Abstract
Background
Chronic diarrhea (CD) is common in dogs, and information on frequency and distribution of primary and secondary causes is lacking.
Objectives
To evaluate underlying causes and predictors of outcome in dogs with CD.
Animals
One hundred and thirty‐six client‐owned dogs with CD (≥3 weeks duration).
Methods
Retrospective review of medical records (Small Animal Clinic, Freie Universität Berlin, Germany, 09/2009‐07/2011). Quantification of final diagnoses and comparison of clinical aspects including disease severity and clinicopathological abnormalities among dogs with clinical remission (either complete [gastrointestinal signs absent] or partial [clinical improvement of gastrointestinal signs and reduced episodes with shortened duration]), and those without recovery.
Results
Ninety percent of dogs were diagnosed with a primary enteropathy: inflammatory (71%; of those 66% dietary responsive, 23% idiopathic, 11% antibiotic responsive), infectious (13%), neoplastic (4%), and in one dog each mechanical disease or systemic vasculitis. Secondary causes were diagnosed in 10% of dogs: exocrine pancreatic (6%), endocrine (2%), and in one dog each hepatic, renal, and cardiac disease. In total, 87% of dogs had clinical remission, whereas 13% died or did not respond to treatment: Lack of recovery was frequently recorded for dogs with primary inflammatory (idiopathic) or neoplastic disease and was significantly associated with increased disease severity scores (P = .005), anemia (hematocrit < 40%, P < .001), severe hypoalbuminemia (serum albumin <2.0 g/dL, P = .008), and severe hypocobalaminemia (serum cobalamin concentration <200 pg/mL, P = .006).
Conclusions and clinical importance
Inflammatory enteropathies and particularly those of dietary origin were the most common causes of CD in dogs. Findings support the usefulness of hematocrit, and serum albumin and cobalamin concentration as prognostic markers in dogs with CD.
Keywords: Enteropathy, Epidemiology, Inflammatory bowel disease, Outcome
Abbreviations
- ARE
antibiotic responsive enteropathy
- BCS
body condition score
- CCECAI
canine chronic enteropathy clinical activity index
- CIBDAI
canine inflammatory bowel disease activity index
- CIE
chronic inflammatory enteropathy
- CR
complete recovery
- DI
diffuse intestinal disease
- FRE
food responsive enteropathy
- IBD
idiopathic inflammatory bowel disease
- LI
large intestinal disease
- NR
no recovery
- PE
primary enteropathy
- PR
partial recovery
- SE
secondary enteropathy
- SI
small intestinal disease
Chronic intermittent or persistent diarrhea is a common clinical sign in dogs with chronic enteropathy and might be a manifestation of gastrointestinal or extragastrointestinal disorders.1 Extragastrointestinal disorders (i.e, secondary enteropathies) include diseases of the exocrine pancreas, the liver, and the kidneys as well as endocrinopathies and diseases of the cardiovascular or central nervous system. The cause of gastrointestinal disorders (i.e, primary enteropathies) can be infectious, neoplastic, mechanical, toxic, or noninfectious inflammatory.1, 2 Noninfectious inflammatory causes include dietary and antibiotic responsive enteropathies as well as idiopathic inflammatory diseases that require anti‐inflammatory or immunosuppressive treatment. The different causes of respective chronic inflammatory enteropathies (CIE) appear phenotypically similar upon histological evaluation of a tissue biopsy and are diagnosed retrospectively by their response to sequential treatment trials involving diet(s), antibiotics, and anti‐inflammatory/immunosuppressive drugs.3, 4, 5, 6, 7
CIE is considered to be the most common cause of chronic gastrointestinal disease in dogs.1, 7, 8 However, information concerning the frequency and distribution of underlying primary and secondary causes of chronic diarrhea in dogs is lacking. Thus, the prevalence of the various causes of CIE is unknown.1,1, 8, 9 Clinicopathological findings and clinical aspects such as the localization to small or large bowel diarrhea might be useful to clarify underlying causes.2, 8
The principal objective of this study was to quantify the final diagnoses in a large number of dogs with chronic diarrhea and to investigate the hypotheses that noninfectious inflammatory enteropathies (CIE) are the most frequent causes of chronic diarrhea. Further objectives were to evaluate clinical and clinicopathological abnormalities associated with an adverse clinical outcome in (1) the overall population of dogs with chronic diarrhea and (2) in dogs with specific diagnoses causing chronic diarrhea.
Materials and Methods
Medical records of dogs with chronic diarrhea that were presented to the Clinic for Small Animals, Freie Universität Berlin, Germany, between September 2009 and July 2011, were retrospectively reviewed. Dogs were included if diarrhea had been present for 3 weeks or longer and a minimum work‐up had been performed, which included hematology, plasma biochemistry profile, and fecal flotation and Giardia ELISA. Other diagnostic modalities included serum analyses for cobalamin, folate, canine trypsin‐like immunoreactivity, canine‐specific pancreatic lipase and cortisol/adrenocorticotropic hormone stimulation test, urinalyses, abdominal radiographs and ultrasound, as well as fecal microbiology and rectal scrapings. Endoscopy of the gastrointestinal tract or laparotomy with collection of tissue biopsies and histopathologic evaluation of intestinal biopsies were recommended in dogs with suspected inflammatory and neoplastic diseases. A stepwise approach was performed to further characterize noninfectious inflammatory enteropathies: initially, dogs were fed an elimination diet of home‐cooked novel protein diet or hypoallergenic diet,2 commercial exclusion diet,3 or a combination thereof. Food responsive enteropathy (FRE) was defined as the positive clinical response to an elimination diet, and the diet was continued for at least 4–8 weeks. If no clinical improvement was observed within 2–4 weeks, it was recommended to change the diet or move to the next therapeutic trial with metronidazole (10 mg/kg BW, PO, q 12 h) or tylosin (25 mg/kg BW, PO, q 12 h) for another empirical treatment period of at least 10 days. A positive clinical response to antibiotic treatment defined the diagnosis of antibiotic responsive enteropathy (ARE). Dogs that neither responded to diet nor to antibiotics, and that required anti‐inflammatory/immunosuppressive treatment such as prednisolone at 0.5–1.0 mg/kg BW, PO, q 12–24 h, or cyclosporine at 5 mg/kg BW, PO, q 24 h were diagnosed as having idiopathic inflammatory bowel disease or steroid responsive enteropathy (IBD). Dogs were excluded from the study if a final diagnosis was not recorded.
Information obtained from the medical records: sex, age, body weight, breed size (criteria were based on breed descriptions from the Federation Cynologique Internationale: small‐sized, which is any breed with a height at withers <30 cm; medium‐sized, which is any breed with a height at withers 30–50 cm; and large‐sized, which is any breed with a height at withers ≥50 cm),10 body condition score (BCS, 5‐integer scale: 1, thin; 2, underweight; 3, ideal weight; 4, overweight; 5, obese),4 feces consistency, frequency, and abnormalities including melena, mucus, or blood in feces and tenesmus, secondary clinical signs such as changes in appetite or activity level, weight loss, vomiting, abdominal pain, borborygmi, flatulence, and pruritus, predominant gastrointestinal site of disease according to presenting clinical signs,1, 2 duration of clinical signs before presentation (3 to 6 weeks, 6 weeks to 6 months, 6 to 12 months, more than 1 year), and clinicopathological abnormalities. The canine IBD activity index (CIBDAI) was used to assess disease severity and was based on the parameters: weight loss, appetite, activity level, feces consistency, feces frequency, and vomiting.11 Furthermore, follow‐up information was obtained from the medical records or by contacting the owners via telephone or e‐mail up to one year after initial presentation (Fig 1). Response to treatment was defined as either complete recovery (CR; marked improvement with the absence of gastrointestinal signs), partial recovery (PR; clinical improvement with reduction of gastrointestinal signs and reduced episodes with shortened duration), or no recovery (NR; no response to treatment, dog died or was euthanized).
Figure 1.
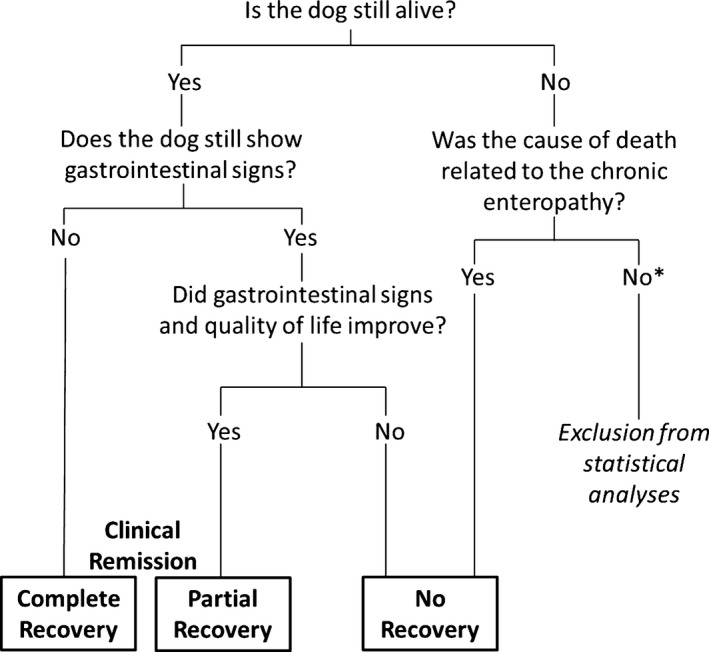
Questionnaire to define outcome groups in dogs with chronic diarrhea. *There was no dog that fulfilled these criteria.
