the minimum number (n = 80) planned in the protocol. In spite of intensive efforts, case recruitment proved to be difficult over the 29‐month inclusion period. As a result, the study was underpowered for the primary endpoint. Calculations indicate that the original target of 80 dogs (40 in each group) should provide 80% power to demonstrate a significant benefit of benazepril on prolonging renal survival time with the treatment effect sizes observed in the subgroups of dogs with UPC > 0.5 or UPC > 0.5 and plasma creatinine ≤440 μmol/L. These subgroups are the optimal target for therapy with ACEIs, that is, mild to moderate CKD with proteinuria.14 Although it would be ideal to conduct a new study with a larger number of dogs, a new placebo‐controlled survival study with ACEIs in dogs with CKD might be difficult because ACEIs have become standard of care in proteinuric dogs with CKD.14
Second, blood pressure was not measured routinely, resulting in data that were too sparse to analyze. As a consequence, we could not evaluate the possible antihypertensive effect of benazepril or study the association between blood pressure and outcomes. Furthermore, as the blood pressure was unknown for most cases and antihypertensive agents other than benazepril were not permitted, control of blood pressure may well have not been optimal in all cases. Systemic hypertension can have an impact on both the magnitude of proteinuria and progression of CKD in dogs.18, 19 Different outcomes might have occurred if benazepril has been used in cases with better control of systemic blood pressure.
Third, oral phosphate binders were not permitted in the protocol in order to reduce variability and because they were not standard of care at the time the study was started. The mean plasma phosphate concentration in both groups was 1.6 mmol/L at baseline, which is higher than the target range of 0.9–<1.5 mmol/L recommended for dogs with CKD.14 Optimum control of plasma phosphate concentrations would be expected to prolong average renal survival times, and therefore the relative effect of benazepril compared to placebo might also be different.
Fourth, the dose of benazepril (range 0.26–0.58 mg/kg administered once daily) tested in our study is at the low end of the range registered and tested in dogs with CHF (0.25–1.0 mg/kg).12 Larger reductions in proteinuria, and possibly greater clinical outcomes, might occur with higher doses of benazepril or twice‐daily dosing, or both.
Fifth, we do not know the frequency of Leishmania or other systemic parasitic infections in the test population.
Finally, the parametric method of RMANCOVA was used to analyze the chemistry, hematology, and urine variables including UPC. This method was preferred because the data were continuous and measured repeatedly. In spite of log transformation of the data, the models for UPC (and many other variables) deviated significantly from a normal distribution. However, it was judged that the distributions for all variables were satisfactory, and analysis of variance models are relatively robust even when the distribution deviates from normality.20 Therefore, we conclude that the statistical analyses were appropriate for the conclusions reached.
Benazepril was tolerated well in this study. The incidence of adverse events was high in both groups, consistent with the fact that the dogs had CKD and were followed for up to 2 years. No differences in frequencies of adverse events were detected between the benazepril and placebo groups.
Plasma creatinine concentrations were moderately but significantly increased by benazepril, with mean values 25 μmol/L higher in the benazepril compared to the placebo group. In humans, ACEIs can induce modest increases in plasma creatinine concentrations at the start of therapy, attributed to reduced glomerular filtration rate as a result of the (beneficial) action of ACEIs in reducing glomerular hypertension.2 Modest increases in plasma creatinine concentrations (up to 45 μmol/L) with ACEIs are therefore not regarded as an adverse drug effect.14
There are risks with introducing ACEIs to clinically unstable dehydrated dogs.14 We did not detect any increased frequency of adverse effects of benazepril compared to placebo in IRIS stage 4 cases, although to our knowledge no dehydrated cases which were unstable were included. In addition, the number of dogs recruited in this study was too low to detect uncommon adverse events. With a total of 24 dogs in the benazepril group, the study had only 71% power to detect adverse events with a true incidence equal to or greater than 5%, and had only 21% power to detect events with a true incidence of 1%.
ACEIs have been reported to increase plasma potassium concentrations and reduce blood erythrocyte counts in humans.4, 21 This study provided no evidence for either of these effects with benazepril in dogs with CKD, as reported previously with benazepril in dogs with CHF.12
In the multivariate CPH analyses, only 2 variables were significantly and independently associated with increased risk of reaching the primary endpoint of treatment failure: higher plasma concentrations of phosphate and protein. The association between hyperphosphatemia and increased risk has been reported previously in cats22, 23, 24 and humans with CKD,25 and an increased serum calcium‐phosphorus concentration product was a negative prognostic indicator for mortality in dogs with CKD.26 The observed association between increased plasma protein and increased risk is presumed to have reflected the presence of dehydration.
Conclusions
In this clinical trial of dogs with CKD, benazepril significantly reduced proteinuria and was well tolerated. Too few dogs were recruited to allow conclusions on renal survival. Further clinical trials are needed with ACEIs in dogs with CKD and proteinuria.
Acknowledgments
The clinical work was conducted in France, Italy, Spain, and United Kingdom, and the data were analyzed in Switzerland. The study was supported by Novartis Animal Health, now owned by Elanco Animal Health, a division of Eli‐Lilly company.
We thank the other investigators including Roberto Santilli (Italy) and Sarah Stonton (United Kingdom) for managing clinical cases, and the following colleagues from former Novartis Animal Health for assistance managing or monitoring the study: Laure Baduel, Fabienne Brovedani, and Philippe Gruet (France) and Debbie Alexander, Cathy Brockman, and Karen Quine (United Kingdom).
Preliminary results from the study were presented at the 1999 European College of Veterinary Internal Medicine meeting.
