Abstract
In this pilot study, 10 dogs with osteosarcoma (OSA) were treated with amputation and subsequent carboplatin chemotherapy (300 mg/m2 IV q3wk × 4 doses) followed by toceranib phosphate (2.75 mg/kg PO q48h starting at day 14 post carboplatin). Monthly clinical monitoring and serum measurements of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9) were acquired. No dogs were removed from the study due to toxicity. Levels of VEGF and MMP-9 did not change over time. Seven dogs died related to local recurrence and/or pulmonary or bone metastasis and the remainder died of other causes. Median OSA-free survival was 238 d with 34% 1-year progression-free survival. Median overall survival was 253 d with 30% alive at 1.5 y and 10% alive at 2 y. Although this regimen was well-tolerated, survival times did not exceed previously published data from dogs treated with amputation plus chemotherapy alone.
Résumé
Dans cette étude pilote, 10 chiens avec un ostéosarcome (OSA) ont été traités par amputation et chimiothérapie subséquente avec du carboplatin (300 mg/m2 IV q3sem × 4 doses) suivi de phosphate de toceranib (2,75 mg/kg PO q48h débutant au jour 14 suivant le carboplatin). Un suivi clinique mensuel et une mesure des taux sériques du facteur de croissance de l’endothélium vasculaire (FCEV) et de la matrice de la métalloprotéinase-9 (MMP-9) ont été obtenus. Aucun chien ne fut retiré de l’étude pour cause de toxicité. Les niveaux de FCEV et MMP-9 n’ont pas changé dans le temps. Sept chiens sont décédés dues à une rechute locale et/ou des métastases osseuses et les autres sont morts d’autres causes. La durée médiane de survie libre d’OSA était de 238 j avec 34 % de survie sans progression de la condition après 1 an. La durée de survie médiane était de 253 j avec 30 % en vie après 1,5 an et 10 % en vie après 2 ans. Bien que ce traitement ait été bien toléré, les temps de survie n’ont pas excédé les résultats publiés antérieurement pour des chiens traités par amputation et chimiothérapie uniquement.
(Traduit par Docteur Serge Messier)
Introduction
An estimated 10 000 cases of canine osteosarcoma (OSA) are diagnosed in pet dogs annually in the United States (1). It is a locally invasive and highly metastatic disease, with micrometastasis present in approximately 75% to 95% of dogs at the time of diagnosis, based on studies of survival following amputation (2,3). While surgery is indicated to remove the painful and functionally limiting primary tumor, amputation is considered palliative therapy and does not result in prolonged survival. Median survival times following amputation alone range from 102 to 168 d, with 1- and 2-year survival rates of 11% to 21% and 2% to 4%, respectively. The administration of adjuvant chemotherapy in an effort to delay or prevent metastasis and improve the outcome, is the current standard of care in veterinary medicine. Three drugs have been identified as having significant activity against osteosarcoma: cisplatin, carboplatin, and doxorubicin (2–4). Median survival times ranging from 290 to 324 d and 1-year survival rates of 45.5% have been reported with the administration of adjuvant cisplatin chemotherapy (3,4). Carboplatin chemotherapy is comparable, with a median survival of 321 d and 1-year survival of 35.4% (5). Similarly, the median survival time following a course of doxorubicin chemotherapy is 366 d with 1- and 2-year survival rates estimated at 50.5% and 9.7%, respectively (6).
A variety of strategies have been attempted in an effort to improve outcomes with chemotherapy. These include altering the time at which chemotherapy is initiated and using cisplatin or carboplatin in combination with doxorubicin (7,8). Unfortunately, these efforts have failed to demonstrate significant improvements in median survival times over those seen with conventional single-agent treatment protocols. Novel therapeutic approaches are needed to control metastatic disease and improve survival.