Statistical analyses were performed by statistical software packages (i.e, SPSS, IBM SPSS statistics campus, Ehningen, Germany; version 20 and GraphPad Prism software, La Jolla, California, USA; version 5). Characteristics, clinical aspects, and outcome were compared between dogs with primary or secondary causes, and among selected groups of dogs with the same final diagnoses with a group size of at least 5 dogs. Final diagnoses with a group size of 3 followed by 4 dogs were descriptively analyzed. Characteristics, clinical, and clinicopathological abnormalities were compared among different groups of dogs based on outcome. Clinicopathological abnormalities identified to be associated with an adverse clinical outcome were compared among dogs with a different predominant site of disease and among groups of dogs with selected final diagnoses. Pearson chi‐square or Fisher's exact tests were used to compare categorical variables, and results are given as percentages. Median and range are presented for quantitative variables and Mann‐Whitney U‐tests or Kruskal‐Wallis tests were used to compare continuous variables between 2 or more groups, respectively. Mann‐Whitney U‐tests with Bonferroni correction of P values were used for posthoc comparisons. Statistical significance was set at P < .05.
Results
Final Diagnoses
Two hundred and nine dogs with chronic intermittent or persistent diarrhea were presented during the 2 year study period, and 136 cases fulfilled the inclusion criteria.
Primary enteropathies (PE) were diagnosed in 123 of 136 dogs (90%), and chronic inflammatory enteropathies were the most frequent causes within this group (n = 97, 79%) (Fig 2): Sixty‐four dogs improved after feeding an elimination diet (home‐cooked novel protein diet, n = 28; hypoallergenic diet,2 n = 25; combined home‐cooked and hypoallergenic diet, n = 5; commercial exclusion diet,3 n = 6) and were therefore diagnosed as FRE. ARE was diagnosed in 11 cases (positive clinical response to metronidazole, n = 9, or tylosin, n = 2), and 22 dogs were diagnosed with IBD. Histopathologic analyses of gastrointestinal biopsies were performed in 34 dogs with chronic inflammatory enteropathies: a lymphocytic‐plasmacytic inflammation was identified in 8 dogs, an eosinophilic inflammation in 2, and a mixed inflammatory cell infiltrate in 18 dogs. In 6 cases, no abnormality was identified (Table 1).
Figure 2.
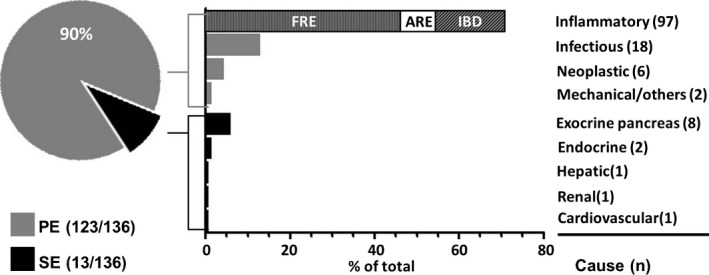
Distribution of primary and secondary causes and frequencies of underlying causes of chronic diarrhea in 136 dogs. Bar graphs representing frequencies of total. PE, primary enteropathy; SE, secondary enteropathy; FRE, food responsive enteropathy (n = 64); ARE, antibiotic responsive enteropathy (n = 11); IBD, idiopathic inflammatory bowel disease (n = 22).
Table 1.
Descriptive results for the histopathologic analyses of gastrointestinal biopsies from 34 dogs with chronic inflammatory enteropathies
| Total (n = 34) | FRE (n = 13) | ARE (n = 5) | IBD (n = 16) | ||
|---|---|---|---|---|---|
| Severity grade | NAD | 6 | 3 | 2 | 1 |
| Mild | 12 | 4 | 2 | 6 | |
| Moderate | 12 | 5 | 1 | 6 | |
| Severe | 4 | 1 | – | 3 | |
| Dominant inflammatory cell type | NAD | 6 | 3 | 2 | 1 |
| Lymphocytic‐plasmacytic | 8 | 2 | – | 6 | |
| Eosinophilic | 1 | – | 1 | – | |
| Eosinophilic and histiocytic | 1 | – | – | 1 | |
| Mixed | 18 | 8 | 2 | 8 |
FRE, food responsive enteropathy; ARE, antibiotic responsive enteropathy; IBD, idiopathic inflammatory bowel disease; NAD, no abnormality detected.
An infectious cause was identified in 18 of 136 dogs (13%): A parasitic infection was diagnosed in 16 dogs, which clinically improved after antiparasitic therapy (Giardia spp., n = 13; mixed infection with Giardia spp., Coccidia, and Toxocara spp., n = 2; Leishmania spp., n = 1). Systemic infection with Prototheca zopfii genotype II was identified in 2 cases.
Lymphoma was diagnosed in 5 of 136 cases (4%) and was the most frequent neoplastic disease. A colorectal adenocarcinoma was identified in one dog.
Colonic intussusception led to chronic bloody diarrhea in a young German Shepherd dog, and systemic vasculitis was diagnosed in one German Shepherd dog.
Secondary enteropathies (SE) were diagnosed in 13 of 136 dogs (10%) (Fig 2): Diseases of the exocrine pancreas were the most frequent extragastrointestinal causes (8 of 136 cases, 6%: exocrine pancreatic insufficiency, n = 4; chronic pancreatitis, n = 3; pancreatic adenocarcinoma, n = 1). Endocrine disorders occurred in two dogs (atypical hypoadrenocorticism and hypothyroidism in one dog each). Hepatic, renal, or cardiovascular diseases were identified in 3 dogs, one with a portosystemic shunt, one with chronic kidney disease, and one with chronic heart failure.
Dog Characteristics
Male dogs were overrepresented in the study population with a male/female ratio of 1.3 in dogs with PE and 2.3 in dogs with SE (Table 2). Middle‐aged dogs (≥2 to <9 years) were most frequently presented (n = 69, 51%), followed by old dogs (9 years and older: n = 37, 27%), and young dogs (less than 2 years: n = 30, 22%; including 4 dogs younger than 1 year). In comparison, dogs diagnosed with PE were younger than dogs with SE. More specifically, dogs diagnosed with Giardia spp. infection were the youngest, followed by dogs with ARE, FRE, and exocrine pancreatic insufficiency (Table 2). Dogs belonged to 47 different breeds with mixed‐breed dogs being most frequently presented (n = 35, 26%). Of those 7 were Terriers, 6 Labrador Retrievers, and 5 German Shepherd mixed‐breeds. Common pure‐bred dogs were German Shepherd dogs (n = 13, 10%), Yorkshire Terriers (n = 9, 7%), Dachshund (n = 8, 6%), and Boxers (n = 5, 4%). There was no breed significantly overrepresented in PE or SE (data not shown). Dogs with PE were often large‐sized (n = 60, 49%), whereas dogs with SE were more frequently medium‐sized (n = 6, 46%) (Table 2). A thin‐to‐underweight body condition score (BCS 1‐2) was common in both groups (PE, n = 74, 60%; SE, n = 9, 69%) (Table 2). Characteristics were not significantly different between dogs with PE and SE, and among selected diagnoses (except age) (Table 2).
Table 2.
