Abstract
Background
Evidence of neurotropic astroviruses has been established using novel genetic methods in cattle suffering from viral encephalitis of previously unknown origin.
Objectives
To describe the clinical signs observed in cattle with astrovirus‐associated encephalitis.
Animals
Eight cattle (4 cows, 3 heifers, and 1 bull of 4 different breeds) admitted to the Clinic for Ruminants for neurologic disease and 1 cow investigated in the field.
Methods
Cases were selected based on neuropathologic diagnosis of nonsuppurative encephalitis, positive in situ hybridization result for astrovirus, and availability of the results of physical and neurologic evaluations. Laboratory results were evaluated if available.
Results
The most frequently observed clinical signs were decreased awareness of surroundings (7), cranial nerve dysfunction (5), and recumbency (5). The cow seen in the field was the only animal that had severe behavioral changes. Cell counts in cerebrospinal fluid (CSF) were increased in 4 animals, and protein concentration was increased in 3 of 5 specimens. In 1 case, the presence of astrovirus could be identified in a CSF sample by reverse transcriptase polymerase chain reaction. Other laboratory abnormalities were nonspecific.
Conclusions and Clinical Importance
Astrovirus infection may be an important differential diagnosis in cattle with clinical signs of brain disease and should be considered after exclusion of other causes. The clinical and epidemiological relevance of encephalitis associated with astrovirus infection should be further investigated.
Keywords: Atypical, Bovine, Central nervous system, Viral
Abbreviations
- BSE
bovine spongiform encephalopathy
- CNS
central nervous system
- CSF
cerebrospinal fluid
- FFPE
formalin‐fixed paraffin‐embedded tissue
- ISH
in situ hybridization
- RT‐PCR
reverse transcriptase polymerase chain reaction
- SBE
sporadic bovine meningoencephalitis
Infectious diseases of the central nervous system (CNS) in cattle can have important economic and public health implications. Some of them, such as rabies, listeriosis, or bovine spongiform encephalopathy (BSE), are zoonoses and thus can threaten human health.1
Examination of brain tissue collected in Switzerland in the time frame of BSE and rabies surveillance indicated that a causative diagnosis could only be made for some, but not all cattle presented with neurologic disease. Several retrospective studies in different countries have aimed at the detection of bacteria, viruses, or parasites as causative agents of neurologic disease, often without success, especially in cases of nonsuppurative encephalitis.2, 3, 4, 5, 6, 7 This type of CNS lesion also has been referred to as European sporadic bovine meningoencephalitis (SBE)8 and has been diagnosed in 9.8% of cattle subjected to histological brain examination as part of the Swiss statutory reporting of rabies and BSE suspects between 1985 and 1994. No etiologic agent could be found in these cases at that time, but brain tissue showed a histological pattern characteristic of SBE. Another 16.9% of the 532 cases of cattle displaying neurologic clinical signs included in that study remained without diagnosis, and histological examination of the brain of those animals showed no common pattern of lesions.5
Bovine astrovirus is a member of the genus Mamastrovirus within the family Astroviridae. Astroviruses are small nonenveloped RNA viruses.9 They are recognized as an important cause of diarrhea in humans, have been found in diarrheic fecal samples of mammals, and also can cause disease in birds.9, 10 In addition to their known gastrointestinal pathogenicity, astroviruses also have been detected in brain tissue of immunocompromised human patients with encephalitis,11, 12 and in an outbreak of shaking mink syndrome.13 By means of unbiased next‐generation sequencing, a neurotropic bovine astrovirus (BoAstV CH13/NeuroS1) has been found in brain tissue of cattle suffering from encephalitis of undetermined origin nearly simultaneously in Switzerland and the United States.14, 15 In the meantime, these viruses have been found in additional bovine cases,16, 17, 18 and diagnostic tools such as in situ hybridization (ISH) or reverse transcriptase polymerase chain reaction (RT‐PCR) have been developed.19, 20 Approximately 25% of the tested brain samples from cattle with nonsuppurative encephalitis of undetermined etiology were positive for BoAstV CH13/NeuroS1,14, 15 and none of the control animals was positive for this virus in case‐control studies, which supports the conclusion that the infection is likely associated with the disease.18, 20
Our aim was to describe the clinical presentation of cattle with neurologic disease associated with the presence of astrovirus in brain samples, so as to allow clinicians to recognize this new entity as a possible diagnosis in cattle with corresponding signs of neurologic disease.