Angiogenesis is the process by which tumors induce new blood vessel formation and is essential for continued tumor growth and metastasis. In the absence of angiogenesis, tumors are restricted to sizes ranging from several microns up to 1 to 2 mm (9). Endogenous angiogenesis inhibitors have been detected in the urine of OSA tumor-bearing dogs and these factors demonstrated potent inhibition of endothelial cell proliferation (10). These endogenous factors were absent in urine collected from the same dogs 1 to 12 wk after surgical amputation of the limb bearing the primary tumor. The pro-angiogenic shift that occurs after primary tumor removal suggests that a strategy designed to pharmacologically control angiogenesis should be evaluated for its capacity to suppress the rapid progression of microscopic metastases to life-threatening metastatic disease. The use of angiogenesis inhibitors has emerged as a clinically valid therapeutic approach in human oncology. This strategy may also have applicability to veterinary oncology, and is particularly appealing for OSA where death is the result of progressive enlargement of metastatic foci.
Anti-angiogenic treatment strategies fall into 2 categories: i) those that are directly cytotoxic to endothelial cells, and ii) those acting indirectly by eliminating critical pro-angiogenic factors [e.g., vascular endothelial growth factor (VEGF)] and promoting anti-angiogenic factors. Vascular endothelial growth factor is a 45-kDa homodimer with pluripotent effects in angiogenesis — stimulating the proliferation, invasion, and migration of endothelial cells. In addition, VEGF is a key target of several angiogenic factors and proteases such as matrix metalloproteinases (MMPs), with roles in tumor metastasis (11). Matrix metalloproteinases are enzymes that degrade structural elements outside of cells and play a critical role in tissue remodeling during tumor invasion, angiogenesis, and metastasis. Among MMPs, MMP-9 is of particular interest. Matrix metalloproteinase-9 is induced via VEGF receptor (VEGF-R) activation and levels of this downstream factor may be useful as a surrogate marker of VEGF-R activity (12); however, there have been no studies directly correlating serum MMP-9 levels with VEGF-R activity in serum or tissues of OSA-bearing dogs.
Vascular endothelial growth factor is expressed by a variety of tumors, and therapies designed to decrease production or otherwise block VEGF activity are being explored as a means of inhibiting tumor proliferation. In an evaluation of 24 dogs with OSA, pretreatment platelet-corrected serum VEGF levels correlated significantly with disease-free interval (13). Results of this study and similar human studies support an important role for VEGF in the development and progression of metastatic disease in OSA. We hypothesize that the inhibition of VEGF activity will delay the progression of OSA micrometastases.
Toceranib phosphate [TP (Palladia; Zoetis, Durham, North Carolina, USA)], an FDA-approved drug for use in dogs, is a selective inhibitor of several members of the receptor tyrosine kinase family, including vascular endothelial growth factor receptor (VEGF-R), KIT, FLT-3, and platelet-derived growth factor receptor (PDGF-R). c-KIT wild type receptor overexpression was identified in a study of 74 human pediatric high-grade OSA, indicating a potential role of the inhibition of KIT signaling in OSA (14). While initially developed for its activity against KIT — a receptor mutated in approximately 30% to 50% of canine mast cell tumors (MCT) — the activity of TP against other members of this receptor tyrosine kinase family, such as VEGF-R, suggests a potentially wider spectrum of activity. Anti-tumor responses are reported in MCT lacking a KIT mutation, providing support for this theory and suggesting that other inhibited receptors, such as VEGF-R, play an important role in tumor progression and regression (15). Toceranib phosphate exerts antiproliferative effects on endothelial cells in vitro, suggesting a role in angiogenesis, and produces clinically meaningful disease stabilization in vivo in dogs with osteosarcoma (16–18). As part of a larger study of dogs with solid tumors being treated with TP, 11/23 (48%) dogs with metastatic OSA had stabilization of their disease (18). The oral dosing of TP on an alternate-day schedule ideally lends itself to chronic inhibition of tumor-promoted angiogenesis to prevent the progression of OSA micrometastases.