Characteristics, clinical aspects, and outcome in dogs with chronic diarrhea
| Disease Group | Characteristics | Clinical aspects | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex | Age | Weight/Breed size | Body condition | N | CIBDAI | Localization | Outcome | |||||||||
| In years | Group | Weight in kg | Size | Score (1–5 points) | Group | Score (1–18 points) | Group | Group | Group | ||||||||
| M/MN/F/FN | Median | Range | YO/MA/OL | Median | Range | SM/ME/LA | Median | Range | T/U/I/O/A | Median | Range | IN/MI/MO/SV | SI/LI/DI | CR/PR/NR | |||
| Total | 136 | 60/19/33/24 | 5.5 | 0.5–15.0 | 30/69/37 | 20.1 | 2.0–70.5 | 31/42/63 | 2 | 1–5 | 17/66/46/6/1 | 127 | 7 | 2–16 | 5/24/54/44 | 42/36/49 | 68/43/16 |
| PE vs. SE (P valuea) | .488 | .113 | .688 | .066 | .190 | .454 | .962 | .501 | .315 | .001 | 1.000 | ||||||
| PE | 123 | 53/17/32/21 | 5.0 | 0.5–13.5 | 28/63/32 | 21.8 | 2.0–70.5 | 27/36/60 | 2 | 1–5 | 15/59/42/6/1 | 116 | 7 | 2–16 | 5/24/47/40 | 33/36/47 | 62/39/15 |
| SE | 13 | 7/2/1/3 | 8.0 | 1.0–15.0 | 2/6/5 | 9.5 | 2.0–32.0 | 4/6/3 | 2 | 1–3 | 2/7/4/0/0 | 11 | 7 | 6–12 | 0/0/7/4 | 9/0/2 | 6/4/1 |
| Selected diagnoses with PE (P valuea) | .328 | <.001 | .006 | .462 | .076 | .008 | .155 | <.001 | <.001 | .071 | <.001 | ||||||
| FRE | 64 | 28/7/16/13 | 5.3a | 0.5–13.5 | 13/36/15 | 17.3 | 3.1–51.3 | 15/25/24 | 2 | 1–5 | 4/29/26/4/1 | 61 | 7a | 2–14 | 5/14/30/12 | 17/20/24 | 40/21/0 |
| ARE | 11 | 5/2/4/0 | 4.0a,b | 1.0–12.0 | 2/7/2 | 26.0 | 3.8–68.5 | 4/1/6 | 2 | 2–4 | 0/7/3/1/0 | 10 | 7a,b | 4–13 | 0/3/5/4 | 1/4/5 | 6/4/0 |
| IBD | 22 | 8/3/5/6 | 8.0a | 1.0–12.5 | 4/8/10 | 28.8 | 2.0–70.5 | 6/2/14 | 2 | 1–3 | 6/11/5/0/0 | 21 | 10b | 4–16 | 0/1/5/15 | 8/1/12 | 4/10/7 |
| LYM | 5 | 2/2/0/1 | 9.0a | 7.0–13.0 | 0/2/3 | 30.0 | 24.0–35.0 | 0/1/4 | 2 | 1–2 | 2/3/0/0/0 | 5 | 11a,b | 5–15 | 0/1/0/4 | 3/1/1 | 0/0/5 |
| Giardia | 15 | 7/2/6/0 | 1.5b | 0.5–6.5 | 9/6/0 | 22.0 | 2.4–40.5 | 2/5/8 | 3 | 1–4 | 1/6/7/1/0 | 13 | 6a | 4–12 | 0/4/8/1 | 3/7/3 | 9/4/0 |
| Selected diagnoses with SEb | |||||||||||||||||
| EPI | 4 | 3/0/1/0 | 5.5 | 1.0–14.0 | 1/2/1 | 16.5 | 5.7–27.0 | 2/0/2 | 2 | 1–2 | 2/2/0/0/0 | 4 | 7 | 6–12 | 0/0/3/1 | 4/0/0 | 3/1/0 |
| CP | 3 | 0/0/0/3 | 10.0 | 6.5–11.0 | 0/1/2 | 20.1 | 4.6–32.0 | 1/1/1 | 3 | 2–3 | 0/1/2/0/0 | 3 | 10 | 9–12 | 0/0/0/3 | 2/0/1 | 2/1/0 |
| Outcome (P valuea) | .858 | .018 | .081 | <.001 | .010 | .132 | .541 | .005 | .056 | .241 | |||||||
| CR | 68 | 32/10/16/10 | 4.5a | 0.5–14.0 | 20/32/16 | 17.0a | 2.0–47.0 | 20/24/24 | 2 | 1–4 | 7/32/25/4/0 | 68 | 7a | 3–15 | 3/15/31/19 | 21/22/25 | |
| PR | 43 | 17/5/12/9 | 5.5a | 0.5–13.5 | 7/26/10 | 22.5a,b | 2.0–68.5 | 7/14/22 | 2 | 1–5 | 5/20/15/2/1 | 43 | 7a | 2–16 | 2/8/20/13 | 12/12/19 | |
| NR | 16 | 6/3/3/4 | 8.8b | 1.0–13.0 | 1/7/8 | 30.1b | 13.0–70.5 | 0/3/13 | 2 | 1–3 | 5/7/4/0/0 | 16 | 11b | 5–15 | 0/1/3/12 | 9/2/5 | |
P values based on Pearson chi‐square or Fisher's exact tests to compare categorical variables and Kruskal‐Wallis comparison of differences among medians. Medians without superscripts in common are statistically different based on Mann‐Whitney U‐tests and Bonferroni adjustment for multiple comparisons. Significance < .05.
Data for selected diagnoses with a group size of 3 or 4 dogs were descriptively analyzed. M, male; MN, male neutered; F, female; FN, female spayed; YO, young (less 2 years); MA, middle‐aged (≥2 to <9 years); OL, old (9 years and older); SM, small‐size breed; ME, medium‐size breed; LA, large‐size breed; T, thin; U, underweight; I, ideal weight; O, overweight; A, adipous; CIBDAI, canine inflammatory bowel disease activity index; IN, clinically insignificant disease; MI, mild disease; MO, moderate disease; SV, severe disease; SI, small intestinal disease; LI, large intestinal disease; DI, diffuse intestinal disease; CR, complete recovery; PR, partial recovery; NR, no recovery; PE, primary enteropathy; SE, secondary enteropathy; FRE, food responsive enteropathy; ARE, antibiotic responsive enteropathy; IBD, idiopathic inflammatory bowel disease; LYM, intestinal lymphoma; Giardia, infection with Giardia spp.; EPI, exocrine pancreatic insufficiency; CP, chronic pancreatitis.
Outcome and Clinical Findings
Clinical findings on the time of first presentation and outcome were known for 127 dogs (PE, n = 116; SE, n = 11). Two‐thirds of dogs were rechecked at the clinic, whereas for the remainder of the dogs follow‐up information was obtained by contacting the owners via telephone or e‐mail: One hundred and eleven dogs (87%) achieved clinical remission with 68 dogs being classified as complete recovery (CR) and 43 cases as partial recovery (PR). The remaining 16 dogs (13%) did not respond to treatment (n = 2), died, or were euthanized due to uncontrolled disease (n = 14), respectively. Dogs with no recovery (NR) had PE (n = 15) or SE (n = 1), and the following final diagnoses were identified: IBD (n = 7), intestinal lymphoma (n = 5), protothecosis (n = 2), systemic vasculitis, and pancreatic adenocarcinoma (both n = 1). Dogs with NR were significantly older than dogs in clinical remission (P = .018, Table 2).
A moderate disease severity (CIBDAI score 6‐8) was most frequently identified in the study population (n = 54, 42%) followed by severe (CIBDAI score ≥9: n = 44, 35%), mild (CIBDAI score 4‐5: n = 24, 19%), and clinically insignificant disease (CIBDAI score ≤3: 4%) (Table 2). Severe disease was significantly more common in dogs with IBD and intestinal lymphoma and was furthermore recorded in all 3 dogs with chronic pancreatitis (Table 2). Disease severity was not significantly different between dogs with PE or SE (Table 2). Disease severity scores were significantly higher in dogs with NR when compared to dogs in remission (P = .005; Fig 3A, Table 2).
Figure 3.
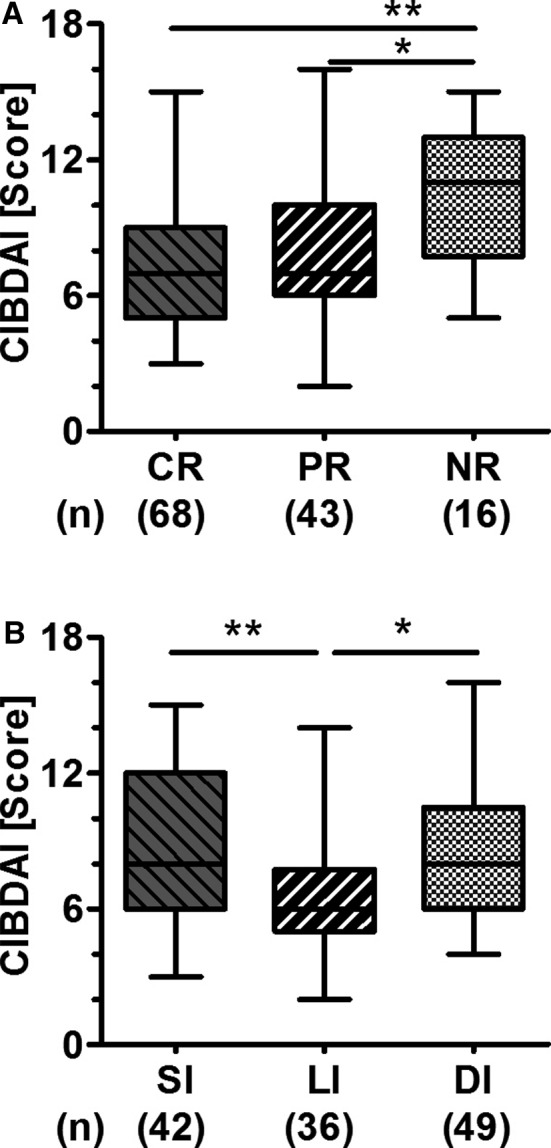
Disease severity in dogs with chronic diarrhea according to outcome (A) and predominant site of disease (B). Box‐and‐whiskers plots showing median, range, and 25th to 75th percentiles. *P < .05 and **P < .01 for Kruskal‐Wallis comparison of differences among medians followed by Mann‐Whitney U‐tests and Bonferroni adjustment for multiple comparisons. CIBDAI, canine inflammatory bowel disease activity index; CR, complete recovery; PR, partial recovery; NR, no recovery; SI, small intestinal disease; LI, large intestinal disease; DI, diffuse intestinal disease.
