ABSTRACT
The oral cavity is a major entry point for bacteria and other microorganisms. Oral biofilms are formed by mixed communities of microorganisms embedded in an exopolysaccharide matrix. Biofilms forming on dental hard or soft tissue are the major cause of caries and endodontic and periodontal disease. Human oral biofilms exhibit high resistance to antimicrobial agents. Antibiofilm peptides constitute a diverse class of host-defense molecules that act to combat invasion and infection with biofilms. Different in vitro and in vivo biofilm models with quantitative analysis have been established to provide predictable platforms for the evaluation of the antibiofilm effect of oral antibiofilm peptides. These peptides have engendered considerable interest in the past decades as potential alternatives to traditional disinfecting agents due to their ability to target bacterial biofilms specifically, leading to the prevention of biofilm formation and destruction of pre-existing biofilms by Gram-positive and -negative bacterial pathogens and fungi. At the same time, challenges associated with the application of these antibiofilm peptides in dental practice also exist. The production of effective, nontoxic, and stable antibiofilm peptides is desired in both academic and industrial fields. This review focuses on the antibiofilm properties of current synthetic peptides and their application in different areas of dentistry.
KEYWORDS: Antibiofilm peptide, antifungal effect, biofilm, cariology, endodontics, implant surface, plaque
Introduction
Human oral biofilms are communities with complex three-dimensional structures consisting of a broad range of multi-species microorganisms formed on colonizable surfaces [1]. The colonization of biofilm on the oral surfaces has been considered as the leading cause of varies infectious diseases in different fields of dentistry, including cariology, endodontics, and periodontics [2]. Over the past a few decades, many studies have been conducted on combating biofilm development with the goal of controlling pathogenic oral microflora [3,4]. However, biofilms provide protection against antibiotics and the human host-defense system by formation of extracellular matrix as a physical barrier and production of own biofilm enzymes for physiological adaptation, which makes biofilms quite recalcitrant to various conventional antimicrobial therapies [5].
Antibiofilm peptides have recently received a great deal of attention for possible use in a number of therapeutic applications against dental biofilms. Many studies have identified antibiofilm peptides as the potential next-generation alternative to traditional antimicrobial therapy in the oral cavity [6,7]. At the same time, challenges associated with the application of these antibiofilm peptides in dental practice also exist [8]. The current knowledge of peptide design for antibiofilm purposes presents opportunities for future dental treatments that offer promotion of oral health.
This review provides an overview of recent advances describing the antibiofilm properties of synthetic peptides and their application in different fields of dentistry.
Synthesis of antibiofilm peptides
Natural antimicrobial peptides
Most living organisms, including the human body, have the ability to synthesize short chains of amino acid monomers to inhibit bacterial infection [9]. These small compounds are recognized as antimicrobial peptides (AMPs) or host-defense peptides (HDPs), which are part of the innate immune system of animals and plants or can be isolated from bacteria and fungi [10]. Nisin is one of the first cationic peptides discovered in 1928 from Lactobacillus lactis [11]. Nisin is stable, with high antimicrobial activity at room temperature and a pH range of 2–6 [12]. Polylysine, a natural AMP isolated from Streptomyces albulus 346, is commercially being produced for food applications [13]. In dentistry, nisin and polylysine were found to be synergistic to inhibit Streptococcus mutans in vitro [14]. Mammalian AMPs are generally expressed and induced in epithelial surfaces to repel assault by bacteria, viruses, fungi, and parasites [11,15]. These AMPs molecules exhibit multiple mechanisms of action for the resistance in bacteria. Natural AMPs have been recently used as templates for the design of synthetic peptide libraries [16].
Synthetic antibiofilm peptides
Modification on the natural peptide templates in terms of biological function and size have been applied in recent years, aiming to optimize their antibiofilm function. Various physiochemical modifications were applied to the naturally occurring peptides to overcome their limitations, including high production cost, degradation by the host, and difficulty in understanding the structure–function relationship [17].
Strategies to enhance antibiofilm properties such as deletion, substitution of amino acids, and cyclization were commonly used. A recent study showed the removal of four N-terminal amino acids displayed bactericidal effects for a frog-secreted antibacterial peptide B1CTcu5 [18]. Another investigation showed the substitution of fatty acids for amino acids in a dermaseptin S4 peptide may be useful for controlling pathogens associated with oral diseases [19].
Other effective strategies such as the incorporation of retro-inverso peptides and D-enantiomer amino acids showed potent antibiofilm effects. Specific D-enantiomeric peptides (DJK-5 and 6) were shown to inhibit Pseudomonas aeruginosa biofilm development strongly and to eradicate preformed biofilms of several other wild-type and antibiotic-resistant Gram-negative pathogens [20]. Moreover, AMPs containing D-enantiomeric amino acids (D-LAK peptides) were reported to be effective against clinical strains of Mycobacterium tuberculosis both in vitro and ex vivo [21].
Sequence truncations is another approach to reduce the length of the AMPs that are expensive to synthesize. Appropriate truncation on different fragments of the original long-length peptide can make the modified peptides more efficient, stable, and safe. It has been reported that truncated fragments of LL-37 exhibited antibiofilm effects on Burkholderia thailandensis and Burkholderia pseudomallei [22]. Removal of the first 12 N-terminal residues decreased the toxicity of LL-37 peptide toward human peripheral blood monocytes and rabbit erythrocytes without affecting its antifungal activity [23]. A further investigation done by Nagant et al. showed that when truncation was performed on both sides of LL-37 peptides, the antimicrobial effect of the fragments remained significant [24].
Antibiofilm peptides can also be synthesized through the construction of hybrids. The hybrid peptides, which are composed of the N-terminal regions of Cecropin A and melittin, have been known to have improved antibacterial activity, with a broader spectrum without hemolytic activity when compared to their parental peptides [25]. Most of the antibiofilm application of a hybrid peptide has been proven in the field of medicine (against Acinetobacter baumannii) [25–27]. One study reported that a hybrid peptide exerts its antifungal effect on Candida albicans by damaging the plasma membrane of the yeast cell [28].
The process of optimizing peptide antimicrobial activity and specificity using large peptide libraries is both tedious and expensive [29]. Computer-aided predictions of the antibiofilm activity of peptides using soft independent modeling and quantitative structure–activity relationship descriptors have demonstrated success in antibiofilm production [29,30]. The synthesis of next-generation peptides can be obtained from the high-throughput screening matrixes with enhanced biological activity compared to the parent peptides [16].
Mechanisms of action
Antibiofilm peptides exert activity against a broad spectrum of microorganisms, including Gram-negative and -positive bacteria, drug-resistant strains, and even fungi [8,31,32]. Generally, most AMPs permeabilize the membrane of the bacterial cells, resulting in either large-scale damage or small defects that dissipate the transmembrane potential, finally leading to cell death [8,33]. More specifically, the mechanisms of action can be explained by pore and nonpore models.
Two theories have been proposed for the pore model: the barrel stave pore model and the toroidal pore model [33]. For the barrel stave pore model [34], AMPs interact with the bacterial cell membrane to form a hydrophilic channel. For the toroidal pore model [35], AMPs affect the curvature of the membrane. A number of models for the nonpore theory have been proposed, including the carpet model [36], the detergent model [37], the molecular shape model [38], and so on. The carpet model is the most-cited model, which demonstrates parallel deposition of AMPs on the cell membrane, causing bilayer destabilization [36]. With all of these theories for explaining the mechanism action of antibiofilm peptides, they imply the need to reach a certain threshold concentration of peptides in the cell membrane prior to disruption. These peptides targeting the cell membrane are suited for the application in surface coating for medical or dental purposes [39].
