ABSTRACT
Human immuodeficency virus (HIV)-infected patients receiving highly active antiretroviral therapy (HAART) and community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) have increased in recent years in Taiwan. This study was undertaken to determine the prevalence of and risk factors for nasal and oral S. aureus and MRSA colonization among contemporary HIV-infected populations. Clinical variables for S. aureus and MRSA colonization among HIV-infected outpatients from three hospitals were analyzed and compared with those for oral Candida colonization. Genetic characteristics of MRSA isolates were analyzed. A total of 714 patients were screened for nasal S. aureus colonization, and a subset of 457 patients were also screened for oral S. aureus colonization. Of all patients, 79.4% were receiving HAART, and their mean CD4 count was 472 cells/mm3. The colonization rates in the oral cavity, nasal cavity, and at either site were 18.8%, 31.7%, and 36.8%, respectively, for S. aureus, and 3.1%, 4.4%, and 5.5%, respectively, for MRSA. These rates were all much lower than the previously reported rate of oral Candida colonization (52.4%). By multivariate analysis, a suppressed viral load (<200 copies/mL) protected against oral S. aureus, MRSA, and Candida colonization, and recent use of antibacterial agents protected against oral and nasal S. aureus colonization. Recent incarceration increased the risk of nasal MRSA colonization, while recent hospitalization, tuberculosis, older age, and intravenous drug use increased the risk of oral Candida colonization. Candida spp. did not augment S. aureus or MRSA colonization in the oral cavity. Most of the 41 MRSA isolates recovered belonged to the SCCmec IV/pvl-negative (51.2%) and VT/pvl-positive (26.8%) ST59 local prevalent CA-MRSA clones. Distinct carriage rates demonstrated here suggested that mucosal immunity against colonization might differ in terms of microbes and sites. A decreased risk in oral carriage of MRSA and Candida might be a benefit of HAART.
KEYWORDS: HIV, Staphylococcus aureus, MRSA, Candida, oral colonization, HAART
Introduction
Staphylococcus aureus and Candida species, both existing as common commensals colonizing human mucosal surfaces, are the leading opportunist pathogens causing human infections. In one study enrolling human immunodeficiency virus (HIV) patients with bacteremia attending the emergency department, S. aureus was found to be the most common (44.7%) causative pathogen [1]. Among S. aureus, methicillin-resistant S. aureus (MRSA) is most noteworthy because it is responsible for an increasing number of hospital- and community-acquired infections worldwide and because of its association with multidrug resistance [2]. Studies have revealed synergistic interactions between S. aureus and Candida albicans in dual-species biofilms, which is consistent with clinical findings of frequent co-isolation of both species from bloodstream infections and biofilm-associated diseases [3,4]. HIV-infected patients who are colonized with opportunistic pathogens are at a higher risk for subsequent infections, as oral Candida colonization has been identified as a predictor for the occurrence of oral candidiasis, and individuals colonized with MRSA are more likely to develop MRSA infections, usually by the same colonizing strains, than those who are not colonized [5,6]. However, little is known about the frequency of co-colonization by both species in the HIV-infected population.
Earlier work examining 162 HIV-infected patients (median CD4 count 205 cells/mm3) in 1999 found a 6% prevalence of nasal MRSA carriage, with ciprofloxacin use and reduced CD4 count being two risk factors for carriage [7]. In Taiwan, the number of HIV-infected patients receiving highly active antiretroviral therapy (HAART) with a favorable CD4 count and suppressed viral load has increased in the recent decade due to the availability of free HAART and implementation of a case management program [8]. Since 1999, the authors have examined the prevalence of and risk factors for oral Candida colonization among HIV-infected outpatients at three hospitals during 2009–2010 in three studies [9–11].
In Taiwan, community-associated MRSA (CA-MRSA) emerged around 2002 and has spread to healthcare settings [2,12]. For the epidemiological surveillance of MRSA, HIV-infected outpatients may serve as a sentinel population because of their frequent contact with both hospital and community settings and exposure to antimicrobial agents. Taken together, these epidemiological changes suggested the need for reevaluating the prevalence of and risk factors for MRSA carriage among the contemporary HIV-infected population. Here, the same study population from 2009–2010 [9–11] was enrolled to investigate (1) the prevalence of and risk factors for nasal and oral S. aureus and MRSA colonization in order to compare with those for oral Candida colonization, and (2) the microbiologic characteristics and clonality of the MRSA colonizing strains. The study results could be helpful for targeted MRSA screening and transmission prevention in this population and expanding our knowledge about the interplay between S. aureus and Candida species in the oral cavity.
Material and methods
Study population and sample collection
This prospective, cross-sectional, multicenter study enrolled HIV-infected outpatients who attended the clinics at China Medical University Hospital, E-Da hospital, and National Cheng Kung University Hospital from October 2009 to February 2010. The hospitals are located in three different counties in Taiwan and are 25–122 miles apart. The Institutional Review Board of each participating hospital approved the study.
After informed consent, the patients were tested for nasal MRSA and oral Candida colonization simultaneously. In addition, oropharyngeal culture for S. aureus was performed on a subset of these patients. All specimens were obtained using dry sponge swabs (EZ Culturette; Becton Dickinson, Sparks, MD), maintained at room temperature, and transported to the central laboratory at the National Health Research Institutes within 24 h where they were plated on solid medium within 4 h of arrival.