Disease was localized to the small intestine (SI, n = 42, 33%) or the large intestine (LI, n = 36, 28%) or was diffuse (DI, n = 49, 39%) (Table 2). Disease severity scores were significantly lower in dogs with predominantly large bowel diarrhea (CIBDAI score median/range: SI, 8/3‐15; LI, 6/2‐14; DI, 8/4‐16; P = .002; Fig 3B). LI was common in PE (Fig 4A) and was most frequently recorded in dogs with Giardia spp. infection, ARE, or FRE (Table 2). Clinical signs predominantly involving the small intestine were significantly more common in dogs with SE (PE: SI, n = 33, 28%; LI, n = 36, 31%; DI, n = 47, 41%; SE: SI, n = 9, 82%; DI, n = 2, 18%; P = .001; Fig 4A, Table 2). Moderate‐to‐severe vomiting was identified to be significantly more frequent in dogs with SE (PE, n = 32, 28%; SE, n = 7, 64%; P = .034). However, vomiting in general was commonly recorded in both dogs with PE and SE (PE, n = 82, 71%; SE, n = 8, 73%; P = 1.000). No other evaluated clinical sign was associated with PE or SE (data not shown). Disease localization was not significantly different among the 3 groups of dogs based on outcome (P = .241, Fig 4B, Table 2). However, a few clinical signs predominantly involving the small intestine were significantly associated with NR: watery diarrhea (CR n = 36, 53%; PR n = 20, 47%; NR n = 14, 88%; P = .017), weight loss (CR, n = 25, 37%; PR, n = 17, 40%; NR, n = 14, 88%; P = .001), and lethargy (CR, n = 1, 2%; PR, n = 1, 2%; NR, n = 3, 20%; P = .019).
Figure 4.
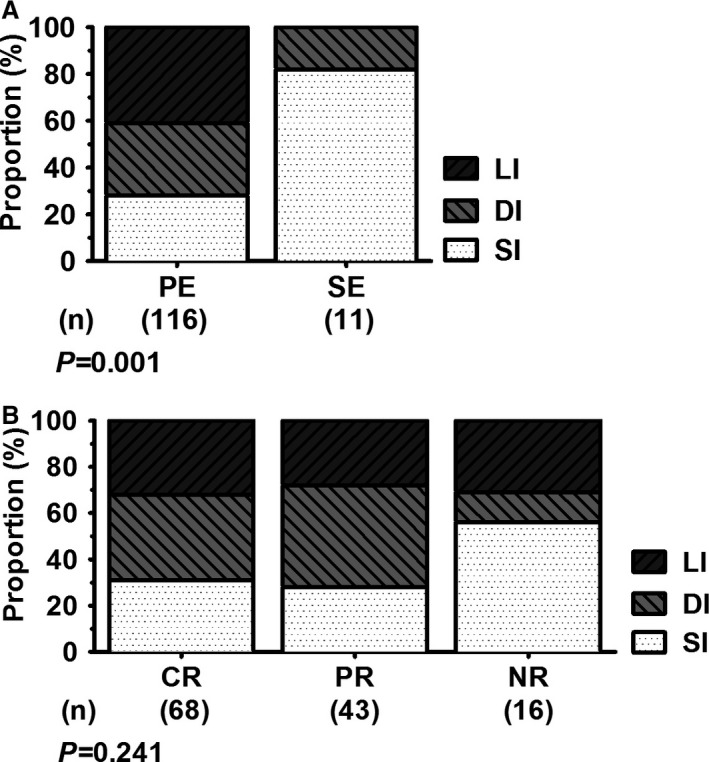
Comparison of predominance of clinical signs between groups of dogs with either primary or secondary enteropathy (A) and among outcome in dogs with chronic diarrhea (B). Pearson chi‐square or Fisher's exact tests were used to compare categorical variables. Statistical significance was set at P < .05. PE, primary enteropathy; SE, secondary enteropathy; CR, complete recovery; PR, partial recovery; NR, no recovery; SI, small intestinal disease; DI, diffuse intestinal disease; LI, large intestinal disease.
Clinical signs had been present for 3 to 6 weeks in 21% of dogs (n = 26), up to 6 months in 31% (n = 40), up to 1 year in 16% (n = 20), or more than 1 year in 32% of dogs (n = 41). Duration of disease was not significantly different between PE and SE, or among the 3 outcome groups (P = .514, resp. P = .260; data not separately shown). Vomiting and pruritus were associated with duration of disease: Dogs with moderate‐to‐severe vomiting were commonly presented earlier than dogs with mild or without vomiting (moderate‐to‐severe vomiting, n = 39: 3 to 6 weeks, n = 14, 36%; up to 6 months, n = 9, 23%; up to 1 year, n = 8, 20%; more than 1 year, n = 8, 20%; mild or no vomiting, n = 88: 3 to 6 weeks, n = 12, 14%; up to 6 months, n = 31, 35%; up to 1 year, n = 12, 14%; more than 1 year, n = 33, 38%; P = .012). Pruritus was recorded for 49 of 108 dogs (45%), and prolonged disease duration of more than 6 months was common in these dogs (3 to 6 weeks, n = 7, 14%; up to 6 months, n = 10, 20%; up to 1 year, n = 13, 27%; more than 1 year, n = 19, 39%; P = .011). Pruritus was frequently recorded in dogs with ARE and IBD, but differences among selected diagnoses were not statistically significant (FRE, n = 21, 38%; IBD, n = 11, 70%; ARE, n = 7, 70%; Giardia spp., n = 4, 50%; intestinal lymphoma, n = 1, 20%; P = .069). The presence of pruritus was not significantly different between outcome groups, disease severity groups, or between localization groups (outcome: CR, n = 26, 45%; PR. n = 20, 54%; NR. n = 3, 23%; P = .157; disease severity: clinically insignificant, n = 1, 20%; mild, n = 13, 62%; moderate, n = 18, 38%; severe, n = 17, 49%; P = .206; localization: SI, n = 14, 44%; LI, n = 11, 36%; DI, n = 24, 53%; P = .296).
Clinicopathological Findings
Hematocrit and serum albumin, calcium, and cobalamin concentration were significantly lower in dogs with no remission than in dogs that responded to treatment (Fig 5, Table 3). Thus anemia (hematocrit <40%), severe hypoalbuminemia (serum albumin concentration <2.0 g/dL), and severe hypocobalaminemia (serum cobalamin concentration <200 pg/mL) were significantly associated with a poor clinical outcome (anemia: CR, n = 5, 7%; PR, n = 3, 7%; NR, n = 9, 56%; P < .001; severe hypoalbuminemia in CR, n = 1, 2%; PR, n = 5, 12%; NR, n = 5, 31%; P = .008; and severe hypocobalaminemia: CR, n = 7, 11%; PR, n = 9, 21%; NR, n = 6, 55%; P = .003). Hypocalcemia (total calcium <2.5 mmol/L) was recorded in 26 of 123 dogs (median: 2.23 mmol/L; range: 1.46–2.44 mmol/L). Initial statistical analyses revealed a significant association of hypocalcemia and poor clinical outcome (CR, n = 9, 14%; PR, n = 10, 23%; NR, n = 7, 44%; P = .031; Table 3), but after the correction for hypoalbuminemia, the serum calcium concentration was only subnormal in one dog with IBD and partial remission (no ionized calcium measurement was available for that dog). No further clinicopathological abnormalities were significantly different among the 3 outcome groups (Fig 5).
Figure 5.
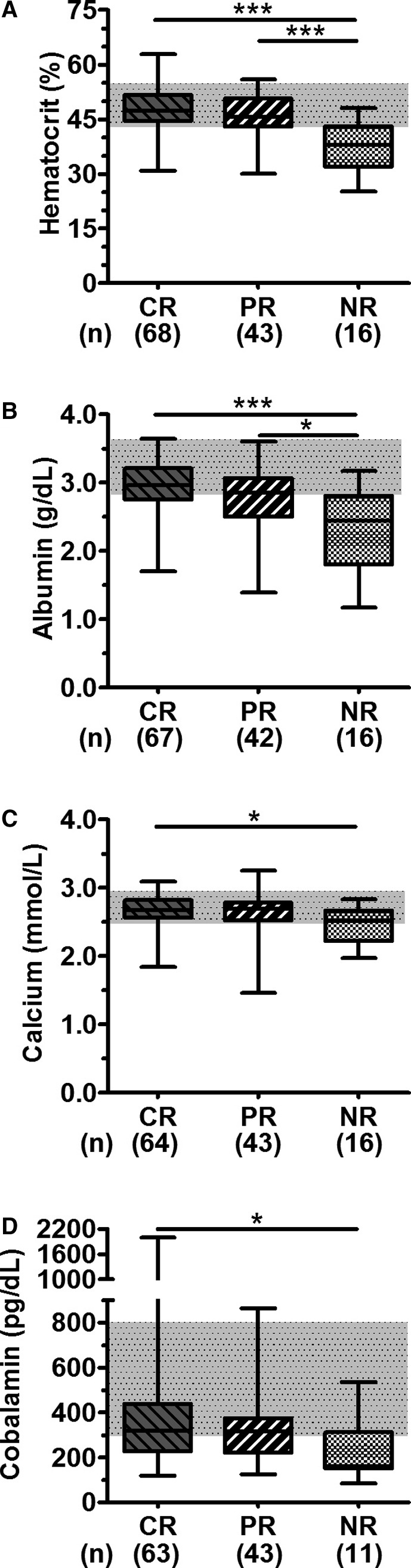
Comparison of clinicopathological abnormalities among outcome groups. Box‐and‐whiskers plots showing median, range and 25th to 75th percentiles for hematocrit (A), serum albumin (B), total serum calcium (C), and cobalamin (D). *P < .05 and ***P < .001 for Kruskal‐Wallis comparison of differences among medians followed by Mann‐Whitney U‐tests and Bonferroni adjustment for multiple comparisons. CR, complete recovery; PR, partial recovery; NR, no recovery.