Although the membrane damage models vary, most of them are linked to each other. Brogden [40] pointed out that the mechanisms of membrane damage by peptides do not appear independently but are correlated and appear gradually. Factors that are closely associated with the effectiveness and specificity of the peptide include the size, the sequence of the amino acid, conformation and structure, hydrophobicity, and amphipathicity [2].
Besides all the models mentioned above, some exceptional peptides act on the antibiofilm function in an alternative way. These peptides can inhibit bacterial cell-wall formation, breaking down DNA or RNA in the cell plasma, causing protein defragmentation or degradation intracellularly, inducing autolysin effect, and inhibiting enzyme activity [31].
Besides the direct antimicrobial activity, antibiofilm peptides can also exhibit further characteristics such as the mineralization effect and the anti-resorptive effect, which may play a role in the complex oral microbial environment under peptide treatment. Kraus et al. [41] found that human β-defensins (HBDs) can affect maturation and proliferation of osteoblast-like cells. The differentiation of osteoblast-like cells was promoted by HBD-2 and HBD-3 with increased transcript levels of osteogenic markers, upregulated ALP enzyme activity, and enhanced mineralized nodule formation. Another strategy through the mineralization approach is to develop self-assembled peptide nanofibers. Sone and Stupp [42] developed histidine-rich peptide-amphiphile fibers to induce biomineralization of magnetosome to replicate the level of control achieved by magnetotactic bacteria. Wang et al. [43] applied silver mineralization on peptide scaffolds to extend antibacterial activity against both Gram-positive and -negative bacteria.
Some AMPs secreted by host cells play a pivotal role in oral wound healing by inhibiting osteoclastogenesis [44]. Peptides in the cathelicidin family are typical osteoblast-derived protectors in infection-induced osteoclastic bone resorption [45]. Cathelicidin-related peptide (e.g. LL-37) can prevent alveolar bone destruction in periodontitis by inhibiting calcineurin activity and nuclear translocation of T-cells [44]. Moreover, a newly developed peptide (microglial healing peptide) was reported to be able to inhibit RANKL-induced osteoclast differentiation [46].
Antibiofilm peptide applications in cariology
Dental caries is a multifactorial disease that remains a prevalent global health concern. Although treatment on dental caries has improved in the last few decades, caries still represents one of the most severe global health burdens [47]. The onset of caries is a result of a shift in the dental plaque microflora toward dominance by acidogenic and aciduric organisms [48]. Even though treatments such as the use of topical antibacterial agents and exposure to fluoride have been applied and have reduced the prevalence of caries over the past few decades, the consequence of dental caries continues to grow [11]. New treatments should attempt to target the causative bacteria with minimum side effects. Many recent studies have indicated great potential for using antibiofilm peptides in the oral cavity, as they do not produce staining or irritation and can be odorless, colorless, and tasteless [11,49,50].
Minimum inhibitory concentration (MIC), scanning electron microscopy (SEM), and live/dead viability staining followed by confocal scanning are the major approaches for the evaluation of the antibiofilm activities in dental research [51–53].
Peptides against single-species biofilm
The majority of studies on the peptides application in cariology have focused on single-species biofilms, as they are easier to obtain, isolate, and culture than multi-species biofilms are. In the caries process, S. mutans and Streptococcus sobrinus deserve particular attention. Although they are not the original colonizers of cariogenic biofilm, S. mutans and S. sobrinus are regarded as the main actors responsible for tooth demineralization due to their ability to produce acid [17].
Among the synthetic peptides, the α-helical synthetic peptide has immense potential, as its antibiofilm activity on dental caries–related strains has been widely reported. KSL (KKVVFKVKFK) is a typical α-helical peptide with broad range of antibacterial activity. KSL acts by targeting the bacterial membrane through electrostatic interactions and causing destabilization in a dose-dependent manner [54].
Previous studies have used KSL to test its antibiofilm activity on 13 types of oral bacteria strains, including S. mutans, S. sobrinus, and Streptococcus sanguinis [55,56]. A MIC test on KSL for the majority of oral bacteria tested in vitro ranged from 3 to 100 μg/mL. Minimal bactericidal concentrations of KSL were within one to two dilutions of the MICs. KSL at 6.25 μg/mL exhibited a 3-log reduction in viable count in selected strains of Lactobacillus salivarius, S. mutans, Streptococcus gordonii, and Actinobacillus actinomycetemcomitans [55]. However, KSL can be degraded by human saliva and gastric fluid in minutes [57]. A modified KSL (KSL-W) has been developed by replacing Lys6 with Trp at the Lys6-Val7 cleavage site [58], which improved the stability in saliva while maintaining the antibiofilm activity. Recently, more α-helical synthetic peptides have been developed with stable activity in saliva. Tu et al. [53] showed α-helical synthetic peptide GH12 (GLLWHLLHHLLH-NH2) had rapid and strong antimicrobial activity against oral streptococci, especially for S. mutans, S. sobrinus, and S. salivarius in vitro, with MICs ranging from 6.7 to 32.0 μg/mL [53].
Besides α-helical synthetic peptides, a variety of synthetic peptides have been developed from different resources. Peptide Lys-a1 was isolated from frog species Hypsiboas albopunctatus. A recent study showed that Lys-a1 exhibited a remarkable antimicrobial effect by inhibiting planktonic and biofilm growth of different strains of oral streptococci [52]. L-K6 peptide was isolated from the skin secretions of Rana chensinesis. A study using confocal laser scanning microscopy demonstrated L-K6 significantly reduced cell viability within S. mutans biofilms [59]. The mechanism of the antibiofilm activity is associated with the inhibition of the bioactivity of lipopolysaccharide (LPS) and neutralization of LPS-induced proinflammatory responses [59]. Chrysophsin-1 is a cationic AMP having broad-spectrum bactericidal activity against both Gram-positive and -negative bacteria. An earlier study used confocal scanning laser microscopy to show that Chrysophsin-1 had a significantly lethal effect on S. mutans biofilm [51]. Cytotoxicity study showed Chrysophsin-1 had little toxicity on human gingival fibroblast at concentrations between 8 and 32 μg/mL [51].
Other than killing the biofilm bacteria, inhibition on the biofilm growth is another, more preventive approach for biofilm control in the oral cavity. A broad-spectrum peptide, Lacticin 3147, has been reported to be effective against S. mutans by substantially attenuating its biofilm formation ability [60]. GL13K, a peptide developed from human salivary protein BPIFA2, prevented formation and growth of S. gordonii biofilms in a drip-flow bioreactor and under regular mild-agitation conditions [61]. SspB peptides have been found to bind significantly with salivary components and inhibit the binding of S. mutans and S. gordonii to saliva-coated hydroxyapatite disks [62]. Ding et al. [63] generated S. mutans biofilm in a BioFlux system under a controlled flow, and the results showed the peptide used in the study (Bac8c) remarkably reduced the viability of cells in biofilms.
Among all the antibiofilm studies in dentistry using peptide treatment, S. mutans is the most frequently used species for the evaluation of antibiofilm property, as it is the key etiological agent of dental caries [50,64]. The susceptibility of S. mutans to AMPs has been reported to be regulated by dltC gene expression in biofilm cells [65].