Clinical data collected included demographic characteristics, clinical and laboratory information within 6 months prior to enrollment such as history of incarceration, the latest CD4 cell count and plasma HIV viral load, antibacterial or antifungal treatments for ≥1 day and antiretroviral agents for ≥2 weeks, and history of hospitalization within 1 year prior to enrollment. A suppressed HIV viral load is defined as plasma HIV RNA level <200 copies/mL [13]. Data on oral Candida colonization from these patients in the three hospitals have been published separately [9–11] and were merged in this study in order to determine if the risk factors for S. aureus, MRSA, and Candida colonization differed.
Microbiological processing
Both the nasal and oral swabs were plated on sheep blood and ChromAgar S. aureus plates (BBL Microbiology System, Cockeysville, MD), plus Trypticase soy enrichment broth containing 7.5% NaCl. After overnight incubation at 35ºC, 10 µL of the enrichment broth was subcultured to ChromAgar S. aureus and ChromAgar MRSA plates. All colonies suspected to be S. aureus were checked by catalase and Gram stain as necessary. All S. aureus isolates were confirmed by coagulase/protein A latex agglutination. Semi-quantification of the bacterial load was recorded as 1+ to 4+ based on number of colonies in the streaked four quadrant areas on primary culture plates, and as ‘rare’ if isolates were recovered only from enrichment broth subculture. All S. aureus isolates were screened for oxacillin (methicillin) resistance using a cefoxitin disk diffusion test following the protocol of Clinical and Laboratory Standards Institute (CLSI) [14]. Susceptibility to different agents was then determined on all MRSA isolates by broth microdilution following the CLSI guidelines using custom-designed Sensititre plates (Trek Diagnostics, East Grinstead, United Kingdom) [14]. The CLSI breakpoints (MICs in μg/mL) for non-susceptibility to chloramphenicol (>8), ciprofloxacin (>1), clindamycin (>0.5), erythromycin (>0.5), gentamicin (>4), rifampin (>1), tetracycline (>4), co-trimoxazole (>2/38), daptomycin (>1), linezolid (>4), teicoplanin (>8), and vancomycin (>2) were used [14]. All MRSA isolates were subject to SCCmec typing and Panton-Valentine leukocidin (PVL) gene detection, as described previously [15]. Clonal relatedness of the MRSA isolates was also investigated by pulsed-field gel electrophoresis (PFGE), and pulsotypes were assigned to clusters of isolates having >80% similarity from the dendrograms [16]. Multilocus sequence typing (MLST) was performed on isolates selected from each pulsotype, and sequence type (ST) was assigned by using the MLST database Web site (www.mlst.net) [17]. Detailed protocols for the culture and identification of Candida species have been described previously [9–11].
Risk factors and statistical analysis
Statistical analyses were performed with the SPSS Statistics for Windows v17.0 (SPSS Inc., Chicago, IL). All categorical variables presented in Table 1 (except the individual antiretroviral agent) were tested for their association with colonization. Variables with a p-value of ≤0.10 in univariate analysis were included in multivariate analysis. A p-value of <0.05 was considered statistically significant, and all tests were two tailed.
Table 1.
Characteristics of HIV-infected patients with S. aureus, MRSA, and Candida colonization.
| Characteristic (mean ± SD) or n (%) | All (714) patients |
|---|---|
| Age, years | 38.2 ± 11.7 |
| Age >50 years | 107 (15.0) |
| Gender, male | 635 (91.5) |
| Transmission type | |
| Heterosexual | 193 (27.0) |
| IDU | 87 (12.2) |
| MSM | 419 (58.7) |
| Comorbidity | |
| Chronic kidney diseases | 2 (0.3) |
| Diabetic mellitus | 20 (2.8) |
| Hypertension | 27 (3.8) |
| Hospitalization within 1 year | 68 (9.5) |
| Incarceration within 6 months | 13 (1.8) |
| Period of HIV infection, years | 5.2 ± 4.0 |
| CD4, cells/mm3 | 472 ± 257 |
| CD4 count <200 | 93 (13.0) |
| CD4 count >500 | 293 (41.0) |
| HIV viral load, log (copies/mL)a | 2.3 ± 1.3 |
| HIV viral load <200 copies/mLa | 481 (67.5) |
| Medications within 6 months | |
| Antiretroviral therapy | 567 (79.4) |
| 3TC or 3TC/AZT | 550 (77.0) |
| Abacavir | 197 (27.6) |
| AZT or 3TC/AZT | 281 (39.4) |
| NNRTIb | 263 (36.8) |
| PIc | 308 (43.1) |
| Antibacterialsd | 65 (9.1) |
| Co-trimoxazole | 46 (6.4) |
| Cephalosporins | 13 (1.8) |
| Anti-tuberculosis regimense | 14 (2.0) |
| Antifungals | |
| Amphotericin B | 3 (0.4) |
| Fluconazole | 12 (1.7) |
aData for two patients were not available.
bNNRTIs: efavirenz or nevirapine.
cPIs: azatanavir/ritonavir, indinavir, or lopinavir/ritonavir.
dAntibacterial agents, i.e. clindamycin, fluoroquinolones, macrolides, and penicillin derivatives, each of which was used by fewer than five patients and which was not associated with colonization by either pathogen, were also included.
eOf 14 patients, 13, 3, 8, and 11 received ethambutol, isoniazid, pyrazinamide, rifabutin/rifampin, respectively.
HIV, human immunodeficiency virus; S. aureus, Staphylococcus aureus; MRSA, methicillin-resistant S. aureus; SD, standard deviation; 3TC, lamivudine; AZT, zidovudine; IDU, intravenous drug use; MSM, men having sex with men or bisexual.