Materials and Methods
Formalin‐fixed paraffin‐embedded (FFPE) and frozen tissue from cattle with histologically confirmed nonsuppurative encephalitis was collected from the archives of the Vetsuisse Faculty Bern, Switzerland. Only those cases for which neither histological examination of brain tissue by an expert neuropathologist nor follow‐up diagnostic tests had allowed for an etiological diagnosis were selected. All available CNS regions of brain tissue of affected animals were tested for the presence of BoAstV‐CH13/ NeuroS1 by ISH,19 RT‐PCR,21 or both. An example of a positive ISH result is shown in Figure 1.
Figure 1.
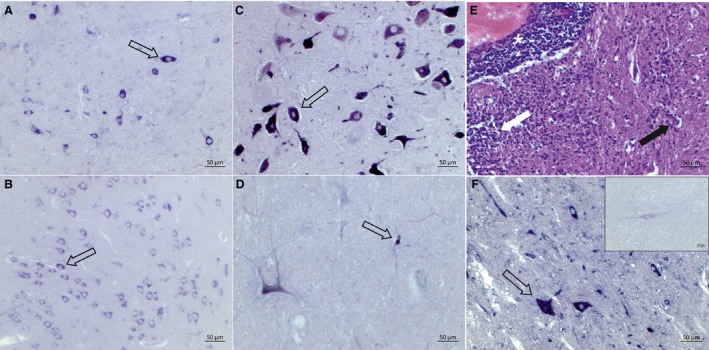
Detection of the BoAstV‐CH13/Neuro‐S1 on RNA level by in situ hybridization (ISH). Arrows indicate positive neurons. Like most BoAstV‐CH13/Neuro‐S1‐positive cattle, case # 4 shows a diffuse positive ISH signal in different regions of the central nervous system, for example, cerebellum (A) and the cerebral cortex (B). Animals # 2 (C) and # 8 (D) are BoAstV‐CH13/Neuro‐S1 positive mainly in the brainstem. The hematoxylin and eosin staining shows histopathologic lesions typical for nonsuppurative encephalitis including perivascular cuffs (asterisk), neuronal degeneration (black arrow), and gliosis (white arrow) in the brainstem of case # 8 (E). The staining of the cases tested for this study is comparable to what we observed in positive controls that were previously tested positively for the presence of the BoAstV‐CH13/Neuro‐S1 (F), while negative controls from healthy animals clearly remain unstained (F, inset).
Cattle that had been referred to the Clinic for Ruminants were selected from the ISH‐positive cases. These animals had received a standardized clinical examination including complete neurologic examination. Further laboratory diagnostic investigations had been performed depending on the individual case.
One case (# 9) had not been examined at the clinic, but the observations of the veterinary practitioner who had provided samples for neuropathologic examination were available. No further clinical laboratory data were available for this animal.
All available retrospective data (results of physical examination, laboratory analyses, and necropsy) were compiled and used as a basis for the description of cattle with neurologic disease associated with astrovirus infection.
Results
Study Group and Available Data
Including the last cow seen in the field, the study involved 9 cattle, 8 females, and 1 male. Ages ranged from 1.5 to 8 years (mean, 3.14 years; median, 2.5 years). The study group included 4 Simmental, 2 Simmental × Red Holstein crosses, and 2 Brown Swiss female cattle, as well as 1 young Eringer bull. Three animals were heifers, and the cows were 2–3 months (3 animals), 8 months, or >12 months (1 each, of which the first was 2 months and the second 7 months pregnant) after parturition, and in their first (3 animals), second, or fourth (1 each) lactation. The first case was seen in 1989 and the last in 2015. No seasonal pattern was evident, with 2 animals presented in the first trimester of the year, 3 in the second, 3 in the third, and 1 in the last trimester (Table S1).