Besides broad-spectrum peptides, another prevailing trend is the rational design of novel fusion peptides to achieve narrow-spectrum activity against specific pathogens [11]. Specifically targeted AMPs (STAMPs) have been designed with specificity by exploiting species-specific competence-stimulating peptide (CSP) domains. CSP and a two-component signal transduction system (ComDE) are involved in the quorum sensing system of S. mutans [50]. Qi et al. [66] found that the addition of exogenous CSP beyond the levels necessary for competence inhibited the growth of S. mutans in a ComDE-dependent manner. However, competition and coexistence exist in multiple species [67]. Tamura et al. [68] suggested that the regulation of CSP in S. mutans and CSP inactivation by S. salivarius are important for cell-to-cell communication between biofilm bacteria and oral streptococci. Four STAMPs (C16G2, M8G2, C16–33, and M8–33) have been reported to have two- to threefold lower MIC ranges against S. mutans (2.5–15 μg/mL) in planktonic culture than S. sobrinus and S. sanguinis have [69].
In addition, as an alternative to AMPs, the novel mimetic meta-phenylene ethynylene (mPE) was constructed to retain the amphiphilic structure and physiochemical characteristics of magainins [49]. Earlier study showed that mPE demonstrates rapid bactericidal activity against S. mutans by inhibiting LPS bioactivity and binding bacterial DNA at equimolar ratios [49].
Against multi-species biofilm
Dental plaque is considered an oral biofilm with a complex bacterial community structure. Bacteria in dental caries originate from tooth surface plaque [70]. The structural and functional architecture of the biofilm influence the metabolic processes and reduce the susceptibility to antimicrobial agents [3]. Antibiofilm peptides have also been applied on multi-species oral biofilms. Helmerhorst et al. [71] showed a series of histatin-derived peptide analogs were able to cause a significant reduction of viable counts for multi-species biofilm from saliva and plaque. Leung et al. [1] tested the effect of KSL on the development of oral multi-species biofilms isolated from human saliva using a dual-flow cell system. The colony-forming units (CFU) and confocal microscopy results showed KSL effectively blocked biofilm growth in a 45 h culturing period with 1.05 log units reduction and significantly reduced the viability of biofilm cells [1]. Moreover, the modified KSL (KSL-W) decapeptide showed excellent inhibition effect on multi-species oral biofilm isolated from saliva [72]. A more recent study investigated the ability of a cationic anti-biofilm peptide 1018 to induce killing of bacterial cells present within oral multi-species plaque biofilms [73]. The results showed that peptide 1018 was able to prevent biofilm formation significantly over 3 days, and the activity of the peptide on preformed biofilms was found to be concentration dependent.
In addition, an earlier study showed that the administration of STAMPs during the early development of multi-species biofilms inhibits S. mutans colonization and preferentially favors establishment of S. sanguinis as the dominant inhabitant [69]. STAMPs also demonstrated selective killing against S. mutans in mixed-culture biofilms [69].
Antibiofilm peptides application in endodontics
The role of biofilm in the initiation and perpetuation of endodontic disease has been well established [74]. Free-floating microorganisms in the root-canal space can attach to each other and grow into biofilm as a microbial community [75]. The maturity of the biofilm is known to influence its resistance to being killed by antibiofilm agents [76]. Bacteria in mature biofilm can resist the action of irrigants and are remarkably difficult to eradicate [77]. As different antibiofilm peptides have been developed and applied in many other medical and dental-related aspects [10,54], endodontics should also be recognized as a potential area for antibiofilm peptide application.
Biofilms grown in an open-culture model
Most endodontic biofilms grow on the dentin walls in the root-canal system. To simulate such biofilm infection, different in vitro models have been developed. Biofilms can be formed on the bottom of the 96-well plates, according to previous studies [78,79], or on collagen-coated hydroxyapatite disks (Figure 1) [5,80], both of which allow direct access by disinfecting agents.
Figure 1.
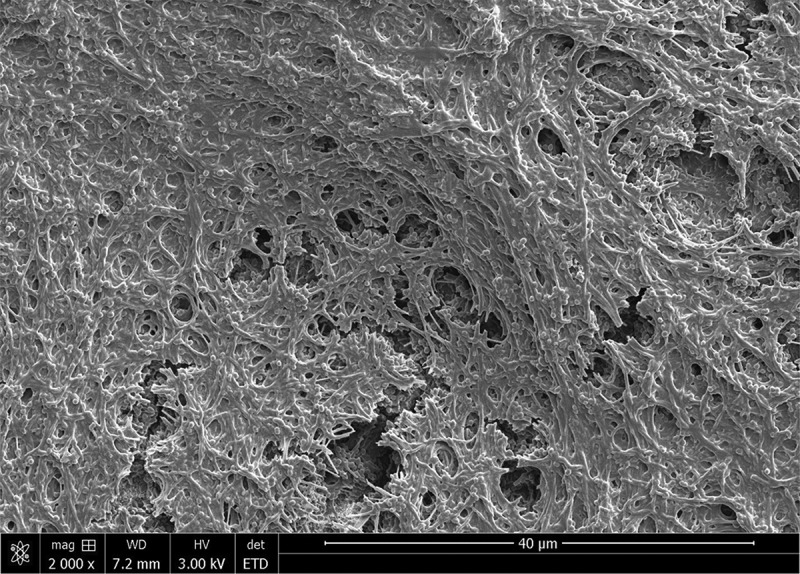
Scanning electron microscope (SEM) image of mature plaque biofilm grown on collagen-coated hydroxyapatite disks.
Enterococcus faecalis is a commonly isolated species that may play a role in persistent endodontic infections [81]. The development of these infections may be caused by E. faecalis having inherent antimicrobial resistance and the ability to adapt to harsh environmental changes [82]. Irrigation solution such as chlorhexidine has been widely used as an intra-canal medicament in endodontics (Figure 2). However, the penetration and killing effectiveness of different endodontic antimicrobial agents seem to be less than optimal in clinical reality [83]. Therefore, alternative intra-canal medicaments should be explored to maximize the eradication and biofilm disruption of therapy-resistant flora [84]. Several studies so far have used antibiofilm peptides to evaluate their efficacy against microbes often found in endodontic infections. Liu et al. [84] tested the antimicrobial efficacy of a casein peptide against E. faecalis in both planktonic and biofilm cultures. Both the glycosylated and non-glycosylated forms of the kappa-casein peptide significantly inhibited planktonic growth of E. faecalis. The glycosylated form of the kappa-casein peptide effectively inhibited E. faecalis in biofilm formation and may therefore have the potential to promote the efficacy of traditional antiseptic agents [84]. Another study tested the antibiofilm activity of four synthetic lipopeptides on a 3-day old E. faecalis biofilm. Confocal microscopy and MIC results showed that the lipopeptide formulated in the biohybrid polymer medium had some antibiofilm effect against E. faecalis [85].
Figure 2.