Results
Study population
A total of 714 patients were studied for nasal S. aureus colonization, and a subset of 457 patients were also screened for oral S. aureus colonization. The mean age of all patients was 38.2 years (range 18–85 years), and the majority (91.9%) were male. Men who have sex with men (MSM) comprised the largest proportion (58.7%), followed by heterosexual patients (27.0%) and intravenous drug users (IDUs; 12.2%). Thirteen patients, all IDUs, had a history of recent incarceration. At enrollment, most patients (79.4%) were receiving HAART, and the mean CD4 count was 472 cells/mm3 (Table 1). The demographic data between the 714 all-patient group and the 457 patient subgroup were not statistically different, except that more patients in the all-patient group had hypertension (p = 0.025; data not shown).
Prevalence of colonization
Among the 457-patient subgroup, oral colonization by S. aureus, MRSA, and Candida species was found in 86 (18.8%), 14 (3.0%), and 231 (50.6%) patients, respectively, while nasal colonization by S. aureus and MRSA was found in 145 (31.7%) and 20 (4.4%) patients, respectively. Oral only, nasal only, both oral and nasal colonization, and colonization at any site by S. aureus was found in 23 (5%), 82 (17.9%), 63 (13.8%), and 168 (36.8%) patients, respectively, and by MRSA in 5 (1.1%), 11 (2.4%), 9 (2.0%), and 25 (5.5%) patients, respectively. The added yield for MRSA carriage from oral screening was 25.0%. Both oral S. aureus and MRSA colonization was positively associated with their nasal colonization (p < 0.001 for both). The all-patient group had a similar prevalence of nasal colonization by S. aureus (228; 31.9%) and MRSA (28; 3.9%), among which 85.5% (195/228) and 85.7% (24/28) had ≥1+ growth, including 71.1% (162/228) and 71.4% (20/28), respectively, having 2+ or more growth on primary culture plates. In contrast, all S. aureus and MRSA from the oral cavity were recovered in a rare amount, since they were detected from enrichment subculture only. The overall oral Candida colonization was 52.4%, with C. albicans being the most common species identified (73.8%). Of the 457-patient subgroup, 9.2 and 1.5% of patients had Candida co-colonization with S. aureus and MRSA, respectively. Oral Candida (and C. albicans; data not shown) colonization was not correlated with either oral or nasal colonization by S. aureus or MRSA (Table 2).
Table 2.
Prevalence of colonization by organisms and sites among 457 HIV-infected outpatients.
| Oral Candida colonization |
p | ||
|---|---|---|---|
| Positive, n = 231 | Negative, n = 226 | ||
| Oral colonization by S. aureus | |||
| Positive, n = 86 | 42 (9.2) | 44 (9.6) | 0.725 |
| Negative, n = 371 | 189 (41.4) | 182 (39.8) | |
| Oral colonization by MRSA | |||
| Positive, n = 14 | 7 (1.5) | 7 (1.5) | 0.967 |
| Negative, n = 443 | 224 (49.0) | 219 (47.9) | |
| Nasal colonization by S. aureus | |||
| Positive, n = 145 | 76 (16.6) | 69 (15.1) | 0.586 |
| Negative, n = 312 | 155 (33.9) | 157 (34.4) | |
| Nasal colonization by MRSA | |||
| Positive, n = 20 | 13 (2.8) | 7 (1.5) | 0.186 |
| Negative, n = 437 | 218 (47.7) | 219 (47.9) | |
Data are shown as n (%).
Patients who were recently incarcerated had a higher risk for MRSA carriage at any site than those who had not been incarcerated (23.1% [3/13] vs. 4.3% [30/701]; p = 0.030). The prevalence of oral S. aureus, MRSA, and Candida colonization was lower among patients with a suppressed viral load than among patients with a higher viral load (≥200 copies/mL; 15.8% vs. 24.7%, p = 0.022; 1.7% vs. 5.7%, p = 0.024; and 46.5% vs. 57.6%, p = 0.024, respectively).
Microbiologic characteristics and clonality of MRSA
A total of 41 MRSA isolates were recovered from 33 patients, including eight pairs of nasal and oral isolates (each pair from the same patient) and an additional 19 nasal and 6 oral isolates from different patients. Based on PFGE, the majority of the isolates belonged to two main clusters: pulsotype B (21 isolates, 51.2%; 16 patients, 48.5%) and pulsotype C (11 isolates, 26.8%; 9 patients, 27.3%). Three isolates (7.3%) each belonged to pulsotypes A (from two patients) and K (from three patients), and the remaining three isolates had PFGE patterns distinct from pulsotypes A–C and K (Figure 1). The nasal and oral isolates from the same patient all had indistinguishable PFGE patterns.
Figure 1.
