Summary
Induction of allograft tolerance has been considered the ultimate goal in organ transplantation. Although numerous protocols to induce allograft tolerance have been reported in mice, a chimerism‐based approach through donor haematopoietic stem cell transplantation has been the only approach to date that induced allograft tolerance reproducibly following kidney transplantation in man. Renal allograft tolerance has been achieved by induction of either transient mixed chimerism or persistent full donor chimerism. Although the risk of rejection may be low in tolerance achieved via durable full donor chimerism, the development of graft‐versus‐host disease (GVHD) has limited the wider clinical application of this approach. In contrast, tolerance induced by transient mixed chimerism has not been associated with GVHD, but the risk of allograft rejection is more difficult to predict after the disappearance of haematopoietic chimerism. Current efforts are directed towards the development of more clinically feasible and reliable approaches to induce more durable mixed chimerism in order to widen the clinical applicability of these treatment regimens.
Keywords: chimerism, cyclophosphamide, durable mixed chimerism, full donor chimerism, GVHD, haematopoietic stem cell transplantation, living‐donor kidney transplantation, NHP, persistent mixed chimerism, TBI, TI, TLI, transient mixed chimerism, transplantation tolerance induction
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immune tolerance in transplantation. Clinical and Experimental Immunology 2017, 189: 133–4.
Transplantation tolerance: the big picture. Where do we stand, where should we go? Clinical and Experimental Immunology 2017, 189: 135–7.
Operational tolerance in kidney transplantation and associated biomarkers. Clinical and Experimental Immunology 2017, 189: 138–57.
Immune monitoring as prerequisite for transplantation tolerance trials. Clinical and Experimental Immunology 2017, 189: 158–70.
Transplantation tolerance: don't forget about the B cells. Clinical and Experimental Immunology 2017, 189: 171–80.
Murine models of transplantation tolerance through mixed chimerism: advances and roadblocks. Clinical and Experimental Immunology 2017, 189: 181–9.
Regulatory T cells: tolerance induction in solid organ transplantation. Clinical and Experimental Immunology 2017, 189: 197–210.
Introduction
As the development of highly efficacious immunosuppressive agents has prevented or treated acute allograft rejection successfully, the short‐term survival of organ transplants has improved significantly, making solid organ transplantation the therapy of choice for most end‐stage organ diseases 1, 2. However, the current requirement for lifelong immunosuppression results in significantly increased risks of cardiovascular disease 3, 4, 5, de‐novo diabetes 6, 7, 8, dyslipidaemia 9, 10, 11, 12 and malignancies 13, 14, 15, which lead to patient death with functioning graft as high as 25% by 10 years after kidney transplantation (KTx) 16. Unfortunately, despite these toxicities, the development of chronic rejection is not prevented consistently by currently available immunosuppressive regimens. Immune tolerance induction is the ultimate solution to these limitations associated with long‐term immunosuppression in transplanted patients. Except for a limited report of induction of liver allograft tolerance through infusion of regulatory cells 17, induction of chimerism through donor haematopoietic stem cell transplantation (HSCT) has been the only approach to date that reproducibly achieved allograft tolerance in clinical KTx.