Animals were humanely euthanized and the brain submitted for neuropathologic examination. In 2 animals (#s 4 and 6), the spinal cord also was evaluated. Six animals (#s 1 and 4–8) underwent complete necropsy.
History
The onset of clinical signs as observed by the owners ranged from the previous day to 3 weeks (mean, 1 week; median, 2.5 days). Some reported problems were nonspecific (eg, anorexia, apathy, decreased general condition) or were first attributed to other diseases such as traumatic reticuloperitonitis or enzootic pneumonia (4 cases, #s 2, and 5–7). The clinical signs observed in the remaining 5 animals, such as tremor, coordination deficits, dysphagia, compulsive walking, or aggressive behavior, were suggestive of neurologic disorders (Table S1).
Results of Clinical Examination
All animals were presented in poor general condition. Specific signs of disease reported included apathy (#s 4 and 7) or decreased peripheral temperature (# 3), but in most cases, only an overall statement of poor general condition was noted. Decreased digestive activity also was noted all animals; feces were more liquid than normal in cows # 2 and # 9 and appeared well digested and of sticky consistency in cow # 1. Respiratory signs were noted in 2 animals: Cow # 1 had increased inspiratory sounds on lung auscultation (a history of enzootic pneumonia had been recorded on this animal's farm before referral), and cow # 3 had a productive cough.
The most commonly observed neurologic signs, in decreasing order of frequency, were decreased awareness of the surroundings (7), signs indicative of cranial nerve dysfunction (5), and recumbency (5) (Table S1).
All but 1 animal (# 4) exhibited decreased awareness of their surroundings (ie, obtundation).
Four animals were recumbent upon arrival to the clinic, and 1 (# 8) became recumbent shortly thereafter. All 3 nonrecumbent animals examined at the Clinic for Ruminants showed gait abnormalities (ie, short steps especially in the pelvic limbs [# 3], ataxia and falling to 1 side [# 6], and unsteady gait [# 7]). Proprioception could not be used to assess clinical signs associated with astrovirus infection in our study, because 5 of 8 affected animals were recumbent and 2 showed loss of balance.
Five of the 8 animals (62.5%) showed at least 1 sign indicative of cranial nerve dysfunction, including dysphagia in 1 animal (# 5), decreased lingual tone in 2 animals (#s 1 and 5), and jaw tone also was decreased in cow # 5. The menace response was decreased in 3 animals (#s 1, 5, and 8), the palpebral reflex in 2 (#s 1 and 5), and ear sensitivity in 3 animals (#s 5, 6, and 8). The corneal reflex was absent in cow # 1, and a ventral rotation of the eye also was noted in cow # 6. Facial sensitivity was completely absent in cow # 8. Cranial nerve deficits without further specification were noted in cow # 7.
Lateralization of signs was observed in 50% of the animals, meaning that they either were present on 1 side only or more severe on 1 side, or that the animal leaned to 1 side in its gait or posture. Heifer # 1 always held its head to the left; in cow # 5, the menace and palpebral reflexes as well as ear sensitivity were only decreased on the left side; cow # 6 had a head tilt to the right and fell on its left side after manipulation of the neck; and the unspecified cranial nerve deficits in cow # 7 were observed to be more severe on the right side.
Seizure Activity was Observed in 2 Animals (#s 2 and No 8).
Finally, the last animal observed in the field (# 9) is reported separately because complete clinical examination was not possible because of its aggressive behavior. It was the only animal with behavioral changes as the main clinical sign. The cow showed mainly hyperexcitability and aggressive behavior. A neurologic examination could not be performed.
Laboratory Findings
A CBC with differential cell count was available for 6 animals (#s 2 and 4–8), whereas only the total leukocyte count was available for cow # 3. The total leukocyte count was markedly increased at 23.54 × 103/μL, due to neutrophilia with a regenerative left shift in 1 animal (# 8). No or slight and nonspecific changes were present in the other available CBCs (Table S2).