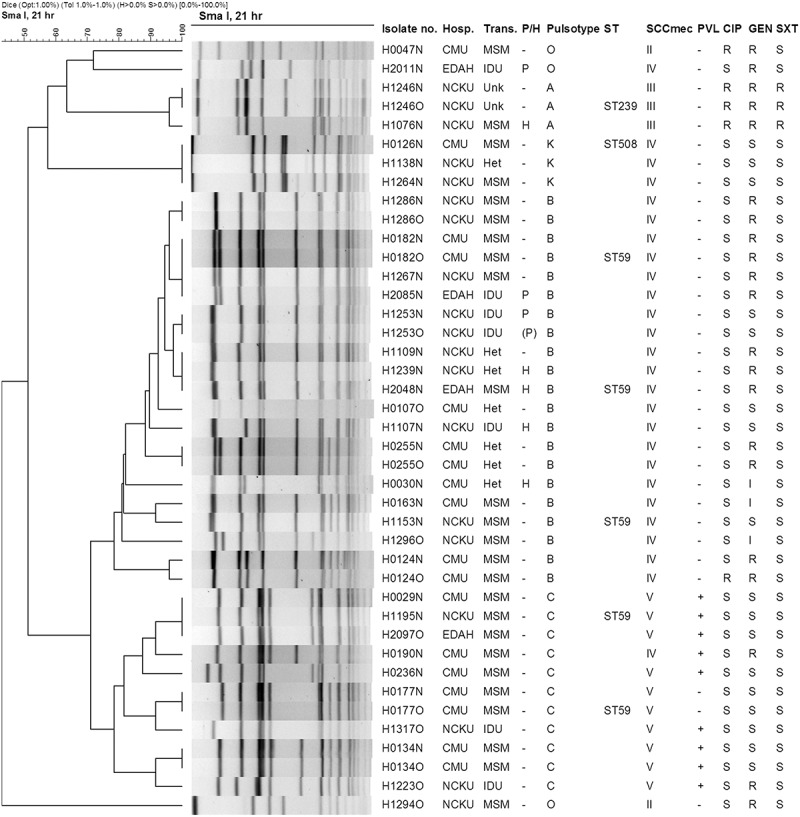
PFGE dendrogram with molecular characterization of nasal and oral MRSA isolates from HIV-infected patients. PFGE cluster was assigned to isolates having ≥80% similarity from the dendrograms. Isolate number indicates the strain number, in which the last letter N and O represent nasal and oral isolates, respectively. Isolates with an identical strain number irrespective of N or O were from the same patient. CIP, ciprofloxacin; Gen, gentamicin; H, hospitalizations; Het, heterosexual; HIV, human immunodeficiency virus; I, intermediate; IDU, intravenous drug user; MRSA, methicillin-resistant S. aureus; MSM, men who have sex with men; P, in jails or prisons; FFGE, pulsed-field gel electrophoresis; PVL, Panton-Valentine leukocidin; R, resistant; S, susceptible; ST, sequence type determined by multi-locus sequence typing; SXT, trimethoprim/sulfamethoxazole (co-trimoxazole); Trans, transmission route of HIV in indicated patient.
Both pulsotypes B and C are associated with ST59 and contained genetically identical or closely related strains from the three hospitals. Pulsotype B isolates were PVL-negative but carried SCCmec IV and were recovered from patients who were MSM, heterosexual, IDUs, or recently incarcerated. Pulsotype C isolates were mostly PVL-positive, carried SCCmec VT, and were recovered from patients who were MSM or IDUs. Pulsotype A isolates are ST239 and carried SCCmec III, while pulsotype K isolates are ST508 and carried SCCmec IV.
Rates of non-susceptibility to chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, rifampin, tetracycline, and co-trimoxazole were 63.4%, 9.8%, 82.9%, 85.4%, 58.5%, 2.4%, 51.2%, and 7.3% (three isolates), respectively, while all isolates were susceptible to daptomycin, linezolid, teicoplanin, and vancomycin. The three co-trimoxazole-resistant isolates all carrying SCCmec III were recovered from two patients, one of whom was recently hospitalized and receiving co-trimoxazole prophylaxis.
Risk factors for colonization
Risk factors for colonization examined by multivariate analysis are presented in Table 3. A suppressed HIV viral load protected against oral colonization by S. aureus and MRSA but not against their nasal colonization. Recent antibacterial use was associated with a lower risk of both nasal and oral S. aureus colonization, and, in particular, recent co-trimoxazole use was negatively associated with nasal S. aureus colonization. Older age (>50 years) and IDU were another two independent protective factors for nasal S. aureus colonization. Recent incarceration was the only risk factor identified for nasal MRSA colonization. For oral Candida colonization, a low HIV viral load remained an independent protective factor, while recent hospitalization, recent tuberculosis (TB) with anti-TB regimens, older age, and IDU increased risk for colonization.
Table 3.
Summary of predictors for S. aureus, MRSA, and Candida colonization in the nasal and oral cavity of HIV-infected outpatients.
| Status | Predictors | Characteristic | Of 457 subjectsa |
Of all (714) subjectsb |
||
|---|---|---|---|---|---|---|
| p | OR (95% CI) | p | OR (95% CI) | |||
| Oral S. aureus colonization | HIV VL <200 | Protective | 0.010 | 0.53 (0.33–0.86) | NA | – |
| Antibacterial agent use | Protective | 0.045 | 0.22 (0.05–0.97) | NA | – | |
| Oral MRSA colonization | HIV VL <200 | Protective | 0.026 | 0.28 (0.09–0.86) | NA | – |
| Nasal S. aureus colonization | Age >50 years | Protective | NS | – | 0.028 | 0.58 (0.36–0.94) |
| Antibacterial agent use | Protective | 0.028 | 0.34 (0.13–0.89) | NS | – | |
| Co-trimoxazole use | Protective | NS | – | 0.028 | 0.39 (0.17–0.91) | |
| Intravenous drug user | Protective | 0.001 | 0.26 (0.12–0.59) | 0.001 | 0.36 (0.20–0.65) | |
| Nasal MRSA colonization | Incarceration | Risk | NS | – | 0.002 | 8.11 (2.10–30.31) |
| MRSA colonization at any site | None | – | – | NA | – | |
| Oral Candida colonization | Age >50 years | Risk | 0.001 | 2.68 (1.49–4.79) | 0.007 | 1.85 (1.18–2.89) |
| HIV VL <200 | Protective | 0.007 | 0.57 (0.38–0.85) | 0.031 | 0.60 (0.40–0.97) | |
| Hospitalization | Risk | 0.040 | 2.27 (1.04–4.98) | 0.019 | 2.07 (1.12–3.81) | |
| Tuberculosis | Risk | 0.059 | 7.58 (0.93–61.91) | 0.028 | 9.96 (1.28–77.79) | |
| Intravenous drug use | Risk | NS | – | 0.002 | 2.20 (1.35–3.59) | |
aExcluding the 257 patients not tested for oral S. aureus colonization.