Preclinical studies
Since Owen and Medawar's discoveries of mixed chimerism and allograft tolerance in Freemartin Cattle, extensive efforts have been directed towards induction of persistent mixed chimerism in adult experimental animals. Although induction of persistent mixed chimerism has been achieved readily in small animal models, it has been extremely difficult to achieve this in non‐human primates (NHPs) or humans. If the conditioning regimen is intensive, donor haematopoietic cells overwhelm recipient haematopoietic cells, which lead to full donor chimerism. Conversely, if the conditioning regimen is less intensive, donor haematopoietic cells are rejected. These contrasting results observed in rodent versus primate studies may be attributed to the presence of heterologous memory T cells (TMEM) in primates, as Adams et al. reported failure of chimerism induction in mice in which alloreactive TMEM were augmented by multiple lymphocytic choriomeningitis (LCMV) infections 18, 19. Nevertheless, we demonstrated in NHPs that induction of only transient mixed chimerism can induce renal allograft tolerance in major histocompatibility complex (MHC)‐mismatched transplant recipients 20, 21, 22. Continued survival of the kidney allograft despite the loss of chimerism suggested that peripheral mechanisms were involved primarily, and induction of renal allograft tolerance has been improved by adding a short course of co‐stimulatory blockade, such as anti‐CD154 monoclonal antibody (mAb) 21 or cytotoxic T lymphocyte (CTLA4) immunoglobulin (Ig) (belatacept) 22. As our original conditioning regimen required initiation of conditioning 1 week before transplantation, the protocol was applicable only to living donor transplant recipients. To extend our approach to deceased donor transplant recipients, we subsequently developed a ‘delayed tolerance’ strategy in which kidney transplantation is performed first with conventional immunosuppression, followed by conditioning and donor bone marrow transplantation (DBMT) several months later using cryopreserved donor bone marrow cells in NHPs 23, 24. ‘Delayed tolerance’ has the theoretical disadvantage of enhanced donor‐specific TMEM responses elicited despite administration of potent immunosuppressive medications during the interval prior to the DBMT. Indeed, more substantial depletion of CD8+ T cells with anti‐CD8 mAb was necessary to induce mixed chimerism in the delayed tolerance conditioning protocol. Nevertheless, the delayed tolerance approach expands the potential applicability of tolerance induction protocol significantly, as it can be used not only for deceased donor transplant recipients but also for any previous living donor transplant recipient, if their donor is available to provide the haematopoietic stem cells. Although the exact mechanistic pathways leading to tolerance induction via transient mixed chimerism remain to be defined, studies to date have provided a number of important observations. We found in NHPs that tolerant recipients consistently lost anti‐donor CD8+ T cell responses while retaining substantial anti‐donor CD4+ T cell responses in vitro. The majority of these CD4+ T cell responses appeared to be from regulatory T cells (Tregs) which expand significantly more to stimulation with donor antigens than to third‐party antigens. When sorted Tregs and non‐Tregs from tolerant recipients were stimulated with donor antigens in the presence of interleukin (IL)‐2, Treg expansion was observed only from non‐Tregs. Furthermore, the expansion of Tregs in tolerant recipients was inhibited by blocking transforming growth factor (TGF)‐β, which resulted in restoration of anti‐donor CD8+ T cell responses. These observations suggest that specific loss of anti‐donor CD8+ T cell responses are maintained by donor‐specific induced Tregs 25. We also found that Tregs were enriched significantly in the kidney allograft of tolerant recipients. Further studies are in progress to clarify the mechanisms of local enrichment of Tregs in the kidney allograft.
A limitation of our tolerance approach with transient mixed chimerism has been inconsistent stability of allograft tolerance. Approximately 20–30% of NHP recipients who were apparently withdrawn successfully from immunosuppression developed antibody‐mediated chronic rejection later 21, 26. Therefore, improving the stability of tolerance is critically important to widen the application of this approach. One strategy to improve the stability of tolerance, therefore, was to modify the conditioning regimen to achieve more robust mixed chimerism. It is possible that reduced‐intensity conditioning regimens permit a substantial proportion of heterologous TMEM to survive, which may contribute to the loss of allogeneic haematopoietic stem cell engraftment 18, 19, 27. Because Tregs have been reported to be capable of suppressing TMEM function effectively 28, 29, 30, we sought modalities to expand Tregs in vivo. We evaluated IL‐6 blockade with anti‐IL‐6R mAb to determine whether we could expand Tregs in vivo. Although IL‐6 blockade alone failed to expand Tregs in this setting, the expansion was successful when combined with anti‐thymocyte globulin (ATG) 31. Subsequently, we included anti‐IL‐6R mAb in our DBMT conditioning regimen to induce mixed chimerism in a widely recognized ‘tolerance‐resistant’ lung transplant model 32. Interestingly, three of four lung transplant recipients developed prolonged mixed chimerism and achieved robust lung allograft tolerance 32. Unfortunately, when this conditioning regimen was tested in kidney transplant recipients, robust chimerism was not induced (unpublished results). We suggest that IL‐6 blockade may be especially effective in lung transplant recipients via suppression of rejection through Th17 33, and this approach may not be applicable to other organ transplant recipients. Nevertheless, this success in lung transplantation emphasized that robust tolerance is inducible in NHP, even in this typically ‘tolerance‐resistant’ lung allograft model by induction of prolonged mixed chimerism.