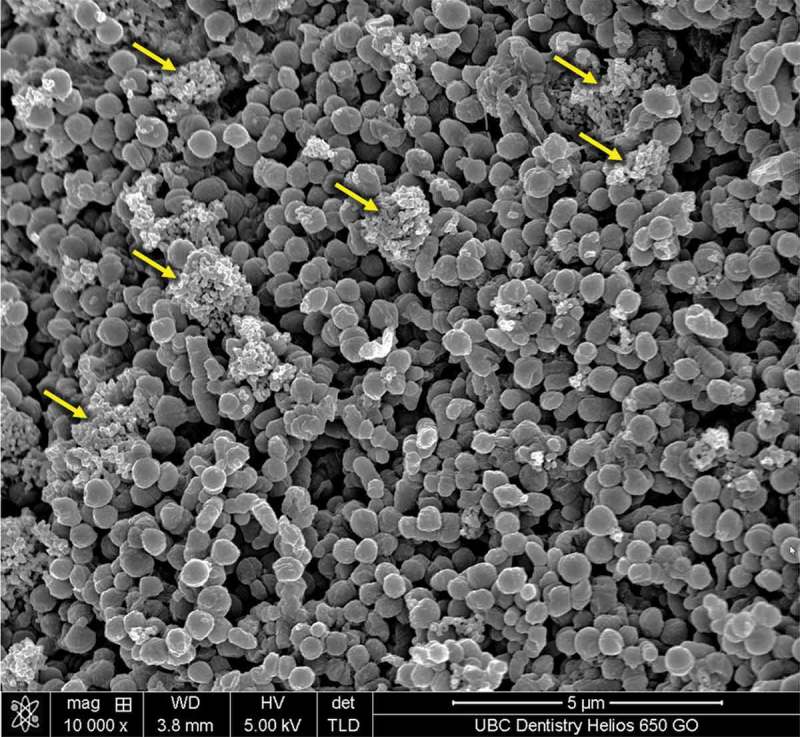
SEM image of plaque biofilm treated by chlorhexidine. Debris from the killed cells has accumulated to the biofilm surface (arrow).
A relatively new peptide 1018 effectively inhibited oral multi-species plaque biofilm growth both in the presence and absence of saliva, which indicates that the peptide was resistant to inhibition or breakdown by salivary factors [73]. Moreover, the combined treatment using low-concentration peptide 1018 (10 μg/mL) and 2% chlorhexidine increased the antibiofilm activity compared to the effect when these were used alone, resulting in >50% killing of the biofilm bacteria [73] (Figure 3). A recent study that compared the antibiofilm efficacy between a novel D-enatiometic peptide (DJK-5) with peptide 1018 on single- and multi-species oral biofilms and DJK-5 showed that the latter was more effective in biofilm inhibition than peptide 1018 was [86]. Lee et al. [87] evaluated antibiofilm efficacy of HBD3 peptide against mixed species biofilms by four different species (Actinomyces naeslundii, L. salivarius, S. mutans, and E. faecalis); HBD3 showed higher bactericidal activity on 3-week-old biofilm than calcium hydroxide and 2% chlorhexidine solution did.
Figure 3.
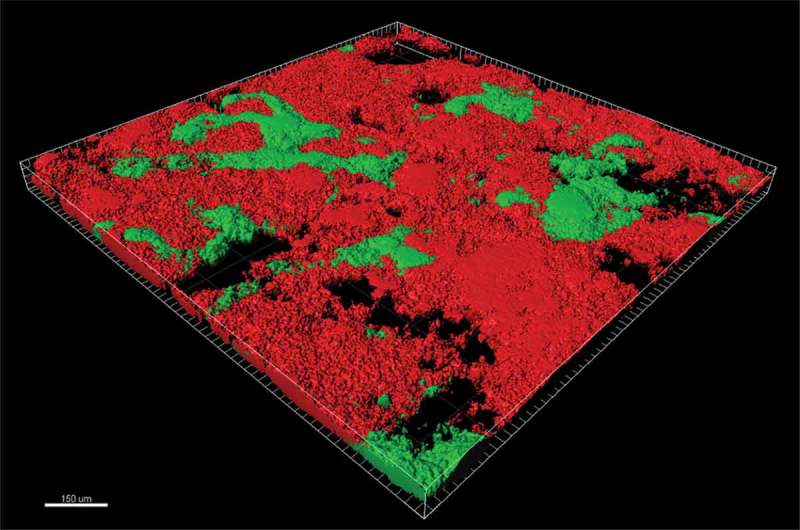
Confocal image (viability staining) of plaque biofilm treated with a combination of chlorhexidine and an antimicrobial peptide. Red areas indicate killed microbes, and green areas indicate viable microbes.
Dentin biofilm model
The inhibition of the antibacterial activity of disinfecting agents, for example by various charged molecules and compounds in the chemical environment of the root canal, contributes to the difficulty of eliminating the biofilm bacteria [88]. Previous studies have shown that dentin has an inhibitory effect on the antibacterial effectiveness of many endodontic disinfectants [89,90]. Therefore, the survival of the bacteria could also be attributed to their invasion into the dentinal tubules where they can be protected from endodontic medicaments [91].
Different dentin infection models have been developed for the evaluation of dentin disinfection in endodontics [88,92]. A recent study used the culturing method to prepare standardized dentin blocks to assess the antibiofilm efficacy of HBD3 against 3-week-old E. faecalis biofilms [84]. The peptide showed significantly higher antimicrobial efficacy than calcium hydroxide and chlorhexidine gel did. E. faecalis biofilm growth was significantly inhibited by HBD3 [84].
Antibiofilm peptide applications in periodontics
Periodontal disease is an inflammatory disease initiated by the formation of multi-species biofilms on teeth, implants, and gingival tissues [93]. Biofilm bacteria and their toxins cause a variety of inflammatory and immune processes that may result in the destruction of gingival tissues, attachment loss, periodontal pocket formation, peri-implantitis, and loss of osseointegration [93,94].
Treatment of periodontitis with antibiotics has had mixed success and does not appear to be effective in the absence of mechanical debridement [95]. Antibiofilm peptides have unique properties that may make them suitable for the prevention or elimination of oral biofilms. Many AMPs exist in human saliva and gingival crevicular fluid in low concentrations [96]. They co-evolve with oral bacteria and do not appear to prevent biofilm formation on their own. However, these peptides may prove to have effective antibiofilm properties when using higher doses, when modifying the original peptide sequence, or when being used as an adjunct to other medications [95].
Biofilm inhibition on implant surfaces
Accumulation of microbial plaque surrounding dental implants may develop into peri-implantitis with bone loss. A previous study indicated that bacteria from periodontal origin can attach to the titanium implant surface [97], which demonstrated the importance of maintaining biofilm-free implant surfaces on both supra- and subgingival portions. One candidate for the prevention of peri-implantitis is the loading of antibiofilm peptides onto the titanium (Ti) implant surface.
Calcium-phosphate coatings, vertically aligned titanium nanotubes, collagen gels, chitosan coatings, and fibrin scaffolds have all been proposed as an antibiofilm peptide delivery system for implants [98–100]. Each approach can result in antimicrobial activity. Yoshinari et al. [101] used a quartz crystal microbalance technique (QCM-D) to bind conjugated molecules consisting of antimicrobial and hexapeptidic Ti-binding peptides (min-TBP) onto Ti surfaces. Results showed that the adenosine triphosphate (ATP) activity of Porphyromonas gingivalis was significantly reduced in the peptide-modified surfaces compared to that in the Ti control. Another more recent study confirmed the antibiofilm activity of min-TBP on other types of biofilm formation (S. gordonii, Streptococcus sanguis, S. mutans, Staphylococcus epidermidis, and so on) on Ti implants [102–104].
One limitation of the traditional peptide-binding techniques is that the peptide exposure time is not enough to promote the soft-tissue healing and osseointegration. An improvement of the exposure time is the appearance of layer-by-layer assembly (LBL) technique [105]. Shi et al. [106] used LBL technique on a pure Ti surface as a delivery system and showed the coating with controlled release of multilayer antibiofilm peptides decreased the growth of both Gram-positive (Staphylococcus aureus) and -negative (P. gingivalis) anaerobes for up to 1 month. This technique has also proven to be a biocompatible approach, showing no cytotoxicity to osteoblast-like cells (MG-63) and with very low red blood cell lysis observed on the implant surface [100].