bAll 714 patients were tested for nasal S. aureus colonization.
CI, confidence interval; MRSA, methicillin-resistant S. aureus; NA, not applicable; NS, not significant; OR, odds ratio; VL, plasma viral load (copies/mL).
Discussion
Although colonization by either MRSA or Candida species among HIV-infected patients has been investigated, this study differed from previous investigations by examining colonization by both species in both the nasal and oral cavities simultaneously in the contemporary HIV-infected population. This study design enabled risk factors to be compared between colonizing organisms and between sites of colonization. Furthermore, a HIV-infected outpatient population was enrolled with a mean CD4 count of 472 cells/mm3, among whom a substantial portion were on HAART (79.4%), with a suppressed viral load (67.5%), and a CD4 count of ≥500 cells/mm3 (41.0%). In general, the normal CD4 count in healthy adults ranges from 500 to 1,500 cells/mm3, and a viral load of ≥200 copies/mL is defined as virologic failure for HIV-infected patients receiving HAART [13,18]. It appeared that most patients enrolled in the present study had their HIV under adequate control and had achieved favorable immunity in terms of these laboratory criteria.
Compared to studies examining MRSA carriage among HIV-infected patients conducted in other parts of world during the past decade, the mean CD4 count of patients in the present study (472 cells/mm3) was comparable to those (416–599 cells/mm3) reported elsewhere [19,20] but higher than the 205 cells/mm3 found in an earlier study by the authors in 1999 [7]. The nasal S. aureus carriage rate (31.9%) found in patients in the present study was also similar to those (26.7–3.6%) of other contemporary HIV-infected patients [19,21]. The nasal and oral MRSA carriage rates (3.9–4.3 and 3.0%) found in this study were within the ranges reported from recent parallel HIV studies (i.e. 0–17.3 and 0–8%, respectively) by other study groups [19,20,22].
In addition, higher rates of nasal S. aureus and MRSA colonization (31.9% and 3.9–4.3%) than oral colonization (18.8 and 3.0%) have been observed in other HIV-infected populations [20,21]. The finding of higher bacterial loads in the nasal than in the oral cavity here was consistent with a recent microbiota analysis showing that Staphylococcaceae constituted 55% of nasal commensals but was comparatively uncommon in the oral cavity [23]. Despite this, several oral-positive/nasal-negative S. aureus and MRSA carriers were identified, resulting in an overall MRSA carriage rate of 5.5%. The added yield for MRSA carriage from oral screening was 25%, similar to the average added yield (17.5%) from screening by throat culture summarized in a meta-analysis [20]. Hence, it should be borne in mind that nasal-only screening would underestimate the MRSA carriage rate.
Although CA-MRSA infection has emerged in Taiwan since 2002, the nasal MRSA carriage rate here (3.9%) did not increase over the years compared with the rate (6%) found in the authors’ 1999 study, and was similar to the rate (3.8%) reported among 3,098 healthy adults in Taiwan in 2007 [7,24]. Different from the United States where the prevalent CA-MRSA clone, USA300 (ST8/SCCmec IV/pvl-positive), which has also been found to be the leading strain colonizing HIV-infected patients [20], the CA-MRSA isolates in Taiwan can be grouped into two distinct clones: the Asian-Pacific clone (ST59/pulsotype B/SCCmec IV/PVL-negative) and the virulent Taiwan clone (ST59/pulsotype C/SCCmec VT/PVL-positive) [2]. Both are susceptible to co-trimoxazole. In the present study, 48.5% of patients were colonized by pulsotype B isolates with genotypes characteristic of the Asian-Pacific clone, and 27.3% were colonized by pulsotype C isolates having the molecular characteristics of the virulent Taiwan clone. Two Taiwanese studies investigating nasal MRSA carriage among healthy adults and children also revealed similar findings [24,25]. These observations indicated that among HIV-infected outpatients comprising a high proportion on HAART, the MRSA carriage rate appeared to be similar to that of the general population, and most of the colonizing isolates belonged to typical CA-MRSA strains circulating locally. However, a few patients were also identified who were colonized by ST239/SCCmec III isolates, the typical healthcare-associated MRSA strains in Taiwan, likely due to recent hospitalization [2].
Three patients were also found who were colonized with ST508/SCCmec IV MRSA. A previous study in Taiwan found ST508 to be the most common nasal carriage clone of methicillin-susceptible S. aureus (MSSA), but it was rarely detected in the MSSA infection strains [26]. In addition, although there have been a few reports of ST508 S. aureus, most are MSSA and rarely MRSA [27]. ST508 is a single locus variant of ST45 belonging to clonal complex 45 (CC45). Since ST45 MRSA has emerged in Taiwan in recent years [28], further studies to determine the association of CC45 MRSA and MSSA might shed light on their evolution.