Kean's group, in Seattle, has reported successful induction of persistent mixed chimerism in NHP recipients of MHC‐matched DBMT. Using a conditioning regimen that consisted of low‐dose total body irradiation (TBI), basiliximab, anti‐CD154 mAb, belatacept and sirolimus, three of nine recipients developed multi‐lineage mixed chimerism for as long as 24 months. Those recipients also achieved prolonged specific acceptance of skin allografts from the bone marrow (BM) donor. However, six of the nine recipients were euthanized because of cytomegalovirus (CMV) reactivation, which suggested that the protocol may be unacceptably immunosuppressive 34. Sykes's group, at Columbia University, also achieved prolonged lymphoid chimerism successfully in a cynomolgus monkey recipient by infusion of ex‐vivo expanded Tregs. However, in this model Treg infusion was also associated with CMV reactivation in a significant number of recipients 35. Thus, more specific suppression of alloimmunity while maintaining anti‐viral immunity is required to develop a conditioning regimen for induction of persistent mixed chimerism.
Clinical trials
Clinical trials to induce renal allograft tolerance through DBMT have been reported from three centres: Stanford, Northwestern and Massachusetts General Hospital (MGH) in the United States.
Stanford approach (Fig. 1a and Table 1)
Figure 1.
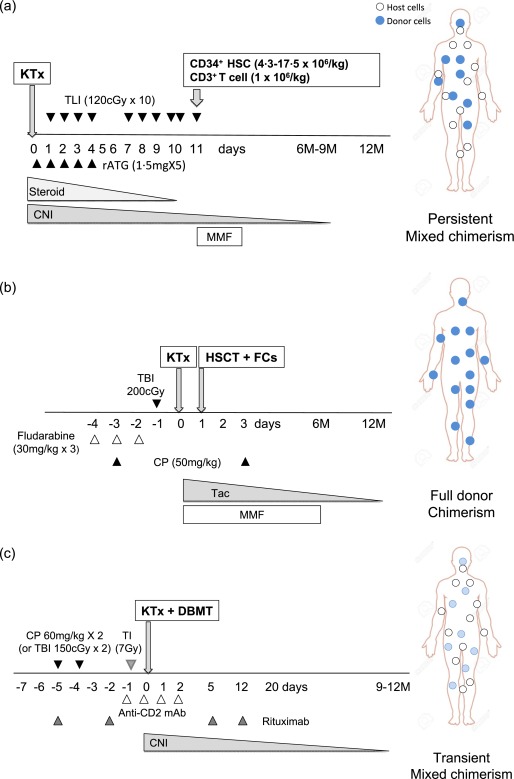
Three pilot studies of renal allograft tolerance induction in human living‐donor kidney transplantation at Stanford, Northwestern and Massachusetts General Hospital (MGH). Stanford human leucocyte antigen (HLA)‐matched conditioning protocol consists of total lymphoid irradiation (TLI) (120 cGy/day, 10 daily doses starting on postoperative day 1) and rabbit anti‐thymocyte globulin (rATG) (1·5 mg/kg/day, five daily doses starting intra‐operatively). Following the last dose of TLI, CD34+‐enriched donor peripheral blood stem cells are infused. The recipients are then maintained on calcineurin inhibitor (CNI)/mycophenolate mofetil (MMF)/steroid therapy until weaning was attempted several months later (a). Northwestern protocol consists of Fludarabine (30 mg/kg on days −4, −3 and −2), cyclophosphamide (CP) (50 mg/kg on days −3 and +3) and total body irradiation (TBI) (200 cGy) on day −1. This is followed by kidney transplantation (KTx), then donor haematopoietic stem cell transplantation (HSCT) on day +1. Immunosuppression consists of MMF and tacrolimus starting on day 0 and tapered off slowly by 1 year. In addition, the Northwestern regimen includes infusion of a unique ‘facilitating cell’ [a mixture of CD8+/T cell receptor (TCR–)] in the attempt to enhance engraftment and reduce further the risk of graft‐versus‐host disease (GVHD) (b). The initial MGH conditioning regimen for human leucocyte antigen (HLA)‐mismatched KTx included CP, TI anti‐CD2 monoclonal antibody (mAb) and post‐transplant CNI administration. To prevent donor‐specific antibody (DSA) development, we add rituximab therapy subsequently around the peri‐transplant period. As acute kidney injury had not been observed in the non‐human primate (NHP) studies that utilized TBI rather than CP in the conditioning regimen, a revised regimen in which low‐dose TBI replaced CP, has been evaluated recently in three recipients (c). Open circles indicate host haematopoietic cells and closed circles indicate donor haematopoietic cells.