Table 3.
Clinicopathologic findings among outcome in dogs with chronic diarrhea
| Variable (reference range) | Complete remission (n = 68) | Partial remission (n = 43) | No remission (n = 16) | P valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (n) | Range | L/E (n) | Median (n) | Range | L/E (n) | Median (n) | Range | L/E (n) | ||
| Leukocytes (6,000–12,000/μL) | 10,510 (68) | 4,010–20,620 | 6/27 | 10,140 (43) | 5,820–50,290 | 1/16 | 10,240 (16) | 7,010–45,720 | 0/5 | .906 |
| Hematocrit (44–55%)a | 47.3a (68) | 30.9–62.9 | 16/8 | 45.6a (43) | 30.1–56.0 | 12/1 | 38.1b (16) | 25.2–48.1 | 13/0 | <.001 |
| Platelets (165–400 G/L) | 260 (68) | 149–1,205 | 3/10 | 239 (43) | 131–760 | 4/4 | 305 (16) | 78–443 | 3/2 | .207 |
| Neutrophils (3,000–9,000/μL) | 7,320 (66) | 6,936–17,939 | 5/19 | 6,794 (41) | 2,328–41,238 | 2/14 | 7031 (16) | 4,673–39,319 | 0/6 | .803 |
| Eosinophils (<600/μL) | 252 (64) | 0–1,789 | –/16 | 503 (41) | 0–2,961 | –/16 | 282 (16) | 0–1,656 | –/5 | .209 |
| Lymphocytes (1,000–3,600/μL) | 1,889 (64) | 206–6,161 | 11/8 | 1,991 (41) | 137–4,844 | 7/4 | 1796 (16) | 231–45,180 | 5/2 | .899 |
| Monocytes (<500/μL) | 540 (61) | 65–2,526 | –/33 | 608 (41) | 0–3,520 | –/24 | 496 (16) | 230–1,841 | –/8 | .674 |
| Protein (5.4–6.6 g/dL) | 6.11 (68) | 3.57–7.36 | 13/13 | 5.97 (43) | 2.88–8.08 | 10/10 | 5.64 (16) | 2.70–7.65 | 7/2 | .212 |
| Albumin (2.8–3.6 g/dL)a | 2.96a (67) | 1.70–3.64 | 21/1 | 2.85a (42) | 1.39–3.60 | 18/0 | 2.44b (16) | 1.17–3.17 | 11/0 | .001 |
| Calcium, total (2.5–2.9 mmol/L)a | 2.7a (64) | 1.8–3.1 | 9/7 | 2.7a (43) | 1.5–3.3 | 10/4 | 2.5b (16) | 2.0–2.8 | 7/0 | .025 |
| Phosphorus (0.96–1.6 mmol/l) | 1.21 (66) | 0.40–2.40 | 13/12 | 1.33 (42) | 0.84–2.79 | 5/9 | 1.32 (16) | 0.66–1.92 | 2/2 | .403 |
| Urea (21–60 mg/dL) | 35.3 (65) | 14.5–83.4 | 8/4 | 36.0 (43) | 19.3–112.3 | 2/5 | 45.9 (16) | 17.1–156.0 | 2/3 | .371 |
| Creatinine (0.6–1.4 mg/dL) | 0.91 (64) | 0.50–1.34 | 4/0 | 0.97 (42) | 0.49–2.26 | 2/3 | 0.96 (16) | 0.63–1.93 | 0/1 | .205 |
| Glucose (81–112 mg/dL) | 102 (66) | 76–135 | 1/16 | 99 (42) | 71–138 | 4/9 | 106 (16) | 76–152 | 2/2 | .531 |
| Sodium (140–150 mmol/L) | 146 (67) | 138–155 | 1/6 | 146 (42) | 127–159 | 1/3 | 147 (16) | 131–154 | 2/3 | .904 |
| Potassium (3.6–4.8 mmol/L) | 3.95 (67) | 2.93–4.93 | 7/2 | 3.93 (41) | 3.37–5.10 | 4/2 | 4.25 (16) | 3.69–4.84 | 0/1 | .147 |
| Alanine aminotransferase (<76 U/L) | 52 (66) | 11–371 | –/19 | 46 (43) | 19–362 | –/11 | 51 (16) | 6–121 | –/5 | .616 |
| Alkaline phosphatase (<97 U/L) | 47 (65) | 7–1,582 | –/11 | 43 (43) | 9–1,412 | –/11 | 65 (16) | 12–279 | –/7 | .204 |
| Aspartate aminotransferase (<41 U/L) | 26 (65) | 7–120 | –/6 | 27 (42) | 5–69 | –/8 | 30 (16) | 8–153 | –/5 | .233 |
| Cobalamin (300–800 pg/mL)a | 319a (63) | 118–2,000 | 28/2 | 317a,b (43) | 125–864 | 17/1 | 160b (11) | 83–536 | 7/0 | .025 |
| Folic acid (3–10 ng/mL) | 12.1 (60) | 4.3–24.0 | 0/40 | 10.1 (36) | 2.2–24.0 | 1/18 | 11.3 (11) | 4.9–17.8 | 0/8 | .439 |
Significance < .05.
P values based on Kruskal‐Wallis comparison of differences among medians. Medians without superscripts in common are statistically different based on Mann‐Whitney U‐tests and Bonferroni adjustment for multiple comparisons. E, blood value elevated (above reference range); L, blood value lowered (below reference range).
Subset analyses revealed that anemia was common, and severe hypoalbuminemia and severe hypocobalaminemia were significantly associated with small intestinal disease (anemia: SI, n = 10, 24%; LI, n = 3, 8%; DI, n = 4, 8%; P = .053; severe hypoalbuminemia: SI, n = 8, 20%; LI, 0%; DI, n = 3, 6%; P = .008; hypocalcemia: SI, n = 15, 37%; LI, n = 2, 6%; DI, n = 9, 19%; P = .005; severe hypocobalaminemia: SI, n = 14, 36%; LI, n = 2, 6%; DI, n = 6, 13%; P = .004, Table 4) and with certain diagnoses: Hematocrit was frequently low in dogs with IBD and intestinal lymphoma (P < .001, Table 4), and in dogs with exocrine pancreatic insufficiency (Table 4). Low serum albumin and calcium concentrations were common in dogs with IBD (P < .001 and P = .001, Table 4). Serum cobalamin concentrations were frequently low in dogs with FRE, IBD, and EPI (Table 4). However, severe hypocobalaminemia was significantly associated with IBD (FRE, n = 8, 13%; ARE, n = 1, 9%; IBD, n = 9, 45%; Giardia sp. infection, n = 3, 21%; P = .016) and was furthermore present in 2 of 4 dogs with exocrine pancreatic insufficiency.
Table 4.