The goals of this study are: i) to evaluate the impact of maintenance antiangiogenic therapy using TP as a sole agent following amputation and carboplatin chemotherapy as a means of improving outcome for dogs with OSA, and ii) to measure circulating levels of endogenous pro-angiogenic factors before and during treatment with TP, which may serve as biomarkers for survival and disease progression. We hypothesize that the administration of TP following amputation and carboplatin chemotherapy will inhibit the progression of metastasis through disruption of receptor tyrosine kinases with subsequent inhibition of angiogenesis. This will lead to prolonged remission duration and survival in dogs with appendicular OSA compared to rates previously achieved in a group of dogs treated with amputation and carboplatin alone (5). We hypothesize that the administration of TP following amputation and carboplatin chemotherapy will decrease circulating levels of pro-angiogenic MMP-9, a downstream factor and potential surrogate marker of VEGF-R inhibition in dogs with appendicular OSA.
Materials and methods
Ten dogs with histologically confirmed appendicular OSA with no radiographic evidence of pulmonary metastasis at the time of enrollment and for whom owner consent was given, were prospectively enrolled. Institutional IACUC approval was obtained. All dogs were evaluated with a physical examination, complete blood (cell) count (CBC), serum biochemistry panel, urinalysis, 3-view thoracic radiographs, and a quality-of-life (QOL) survey completed by the owner prior to treatment. Two weeks following amputation, carboplatin (Paraplatin; Bristol Myers Squibb, New York, New York, USA) chemotherapy was administered intravenously at a dosage of 300 mg/m2 at 3-week intervals for a total of 4 treatments. A CBC was performed immediately before each carboplatin treatment and 7 d and 14 d following the first 2 treatment cycles to monitor for potential hematologic toxicity. A 25% dosage reduction was prescribed for any patient developing grade 3 or higher bone marrow or gastrointestinal toxicity as defined by the Veterinary Co-operative Oncology Group Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v1.0 (19). Three-view thoracic radiographs were repeated prior to the third carboplatin chemotherapy treatment and immediately before initiation of TP. Two weeks following the final carboplatin chemotherapy treatment, patients began TP therapy at a dosage of 2.75 mg/kg body weight (BW), PO, q48h. This drug was to be continued indefinitely unless evidence of toxicity, development of concurrent co-morbidities, death, or owner withdrawal of the pet from the study, occurred. Dogs that developed local recurrence or metastasis were censored from statistical analysis but were allowed to continue to receive TP if the owner elected to do so; owners were also counseled about all other potential treatment options outside of the study. To minimize the likelihood of toxicity, all dogs received omeprazole (Prilosec 0.5 to 1.0 mg/kg BW, PO, q24h; AstraZeneca Pharmaceuticals, Stockholm, Sweden) or famotidine (Pepcid 0.5 to 1.0 mg/kg BW, PO, q12-24h; McNeil Consumer Pharmaceuticals, Fort Washington, Pennsylvania, USA) and, if needed, metoclo-pramide (0.2 to 0.4 mg/kg BW, PO, q8h) and/or maropitant (Cerenia 2 mg/kg BW, PO, q24h × consecutive 5 d each week; Zoetis, Parsippany-Troy Hills, New Jersey, USA). To avoid confounding variables, NSAIDs were discontinued for the duration of the study period. Patients requiring ongoing analgesia received tramadol (1 to 4 mg/kg BW, PO, q6-12h), amantadine (3 to 5 mg/kg BW, PO, q24h), and/or gabapentin (5 to 10 mg/kg BW, PO, q8-12h).
Sample collection
Serum samples for enzyme-linked immunosorbent assay (ELISA) evaluation of VEGF and MMP-9 were collected for analysis: i) immediately before initiation of carboplatin chemotherapy, ii) immediately prior to initiation of TP, and iii) 3 and 6 wk after starting TP, and iv) every 8 wk thereafter for 18 mo or until the time of disease progression if this occurred at less than 18 mo.