The values of PCV, total protein, and albumin concentrations and results of the glutaraldehyde test as an indicator of inflammation were evaluated in relation to each other. Three animals (#s 3, 6, and 8) had slightly to severely increased PCV in combination with increased total protein and albumin concentrations. These animals had enophthalmos (# 3) or were in severely decreased general condition (#s 6 and 8). Three animals (#s 4, 5, and 7) had increased total protein concentrations in combination with low (# 4) or normal (#s 5 and 7) albumin concentrations and normal PCV. These 3 animals had a glutaraldehyde test below 15 min, the normal limit set by the manufacturer.1
A serum biochemistry panel was available for 7 animals (#s 2–8). In all but 1 animal (# 3, which was not recumbent), the activity of creatine kinase and of at least 1 of the routinely measured serum enzymes was increased. No consistent abnormalities were observed in the serum electrolyte concentrations of the 7 animals for which a serum biochemistry panel was performed.
Samples of cerebrospinal fluid (CSF) were collected in 5 animals antemortem (#s 1, 3, 6–8). An increased CSF protein concentration was observed in 3 of 5 samples (#s 1, 3, and 6), and nonsuppurative pleocytosis was observed in 4 of 5 (#s 1 and 6–8; range, 26–141 cells/3 μL; normal, <3 cells/μL). Another set of 4 CSF samples (from animal #s 5–8) was tested for astroviruses by RT‐PCR. The CSF sample of cow # 5 was collected postmortem and submitted for RT‐PCR only. One of these 4 samples (# 7) gave a positive result.
Necropsy and Neuropathologic Examination
Complete necropsy was performed in 6 animals (#s 1 and 4–8). Additional lesions in the form of abscesses were found in cow #s 3 and 4 (the latter also had myocarditis with Sarcocystis). Animal #s 6 and 7 had ulcerative abomasal inflammation (accompanied by cholangitis due to Fasciola hepatica in cow # 7) (Table S2).
Features of nonsuppurative encephalitis and meningitis were observed to a variable extent in all animals. The brainstem was moderately to severely affected in all animals. Nuclei of the cranial nerves were in particular affected in animal #s 2, 5, 7, and 8, for all of which, except cow # 2, cranial nerve deficits had been recorded. Cranial nerve deficits had been recorded for another animal (# 1), but no histopathologic lesions were identified in the brainstem.
The cerebellum generally was less affected than the brainstem, mainly by gliosis and meningitis. In animal #s 3, 7, and 8, the hippocampus had especially severe lesions. Nonsuppurative myelitis was diagnosed in those animals for which spinal cord tissue was available (#s 4 and 6), and ganglioneuritis was evident in 2 animals for which the trigeminal ganglia were available (#s 6 and 7). Besides nonsuppurative inflammation, case # 9 showed severe neuronal vacuolation in the red nucleus of the rostral midbrain. All animals had tested negative for BSE.
Discussion
Nine cattle with neurologic disease and positive for BoAstV CH13/NeuroS1 in brain tissue were identified. The duration of disease before presentation was variable, but this information must be interpreted with caution because it depends on the ability of owners to recognize subtle changes (eg, changes in gait and behavior). All animals referred to the Clinic for Ruminants had poor general condition, possibly because they were presented late in the course of the disease.
The brainstem was moderately to severely affected in all animals, which relates to the most frequent neurologic sign of obtundation and also fits with the frequently observed gait abnormalities. Nuclei of the cranial nerves were affected in cow #s 2, 5, 7, and 8, for all of which, except cow # 2, signs indicative of cranial nerve deficits were recorded. However, cow # 2 was presented in a late state of disease, already showing tremor and seizures; thus, it is unclear whether it really was possible to test for cranial nerve deficits in this animal. In cow #s 3, 7, and 8, the hippocampus had particularly severe lesions. Based on its function in the limbic system, pronounced behavioral abnormalities were expected, but were not noted in these cases. Cow # 9 had severe neuronal vacuolation in the red nucleus of the rostral midbrain. Because it is part of the extrapyramidal motor system, marked gait abnormalities would be expected, but hyperexcitability primarily was noted it this animal, which was seen by a veterinarian in the field. It was thus reported separately because it had a different clinical presentation with mainly behavioral changes, and a thorough clinical examination was not possible because of hyperexcitability.