Selected clinicopathologic findings associated with poor clinical outcome compared among localization and among selected diagnoses in dogs with chronic diarrhea
| Group | Hematocrit (44‐55%) | Albumin (2.8‐3.6 g/dL) | Calcium, total (2.5‐2.9 mmol/L) | Cobalamin (300‐800 pg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (n) | Range | L/E (n) | Median (n) | Range | L/E (n) | Median (n) | Range | L/E (n) | Median (n) | Range | L/E (n) | |
| Localization (P valuea) | .009 | .010 | .027 | .003 | ||||||||
| SI | 44.6a (42) | 25.2–60.5 | 18/2 | 2.75a (41) | 1.49–3.55 | 23/0 | 2.6a (41) | 2.0–3.1 | 15/3 | 229a (39) | 83–2,000 | 26/2 |
| LI | 46.9a,b (36) | 33.6–59.0 | 14/2 | 2.95b (36) | 2.27–3.64 | 12/1 | 2.8b (34) | 2.4–3.0 | 2/4 | 359b (33) | 154–702 | 7/0 |
| DI | 48.1b (49) | 31.7–62.9 | 9/5 | 2.98b (48) | 1.17–3.60 | 15/0 | 2.7a,b (48) | 1.5–3.3 | 9/4 | 319b (45) | 125–864 | 19/1 |
| PE vs. SE (P valuea) | .665 | .373 | .509 | .600 | ||||||||
| PE | 46.6 (123) | 15.0–62.9 | 40/8 | 2.87 (121) | 1.17–3.64 | 49/1 | 2.66 (119) | 1.46–3.25 | 27/9 | 312 (115) | 83–2,000 | 51/3 |
| SE | 45.2 (13) | 30.1–59.8 | 5/2 | 3.01 (13) | 2.06–3.26 | 4/0 | 2.62 (13) | 2.40–2.93 | 2/2 | 248 (9) | 169–450 | 5/0 |
| Selected diagnoses with PE (P valuea) | <.001 | <.001 | .001 | .224 | ||||||||
| FRE | 47.9a (64) | 39.2–62.9 | 11/6 | 2.99a (62) | 1.70–3.64 | 17/1 | 2.72a (61) | 1.82–3.14 | 8/7 | 303 (63) | 118–864 | 31/1 |
| ARE | 45.6a,b (11) | 39.4–59.0 | 5/1 | 2.92a,b (11) | 1.62–3.26 | 4/0 | 2.64a,b (11) | 2.08–2.81 | 2/0 | 353 (11) | 154–898 | 3/1 |
| IBD | 41.9b (22) | 30.3–54.2 | 13/0 | 2.22b (22) | 1.17–3.21 | 15/0 | 2.43b (22) | 1.46–3.25 | 11/1 | 297 (20) | 83–702 | 11/0 |
| LYM | 33.0b (5) | 25.2–47.1 | 4/0 | 2.80a,b (5) | 2.16–3.17 | 2/0 | 2.59a,b (5) | 2.23–2.67 | 2/0 | 311 (3)* | 225–312 | 1/0 |
| Giardia | 49.7a,b (15) | 15.0–55.9 | 3/1 | 2.88a (15) | 2.29–3.42 | 5/0 | 2.74a (14) | 2.40–2.93 | 2/1 | 366 (14) | 108–2,000 | 3/1 |
| Selected diagnoses with SEb | ||||||||||||
| EPI | 41.8 (4) | 30.9–45.2 | 3/0 | 2.81 (4) | 2.29–3.24 | 2/0 | 2.58 (4) | 2.44–2.78 | 1/0 | 224 (4) | 169–308 | 3/0 |
| CP | 47.2 (3) | 44.3–59.8 | 0/1 | 2.87 (3) | 2.43–3.26 | 1/0 | 2.61 (3) | 2.52–2.93 | 0/1 | 233 (1) | – | 1/– |
P values based on Kruskal‐Wallis comparison of differences among medians. Medians without superscripts in common are statistically different based on Mann‐Whitney U‐tests and Bonferroni adjustment for multiple comparisons. Significance < .05.
Data for selected diagnoses with a group size of 3 or 4 dogs were descriptively analyzed. L, blood value lowered (below reference range); E, blood value elevated (above reference range); SI, small intestinal disease; LI, large intestinal disease; DI, diffuse intestinal disease; PE, primary enteropathy (gastrointestinal disease); SE, secondary enteropathy (extragastrointestinal disease); FRE, food responsive enteropathy; ARE, antibiotic responsive enteropathy; IBD, idiopathic inflammatory bowel disease; LYM, intestinal lymphoma; Giardia, infection with Giardia spp.; EPI, exocrine pancreatic insufficiency; CP, chronic pancreatitis.
Discussion
Primary enteropathies were identified with the highest frequency with noninfectious inflammatory enteropathies constituting the most frequent cause of chronic diarrhea in dogs with an overall frequency of 71%. Results of the present study are consistent with the hypothesis that the chronic inflammatory enteropathy is the most common cause of chronic diarrhea, and the findings of the present study are substantiated by recent observations in dogs with various gastrointestinal signs.5,6 Food responsive enteropathy (FRE) was the most frequent final diagnosis in the present study with an overall frequency of 47% (66% of all dogs with a chronic inflammatory enteropathy). FRE was diagnosed based on response to an elimination diet, which has been proven to be the most effective method for diagnosis and treatment.1, 8, 12 Differentiation between food allergy (immunological reaction) and food intolerance (nonimmunological reactions) could not be made as both food allergy and food intolerance manifest themselves with diarrhea or vomiting or both and are hence clinically indistinguishable.1, 8, 12 Thirty‐eight percent of dogs diagnosed with FRE were presented with pruritus, which might be suggestive for an allergic reaction to food.1 However, pruritus might also occur in dogs with food intolerance,1, 12 and thus it remains unknown whether reactions to food were immunological or not. Nevertheless, findings coincide with other studies demonstrating that approximately 1/2 to 2/3 of dogs with chronic inflammatory enteropathies have FRE, with the remainder having antibiotic responsive or idiopathic inflammatory bowel disease (IBD) with an approximately equal frequency of 15–20%.5, 6,5, 13 In accordance with other reports, the findings of the present study demonstrate that idiopathic IBD is common in dogs, but that it is not the most common cause of chronic diarrhea in dogs.1,13 Chronic inflammatory enteropathies are multifactorial disease complexes. An adverse immune response to environmental factors including dietary and microbial antigens is likely to be important in the pathogenesis and might further influence endoparasites.1, 14, 15 The findings of the present study highlight the need for a detailed and stepwise diagnostic work‐up including therapeutic trials to eliminate the possibility of parasitic infections and to exclude diet responsive and antibiotic responsive enteropathies before a suspicion of idiopathic IBD can be substantiated.1, 5, 6,1, 13
In line with other studies, neoplastic causes were less frequent primary enteropathies and of those, intestinal lymphoma was the most frequent gastrointestinal neoplasia with an overall frequency of 4% in the present study.5, 6,16 Histopathologic evaluation of intestinal biopsies remains an important diagnostic tool to differentiate IBD and intestinal lymphoma, but the latter might be a result of chronic lymphocytic‐plasmacytic inflammation, which is the most common type of chronic intestinal inflammation.1, 6, 7, 8, 17, 18, 19, 20, 21 Whether dogs diagnosed with chronic lymphocytic‐plasmacytic or mixed inflammation developed intestinal lymphoma later on was not evaluated in the present study (i.e, postmortem examinations and adjunctive techniques such as immunohistochemistry, flow cytometry, and PCR for antigen receptor rearrangements were not performed),19, 21, 22 and thus underlying intestinal lymphoma could have been missed, which is a limiting factor of the present study.
Parasitic infections were the second leading cause of chronic diarrhea in the dogs in this study. Giardia has been recognized as a common parasitic infection causing gastrointestinal disease and was the predominant infectious cause in the present study with an overall frequency of 11% of the study population, which is lower than previously reported in a European multicenter study (28%).23 However, the frequency of parasitic causes of chronic enteropathies in canine studies ranges from less than 2% to more than 30%.1, 23, 24 The findings of the present study were comparable to recent reports of 9 to 18%.5, 6 Infectious causes evaluated in this study were limited to parasitic or algae infections, and no viral or obligate enteropathogenic bacteria were identified as a primary cause of chronic diarrhea. Potentially pathogenic organisms can be frequently found in the feces of clinically healthy dogs and dogs with chronic enteropathy making it difficult to determine whether a specific organism identified acts as an etiologic factor, is a result of a changing microbiota due to the chronic enteropathy, or is in fact unrelated to the disease process.4, 24, 25, 26, 27, 28, 29 With respect to the multifactorial etiology of chronic enteropathies, the resolution of clinical signs after elimination of the identified organism is essential to determine disease causation.1, 30 Thus a parasitic infection was diagnosed as the primary cause of chronic diarrhea based on fecal testing and clinical response to appropriate antiparasitic therapy (e.g, fenbendazole).1 Routine bacteriological and virological analyses of feces from dogs with diarrhea are not warranted for several reasons, including the usually acute, mild, and self‐limiting character of many bacterial and viral infections, presentation with characteristic clinical or laboratory features, relatively brief period of virus shedding, and difficulties with interpretation of culture results.1, 31 Routine analyses are indicated in dogs with hemorrhagic diarrhea, pyrexia, and an inflammatory leukogram.1, 31 In the present study bacteriological cultures were performed in 51 of 136 dogs (36%) without identifying bacterial infection as a primary cause. During follow‐up, Campylobacter spp. was detected in the feces of one dog with IBD, which had presented with acute watery diarrhea but ultimately with a self‐limiting course of disease. Although findings of the present study are in line with recent observations,5, 6,13 several primary causes might have been missed, as not all dogs had all testing performed.