Besides the LBL techniques, covalent anchoring of biomolecules to the implant surfaces is another feasible approach to create antibacterial passive coatings successfully and has been shown to inhibit bacterial adhesion and growth significantly [107–109]. A group of studies anchored human lactoferrin-derived peptide hLf1-11 to titanium surfaces. An outstanding reduction in the adhesion and early stages of biofilm formation of single- (S. sanguinis and L. salivarius) and multi-species (plaque) biofilm was observed on the biofunctionalized peptide-coated Ti surfaces compared to non-treated control samples [94,110,111]. Moreover, hLf1-11 peptide did not affect human fibroblast viability [111].
Antibiofilm effects on periodontal pathogens
Biofilms in the periodontal pocket are highly resistant to antibiotic treatment, and drug-resistant bacterial strains have emerged due to the abuse of antibiotics [112]. Thus, the development of alternative treatment options such as the application of antibiofilm peptides is important in the effort to remove periodontal pathogens from tooth and implant surfaces [113].
It has been reported that specific natural peptides have been recognized as biomarkers for early detection of periodontitis [114]. Lappin et al. [115] reported that a natural peptide served as a systemic indicator of the inflammatory process and disease severity in subjects with periodontitis. Previous studies have also investigated the antibiofilm activity of synthetic peptides against major pathogenic bacteria strains involved in the periodontal plaque biofilm formation. Wang et al. [116] found that a synthetic cationic AMP Nal-P-113 can inhibit periodontal bacteria and biofilm (S. gordonii, Fusobacterium nucleatum, and P. gingivalis) probably by forming pores within the cytoplasmic membranes and further killing the bacteria. Another study showed a synthetic peptide (BAR) could also influence interactions between bacterial strains [117]. The BAR peptide inhibited P. gingivalis adherence to S. gordonii. However, it could be degraded by P. gingivalis proteases. Limiting its susceptibility to proteolytic degradation became important to maintain the antibiofilm activity of the peptide.
A more recent study followed P. gingivalis infection in mice and examined the efficacy of vaccination by recombinant and native RgpA peptide in modulating the early local anti-inflammatory and immune responses and periodontal bone loss [118]. Results showed that vaccination with recombinant RgpA peptide protected the mice against P. gingivalis–induced bone loss.
Antifungal effects
Oral infections caused by fungi represent an increasing problem in human oral health. HDPs are strong therapeutic candidates as antifungals [119]. However, they are expensive to produce and are often sensitive to protease digestion. Therefore, inexpensive nonpeptidic oligomers and compounds that mimic HDPs in both structure and activity have been developed for the treatment of oral candidiasis [120]. In a study by Ryan et al. [120], the total Candida burdens in tongues of infected mouse were reduced up to 3 logs after single-dose administrations of HDP mimetics.
Histatins are predominantly antifungal both in vitro and in vivo and comprise of three pairs of bands (His-1, His-3, and His-5) [7,121–123]. The antifungal mechanism of histatins occurs in a series of sequence: bonding to bacterial membrane, penetrating the membrane, inhibiting mitochondrial respiration, entering the cell by mobilization of ions, and causing cell death [124]. A particular promising antibiofilm peptide, histatin 5 12-mer P113 (Demegen), has been used in a mouth rinse for oral candidiasis in patients with human immunodeficiency virus [6,93].
Since the common cause of biofilm in dental prosthesis is by colonization of Candida spp., previous studies used denture acrylic as substance for biofilm growth to simulate the clinical reality [125,126]. His-5 showed similar limiting effect on 3-day biofilm in comparison with chlorhexidine [126]. Other synthetic peptides such as HBD3 also demonstrated antifungal activity by killing C. albicans and inhibiting biofilm formation [127].
Challenges of antibiofilm peptides in clinical application
Despite all the advantages that the antibiofilm peptides demonstrate, there are also limitations existing in their therapeutic utility [33]. In the presence of biological fluids such as plasma, serum, or saliva, the antibiofilm activity of the peptides can be significantly reduced compared to their performance in non-physiological conditions [2]. Moreover, antibiofilm peptides are difficult and expensive to manufacture in large quantities [49], mainly due to the complex processes needed for their extraction, isolation, and purification. It is also difficult to use the antibiofilm peptides by the parenteral route, as it is excreted quickly through the kidney [128]. In addition, some antibiofilm peptides display toxicity to the host cells and may induce unwanted pro-inflammatory responses [129].
Conclusion
Antibiofilm peptides have been found to be active against the oral biofilms associated with caries and endodontic, periodontal, and fungal diseases. However, efforts are still needed to focus on exploring more novel peptides that are easier to produce and which may overcome some of the limitations of the current peptides.
Biographies
Dr. Zhejun Wang received his DDS and PhD from Wuhan University in China. During his PhD studies, he completed an electron microscopy specialty training program in the Cavendish Laboratory at the University of Cambridge in 2011. Since 2012, he has been furthering his research training as a Post-Doctoral Fellow at the University of British Columbia (UBC). Since 2015, he started his endodontic residency at UBC Dentistry. Dr. Wang has authored or co-authored over 50 peer-reviewed publications. His areas of interest include endodontic disinfection, oral biofilms, antibiofilm peptides, and the biomineralization of dental materials.
Dr. Ya Shen is an Associate Professor at the Department of Oral Biological & Medical Sciences at the University of British Columbia (UBC) in Vancouver, Canada. She has published more than 120 papers in peer-reviewed journals. Her main research interests are nickel–titanium instrument fracture mechanics, biofilms, and dental material in endodontics.
Dr. Markus Haapasalo is a Professor and Chair of Endodontics at the Department of Oral Biological & Medical Sciences at the University of British Columbia (UBC) in Vancouver, Canada. He has published over 200 peer-reviewed articles. His main research interests are endodontic disinfection, biofilm models, and anti-biofilm strategies.