Clinical laboratory results were nonspecific, with some animals appearing to have chronic inflammation (eg, increased serum protein concentrations, normal or low serum albumin concentration, shortened glutaraldehyde test), 1 animal an acute inflammatory response (cow # 8 with leukocytosis, neutrophilia, and a regenerative left shift), and others only with dehydration (high total protein and albumin concentrations, increased PCV, and corresponding clinical signs). The increased activity of creatine kinase and at least 1 other serum enzyme activity were the most consistent finding in the serum biochemistry panel and likely were related to the recumbency observed in all but 1 animal. Cerebrospinal fluid was altered in all cases (high protein concentration in 3 and pleocytosis in 4 of 5 cases), but not all samples had the same changes, and the number of CSF samples was limited. These results therefore should be interpreted with caution. In 1 of 4 samples, a positive RT‐PCR for BoAstV CH13/NeuroS1 result was obtained.
Two single‐case reports of neurotropic astroviruses described a sudden onset of clinical signs.16, 17 One study that evaluated 21 cases of SBE also described animals with a sudden onset and others with a slower progression of disease.8 So far, no report mentions several diseased animals in 1 herd, and based on available information, such was not the case in the cattle of the present study either.
Clinically, several similarities to SBE could be noted, such as the presence of gait abnormalities, decreased awareness of the surroundings, or signs indicative of cranial nerve dysfunction.8 Brain tissue samples originating from the SBE study recently have been investigated for the presence of neurotropic astroviruses, and 12 of 14 cases were positive by ISH.18
To our knowledge, there are 3 other single‐case reports of neurotropic bovine astrovirus infection. One animal was found in lateral recumbency with opisthotonus and limbs in extensor rigidity,15 possibly showing a terminal stage of the disease as with the 2 animals with tonic‐clonic convulsions seen at our clinic. Another animal showed marked behavioral abnormalities (“suddenly running off in the field”) similar to our field case (# 9), but also presented with “oblique bearing of the head, circling movements, inappetence, and somnolence”16, which were not reported in our case. Lateralization of the signs, however, also was an important trait in the referred cases. The animal in the third case report had gait abnormalities similar to those of our patients and also had hyperesthesia.17 In 3 of our cases (#s 3, 7, and 8), lesions were especially severe in the hippocampus, and in the recent study about astroviruses in SBE cases,18 infection tended to be more severe in this area, which is related to behavior. However, altered behavior was not reported in these animals, and subtle changes might have been missed because all 3 animals were presented in a poor general condition.
Astroviruses are an important cause of diarrhea in humans9, 10 and also have been isolated from fecal samples of cattle in Europe and Asia.22, 23 A link between diarrhea and the presence of astroviruses was not established in these studies. One study noted that the presence of astroviruses was strongly associated with the presence of group A rotaviruses and postulated that age may protect against clinical disease after infection with astroviruses as is known for rotaviruses.23 The genetic sequences identified in astroviruses from fecal samples did not correspond to those of neurotropic astroviruses, indicating a different tissue tropism.22, 23 Only 3 animals in our study had altered fecal consistency (cow #s 1, 2, and 9), and this finding might also be explained by indigestion or excitement. Changes in fecal consistency were not described in the 3 available case reports.15, 16, 17 Another study described diarrhea in only a few animals.8 In the time frame of our study, the herd of cow # 9 was visited several months after euthanasia of the diseased animal, and blood and fecal samples were collected. Neither blood nor feces of herd mates were positive for neurotropic astrovirus by RT‐PCR.