Multivariate analysis revealed that a suppressed HIV viral load protected against oral Candida colonization, consistent with a previous study demonstrating that high HIV viral load was a significant predictor for oral Candida colonization [29]. Further, it was found that a suppressed viral load protected against oral colonization by S. aureus and MRSA as well, which has not been reported previously. It has been shown that the phagocytic activity of monocytes and neutrophils can be directly suppressed by HIV but restored by lowering HIV load [30]. Such direct and other indirect effects exerted by HIV might alter oral mucosal immunity that promotes colonization and infection by commensal or pathogenic organisms [29,30]. In contrast, effective management of HIV infection by HAART, reflected by a decreased viral load, might restore oral immunity and subsequently decrease microbial colonization and infection, as observed in this study [29,30]. Notably, although a CD4 count <200 cells/mm3 was identified as a risk factor for oral Candida colonization in the authors’ previous work in which the viral load was not included for analysis [11], the association between a low CD4 count and oral colonization by either S. aureus or Candida was not demonstrated here. This is in agreement with clinical findings that plasma HIV-RNA levels, regardless of CD4 count, was the only correlate of oral Candida colonization, and oral candidiasis usually resolved after initiation of HAART, even though the CD4 count remained low [29]. These implied that improvements in the immune function after HAART are not reflected solely by an increase of CD4 count, which might lag behind other immunological parameters [29]. On the other hand, a suppressed viral load did not protect against nasal S. aureus and MRSA colonization, as found in most studies [20]. Together with the observation of more abundance of Staphylococcaceae in the nasal cavity, the mucosal immunity against S. aureus colonization appears to differ between the nasal and oral microenvironments.
Synergic interaction between Candida species (and C. albicans) and S. aureus in oral mucosal colonization was not demonstrated here and is rarely mentioned in clinical studies. C. albicans usually appear as non-invasive oral commensals, which, under conditions of immune dysfunction, can rapidly transition to pathogens through morphological switch from the rounded yeast to the invasive hyphal form, causing local or systemic diseases [31]. Furthermore, C. albicans can facilitate the invasion of S. aureus across host mucosal barriers, leading to systemic infection, resulting from the high affinity of S. aureus to the invasive hyphal elements (but not to the yeast form) of C. albicans and the propensity of C. albicans to adhere to and penetrate tissue via its invasive hyphae [31]. Lack of synergy found here might be explained by the fact that most patients enrolled have achieved favorable immunity, which restricted the Candida yeast-to-hyphae transition and subsequent bounding between the Candida hyphae and S. aureus. Though further studies are needed, it seems that under restored immunity, Candida spp. does not augment S. aureus colonization in the oral mucosa, which reduces the possibility of co-colonization and further progression to systemic diseases and may in theory be another benefit of HAART.
Recent antibacterial agent use, particularly co-trimoxazole, was another protective factor against nasal and oral S. aureus colonization. This is consistent with the fact that co-trimoxazole remained active against most community-associated S. aureus isolates, including CA-MRSA in Taiwan found in the present study and elsewhere [24,26]. The positive association of prior incarceration with nasal MRSA colonization has also been documented in several studies [20]. This could be explained by the fact that the nostrils are more susceptible to S. aureus colonization and more frequently in contact with the environmental microbes compared to the oral cavity, and prisons could be a reservoir of CA-MRSA due to overcrowding and unsanitary conditions [20,23]. Hence, adherence to general hygiene and infection-control practices is emphasized in order to reduce MRSA transmission in crowded institutions.
Recent hospitalization, tuberculosis, older age, and IDU were risk factors for oral Candida colonization. Although the association of tuberculosis with colonization has been demonstrated in two other studies [32,33], it is not known whether broad-spectrum antibacterial activity exerted by anti-tuberculosis regimens played a role, or whether tuberculosis served as a confounder reflecting impaired immunity or other unmeasured risk factors for colonization, which merits further studies. Older age and IDU increased the risk of oral Candida colonization, which is similar to previous studies showing that both groups had higher rates of oral Candida colonization [34,35]. Several factors, such as denture use, oral hygiene, smoking, and access to healthcare, should be evaluated in order to delineate the impact of age and IDU on oral Candida colonization.
This study has a few limitations. First, a HIV-uninfected population was not enrolled. However, a separate large-scale surveillance for MRSA carriage among 3,098 healthy adults with a similar average age (mean 39 years) and rate of hospitalization within 1 year (5.3%) that was conducted around the same study period could serve as a comparable reference group [24]. Second, patients in the present study were not followed up for subsequent infections. Therefore, the clinical impact of MRSA colonization by different clones, and that of Candia and S. aureus co-colonization, was not clear.
In conclusion, the finding of a lower rate and bacterial burden of S. aureus oral carriage compared with those of S. aureus nasal carriage and rate of Candida oral carriage suggested that the mucosal immunity against colonization might differ in terms of microbes and sites. Among HIV-infected outpatients, most of whom were receiving HAART, synergic interaction between S. aureus and Candida spp. in the oral mucosa was not demonstrated, and a suppressed viral load protected against S. aureus, MRSA, and Candida oral colonization, both of which might be benefits of HAART. Patients with recent incarceration were at risk for nasal MRSA colonization and hence should be carefully screened if clinically indicated.