Patient monitoring
Disease status was monitored by a physical examination and 3-view thoracic radiographs at 8-week intervals until disease progression was documented. To monitor for potential TP-related toxicity, CBC and body weight were evaluated weekly for the first 6 wk and a chemistry panel and urinalysis were also performed at weeks 1, 3, and 6. Subsequently, a CBC, body weight, chemistry panel, and urinalysis were performed at 8-week intervals. Quality-of-life surveys were also submitted by pet owners at each of these time points. Toxicity was recorded at each time point. A 1-week cessation of TP therapy followed by incremental dosage reductions (0.5 mg/kg BW increments to a minimum of 2.2 mg/kg BW) and/or decreased TP administration frequency (from q48h to Monday, Wednesday, and Friday schedule) was instituted for any dog experiencing > grade 2 adverse event(s).
Sample analysis
Approximately 7 to 10 mL of whole blood was collected into serum and plasma separator tubes by jugular venipuncture. Immediately after collection, blood samples were centrifuged and the serum frozen at −70°C until assayed. Commercially available ELISA test kits were used for VEGF (CAVEOO Canine VEGF Quantikine ELISA Kit; R&D Systems, Minneapolis, Minnesota, USA) and MMP-9 (SEA553CA ELISA Kit for MMP9 Organism species; Cedarlane Labs) and tests were performed according to the manufacturers’ directions. To minimize the impact that VEGF released from platelets contributed to serum VEGF levels, serum VEGF levels were corrected for platelet counts as follows: serum VEGF (pg/mL)/platelet count (× 106/μL) (20,21).
Statistical analysis
A sample size of 10 dogs provides a power of 80% and a 50% level of significance to detect a 47% increase in 1-year survival (i.e., increase the probability of survival at 1 y, from 34.5% to 50.7%) in dogs with appendicular OSA treated with carboplatin chemotherapy followed by TP following amputation. The historical control consisted of a previously reported group of 48 dogs treated with amputation and 4 cycles of carboplatin chemotherapy with a median survival of 321 d and 1-year survival of 34.5% (5). Osteosarcoma-free survival time was defined as the date of amputation to the date of documentation of local recurrence or OSA metastasis. Overall survival (OS) was defined as the date of amputation until death from any cause. A commercially available statistical program was used (GraphPad Prism 6; San Diego, California, USA). The Kaplan-Meier method was used to calculate OSA-free survival and overall survival (OS). A Friedman’s test was used to compare VEGF and MMP values at baseline, pre-TP, and end of study. A Kruskal-Wallis test of repeated measures was used to compare platelet-corrected VEGF values at baseline, pre-TP, and end of study. P < 0.1 was considered significant. A P-value = 0.1 was selected due to the small sample size to try to avoid type II error.
Results
Median age was 9.5 y (range: 5 to 11 y). Median body weight was 31 kg (range: 19 to 48 kg). Seven were spayed females and 3 were castrated males. There were 3 golden retrievers, 1 mixed breed dog, and 1 of each of the following breeds: Belgian tervuren, great Dane, border collie, Rottweiler, Labrador retriever, and St. Bernard. Tumor locations included the distal radius (n = 5), distal femur (n = 2), distal tibia (n = 2), and proximal humerus (n = 1). No patients had CBC abnormalities prior to treatment. Serum biochemical abnormalities included increased alkaline phosphatase in 2 dogs [726 and 164 IU/L (normal < 140)] and in one dog, increased BUN [67 mg/dL (normal < 26)], creatinine [3 mg/dL (normal < 1.5 mg/dL)], phosphorous [6.1 mg/dL (normal < 5.6 mg/dL)], and potassium [5.8 mmol/L (normal < 5.6 mmol/L)] (in this dog, the urine specific gravity was 1.018). Seven dogs were treated with NSAIDs prior to being enrolled in the study for a variable duration of time; these drugs were discontinued on the first day of chemotherapy in 3 dogs and 3 to 35 d prior to initiation of chemotherapy in the remaining dogs.
Chemotherapy administration
Carboplatin chemotherapy was started a median of 16 d post-amputation (range: 11 to 30 d). Eight dogs received the first dose of carboplatin at 300 mg/m2; 1 dog received 239 mg/m2 (unknown reason; given at RDVM) and the dog with renal insufficiency received 150 mg/m2 (arbitrary dose reduction due to renal insufficiency). Four dogs required dose reductions of carboplatin due to neutropenia; no dogs required hospitalization for supportive care. The carboplatin dose was reduced from 300 mg/m2 to 225 mg/m2 after the first dose in 3 dogs and after the second dose in 1 dog (who also had a 1-week treatment delay due to prolonged neutropenia).