No clinical laboratory data were available from other case reports of astrovirus‐positive animals.15, 16, 17 Considering the 8 animals referred to our clinic, leukograms showed only slight alterations, except for cow # 8 with acute inflammatory response. This animal had the shortest disease history with sudden onset and rapid deterioration at the clinic, with seizure activity within 1 day of the first observation of clinical signs. The increase in creatine kinase activity seen in several animals can be explained by muscle damage, especially in recumbent animals.24 Thus, the results of blood tests in animals infected with neurotropic astroviruses seem to be nonspecific and mostly can be explained by accompanying disease such as abscesses or abomasal ulcers.
Samples of CSF were collected from 5 animals, and 3 samples had high protein concentrations and 4 had increased cell counts, consisting mostly of lymphocytes, monocytes, and macrophages. The low number of analyzed samples does not allow for detailed conclusions regarding potential alterations in CSF of animals with astrovirus‐associated encephalitis, but all 5 samples analyzed had altered protein concentration, pleocytosis or both.
In 4 animals, astrovirus RT‐PCR was conducted retrospectively on CSF and was positive in 1 case. The fact that these samples had already been centrifuged and therefore were cell‐free may have influenced the results. Nevertheless, the fact that BoAstV CH13/NeuroS1 can be detected by RT‐PCR in CSF samples suggests that antemortem diagnosis of the infection may be possible.
Interpretation of the results presented here is complicated by the fact that the described cases were seen over a time frame of almost 30 years; thus, different investigators may not have reported the observed clinical signs consistently over the years despite the use of standardized examination protocols, and the laboratory investigations conducted also were variable among the cases. Such drawbacks are common to retrospective studies, but analysis of the data and tissue samples collected over a prolonged period of time allowed for the report of the largest case series of bovine nonsuppurative encephalitis associated with astrovirus infection to date.
The fact that the presence of neurotropic astroviruses has been shown independently by 2 groups on 2 continents,14, 15 and later found in more animals,16, 17 strongly supports the hypothesis of the clinical relevance of CNS infections with astroviruses. In addition, none of the control animals was positive for neurotropic candidate astroviruses in a recent case‐control study.20 However, astrovirus infection may only explain some but not all nonsuppurative encephalitis cases. Because the clinical signs of astrovirus‐associated encephalitis can be similar to those of neurologic diseases of high public health relevance such as rabies or BSE, it is important that additional investigations be conducted in the future to better define the clinical presentation and diagnosis of this disease. Veterinary clinicians should be aware of astrovirus infection as a potential differential diagnosis in cattle with corresponding signs of neurologic disease.
Supporting information
Table S1. Summary of study group, history and neuroclinical signs.
Table S2. Laboratory findings in astrovirus positive animals.
Acknowledgments
The authors thank the referring veterinarian and the owner of 1 animal (case # 9) that was seen in the field.
Grant support: This work was funded in part by the Federal Food Safety and Veterinary Office (T. Seuberlich, grant MON‐108) and by the Swiss National Science Foundation (T. Seuberlich, grant 31003A_163438).
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at the Clinic for Ruminants and the Division of Neurological Sciences.
Footnote
Graeub AG, Bern, Switzerland
References
- 1. World Organization for Animal Health . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th ed Paris, France: World Organization for Animal Health; 2004. [Google Scholar]
- 2. Sánchez S, Clark EG, Wobeser GA, et al. A retrospective study of non‐suppurative encephalitis in beef cattle from western Canada. Can Vet J 2013;54:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 3. Bozzetta E, Caramelli M, Casalone C, et al. BSE surveillance in Italy: Neuropathological findings in cattle in the frame of the passive surveillance programme. J Vet Med A Physiol Pathol Clin Med 2003;50:48–49. [DOI] [PubMed] [Google Scholar]
- 4. Jeffrey M. A neuropathological survey of brains submitted under the bovine spongiform encephalopathy orders in Scotland. Vet Rec 1992;131:332–337. [DOI] [PubMed] [Google Scholar]
- 5. Heim D, Fatzer R, Hörnlimann B, et al. Frequency of neurologic diseases in cattle. Schweiz Arch Tierheilkd 1997;139:354–362. [PubMed] [Google Scholar]
- 6. Bachmann PA, ter Meulen V, Jentsch G, et al. Sporadic Bovine‐Meningo‐Encephalitis—Isolation of a Paramyxovirus. Arch Viro 1975;48:107–20. [DOI] [PubMed] [Google Scholar]
- 7. Theil D, Fatzer R, Schiller I, et al. Neuropathological and aetiological studies of sporadic non‐suppurative meningoencephalomyelitis of cattle. Vet Rec 1998;143:244–249. [DOI] [PubMed] [Google Scholar]
- 8. Fankhauser R. Sporadische Meningo‐Encephalomyelitis beim Rind. Schweiz Arch Tierheilkd 1961;103:225–234. [Google Scholar]
- 9. Méndez E, Murillo A, Velázquez R, et al. Astrovirus Research. Astrovirus Res Essent Ideas, Everyday Impacts, Futur Dir. 2013;19–46.