Biographies
Dr. Chi-Jung Wu is an infectious disease physician who received her residency training at the National Cheng Kung University (NCKU) Hospital and a Ph.D. at the Graduate Institute of Clinical Medicine, NCKU (Taiwan). Her areas of interest include clinical bacteriology and mycology, with focus on antimicrobial resistance, epidemiology and molecular diagnosis of infectious diseases. She is now an assistant investigator at the National Institute of Infectious Diseases and Vaccinology, National Health Research Institutes (Taiwan).
Dr. Wen-Chien Ko is a professor of medicine with special interests in the field of human Aeromonas and Clostridium difficile infections.
Dr. Mao-Wang Ho is a clinical associate professor of medicine with special interests in clinical mycology and opportunistic infection in HIV-infected patients and transplantation recipients.
Dr. Hsi-Hsun Lin is the Director of the Department of Internal Medicine at E-Da Hospital and a professor at I-Shou University in Kaohsiung, Taiwan. Professor Lin obtained his M.D. degree from the National Defense Medical Center and Ph.D. degree from the National Yang-Ming University in Taipei, Taiwan. He completed a research fellowship at the Aaron Diamond AIDS Research Center, Rockefeller University, USA. His research interests include HIV/AIDS, clinical microbiology, and molecular epidemiology, specifically HIV and hepatitis co-infection. Professor Lin is currently the President of Taiwan AIDS Society.
Dr. Yun-Liang Yang received his Ph.D. from Indiana University, Bloomington, Indiana, USA. He was a professor of National Chiao Tung University. His research focused on microbial pathogenesis from the angle of molecular genetics and biochemistry. Pathogenic fungus, Candida, and Flavivirus were the model organisms.
Dr. Jiun-Nong Lin received his M.D./Ph.D. from Kaohsiung Medical University and LL.M. from National Kaohsiung First University of Science and Technology. His areas of interest include infectious diseases, clinical microbiology, critical care medicine, emergency medicine, and medical laws. He is the director of Department of Critical Care Medicine, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan.
Ms. I-Wen Huang is a member of the Taiwan Surveillance of Antimicrobial Resistance (TSAR) research team. She has extensive experience working with MRSA.
Ms. Hui-Ying Wang is a member of the Taiwan Surveillance of Antimicrobial Resistance (TSAR) research team.
Ms. Jui-Fen Lai is a member of the Taiwan Surveillance of Antimicrobial Resistance (TSAR) research team.
Ms. Yih-Ru Shiau is a member of the Taiwan Surveillance of Antimicrobial Resistance (TSAR) research team.
Ms. Li-Yun Hsieh is a research nurse who also assists in statistical analysis on research data.
Ms. Hui-Ting Chen is a master degree student of National Chiao Tung University assisting in antifungal drug susceptibility testing.
Mr. Chih-Chao Lin is a management of medical mycology laboratory arranging this whole survey.
Ms. Wen-Li Chu is a research assistant of medical mycology laboratory assisting in identification of pathogenic yeasts.
Dr. Hsiu-Jung Lo is a principal investigator with special interests in epidemiology and mechanisms of antifungal resistance, and pathogenesis of fungal infections. She also oversees the Taiwan Surveillance of Antimicrobial Resistance of Yeast (TSARY) program. Currently, she is organizing Taiwan Mycology Reference Center.
Dr. Tsai-Ling Lauderdale is a principal investigator whose work focuses on molecular epidemiology and mechanisms of antimicrobial resistance in bacteria. She also oversees the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program.
Funding Statement
This work was supported in part by intramural grants CL-098-PP-04 and ID-099-PP-04 (to Hsiu-Jung Lo), and IV-103-PP01 (to Tsai-Ling Lauderdale) from the National Health Research Institutes, and by grants 98W806 and 99W962 (to Yun-Liang Yang) from the National Chiao Tung University. The funding sources had no involvement in the study design, data collection, analysis and interpretation, writing of the report, or decision to submit the article for publication.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Lee C-C, Chou Y-J, Lin J-N, et al. Clinical predictors of the leading pathogens in human immunodeficiency virus-infected adults with community-onset bacteremia in the emergency department: the importance of transmission routes. J Microbiol Immunol Infect. 2016. pii:S1684-1182(16)30136-0. doi: 10.1016/j.jmii.2016.08.001 [DOI] [PubMed] [Google Scholar]
- [2].Chen CJ, Huang YC.. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20:605–9. [DOI] [PubMed] [Google Scholar]
- [3].Peters BM, Jabra-Rizk MA, Scheper MA, et al. Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Klotz SA, Chasin BS, Powell B, et al. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis. 2007;59:401–406. [DOI] [PubMed] [Google Scholar]
- [5].Vargas KG, Joly S. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J Clin Microbiol. 2002;40:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peters PJ, Brooks JT, McAllister SK, et al. Methicillin-resistant Staphylococcus aureus colonization of the groin and risk for clinical infection among HIV-infected adults. Emerg Infect Dis. 2013;19:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McDonald LC, Lauderdale TL, Lo HJ, et al. Colonization of HIV-infected outpatients in Taiwan with methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Int J STD AIDS. 2003;14:473–477. [DOI] [PubMed] [Google Scholar]