In clear contrast to primary enteropathies, extragastrointestinal causes (i.e, secondary enteropathies) were less frequently recorded, with diseases of the exocrine pancreas being the most common extragastrointestinal diseases. The overall frequency of secondary enteropathies in the dogs evaluated for the current study was 10%, which is lower than other studies in dogs with various gastrointestinal signs (17 and 26%).5, 6 In line with other studies, frequent vomiting was significantly associated with secondary enteropathies.5, 6 Thus differences in the prevalence of primary and secondary enteropathies are attributed to the inclusion criteria that only dogs with diarrhea (either with or without vomiting) had been included into the present study. Exclusion of dogs without a final diagnosis might have had an impact on the distribution of primary and secondary enteropathies within the overall population of dogs with chronic diarrhea and, subsequently, might have biased the distribution of dietary responsive, antibiotic responsive, and idiopathic IBD within the group of dogs with chronic inflammatory enteropathies. During the 2 year study period, 65% of dogs fulfilled the inclusion criteria, and the remainder of dogs was excluded based on the lack of a final diagnosis. Some dogs were lost to follow‐up as they were presented only once for an initial detailed work‐up and were then treated by their general practitioner. The major problems for missing final diagnoses were poor owner or dog compliance, which are important factors affecting diagnostic and therapeutic success of many forms of chronic enteropathies.32, 33 However, findings of the present study are in line with recent observations suggesting that the impact of excluded cases might only be marginal.5, 6,13
Underlying diseases such as systemic protothecosis and leishmaniosis as well as mechanical disorders, endocrinopathies, and diseases of the liver, kidney, and the cardiovascular system were uncommon causes of chronic diarrhea with frequencies less 1% in the present study. Although these conditions appear to be rare in dogs with chronic intermittent or persistent diarrhea, they might become acutely life‐threatening and should therefore not be ignored during the diagnostic work‐up.5, 6,1, 2
Additionally, we evaluated characteristics, outcome, and associated clinical and clinicopathological abnormalities in the overall study population, and in particular in dogs with selected diagnoses (i.e, diagnoses that had been assigned to at least 3 dogs). Results reported in this study coincide largely with other reports.6,5, 13, 17, 34, 35, 36 To date, no sex predisposition had been described in dogs with gastrointestinal disease, although an overrepresentation of intact males followed by spayed females had been described in several studies.6,17, 18, 20, 36, 37, 38, 39 Intact males were clearly overrepresented in the present study. However, a formal comparison to the hospital population over the same time period was not performed, and therefore, the clinical importance of the finding of the present study remains unknown.
The physical appearance of feces as well as the appearance of secondary clinical signs such as vomiting, weight loss, abdominal pain, borborygmi, flatulence, and alterations in appetite might help to differentiate between small and large intestinal disease, which might be useful to clarify the underlying cause.2, 8 In the present study, clinical signs of predominantly small bowel diarrhea were significantly more common in dogs with extragastrointestinal causes when compared to dogs with primary enteropathies. The presence of moderate‐to‐severe vomiting was more common in dogs with secondary enteropathies, and these results are consistent with a recent study.5 These findings suggest that extragastrointestinal disease leads to secondary diarrhea, and vomiting might have been the principal reason for presentation in these dogs. Interestingly, vomiting was observed to be significantly more common in dogs with short disease duration, suggesting that owners might rate vomiting as an alert sign.
Furthermore, small intestinal disease was more common in dogs with a poor clinical outcome, and clinical signs such as watery diarrhea, weight loss, and lethargy appear to be of prognostic value. Overall, poor clinical outcomes had been observed in 13% of cases, which is comparable to previous studies.6,5, 17, 34 Eighty‐seven percent of dogs achieved either complete or partial remission. Craven et al.17 described IBD cases that achieved remission times of 3 years before relapse. In the present study, the duration of follow‐up varied and was limited to 1 year; relapses thereafter might have been missed. Due to the retrospective nature of this study, interpretation of outcome factors needs to be carried out with caution. The clinical activity score (CIBDAI) used in the present study has been shown to be less powerful to accurately predict long‐term disease outcome.5 The assessment of ascites and pruritus, as well as adding low serum albumin concentrations to the CIBDAI increases the predictive ability of clinical disease severity scoring (canine chronic enteropathy clinical activity index = CCECAI).5 Thus, it is possible that the current study could have been improved if the CCECAI would have been used to assess the outcome. However, this was not possible because the information available in the records of the present study for pruritus and ascites did not match the criteria points defined by the CCECAI score. Also, the current study might have been improved if comparisons of findings before and after treatment could have been performed. However, follow‐up information was limited in some dogs for a variety of reasons, including the retrospective study design and the diversity in data acquisition by different clinicians during follow‐up. Therefore, clinical and clinicopathological abnormalities were only analyzed at the time of first presentation, and criteria for treatment outcome were limited to few questions, including whether the dog is still alive and whether gastrointestinal signs improved. Variation in dietary, antibiotic, and anti‐inflammatory/immunosuppressive treatments reduced further the comparability of groups. Response to treatment was associated with the disease classification of chronic inflammatory enteropathies because a diagnosis of FRE and ARE were based on a clinical response (either complete or partial) to dietary or antibiotic treatments, and failure to respond suggests the presence of IBD, lymphoma, or a rare condition. IBD and intestinal lymphoma were the most frequent diagnoses associated with a poor clinical outcome. A further limiting factor of the present study is variations in prior treatments that might have influenced the evaluation of clinical and clinicopathological abnormalities on the time of first presentation. Nevertheless, findings among outcome groups, and in particular among subsequent analyses of selected diagnoses (e.g, chronic inflammatory enteropathies), are largely in agreement with other reports.6,5, 13, 16, 17, 18, 20, 34, 36 Subsequent analyses substantiate original findings by Allenspach and others (2007) that younger dogs with less severe and predominantly large bowel disease are more likely to be diet responsive and have a good prognosis.5 On the contrary, older age, high disease severity scores, and predominantly small intestinal disease were associated with a poor clinical outcome.6,5, 13, 16, 17, 18, 20, 34, 36
Similar to previous studies, clinicopathological abnormalities such as anemia (hematocrit <40%), severe hypoalbuminemia (serum albumin concentration <2.0 g/dL), and severe hypocobalaminemia (serum cobalamin concentration <200 pg/mL) were poor prognostic indicators.6,5, 13, 17, 34, 36, 40 Subsequent analyses demonstrated that these clinicopathological abnormalities were common in dogs with small intestinal disease. Anemia might indicate chronic inflammation or chronic intestinal blood loss, which is common in dogs with IBD or intestinal lymphoma.18, 41 Serum albumin concentrations are routinely measured in dogs with gastrointestinal disease, and it has been shown previously that hypoalbuminemia occurs in dogs with increased disease severity that is associated with a poor clinical outcome.5, 13, 17, 34, 36 In line with other reports, severe hypoalbuminemia was common in dogs with IBD and is most likely attributed to protein‐losing enteropathy, a heterogeneous group of diseases with nonselective and excessive loss of plasma proteins into the intestinal lumen.5, 14, 28, 29, 36 Serum cobalamin is a potentially useful marker of intestinal malabsorption and particularly of small intestinal disease.1, 42 A severe decrease of serum cobalamin concentration was most frequently recorded in dogs with IBD and exocrine pancreatic insufficiency.13, 42, 43 The overall frequency of hypocobalaminemia in dogs with chronic diarrhea was 44% and an approximate frequency of 30% of dogs had a low‐normal serum cobalamin concentration (300–400 pg/mL) confirming that concentrations within the reference interval do not exclude the possibility of intestinal disease.42 Seventy percent of dogs received cobalamin supplementation for approximately 16 weeks, which might have affected the response to treatment and outcome in these cases.5, 44 Measurement of serum cobalamin concentration was often but not always repeated 4 weeks after the last cobalamin injection. Furthermore, follow‐up measurements were not available in most cases with an adverse clinical outcome, thus a comparison of pre‐ and post‐treatment cobalamin concentration was not performed.
Conclusion
This study highlights underlying causes and final diagnoses in a large number of dogs with chronic diarrhea. Chronic inflammatory enteropathies and, in particular, food responsive enteropathies were the most frequent causes of chronic diarrhea in dogs. Diarrhea is the cardinal sign of intestinal dysfunction but dogs might also be presented due to secondary clinical signs that might or might not be accompanied by diarrhea. Moderate‐to‐severe vomiting was significantly associated with secondary enteropathies suggesting that extragastrointestinal disease might lead to secondary diarrhea. Clinical and clinicopathological abnormalities at the time of first presentation were compared among dogs with complete, partial, or no recovery. Clinical signs predominantly involving the small intestine and particularly weight loss and lethargy were significantly associated with an adverse clinical outcome. The findings of this study are consistent with previous studies suggesting that anemia, severe hypoalbuminemia, and severe hypocobalaminemia are associated with a poor prognosis and thus highlight the importance of measuring these parameters in dogs with chronic signs of gastrointestinal disease. These clinical and clinicopathological findings have prognostic, but not diagnostic value.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Clinic for Small Animals, Freie Universität Berlin, Germany.
This work was supported by the Akademie für Tiergesundheit and the Drs. Jutta and Georg Bruns Foundation. Parts of this manuscript were presented as an abstract at the 2015 and 2011 DGK‐DVG congress in Berlin/Germany, the 2015 and 2012 InnLab Forum in Leipzig and Göttingen, Germany, the 2014 ECVIM Forum in Mainz, Germany, and the 2012 ACVIM Forum in New Orleans, LA, USA.
Footnotes
Willard M. Chronic Small Bowel Diarrheas: IBD is Not the Most Common Cause. In: CVC, Washington, D.C. 2014.
Hill`s Prescription Diet z/d
Hill`s Prescription Diet Canine d/d, VetConcept Dog Sana
Rade C. Compliance bei Reduktionsdiäten ‐ So macht der Besitzer mit. kleintierkonkret 2009;4:11‐16.
Baumgart K, Volkmann M, Steiner JM, et al. Final Diagnoses in 155 Dogs with Chronic Vomiting and/or Diarrhea. In: 24th ECVIM‐CA Congress, Mainz 2014;ESCG‐P‐10.
Kuehn M. Retrospektive Analyse chronischer Enteropathien beim Hund. Doctoral thesis, LMU Munich; 2012.