- 10. Moser L, Schultz‐Cherry S. Pathogenesis of astrovirus infection. Viral Immunol 2005;18:4–10. [DOI] [PubMed] [Google Scholar]
- 11. Quan PL, Wagner TA, Briese T, et al. Astrovirus encephalitis in boy with X‐linked agammaglobulinemia. Emerg Infect Dis 2010;16:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naccache SN, Peggs KS, Mattes FM, et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next‐generation sequencing. Clin Infect Dis 2015;60:919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blomström A‐L, Widén F, Hammer A‐S, et al. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J Clin Microbiol 2010;48:4392–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouzalas IG, Wüthrich D, Walland J, et al. Neurotropic astrovirus in cattle with nonsuppurative encephalitis in Europe. J Clin Microbiol 2014;52:3318–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li L, Diab S, McGraw S, et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg Infect Dis 2013;19:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlottau K, Schulze C, Bilk S, et al. Detection of a novel bovine astrovirus in a cow with encephalitis. Transbound Emerg Dis 2016;63:253–259. [DOI] [PubMed] [Google Scholar]
- 17. Anonymous . Bovine astrovirus associated with encephalitis in cattle. Vet Rec 2015;177:91. [DOI] [PubMed] [Google Scholar]
- 18. Selimovic‐Hamza S, Bouzalas IG, Vandevelde M, et al. Detection of astrovirus in historical cases of European sporadic bovine encephalitis, Switzerland 1958–1976. Front Vet Sci 2016;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bouzalas IG, Wüthrich D, Selimovic‐Hamza S, et al. Full‐genome based molecular characterization of encephalitis‐associated bovine astroviruses. Infect Genet Evol 2016;44:162–168. [DOI] [PubMed] [Google Scholar]
- 20. Wüthrich D, Boujon CL, Truchet L, et al. Exploring the virome of cattle with non‐suppurative encephalitis of unknown etiology by metagenomics. Virology 2016;493:22–30. [DOI] [PubMed] [Google Scholar]
- 21. Mittelholzer C, Hedlund KO, Englund L, et al. Molecular characterization of a novel astrovirus associated with disease in mink. J Gen Virol 2003;84:3087–3094. [DOI] [PubMed] [Google Scholar]
- 22. Nagai M, Omatsu T, Aoki H, et al. Full genome analysis of bovine astrovirus from fecal samples of cattle in Japan: Identification of possible interspecies transmission of bovine astrovirus. Arch Virol 2015;160:2491–2501. [DOI] [PubMed] [Google Scholar]
- 23. Sharp CP, Gregory WF, Mason C, et al. High prevalence and diversity of bovine astroviruses in the faeces of healthy and diarrhoeic calves in South West Scotland. Vet Microbiol 2015;178:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stämpfli H, Olimpo O‐E. Clinical chemistry tests In: Smith BP, ed. Large Animal Internal Medicine, 5th ed St. Louis, MO: Elsevier; 2015:350–373. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of study group, history and neuroclinical signs.
Table S2. Laboratory findings in astrovirus positive animals.