Toceranib phosphate administration
Eight dogs received TP at doses ranging from 2.2 to 2.9 mg/kg (median dose: 2.7 mg/kg). After starting TP, 1 dog had two 1-week drug holidays related to grade I diarrhea and 1 dog had dose reductions and an extended dosing interval due to persistent, non-progressive grade I neutropenia.
Toxicity outcomes
No dogs were removed from the study due to toxicity or declining QOL. Quality-of-life surveys were subjectively scored, and scores remained stable throughout the study (scores did not decline from baseline after starting carboplatin or TP) until the patient experienced a clinical decline typically related to disease progression.
Tumor-related outcomes
Seven dogs developed metastasis as the first event. Pulmonary metastasis was suspected based on thoracic radiographs in 6 dogs 60 to 240 d post-amputation (median 180 d) and osseous metastasis to the vertebrae and ribs was suspected in one dog based on radiographs at 575 d after amputation. One dog that developed pulmonary metastasis also concurrently developed an amputation-site stump recurrence 180 d post-amputation. Two of 10 (20%) dogs developed pulmonary metastasis while receiving carboplatin chemotherapy and did not continue on to the TP phase of the study. These 2 dogs were included in outcome analysis on an intent-to-treat basis. Of the 8 dogs that received TP, 3 died of metastasis (pulmonary in 2 dogs, osseous in 1 dog), 1 died of concurrent pulmonary metastasis and stump recurrence, 3 died of other causes [lymphoma that was diagnosed during the study while the dog was receiving TP (n = 1), metastatic hemangiosarcoma that developed while the dog was receiving TP (n = 1), and progressive spinal pain with no definitive diagnosis despite and extensive workup (n = 1)] and 1 dog was withdrawn from the study per the owner’s request with no evidence of OSA (n = 1). Toceranib phosphate was continued in 3 dogs at the owners’ request after metastasis was detected (dogs were censored from analysis on the date metastasis was diagnosed) and these dogs remained on the drug until death. An additional dog received treatment with doxorubicin after pulmonary metastasis was detected. All dogs were followed until the time of their death and all are deceased. Only 1 dog underwent necropsy.
Survival data
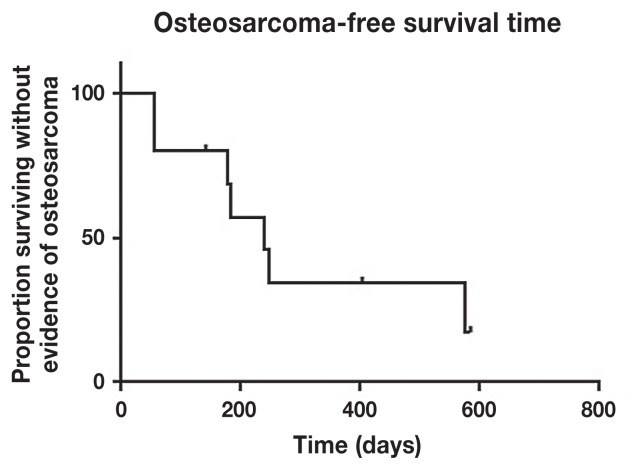
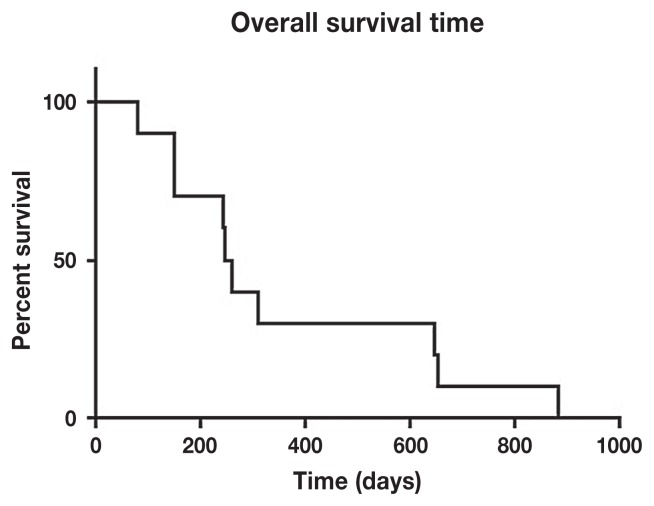
The median OSA-free survival was 238 d (range: 50 to 575 d) with 34% progression-free survival at 1 y (Figure 1). Overall survival was 253 d with 30% alive at 1.5 y and 10% alive at 2 y (Figure 2).