- [8].Ko NY, Chen YC, Lai YY, et al. A prospective study of interactions between a case management program and retention in care on HIV suppression in Taiwan, 2008-2012. AIDS Patient Care St. 2015;29:165–168. [DOI] [PubMed] [Google Scholar]
- [9].Ho MW, Yang YL, Lin CC, et al. Yeast oropharyngeal colonization in human immunodeficiency virus-infected patients in central taiwan. Mycopathologia. 2014;177:309–317. [DOI] [PubMed] [Google Scholar]
- [10].Lin J-N, Lin C-C, Lai C-H, et al. Predisposing factors for oropharyngeal colonization of yeasts in human immunodeficiency virus-infected patients: a prospective cross-sectional study. J Microbiol Immunol Infect. 2013;46:129–135. [DOI] [PubMed] [Google Scholar]
- [11].Wu CJ, Lee HC, Yang YL, et al. Oropharyngeal yeast colonization in HIV-infected outpatients in southern Taiwan: CD4 count, efavirenz therapy and intravenous drug use matter. Clin Microbiol Infect. 2012;18:485–490. [DOI] [PubMed] [Google Scholar]
- [12].Chen FJ, Lauderdale TL, Huang IW, et al. Methicillin-resistant Staphylococcus aureus in Taiwan. Emerg Infect Dis. 2005;11:1760–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services 2016. [cited 2017 March 30]. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- [14].Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing: 25th informational supplement M100-S25. Wayne (PA): Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- [15].Lauderdale TL, Wang JT, Lee WS, et al. Carriage rates of methicillin-resistant Staphylococcus aureus (MRSA) depend on anatomic location, the number of sites cultured, culture methods, and the distribution of clonotypes. Eur J Clin Microbiol Infect Dis. 2010;29:1553–1559. [DOI] [PubMed] [Google Scholar]
- [16].McDougal LK, Steward CD, Killgore GE, et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].World Health Organization WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- [19].Imaz A, Camoez M, Di Yacovo S, et al. Prevalence of methicillin-resistant Staphylococcus aureus colonization in HIV-infected patients in Barcelona, Spain: a cross-sectional study. BMC Infect Dis. 2015;15:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zervou FN, Zacharioudakis IM, Ziakas PD, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV infection: a meta-analysis. Clin Infect Dis. 2014;59:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Crum-Cianflone NF, Shadyab AH, Weintrob A, et al. Association of methicillin-resistant Staphylococcus aureus (MRSA) colonization with high-risk sexual behaviors in persons infected with human immunodeficiency virus (HIV). Medicine (Baltimore). 2011;90:379–389. [DOI] [PubMed] [Google Scholar]
- [22].Popovich KJ, Hota B, Aroutcheva A, et al. Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin Infect Dis. 2013;56:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bassis CM, Tang AL, Young VB, et al. The nasal cavity microbiota of healthy adults. Microbiome. 2014;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang JT, Liao CH, Fang CT, et al. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J Clin Microbiol. 2009;47:2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen CJ, Hsu KH, Lin TY, et al. Factors associated with nasal colonization of methicillin-resistant Staphylococcus aureus among healthy children in Taiwan. J Clin Microbiol. 2011;49:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen FJ, Siu LK, Lin JC, et al. Molecular typing and characterization of nasal carriage and community-onset infection methicillin-susceptible Staphylococcus aureus isolates in two Taiwan medical centers. BMC Infect Dis. 2012;12:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Conceicao T, Coelho C, Silva IS, et al. Staphylococcus aureus in former Portuguese colonies from Africa and the Far East: missing data to help fill the world map. Clin Microbiol Infect. 2015;21:842e841–842 e810. [DOI] [PubMed] [Google Scholar]
- [28].Tsao FY, Kou HW, Huang YC. Dissemination of methicillin-resistant Staphylococcus aureus sequence type 45 among nursing home residents and staff in Taiwan. Clin Microbiol Infect. 2015;21:451–458. [DOI] [PubMed] [Google Scholar]
- [29].Gottfredsson M, Cox GM, Indridason OS, et al. Association of plasma levels of human immunodeficiency virus type 1 RNA and oropharyngeal Candida colonization. J Infect Dis. 1999;180:534–537. [DOI] [PubMed] [Google Scholar]
- [30].Michailidis C, Giannopoulos G, Vigklis V, et al. Impaired phagocytosis among patients infected by the human immunodeficiency virus: implication for a role of highly active anti-retroviral therapy. Clin Exp Immunol. 2012;167:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schlecht LM, Peters BM, Krom BP, et al. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology. 2015;161:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thanyasrisung P, Kesakomol P, Pipattanagovit P, et al. Oral Candida carriage and immune status in Thai human immunodeficiency virus-infected individuals. J Med Microbiol. 2014;63:753–759. [DOI] [PubMed] [Google Scholar]
- [33].Owotade FJ, Patel M, Ralephenya TR, et al. Oral Candida colonization in HIV-positive women: associated factors and changes following antiretroviral therapy. J Med Microbiol. 2013;62:126–132. [DOI] [PubMed] [Google Scholar]
- [34].Zaremba ML, Daniluk T, Rozkiewicz D, et al. Incidence rate of Candida species in the oral cavity of middle-aged and elderly subjects. Adv Med Sci. 2006;51 Suppl 1:233–236. [PubMed] [Google Scholar]
- [35].Ceballos Salobreña AGCL, Ruesga MT, Ceballos García L, et al. Prevalence of oral lesions by Candida sp.: their varieties and serotypes in a population of patients with AIDS under a highly active antiretroviral therapy. Revista Iberoamericana Micología. 1998;15:141–145. [PubMed] [Google Scholar]