Funding Statement
This work was supported by the Canada Foundation for Innovation under [Grant number 32623].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Leung K-P, Crowe TD, Abercrombie JJ, et al. Control of oral biofilm formation by an antimicrobial decapeptide. J Dent Res. 2005;84:1172–11. [DOI] [PubMed] [Google Scholar]
- [2].da Silva BR, de Freitas VA, Nascimento-Neto LG, et al. Antimicrobial peptide control of pathogenic microorganisms of the oral cavity: a review of the literature. Peptides. 2012;36:315–321. [DOI] [PubMed] [Google Scholar]
- [3].Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. [DOI] [PubMed] [Google Scholar]
- [4].Prada-López I, Quintas V, Vilaboa C, et al. Devices for in situ development of non-disturbed oral biofilm. A systematic review. Front Microbiol. 2016;7:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shen Y, Zhao J, de la Fuente-Núñez C, et al. Experimental and theoretical investigation of multispecies oral biofilm resistance to chlorhexidine treatment. Sci Rep. 2016;6:27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gorr S-U. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. [DOI] [PubMed] [Google Scholar]
- [7].Khurshid Z, Naseem M, Sheikh Z, et al. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J. 2016;24:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wimley WC, Hristova K. Antimicrobial peptides: successes, challenges and unanswered questions. J Membr Biol. 2011;239:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fjell CD, Hiss JA, Hancock RE, et al. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11:37–51. [DOI] [PubMed] [Google Scholar]
- [10].de la Fuente-Núñez C, Cardoso MH, de Souza Cândido E, et al. Synthetic antibiofilm peptides. Biochim Biophys Acta. 2016;1858:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pepperney A, Chikindas ML. Antibacterial peptides: opportunities for the prevention and treatment of dental caries. Probiotics Antimicrob Proteins. 2011;3:68–96. [DOI] [PubMed] [Google Scholar]
- [12].Rollema HS, Kuipers OP, Both P, et al. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl Environ Microbiol. 1995;61:2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yoshida T, Nagasawa T. Epsilon-Poly-L-lysine: microbial production, biodegradation and application potential. Appl Microbiol Biotechnol. 2003;62:21–26. [DOI] [PubMed] [Google Scholar]
- [14].Badaoui Najjar M, Kashtanov D, Chikindas ML. Natural antimicrobials ε-Poly-L-lysine and Nisin A for control of oral microflora. Probiotics Antimicrob Proteins. 2009;1:143–147. [DOI] [PubMed] [Google Scholar]
- [15].Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hilpert K, Volkmer-Engert R, Walter T, et al. High-throughput generation of small antibacterial peptides with improved activity. Nat Biotechnol. 2005;23:1008–1012. [DOI] [PubMed] [Google Scholar]
- [17].Silva ON, Alves ES, de la Fuente-Núñez C, et al. Structural studies of a lipid-binding peptide from tunicate hemocytes with anti-biofilm activity. Sci Rep. 2016;6:27128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Abraham P, Sundaram A, Asha R, et al. Structure-activity relationship and mode of action of a frog secreted antibacterial peptide B1CTcu5 using Synthetically and Modularly Modified or Deleted (SMMD) peptides. PLoS One. 2015;10:e0124210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Porat Y, Marynka K, Tam A, et al. Acyl-substituted dermaseptin S4 derivatives with improved bactericidal properties, including on oral microflora. Antimicrob Agents Chemother. 2006;50:4153–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de la Fuente-Núñez C, Reffuveille F, Mansour SC, et al. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 2015;22:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lan Y, Lam JT, Siu GK, et al. Cationic amphipathic D-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against drug-resistant Mycobacterium tuberculosis. Tuberculosis (Edinb). 2014;94:678–689. [DOI] [PubMed] [Google Scholar]
- [22].Kanthawong S, Bolscher JG, Veerman EC, et al. Antimicrobial and antibiofilm activity of LL-37 and its truncated variants against Burkholderia pseudomallei. Int J Antimicrob Agents. 2012;39:39–44. [DOI] [PubMed] [Google Scholar]
- [23].Kanthawong S, Bolscher JG, Veerman EC, et al. Antimicrobial activities of LL-37 and its truncated variants against Burkholderia thailandensis. Int J Antimicrob Agents. 2010;36:447–452. [DOI] [PubMed] [Google Scholar]
- [24].Nagant C, Pitts B, Nazmi K, et al. Identification of peptides derived from the human antimicrobial peptide LL-37 active against biofilms formed by Pseudomonas aeruginosa using a library of truncated fragments. Antimicrob Agents Chemother. 2012;56:5698–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shin SY, Lee MK, Kim KL, et al. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J Pept Res. 1997;50:279–285. [DOI] [PubMed] [Google Scholar]
- [26].Saugar JM, Rodríguez-Hernández MJ, de la Torre BG, et al. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother. 2006;50:1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alarcón T, López-Hernández S, Andreu D, et al. In vitro activity of CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide, against multiresistant Acinetobacter baumannii strains. Rev Esp Quimioter. 2001;14:184–190. [PubMed] [Google Scholar]
- [28].Park Y, Lee DG, Hahm K-S. HP(2-9)-magainin 2 (1-12),a synthetic hybrid peptide, exerts its antifungal effect on Candida albicans by damaging the plasma membrane. J Pept Sci. 2004;10:204–209. [DOI] [PubMed] [Google Scholar]
- [29].Jenssen H, Fjell CD, Cherkasov A, et al. QSAR modeling and computer-aided design of antimicrobial peptides. J Pept Sci. 2008;14:110–114. [DOI] [PubMed] [Google Scholar]
- [30].Cherkasov A, Hilpert K, Jenssen H, et al. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem Biol. 2009;4:65–74. [DOI] [PubMed] [Google Scholar]
- [31].Sang Y, Blecha F. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev. 2008;9:227–235. [DOI] [PubMed] [Google Scholar]
- [32].Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J. 2011;40:387–397. [DOI] [PubMed] [Google Scholar]
- [33].Jorge P, Lourenço A, Pereira MO. New trends in peptide-based anti-biofilm strategies: a review of recent achievements and bioinformatic approaches. Biofouling. 2012;28:1033–1061. [DOI] [PubMed] [Google Scholar]
- [34].Rapaport D, Shai Y. Interaction of fluorescently labeled pardaxin and its analogues with lipid bilayers. J Biol Chem. 1991;266:23769–23775. [PubMed] [Google Scholar]
- [35].Ludtke SJ, He K, Heller WT, et al. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. [DOI] [PubMed] [Google Scholar]
- [36].Gazit E, Miller IR, Biggin PC, et al. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J Mol Biol. 1996;258:860–870. [DOI] [PubMed] [Google Scholar]
- [37].Ostolaza H, Bartolomé B, Ortiz de Zárate I, et al. Release of lipid vesicle contents by the bacterial protein toxin alpha-haemolysin. Biochim Biophys Acta. 1993;1147:81–88. [DOI] [PubMed] [Google Scholar]
- [38].Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1529–1539. [DOI] [PubMed] [Google Scholar]
- [39].Bagheri M, Beyermann M, Dathe M. Mode of action of cationic antimicrobial peptides defines the tethering position and the efficacy of biocidal surfaces. Bioconjug Chem. 2012;23:66–74. [DOI] [PubMed] [Google Scholar]
- [40].Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. [DOI] [PubMed] [Google Scholar]
- [41].Kraus D, Deschner J, Jäger A, et al. Human beta-defensins differently affect proliferation, differentiation, and mineralization of osteoblast-like MG63 cells. J Cell Physiol. 2012;227:994–1003. [DOI] [PubMed] [Google Scholar]
- [42].Sone E, Stupp S. Bioinspired magnetite mineralization of peptide−amphiphile nanofibers. Chem Mater. 2011;23:2005–2007. [Google Scholar]
- [43].Wang Y, Cao L, Guan S, et al. Silver mineralization on self-assembled peptide nanofibers for long term antimicrobial effect. J Mater Chem. 2012;22:2575–2581. [Google Scholar]