Figure 1.
Median osteosarcoma-free survival time was 238 days with 34.3% event-free at 1 year.
Figure 2.
Median overall survival time was 253.5 days with 30% alive at 1.5 years and 10% alive at 2 years.
VEGF and MMP-9 data
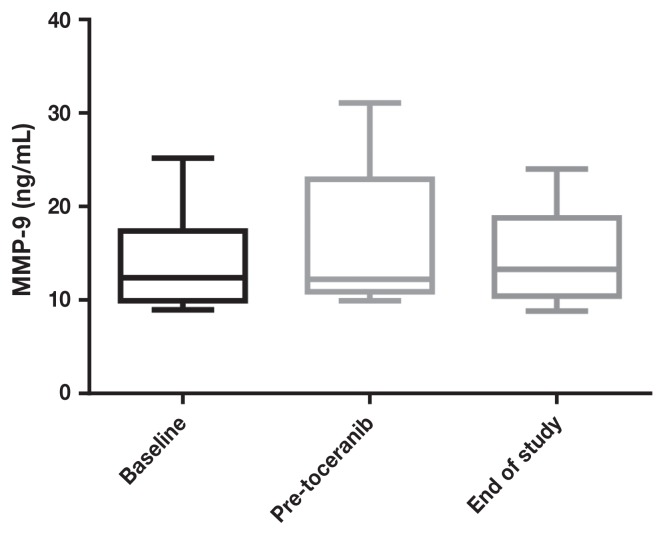
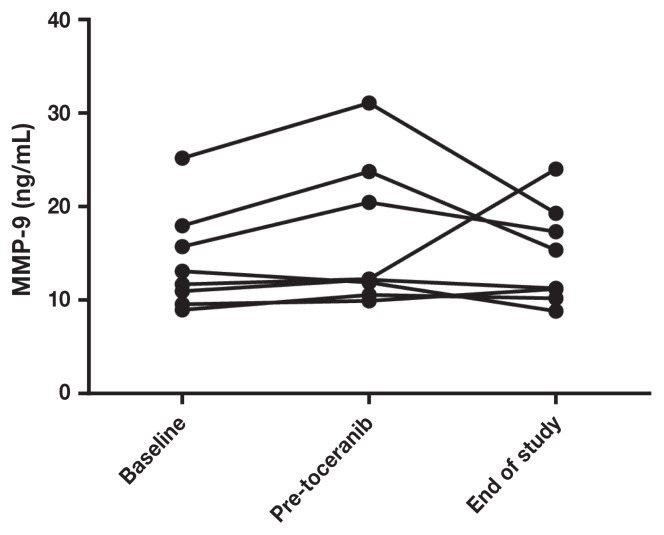
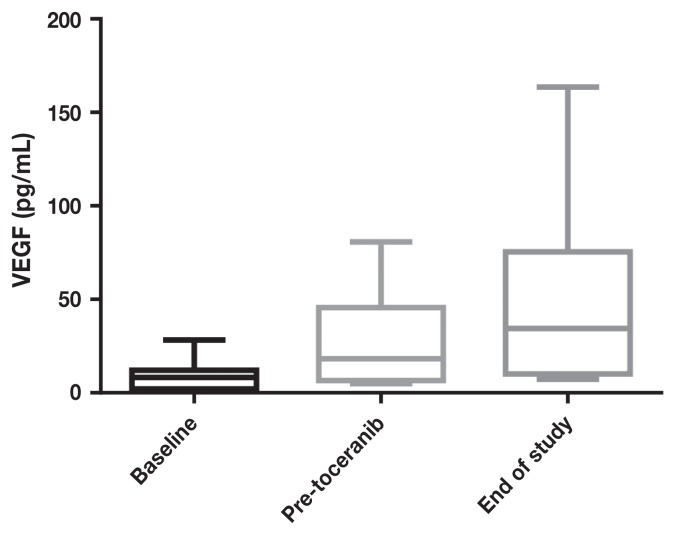
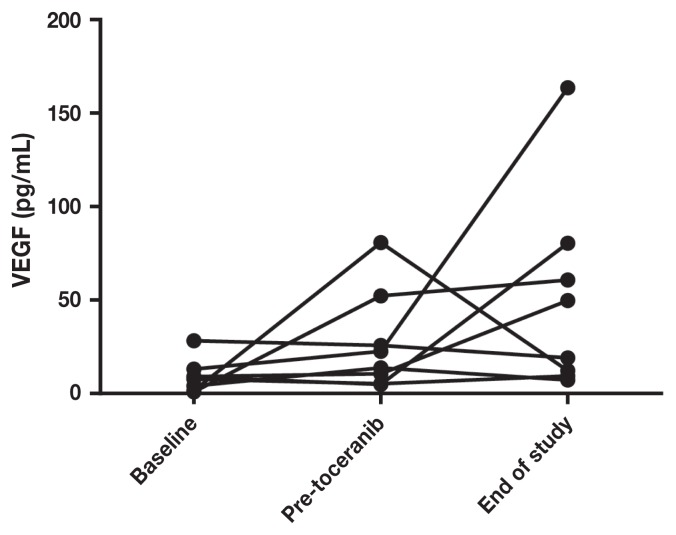
Pre-treatment serum VEGF levels ranged from 0.8 to 20.4 (median: 6.5) and MMP-9 levels ranged from 8.9 to 25.2 (median: 12), with wide variations in intra- and inter-patient variability. There was no significant difference in MMP (Figures 3 and 5) or VEGF [absolute (Figures 4 and 6) or platelet-corrected] values from baseline and pre-TP, baseline and TP discontinuation, or pre-TP to TP discontinuation.
Figure 3.
Metalloproteinase-9 concentrations at baseline, before toceranib phosphate, and at time of discontinuation of toceranib phosphate.
Figure 5.
Individual patient metalloproteinase-9 concentrations at baseline, before toceranib phosphate, and at time of discontinuation of toceranib phosphate.
Figure 4.
Vascular endothelial growth factor concentrations at baseline, before toceranib phosphate, and at time of discontinuation of toceranib phosphate.
Figure 6.
Individual patient vascular endothelial growth factor concentrations at baseline, before toceranib phosphate, and at time of discontinuation of toceranib phosphate.
Discussion