Table 1.
Outcomes of pilot studies of tolerance induction for living donor kidney transplantation in Stanford, Northwestern and Massachusetts General Hospital (MGH)
| Stanford | Northwestern | MGH | ||
|---|---|---|---|---|
| HLA | Matched | Mismatched | Mismatched | |
| Number | 22 | 31 | 12* | |
| Off immunosuppression | 17 † | 16 | 8 | |
| Death (related directly to the regimen) | 0 | 1 ‡ | 0 | |
| Rejection | 3 | 3 | 3 § | |
| GVHD | 0 | 2 | 0 | |
| Chimerism | ||||
| Induction | 21 | 30 | 11 | |
| Transient | 9 | 5 | 11 | |
| Stable mix | 7 | 3 | 0 | |
| Full | 0 | 16 | 0 | |
*10 patients received the cyclophosphamide‐based conditioning regimen and two received total body irradiation (TBI)‐based regimen. †One patient is in the midst of immunosuppressive drug tapering. ‡One patient died due to graft‐versus‐host disease (GVHD). §Two patients developed chronic rejection after 5 and 8 years. One patient developed acute rejection at 9 months. HLA = human leucocyte antigen.
In 1989, Strober et al. reported successful induction of renal allograft tolerance in three human leucocyte antigen (HLA)‐mismatched kidney transplant recipients using total lymphoid irradiation (TLI) and rabbit ATG (rATG), but without DBMT 36. However, two of these three recipients eventually lost renal allograft function due to chronic rejection and ureteral stricture 37. Based on this initial experience with TLI, HSCT was combined with TLI and rATG to induce mixed chimerism. Their conditioning protocol consists of TLI (80–120 cGy/day 10 daily doses starting on post‐operative day 1) and rATG (1·5 mg/kg/day, five daily doses starting on day 0). Following the last dose of TLI, CD34+‐enriched donor peripheral blood stem cells were infused. These cells were collected by one or two aphereses from the donor after treatment with granulocyte–colony‐stimulating factor for 5–6 days. The recipients were maintained with calcineurin inhibitor (CNI), mycophenolate mofetil (MMF) and steroid immunosuppression. The advantage of this protocol is its clinical applicability to deceased donor transplantation, because all treatments are initiated after transplantation. This group has described three cohorts that underwent this protocol in a recent summary of their experience 38. The first cohort (2000–03) included six HLA‐mismatched renal allograft recipients. Only two of the six recipients developed transient chimerism for 2–3 months. Weaning of immunosuppression was attempted in these two recipients, but both developed mild rejection (Banff I) at 3 and 5 months after immunosuppression withdrawal, leading to reinstitution of immunosuppression 39. The second cohort (2005–13) included only HLA‐matched allograft recipients. Immunosuppression withdrawal criteria were modified in this group, requiring persistent chimerism for at least 6 months, absence of rejection on protocol biopsy and no evidence of graft‐versus‐host disease (GVHD) (Fig. 1a and Table 1). Chimerism was induced initially in 21 of 22 subjects studied, and 18 patients met the immunosuppression withdrawal criteria. Among these 18 recipients, 16 (seven with stable chimerism and nine with transient chimerism) continued to be off immunosuppression for 2–66 months. Immunosuppression was reinstituted in one recipient due to lupus flare, and one recipient is still in the midst of immunosuppression withdrawal. Three did not meet the immunosuppression withdrawal criteria despite development of chimerism, because of clinical or biopsy‐proven rejection 38. The third cohort included 10 recipients of HLA‐haplotype‐matched kidneys. An escalating dose of infused CD34+ and CD3+ T cells (3, 10, 20 and 50 × 106/kg compared to 1 × 106/kg in the prior two cohorts) was used in the effort to promote mixed chimerism induction. Persistent chimerism for at least 12 months was achieved in two patients. In these two patients MMF was discontinued at 9 months, after which the patients remained on tacrolimus monotherapy which continued at the time of this report. The remaining eight recipients developed transient chimerism or no chimerism, and their immunosuppression was not tapered 38. In summary, with the Stanford protocol, durable or transient chimerism was induced in the majority of HLA‐matched transplant recipients and immunosuppression was discontinued successfully in approximately 70% of the patients. Induction of chimerism has been more difficult in HLA‐mismatched transplant recipients, and none of these recipients has achieved complete withdrawal of immunosuppression to date.