- [44].Supanchart C, Thawanaphong S, Makeudom A, et al. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dent Res. 2012;91:1071–1077. [DOI] [PubMed] [Google Scholar]
- [45].Horibe K, Nakamichi Y, Uehara S, et al. Roles of cathelicidin-related antimicrobial peptide in murine osteoclastogenesis. Immunology. 2013;140:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kurinami H, Shimamura M, Nakagami H, et al. A novel therapeutic peptide as a partial agonist of RANKL in ischemic stroke. Sci Rep. 2016;6:38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31 Suppl 1:3–23. [DOI] [PubMed] [Google Scholar]
- [48].Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Beckloff N, Laube D, Castro T, et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 2007;51:4125–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leung V, Dufour D, Lévesque CM. Death and survival in Streptococcus mutans: differing outcomes of a quorum-sensing signaling peptide. Front Microbiol. 2015;6:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang W, Tao R, Tong Z, et al. Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides. 2012;33:212–219. [DOI] [PubMed] [Google Scholar]
- [52].da Silva BR, de Freitas VA, Carneiro VA, et al. Antimicrobial activity of the synthetic peptide Lys-a1 against oral streptococci. Peptides. 2013;42:78–83. [DOI] [PubMed] [Google Scholar]
- [53].Tu H, Fan Y, Lv X, et al. Activity of synthetic antimicrobial peptide GH12 against oral Streptococci. Caries Res. 2016;50:48–61. [DOI] [PubMed] [Google Scholar]
- [54].Leung K-P, Abercrombie JJ, Campbell TM, et al. Antimicrobial peptides for plaque control. Adv Dent Res. 2009;21:57–62. [DOI] [PubMed] [Google Scholar]
- [55].Concannon SP, Crowe TD, Abercrombie JJ, et al. Susceptibility of oral bacteria to an antimicrobial decapeptide. J Med Microbiol. 2003;52:1083–1093. [DOI] [PubMed] [Google Scholar]
- [56].Liu Y, Wang L, Zhou X, et al. Effect of the antimicrobial decapeptide KSL on the growth of oral pathogens and Streptococcus mutans biofilm. Int J Antimicrob Agents. 2011;37:33–38. [DOI] [PubMed] [Google Scholar]
- [57].Na DH, Faraj J, Capan Y, et al. Chewing gum of antimicrobial decapeptide (KSL) as a sustained antiplaque agent: preformulation study. J Control Release. 2005;107:122–130. [DOI] [PubMed] [Google Scholar]
- [58].Na DH, Faraj J, Capan Y, et al. Stability of antimicrobial decapeptide (KSL) and its analogues for delivery in the oral cavity. Pharm Res. 2007;24:1544–1550. [DOI] [PubMed] [Google Scholar]
- [59].Shang D, Liang H, Wei S, et al. Effects of antimicrobial peptide L-K6, a temporin-1CEb analog on oral pathogen growth, Streptococcus mutans biofilm formation, and anti-inflammatory activity. Appl Microbiol Biotechnol. 2014;98:8685–8695. [DOI] [PubMed] [Google Scholar]
- [60].Dobson A, O’Connor PM, Cotter PD, et al. Impact of the broad-spectrum antimicrobial peptide, lacticin 3147, on Streptococcus mutans growing in a biofilm and in human saliva. J Appl Microbiol. 2011;111:1515–1523. [DOI] [PubMed] [Google Scholar]
- [61].Chen X, Hirt H, Li Y, et al. Antimicrobial GL13K peptide coatings killed and ruptured the wall of Streptococcus gordonii and prevented formation and growth of biofilms. PLoS One. 2014;9:e111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Okuda K, Hanada N, Usui Y, et al. Inhibition of Streptococcus mutans adherence and biofilm formation using analogues of the SspB peptide. Arch Oral Biol. 2010;55:754–762. [DOI] [PubMed] [Google Scholar]
- [63].Ding Y, Wang W, Fan M, et al. Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides. 2014;52:61–67. [DOI] [PubMed] [Google Scholar]
- [64].Wei GX, Campagna AN, Bobek LA. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother. 2006;57:1100–1109. [DOI] [PubMed] [Google Scholar]
- [65].Mazda Y, Kawada-Matsuo M, Kanbara K, et al. Association of CiaRH with resistance of Streptococcus mutans to antimicrobial peptides in biofilms. Mol Oral Microbiol. 2012;27:124–135. [DOI] [PubMed] [Google Scholar]
- [66].Qi F, Kreth J, Lévesque CM, et al. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol Lett. 2005;251:321–326. [DOI] [PubMed] [Google Scholar]
- [67].Moye ZD, Son M, Rosa-Alberty AE, et al. Effects of carbohydrate source on genetic competence in streptococcus mutans. Appl Environ Microbiol. 2016;82:4821–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tamura S, Yonezawa H, Motegi M, et al. Inhibiting effects of Streptococcus salivarius on competence-stimulating peptide-dependent biofilm formation by Streptococcus mutans. Oral Microbiol Immunol. 2009;24:152–161. [DOI] [PubMed] [Google Scholar]
- [69].Li L-N, Guo L-H, Lux R, et al. Targeted antimicrobial therapy against Streptococcus mutans establishes protective non-cariogenic oral biofilms and reduces subsequent infection. Int J Oral Sci. 2010;2:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jakubovics NS, Kolenbrander PE. The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 2010;16:729–739. [DOI] [PubMed] [Google Scholar]
- [71].Helmerhorst EJ, Hodgson R, van ‘t Hof W, et al. The effects of histatin-derived basic antimicrobial peptides on oral biofilms. J Dent Res. 1999;78:1245–1250. [DOI] [PubMed] [Google Scholar]
- [72].Bernegossi J, Calixto GM, Sanches PR, et al. Peptide KSL-W-loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules. 2015;21:E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang Z, de la Fuente-Núñez C, Shen Y, et al. Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One. 2015;10:e0132512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nair PN. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39:249–281. [DOI] [PubMed] [Google Scholar]
- [75].Haapasalo M, Endal U, Zandi H, et al. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics. 2005;10:77–102. [Google Scholar]
- [76].Shen Y, Stojicic S, Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod. 2011;37:657–661. [DOI] [PubMed] [Google Scholar]
- [77].Dunavant TR, Regan JD, Glickman GN, et al. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 2006;32:527–531. [DOI] [PubMed] [Google Scholar]
- [78].Kristich CJ, Li Y-H, Cvitkovitch DG, et al. Esp-independent biofilm formation by Enterococcus faecalis. J Bacteriol. 2004;186:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tendolkar PM, Baghdayan AS, Gilmore MS, et al. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:6032–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shen Y, Stojicic S, Qian W, et al. The synergistic antimicrobial effect by mechanical agitation and two chlorhexidine preparations on biofilm bacteria. J Endod. 2010;36:100–104. [DOI] [PubMed] [Google Scholar]
- [81].Liu H, Wei X, Ling J, et al. Biofilm formation capability of Enterococcus faecalis cells in starvation phase and its susceptibility to sodium hypochlorite. J Endod. 2010;36:630–635. [DOI] [PubMed] [Google Scholar]
- [82].Peciuliene V, Balciuniene I, Eriksen HM, et al. Isolation of Enterococcus faecalis in previously root-filled canals in a Lithuanian population. J Endod. 2000;26:593–595. [DOI] [PubMed] [Google Scholar]
- [83].Chávez de Paz LE, Bergenholtz G, Svensäter G. The effects of antimicrobials on endodontic biofilm bacteria. J Endod. 2010;36:70–77. [DOI] [PubMed] [Google Scholar]
- [84].Lee J-K, Park Y-J, Kum K-Y, et al. Antimicrobial efficacy of a human β-defensin-3 peptide using an Enterococcus faecalis dentine infection model. Int Endod J. 2013;46:406–412. [DOI] [PubMed] [Google Scholar]
- [85].Eckhard LH, Sol A, Abtew E, et al. Biohybrid polymer-antimicrobial peptide medium against Enterococcus faecalis. PLoS One. 2014;9:e109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhang T, Wang Z, Hancock RE, et al. Treatment of oral biofilms by a D-enantiomeric peptide. PLoS One. 2016;11:e0166997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lee J-K, Chang SW, Perinpanayagam H, et al. Antibacterial efficacy of a human beta-defensin-3 peptide on multispecies biofilms. J Endod. 2013;39:1625–1629. [DOI] [PubMed] [Google Scholar]