In this study, continuous administration of TP was well-tolerated in dogs with OSA treated with amputation and adjuvant carboplatin chemotherapy, although a survival benefit was not shown. In the present study, OS was 253 d with 30% alive at 1.5 y post-amputation, which is comparable to a historical group treated with amputation and adjuvant carboplatin chemotherapy in which OS was 321 d with 35% alive at 1 y (5). To date, 2 previously published studies of dogs with OSA treated with carboplatin chemotherapy and metronomic chemotherapy (MC) have failed to demonstrate improved survival times versus historical publications of dogs treated with carboplatin alone. In one publication, as part of a larger study of dogs with OSA treated with chemotherapy + MC [piroxicam and oral cyclophosphamide (10 to 12 mg/m2 per day)], 14 dogs were treated with carboplatin concurrent with MC (22). Three dogs were removed from the study due to toxicity (gastrointestinal). The overall disease-free interval was 192 d, with a median survival time of 217 d. The lack of improvement with post-chemotherapy MC (including TP in some dogs) was also confirmed in a recent, larger study (23). One hundred twenty-six dogs with appendicular OSA were treated with amputation followed by 4 doses of carboplatin chemotherapy and then randomized to receive MC [piroxicam/cyclophosphamide (10 mg/m2 q48h)] with or without addition of TP [MC + TP (2.75 mg/kg PO q48h)]. Thirty-two dogs developed metastasis before the onset of MC or MC + TP, and a total of 35 received MC and 46 received MC + TP. Toxicities were greater in the MC + TP group, but most were mild and resolved with supportive care. The median DFI was 215 and 233 d and the OS was 242 and 318 d, respectively, for the MC versus MC + TP groups, which did not differ significantly. The 1-y survival rate was 35% and 38% for the MC versus MC + TP groups, respectively.