Northwestern approach (Fig. 1b and Table 1)
Until recently HLA‐mismatched allogeneic DBMT (complete chimera) was associated with a mortality rate exceeding 50%, mainly as a result of GVHD 40. The Johns Hopkins group developed a novel conditioning regimen for HLA‐haploidentical allogeneic DBMT, which provided no incidence of GVHD among 13 sickle cell disease patients 41. The most important component of this regimen is post‐transplant cyclophosphamide (CP) on days 3 or 4, designed to delete alloreactive T cells elicited after DBMT 41. The conditioning regimen developed by the Northwestern group also includes post‐Tx CP. Their regimen consisted of fludarabine (30 mg/kg on days −4, −3 and −2), CP (50 mg/kg on day −3) and TBI (200 cGy on day −1), followed by KTx on day 0 and HSCT on day +1. Post‐Tx CP is administered on day +3, but they also added infusion (day + 1 with HSCT) of a unique recipient cell population named ‘facilitating cells’, which have tolerogenic features of CD8+/T cell receptor (TCR−) and a heterogeneous population composed predominantly of plasmacytoid DC 42, 43, on day +1 in an effort to enhance engraftment of haematopoietic stem cells and to reduce further the risk of GVHD 44. MMF and tacrolimus are administered after transplant and are tapered off by 6 and 12 months, respectively. A total of 31 patients have been enrolled into their clinical trial and 30 exhibited donor chimerism at 1 month after KTx. Nineteen recipients achieved durable chimerism, 16 were completely withdrawn from immunosuppressant for 3–65 months and the remaining three were weaning with all stable chimeric conditions. Two of 30 subjects lost their allograft due to infectious complications. Although the incidence of GVHD was reduced significantly, even in HLA‐mismatched transplantation, two of 30 patients developed GVHD and one was dead 45. The state of full chimerism has been considered immuno‐incompetent 46, 47, 48, and two serious infectious complications that resulted in graft loss have been reported. Another death due to malignancy has also been reported 45, but its relevance to the state of full chimerism is not known.
The Northwestern group is pursuing another tolerance induction strategy for HLA‐matched living donor KTx recipients. This regimen includes alemtuzumab induction, donor HSC infusion, MMF and tacrolimus, with tacrolimus being converted to sirolimus after 3 months and then tapered off slowly by 24 months post‐transplantation. Twenty recipients were enrolled originally, but five recipients were excluded due to positive pre‐Tx cross‐match (n = 1), non‐compliance (n = 1) and disease recurrences (n = 3). Among the 15 remaining recipients who completed 36 months post‐Tx follow‐up, six recipients achieved successful immunosuppression withdrawal for 32–64 months. Immunosuppression was not discontinued in the other nine recipients due to rejection detected in the protocol biopsies 49.