Conflict of Interest Declaration: The study was supported by Novartis Animal Health, now owned by Elanco Animal Health, a division of Eli‐Lilly company, which manufactures and distributes benazepril hydrochloride tablets (Fortekor®).
J.N. King and W. Seewald are employed by Elanco Animal Health, and G. Strehlau is retired from Novartis. Sponsorship was supplied by Novartis to A. Font for further education, and by Novartis and Elanco to C. Brovida to be a member of the IRIS Group. The other authors declare no other conflict of interest.
Off‐label Antimicrobial Declaration: As the in‐life phase of this study ran from 1997 to 2000, we cannot declare that there was no off‐label use of antimicrobials.
Footnotes
VICH GL9, CVMP:VICH/595/98, 2000
Hills k/d diet
Léorénil Renal diet
Waltham Low Protein diet
Vetalim Software, F.Enjalbert, D.Grandjean, B.M.Paragon, Vetocom Sarl, France
Fortekor® 5 and 20 mg, Elanco Animal Health, Huningue, France
SAS® Software, version 8.2, Cary, NC
References
- 1. Brown SA, Crowell WA, Brown CA, et al. Pathophysiology and management of progressive renal disease. Vet J 1997;154:93–109. [DOI] [PubMed] [Google Scholar]
- 2. Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin‐converting‐enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 1996;334:939–945. [DOI] [PubMed] [Google Scholar]
- 3. GISEN Group . Randomised placebo‐controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non‐diabetic nephropathy. Lancet 1997;349:1857–1863. [PubMed] [Google Scholar]
- 4. Lefebvre HP, Toutain PL. Angiotensin‐converting enzyme inhibitors in the therapy of renal diseases. J Vet Pharmacol Therap 2004;27:265–281. [DOI] [PubMed] [Google Scholar]
- 5. Grauer GF, Greco DS, Getzy DM, et al. Effects of enalapril versus placebo as a treatment for canine idiopathic glomerulonephritis. J Vet Intern Med 2000;14:526–533. [DOI] [PubMed] [Google Scholar]
- 6. Zatelli A, Roura X, D'Ippolito PD, et al. The effect of renal diet in association with enalapril or benazepril on proteinuria in dogs with proteinuric chronic kidney disease. Open Vet J 2016;6:121–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grodecki KM, Gains MJ, Baumal R, et al. Treatment of X‐linked hereditary nephritis in Samoyed dogs with angiotensin converting enzyme (ACE) inhibitor. J Comp Pathol 1997;117:209–225. [DOI] [PubMed] [Google Scholar]
- 8. Brown SA, Finco DR, Brown CA, et al. Evaluation of the effects of inhibition of angiotensin converting enzyme with enalapril in dogs with induced chronic renal insufficiency. Am J Vet Res 2003;64:321–327. [DOI] [PubMed] [Google Scholar]
- 9. Mishina M, Watanabe T. Development of hypertension and effects of benazepril hydrochloride in a canine remnant kidney model of chronic renal failure. J Vet Med Sci 2008;70:455–460. [DOI] [PubMed] [Google Scholar]
- 10. Tenhündfeld J, Wefstaedt P, Nolte JA. A randomized controlled clinical trial of the use of benazepril and heparin for the treatment of chronic kidney disease in dogs. J Am Vet Med Assoc 2009;234:1031–1037. [DOI] [PubMed] [Google Scholar]
- 11. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials 2010;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The BENCH (Benazepril in Canine Heart Disease) Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a prospective, randomized, double‐blinded, placebo‐controlled clinical trial. J Vet Cardiol 1999;1:7–18. [DOI] [PubMed] [Google Scholar]
- 13. Lefebvre HP, Laroute V, Concordet D, et al. Effects of renal impairment on the disposition of orally administered enalapril, benazepril, and their active metabolites. J Vet Intern Med 1999;13:21–27. [PubMed] [Google Scholar]
- 14. IRIS . 2013 Treatment recommendations for CKD in dogs. Available at www.IRIS-kidney.com.
- 15. King JN, Gunn‐Moore D, Tasker S, et al. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006;20:1054–1064. [DOI] [PubMed] [Google Scholar]
- 16. Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non‐diabetic renal disease. Kidney Int 2001;60:1131–1140. [DOI] [PubMed] [Google Scholar]
- 17. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc 2005;226:393–400. [DOI] [PubMed] [Google Scholar]
- 18. Wehner A, Hartmann J, Hirschberger J. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non‐renal diseases. Vet Rec 2008;162:141–147. [DOI] [PubMed] [Google Scholar]
- 19. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003;222:322–329. [DOI] [PubMed] [Google Scholar]
- 20. Khan A, Rayner GD. Robustness to non‐normality of common tests for the many‐sample location problem. J App Math Dec Sci 2003;7:187–206. [Google Scholar]
- 21. Morrone LF, Di Paolo S, Logoluso F, et al. Interference of angiotensin‐converting enzyme inhibitors on erythropoiesis in kidney transplant recipients. Transplantation 1997;64:913–918. [DOI] [PubMed] [Google Scholar]
- 22. King JN, Tasker S, Gunn‐Moore D, et al. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007;21:906–916. [PubMed] [Google Scholar]
- 23. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000‐2002). J Vet Intern Med 2008;22:1111–1117. [DOI] [PubMed] [Google Scholar]
- 24. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012;26:275–281. [DOI] [PubMed] [Google Scholar]
- 25. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1–S201. [PubMed] [Google Scholar]
- 26. Lippi I, Guidi G, Marchetti V, et al. Prognostic role of the product of serum calcium and phosphorus concentrations in dogs with chronic kidney disease: 31 cases (2008–2010). J Am Vet Med Assoc 2014;245:1135–1140. [DOI] [PubMed] [Google Scholar]