References
- 1. Hall EJ, German AJ. Diseases of the Small Intestine In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Medicine ‐ Diseases of the Dog and the Cat, 7 ed Philadelphia: Saunders; 2010:1526–1572. [Google Scholar]
- 2. Marks SL. Diarrhea In: Washabau RJ, Day MJ, eds. Canine and Feline Gastroenterology. St.Louis, Missouri: Elsevier Saunders; 2013:99–108. [Google Scholar]
- 3. Schreiner NM, Gaschen F, Grone A, et al. Clinical signs, histology, and CD3‐positive cells before and after treatment of dogs with chronic enteropathies. J Vet Intern Med 2008;22:1079–1083. [DOI] [PubMed] [Google Scholar]
- 4. Allenspach K. Tests to investigate gastrointestinal diseases in dogs–which markers are actually useful for the practitioner? J Small Anim Pract 2007;48:607–608. [DOI] [PubMed] [Google Scholar]
- 5. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 6. Jergens AE, Moore FM, Haynes JS, et al. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990). J Am Vet Med Assoc 1992;201:1603–1608. [PubMed] [Google Scholar]
- 7. Cave NJ. Chronic inflammatory disorders of the gastrointestinal tract of companion animals. N Z Vet J 2003;51:262–274. [DOI] [PubMed] [Google Scholar]
- 8. Allenspach K, Gaschen F. Chronic intestinal diseases in the dog: A review. Schweiz Arch Tierheilkd 2003;145:209–219, 221–202. [DOI] [PubMed] [Google Scholar]
- 9. Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci (Elite Ed) 2012;4:1404–1419. [DOI] [PubMed] [Google Scholar]
- 10. Beck S, Schreiber C, Schein E, et al. Tick infestation and prophylaxis of dogs in northeastern Germany: A prospective study. Ticks Tick Born Dis 2014;5:336–342. [DOI] [PubMed] [Google Scholar]
- 11. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 12. Kennis RA. Food allergies: Update of pathogenesis, diagnoses, and management. Vet Clin North Am Small Anim Pract 2006;36:175–184, vii‐viii. [DOI] [PubMed] [Google Scholar]
- 13. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec 2016;178:368. [DOI] [PubMed] [Google Scholar]
- 14. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- 15. Dijkstra M, Kraus JS, Bosje JT, et al. Protein‐losing enteropathy in Rottweilers. Tijdschr Diergeneeskd 2010;135:406–412. [PubMed] [Google Scholar]
- 16. Frank JD, Reimer SB, Kass PH, et al. Clinical outcomes of 30 cases (1997–2004) of canine gastrointestinal lymphoma. J Am Anim Hosp Assoc 2007;43:313–321. [DOI] [PubMed] [Google Scholar]
- 17. Craven M, Simpson JW, Ridyard AE, et al. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract 2004;45:336–342. [DOI] [PubMed] [Google Scholar]
- 18. Couto CG, Rutgers HC, Sherding RG, et al. Gastrointestinal lymphoma in 20 dogs. A retrospective study. J Vet Intern Med 1989;3:73–78. [DOI] [PubMed] [Google Scholar]
- 19. Burkhard MJ, Bienzle D. Making sense of lymphoma diagnostics in small animal patients. Vet Clin North Am Small Anim Pract 2013;43:1331–1347, vii. [DOI] [PubMed] [Google Scholar]
- 20. Mancho C, Sainz A, Garcia‐Sancho M, et al. Evaluation of perinuclear antineutrophilic cytoplasmic antibodies in sera from dogs with inflammatory bowel disease or intestinal lymphoma. Am J Vet Res 2011;72:1333–1337. [DOI] [PubMed] [Google Scholar]
- 21. Carrasco V, Rodriguez‐Bertos A, Rodriguez‐Franco F, et al. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet Pathol 2015;52:668–675. [DOI] [PubMed] [Google Scholar]
- 22. Thalheim L, Williams LE, Borst LB, et al. Lymphoma immunophenotype of dogs determined by immunohistochemistry, flow cytometry, and polymerase chain reaction for antigen receptor rearrangements. J Vet Intern Med 2013;27:1509–1516. [DOI] [PubMed] [Google Scholar]
- 23. Epe C, Rehkter G, Schnieder T, et al. Giardia in symptomatic dogs and cats in Europe–results of a European study. Vet Parasitol 2010;173:32–38. [DOI] [PubMed] [Google Scholar]
- 24. Claerebout E, Casaert S, Dalemans AC, et al. Giardia and other intestinal parasites in different dog populations in Northern Belgium. Vet Parasitol 2009;161:41–46. [DOI] [PubMed] [Google Scholar]
- 25. Cave NJ, Marks SL, Kass PH, et al. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J Am Vet Med Assoc 2002;221:52–59. [DOI] [PubMed] [Google Scholar]
- 26. Guest CM, Stephen JM, Price CJ. Prevalence of Campylobacter and four endoparasites in dog populations associated with Hearing Dogs. J Small Anim Pract 2007;48:632–637. [DOI] [PubMed] [Google Scholar]
- 27. Weese JS, Staempfli HR, Prescott JF, et al. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med 2001;15:374–378. [PubMed] [Google Scholar]
- 28. Burnens AP, Angeloz‐Wick B, Nicolet J. Comparison of Campylobacter carriage rates in diarrheic and healthy pet animals. Zentralbl Veterinarmed B 1992;39:175–180. [DOI] [PubMed] [Google Scholar]
- 29. Schulz BS, Strauch C, Mueller RS, et al. Comparison of the prevalence of enteric viruses in healthy dogs and those with acute haemorrhagic diarrhoea by electron microscopy. J Small Anim Pract 2008;49:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tzannes S, Batchelor DJ, Graham PA, et al. Prevalence of Cryptosporidium, Giardia and Isospora species infections in pet cats with clinical signs of gastrointestinal disease. J Feline Med Surg 2008;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matz ME, Guilford WG. Laboratory procedures for the diagnosis of gastrointestinal tract diseases of dogs and cats. N Z Vet J 2003;51:292–301. [DOI] [PubMed] [Google Scholar]
- 32. Sturgess K. Diagnosis and management of idiopathic inflammatory bowel disease in dogs and cats. In Practice 2005;27:293–301. [Google Scholar]
- 33. Rosser EJ Jr. Diagnosis of food allergy in dogs. J Am Vet Med Assoc 1993;203:259–262. [PubMed] [Google Scholar]
- 34. Munster M, Horauf A, Bilzer T. Assessment of disease severity and outcome of dietary, antibiotic, and immunosuppressive interventions by use of the canine IBD activity index in 21 dogs with chronic inflammatory bowel disease. Berl Munch Tierarztl Wochenschr 2006;119:493–505. [PubMed] [Google Scholar]
- 35. Bostrom BM, Xenoulis PG, Newman SJ, et al. Chronic pancreatitis in dogs: A retrospective study of clinical, clinicopathological, and histopathological findings in 61 cases. Vet J 2013;195:73–79. [DOI] [PubMed] [Google Scholar]
- 36. Munster M, Suchodolski JS, Bilzer T, et al. Influence of physiological disturbances on treatment success of dietary therapy in dogs with chronic enteropathies. Berl Munch Tierarztl Wochenschr 2010;123:74–82. [PubMed] [Google Scholar]
- 37. Allenspach K, Steiner JM, Shah BN, et al. Evaluation of gastrointestinal permeability and mucosal absorptive capacity in dogs with chronic enteropathy. Am J Vet Res 2006;67:479–483. [DOI] [PubMed] [Google Scholar]
- 38. Burgener IA, Konig A, Allenspach K, et al. Upregulation of toll‐like receptors in chronic enteropathies in dogs. J Vet Intern Med 2008;22:553–560. [DOI] [PubMed] [Google Scholar]
- 39. Heilmann RM, Grellet A, Allenspach K, et al. Association between fecal S100A12 concentration and histologic, endoscopic, and clinical disease severity in dogs with idiopathic inflammatory bowel disease. Vet Immunol Immunopathol 2014;158:156–166. [DOI] [PubMed] [Google Scholar]
- 40. Titmarsh H, Gow AG, Kilpatrick S, et al. Association of vitamin D status and clinical outcome in dogs with a chronic enteropathy. J Vet Intern Med 2015;29:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hall EJ. Clinical laboratory evaluation of small intestinal function. Vet Clin North Am Small Anim Pract 1999;29:441–469, vi. [PubMed] [Google Scholar]
- 42. Batt RM, Morgan JO. Role of serum folate and vitamin‐B12 concentrations in the differentiation of small intestinal abnormalities in the dog. Res Vet Sci 1982;32:17–22. [PubMed] [Google Scholar]
- 43. Batchelor DJ, Noble PJ, Taylor RH, et al. Prognostic factors in canine exocrine pancreatic insufficiency: Prolonged survival is likely if clinical remission is achieved. J Vet Intern Med 2007;21:54–60. [DOI] [PubMed] [Google Scholar]
- 44. Toresson L, Steiner JM, Suchodolski JS, et al. Oral cobalamin supplementation in dogs with chronic enteropathies and hypocobalaminemia. J Vet Intern Med 2016;30:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