MGH approach (Fig. 1c and Table 1)
Based on decades‐long studies in NHPs 20, 21, 22, we have performed clinical trials to induce allograft tolerance in HLA‐matched 50 and ‐mismatched living donor KTx 51, 52, 53. The initial conditioning regimen for HLA‐mismatched KTx included CP, thymic irradiation (TI), anti‐CD2 mAb and post‐transplant CNI administration. Because of humoral responses observed in the second and third patients, perioperative administration of rituximab was added after the fourth recipient. Of the 10 recipients enrolled into the studies, all developed transient mixed chimerism and immunosuppression was discontinued in eight recipients by 9–14 months post‐transplant. One of the eight developed acute rejection and required retransplantation 2 years later despite reinstitution of immunosuppression. After a follow‐up period of 7–14 years, four of the remaining seven remained immunosuppression free for 14, 7, 6 and 6 years, while three resumed immunosuppression at 5, 7 and 8 years after KTx as a result of original kidney disease recurrence or chronic rejection 52, 53. An unexpected adverse event, acute kidney injury, was observed between 10 and 20 days post‐transplant in nine of these 10 subjects. It was associated with haematopoietic cell recovery and then rapid loss of chimerism. As acute kidney injury had not been observed in the NHP studies that received low‐dose TBI rather than CP, we performed a clinical pilot study more recently using a conditioning regimen in which CP was replaced with low dose TBI. Both recipients have done well, without evidence of the acute kidney injury, and immunosuppression in the first patient has been discontinued for > 3 years. As anti‐CD2 mAb (MEDI‐507 mAb) included in the initial conditioning regimen is not a Food and Drug Administration (FDA)‐approved drug and its future clinical availability is uncertain, further clinical trials are planned using a new regimen with belatacept, which is developed based on an NHP study 22.
We compared postoperative complications and quality of life (QoL) of five tolerance recipients (tolerant group) with 31 comparable live donor kidney recipients on conventional immunosuppression (conventional group). Patients in the tolerant group required significantly less treatment after transplant for hypertension and no medications for diabetes (P < 0·01). There was no diabetes, dyslipidaemia or malignancy in the tolerant group, while these were observed in 12·5, 40·6 and 11·8% of the conventional group, respectively. Tolerant patients experienced better overall health (P < 0·01) and scored higher on kidney transplant‐targeted scales and healthy survey scales than patients in the conventional group according to the KDQOL SF‐36 (P < 0·05). Tolerant patients were less likely to experience depression, dyspnoea, excessive appetite/thirst, flatulence, hearing loss, itching, joint pain, lack of energy, muscle cramps and lack of libido than conventional patients, according to the MTSOSD‐59R (P < 0·05) 54. These observations provide the proof of principle that induction of tolerance is ideal for maintenance of overall QOL.
Conclusion
Tolerance induction is now a clinical reality in humans, at least for patients undergoing living donor KTx. The three major centres performing these studies continue to obtain promising results, and hopefully can soon expand their tolerance approaches to deceased donor transplants or non‐renal organs. Improving the consistency and safety of tolerance induction will be a next crucial step to bringing tolerance to a wider range of clinical applications.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose. This paper was also not prepared or funded by any commercial organization.