- [88].Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–1379. [DOI] [PubMed] [Google Scholar]
- [89].Haapasalo HK, Sirén EK, Waltimo TM, et al. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J. 2000;33:126–131. [DOI] [PubMed] [Google Scholar]
- [90].Portenier I, Haapasalo H, Rye A, et al. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001;34:184–188. [DOI] [PubMed] [Google Scholar]
- [91].Wang Z, Shen Y, Haapasalo M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2012;38:1376–1379. [DOI] [PubMed] [Google Scholar]
- [92].Ma J, Wang Z, Shen Y, et al. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37:1380–1385. [DOI] [PubMed] [Google Scholar]
- [93].Gorr S-U. Antimicrobial peptides in periodontal innate defense. Front Oral Biol. 2012;15:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Godoy-Gallardo M, Mas-Moruno C, Yu K, et al. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: a comparison study between silanization and surface initiated polymerization. Biomacromolecules. 2015;16:483–496. [DOI] [PubMed] [Google Scholar]
- [95].Gorr S-U, Abdolhosseini M. Antimicrobial peptides and periodontal disease. J Clin Periodontol. 2011;38 Suppl 11:126–141. [DOI] [PubMed] [Google Scholar]
- [96].Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. [DOI] [PubMed] [Google Scholar]
- [97].Sumida S, Ishihara K, Kishi M, et al. Transmission of periodontal disease-associated bacteria from teeth to osseointegrated implant regions. Int J Oral Maxillofac Implants. 2002;17:696–702. [PubMed] [Google Scholar]
- [98].Kazemzadeh-Narbat M, Kindrachuk J, Duan K, et al. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials. 2010;31:9519–9526. [DOI] [PubMed] [Google Scholar]
- [99].Ma M, Kazemzadeh-Narbat M, Hui Y, et al. Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. J Biomed Mater Res A. 2012;100:278–285. [DOI] [PubMed] [Google Scholar]
- [100].Kazemzadeh-Narbat M, Lai BF, Ding C, et al. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials. 2013;34:5969–5977. [DOI] [PubMed] [Google Scholar]
- [101].Yoshinari M, Kato T, Matsuzaka K, et al. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling. 2010;26:103–110. [DOI] [PubMed] [Google Scholar]
- [102].Liu Z, Ma S, Duan S, et al. Modification of titanium substrates with chimeric peptides comprising antimicrobial and titanium-binding motifs connected by linkers to inhibit biofilm formation. ACS Appl Mater Interfaces. 2016;8:5124–5136. [DOI] [PubMed] [Google Scholar]
- [103].Yazici H, O’Neill MB, Kacar T, et al. Engineered chimeric peptides as antimicrobial surface coating agents toward infection-free implants. ACS Appl Mater Interfaces. 2016;8:5070–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yucesoy DT, Hnilova M, Boone K, et al. Chimeric peptides as implant functionalization agents for titanium alloy implants with antimicrobial properties. JOM (1989). 2015;67:754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Choi JH, Park YW, Park TH, et al. Fuzzy nanoassembly of polyelectrolyte and layered clay multicomposite toward a reliable gas barrier. Langmuir. 2012;28:6826–6831. [DOI] [PubMed] [Google Scholar]
- [106].Shi J, Liu Y, Wang Y, et al. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci Rep. 2015;5:16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Costa F, Carvalho IF, Montelaro RC, et al. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7:1431–1440. [DOI] [PubMed] [Google Scholar]
- [108].Onaizi SA, Leong SS. Tethering antimicrobial peptides: current status and potential challenges. Biotechnol Adv. 2011;29:67–74. [DOI] [PubMed] [Google Scholar]
- [109].Holmberg KV, Abdolhosseini M, Li Y, et al. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013;9:8224–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Godoy-Gallardo M, Wang Z, Shen Y, et al. Antibacterial coatings on titanium surfaces: a comparison study between in vitro single-species and multispecies biofilm. ACS Appl Mater Interfaces. 2015;7:5992–6001. [DOI] [PubMed] [Google Scholar]
- [111].Godoy-Gallardo M, Mas-Moruno C, Fernández-Calderón MC, et al. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014;10:3522–3534. [DOI] [PubMed] [Google Scholar]
- [112].Chastre J. Evolving problems with resistant pathogens. Clin Microbiol Infect. 2008;14 Suppl 3:3–14. [DOI] [PubMed] [Google Scholar]
- [113].Hua J, Scott RW, Diamond G. Activity of antimicrobial peptide mimetics in the oral cavity: II. Activity against periopathogenic biofilms and anti-inflammatory activity. Mol Oral Microbiol. 2010;25:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Güncü GN, Yilmaz D, Könönen E, et al. Salivary antimicrobial peptides in early detection of periodontitis. Front Cell Infect Microbiol. 2015;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lappin DF, Murad M, Sherrabeh S, et al. Increased plasma levels epithelial cell-derived neutrophil-activating peptide 78/CXCL5 in periodontitis patients undergoing supportive therapy. J Clin Periodontol. 2011;38:887–893. [DOI] [PubMed] [Google Scholar]
- [116].Wang H-Y, Cheng J-W, Yu H-Y, et al. Efficacy of a novel antimicrobial peptide against periodontal pathogens in both planktonic and polymicrobial biofilm states. Acta Biomater. 2015;25:150–161. [DOI] [PubMed] [Google Scholar]
- [117].Daep CA, Novak EA, Lamont RJ, et al. Selective substitution of amino acids limits proteolytic cleavage and improves the bioactivity of an anti-biofilm peptide that targets the periodontal pathogen, Porphyromonas gingivalis. Peptides. 2010;31:2173–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wilensky A, Potempa J, Houri-Haddad Y, et al. Vaccination with recombinant RgpA peptide protects against Porphyromonas gingivalis-induced bone loss. J Periodontal Res. 2017;52:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Diamond G, Beckloff N, Weinberg A, et al. The roles of antimicrobial peptides in innate host defense. Curr Pharm Des. 2009;15:2377–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ryan LK, Freeman KB, Masso-Silva JA, et al. Activity of potent and selective host defense peptide mimetics in mouse models of oral candidiasis. Antimicrob Agents Chemother. 2014;58:3820–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Komatsu T, Salih E, Helmerhorst EJ, et al. Influence of histatin 5 on Candida albicans mitochondrial protein expression assessed by quantitative mass spectrometry. J Proteome Res. 2011;10:646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sun JN, Li W, Jang WS, et al. Uptake of the antifungal cationic peptide Histatin 5 by Candida albicans Ssa2p requires binding to non-conventional sites within the ATPase domain. Mol Microbiol. 2008;70:1246–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kong EF, Tsui C, Boyce H, et al. Development and in vivo evaluation of a novel histatin-5 bioadhesive hydrogel formulation against oral candidiasis. Antimicrob Agents Chemother. 2016;60:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Xu T, Levitz SM, Diamond RD, et al. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hua J, Yamarthy R, Felsenstein S, et al. Activity of antimicrobial peptide mimetics in the oral cavity: I. Activity against biofilms of Candida albicans. Mol Oral Microbiol. 2010;25:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Pusateri CR, Monaco EA, Edgerton M. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch Oral Biol. 2009;54:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lim S-M, Ahn K-B, Kim C, et al. Antifungal effects of synthetic human beta-defensin 3-C15 peptide. Restor Dent Endod. 2016;41:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Baltzer SA, Brown MH. Antimicrobial peptides: promising alternatives to conventional antibiotics. J Mol Microbiol Biotechnol. 2011;20:228–235. [DOI] [PubMed] [Google Scholar]
- [129].Batoni G, Maisetta G, Brancatisano FL, et al. Use of antimicrobial peptides against microbial biofilms: advantages and limits. Curr Med Chem. 2011;18:256–279. [DOI] [PubMed] [Google Scholar]