Acknowledgements
The present work was supported in part by NIH/NIAID NO1 AI1541, the Immune Tolerance Network (ITN) and grant 5U19AI102405, part of the NIH NHP Transplantation Tolerance Cooperative Study Group and sponsored by the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1. Meier‐Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4:378–83. [DOI] [PubMed] [Google Scholar]
- 2. Lodhi SA, Lamb KE, Meier‐Kriesche HU. Solid organ allograft survival improvement in the United States: the long‐term does not mirror the dramatic short‐term success. Am J Transplant 2011; 11:1226–35. [DOI] [PubMed] [Google Scholar]
- 3. Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2002; 2:807–18. [DOI] [PubMed] [Google Scholar]
- 4. Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol 2005; 16:496–506. [DOI] [PubMed] [Google Scholar]
- 5. Morales JM, Dominguez‐Gil B. Cardiovascular risk profile with the new immunosuppressive combinations after renal transplantation. J Hypertens 2005; 23:1609–16. [DOI] [PubMed] [Google Scholar]
- 6. Scantlebury V, Shapiro R, Fung J et al New onset of diabetes in FK 506 vs cyclosporine‐treated kidney transplant recipients. Transplant Proc 1991; 23:3169–70. [PMC free article] [PubMed] [Google Scholar]
- 7. Jindal RM, Sidner RA, Milgrom ML. Post‐transplant diabetes mellitus. The role of immunosuppression. Drug Saf 1997; 16:242–57. [DOI] [PubMed] [Google Scholar]
- 8. Marchetti P, Navalesi R. The metabolic effects of cyclosporin and tacrolimus. J Endocrinol Invest 2000; 23:482–90. [DOI] [PubMed] [Google Scholar]
- 9. Nemunaitis J, Deeg HJ, Yee GC. High cyclosporin levels after bone marrow transplantation associated with hypertriglyceridaemia. Lancet 1986; 2:744–5. [DOI] [PubMed] [Google Scholar]
- 10. Jevnikar AM, Petric R, Holub BJ, Philbrick DJ, Clark WF. Effect of cyclosporine on plasma lipids and modification with dietary fish oil. Transplantation 1988; 46:722–5. [DOI] [PubMed] [Google Scholar]
- 11. Abouljoud MS, Levy MF, Klintmalm GB. Hyperlipidemia after liver transplantation: long‐term results of the FK506/cyclosporine A US Multicenter Trial. US Multicenter Study Group. Transplant Proc 1995; 27:1121–3. [PubMed] [Google Scholar]
- 12. McCune TR, Thacker LR II, Peters TG et al Effects of tacrolimus on hyperlipidemia after successful renal transplantation: a Southeastern Organ Procurement Foundation multicenter clinical study. Transplantation 1998; 65:87–92. [DOI] [PubMed] [Google Scholar]
- 13. Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc 1969; 1:106–12. [PMC free article] [PubMed] [Google Scholar]
- 14. Schneck SA, Penn I. De‐novo brain tumours in renal‐transplant recipients. Lancet 1971; 1:983–6. [DOI] [PubMed] [Google Scholar]
- 15. Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl 1998; 147–58. [PubMed] [Google Scholar]
- 16. Hart A, Smith JM, Skeans MA et al Kidney. Am J Transplant 2016; 16:11–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todo S, Yamashita K, Goto R et al A pilot study of operational tolerance with a regulatory T‐cell‐based cell therapy in living donor liver transplantation. Hepatology 2016; 64:632–43. [DOI] [PubMed] [Google Scholar]
- 18. Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev 2003; 196:147–60. [DOI] [PubMed] [Google Scholar]
- 19. Adams AB, Williams MA, Jones TR et al Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 2003; 111:1887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawai T, Cosimi AB, Colvin RB et al Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 1995; 59:256–62. [PubMed] [Google Scholar]
- 21. Kawai T, Sogawa H, Boskovic S et al CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant 2004; 4:1391–8. [DOI] [PubMed] [Google Scholar]
- 22. Yamada Y, Ochiai T, Boskovic S et al Use of CTLA4Ig for induction of mixed chimerism and renal allograft tolerance in nonhuman primates. Am J Transplant 2014; 14:2704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koyama I, Nadazdin O, Boskovic S et al Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant 2007; 7:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamada Y, Boskovic S, Aoyama A et al Overcoming memory T‐cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant 2012; 12:330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hotta K, Aoyama A, Oura T et al Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI Insight 2016; 1:e86419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boskovic S, Kawai T, Smith RN et al Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long‐term observations in nonhuman primates. Transplantation 2006; 82:819–25. [DOI] [PubMed] [Google Scholar]
- 27. Wu Z, Bensinger SJ, Zhang J et al Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med 2004; 10:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai Z, Li Q, Wang Y et al CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30‐dependent mechanism. J Clin Invest 2004; 113:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of Treg‐mediated T cell suppression. Front Immunol 2012; 3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T‐cell effector differentiation by modulating IL‐2 homeostasis. Proc Natl Acad Sci USA 2011; 108:7529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aoyama A, Tonsho M, Ng CY et al Long‐term lung transplantation in nonhuman primates. Am J Transplant 2015; 15:1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tonsho M, Lee S, Aoyama A et al Tolerance of lung allografts achieved in nonhuman primates via mixed hematopoietic chimerism. Am J Transplant 2015; 15:2231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burlingham WJ, Love RB, Jankowska‐Gan E et al IL‐17‐dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 2007; 117:3498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng HB, Watkins B, Tkachev V et al The knife's edge of tolerance: inducing stable multilineage mixed chimerism but with a significant risk of CMV reactivation and disease in Rhesus macaques. Am J Transplant 2016; 8:14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duran‐Struuck R, Sondermeijer HP, Buhler L et al Effect of ex vivo expanded recipient regulatory T cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in Cynomolgus macaques. Transplantation 2017; 101:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strober S, Dhillon M, Schubert M et al Acquired immune tolerance to cadaveric renal allografts. A study of three patients treated with total lymphoid irradiation. N Engl J Med 1989; 321:28–33. [DOI] [PubMed] [Google Scholar]
- 37. Strober S, Benike C, Krishnaswamy S, Engleman EG, Grumet FC. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: studies of chimerism and anti‐donor reactivity. Transplantation 2000; 69:1549–54. [DOI] [PubMed] [Google Scholar]
- 38. Scandling JD, Busque S, Shizuru JA et al Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant 2015; 15:695–704. [DOI] [PubMed] [Google Scholar]
- 39. Millan MT, Shizuru JA, Hoffmann P et al Mixed chimerism and immunosuppressive drug withdrawal after HLA‐mismatched kidney and hematopoietic progenitor transplantation. Transplantation 2002; 73:1386–91. [DOI] [PubMed] [Google Scholar]
- 40. Shaw PJ, Kan F, Woo Ahn K et al Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood 2010; 116:4007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bolanos‐Meade J, Fuchs EJ, Luznik L et al HLA‐haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 2012; 120:4285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow‐derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 1994; 84:2436–46. [PubMed] [Google Scholar]
- 43. Fugier‐Vivier IJ, Rezzoug F, Huang Y et al Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med 2005; 201:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leventhal J, Abecassis M, Miller J et al Chimerism and tolerance without GVHD or engraftment syndrome in HLA‐mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 2012; 4:3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leventhal J, Galvin J, Stare D et al Seven year follow‐up of a phase 2 clinical trial to induce tolerance in living donor renal transplant recipients. Am J Transplant 2016; 16(suppl 3). [Google Scholar]
- 46. Sykes M, Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol Today 1988; 9:23–7. [DOI] [PubMed] [Google Scholar]
- 47. Zinkernagel RM, Althage A, Callahan G, Welsh RM Jr. On the immunocompetence of H‐2 incompatible irradiation bone marrow chimeras. J Immunol 1980; 124:2356–65. [PubMed] [Google Scholar]
- 48. Speiser DE, Roosnek E, Jeannet M, Zinkernagel RM. T‐cell immunoincompetence in allogeneic chimerism. N Engl J Med 1992; 326:1028–9. [DOI] [PubMed] [Google Scholar]
- 49. Leventhal JR, Mathew JM, Salomon DR et al Nonchimeric HLA‐identical renal transplant tolerance: regulatory immunophenotypic/genomic biomarkers. Am J Transplant 2016; 16:221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spitzer TR, Sykes M, Tolkoff‐Rubin N et al Long‐term follow‐up of recipients of combined human leukocyte antigen‐matched bone marrow and kidney transplantation for multiple myeloma with end‐stage renal disease. Transplantation 2011; 91:672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kawai T, Cosimi AB, Spitzer TR et al HLA‐mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA‐mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2013; 368:1850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawai T, Sachs DH, Sprangers B et al Long‐term results in recipients of combined HLA‐mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 2014; 14:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Madariaga ML, Spencer PJ, Shanmugarajah K et al Effect of tolerance versus chronic immunosuppression protocols on the quality of life of kidney transplant recipients. JCI Insight 2016; 1:e87019. [DOI] [PMC free article] [PubMed] [Google Scholar]