Although MC administration has not yet been shown to improve outcomes in dogs with OSA when used in combination with traditional maximally tolerated dose (MTD) chemotherapy, its potential usefulness in highly metastatic diseases such as OSA, continues to be explored. In an attempt to further elucidate the tolerability and mechanism of action of MC, a recent study evaluated toxicity and lymphocyte profiles in tumor-bearing dogs treated with MTD doxorubicin (MTD-dox) only and those treated with MTD-dox alone and in combination with metronomic cyclophosphamide (MCTX) (24). A phase I study was performed and established that the standard doxorubicin dose of 30 mg/m2 (IV q21d) and a previously published MC dose of cyclophosphamide of 15 mg/m2 per day was tolerable when administered concurrently. Dogs were then randomized to receive MTD-dox (n = 8) or MTD-dox + MCTX (n = 8). Both treatment groups showed declines in both total and T-regulatory lymphocytes (Tregs) by day 7 after MTD-dox, and there was no statistically significant difference between the groups in either total lymphocyte count or Tregs. In contrast to previous studies demonstrating that Tregs were selectively inhibited by CTX (25), there was no selective depletion of Tregs. The authors theorized that concurrent administration of MTD-dox and MC may not be beneficial if the MTD chemotherapy dampens the inhibition of the selective Treg depletion seen with MCTX alone. Although this study used doxorubicin and the current study as well as a previously published larger study used carboplatin, this theory could explain why MTD carboplatin + MC did not yield prolonged survival times in dogs with OSA. Other potential mechanisms of efficacy of MC include other immunomodulatory effects (e.g., restoring NK effector function), anti-angiogenic effects [including decreased tumor microvessel density and decreased mobilization of circulating endothelial precursors (CEPs)], inhibition of normal stromal cells that support growing cancer cells, and possibly direct anti-tumor effects. The use of NSAIDs in MC can serve as a confounding factor when comparing studies since they inhibit COX-2, which may result in an inability of CEPs to survive and proliferate within the tumor microenvironment (24); therefore, the contribution of metronomic chemotherapy drugs such as cyclophosphamide to the outcome can be difficult to determine when NSAIDs are used concurrently. It is likely that the full potential of MC in addition to MTD chemotherapy for OSA will only be realized with clinical trials that compare use of standardized MTD chemotherapy and MC regimens both concurrently and sequentially.
One aspect of the present study that is unique, is the attempt to quantify the degree of antiangiogenesis via measurement of serum MMP-9, VEGF, and platelet-corrected VEGF levels at baseline (post-amputation), pre-TP (after 4 cycles of carboplatin), over time during TP administration, and at the time when TP was discontinued. We anticipated changes in VEGF and MMP-9 levels concomitant with changes in disease status. If changes were repeatable and consistent among the study dogs, these serum markers may have served a surrogate markers for the remission status of OSA. In a study of dogs with OSA treated with amputation, pre-amputation platelet-corrected VEGF was significantly correlated with DFI (13). In a study of dogs treated with metronomic chemotherapy (NSAID + cyclophosphamide) (26) for various metastatic tumors, VEGF levels were measured before (but not during) treatment, and dogs with lower VEGF levels had longer survival times and were more likely to respond to treatment than dogs with levels above the median. Unfortunately, in the current group of dogs, the MMP-9 and VEGF (absolute or platelet-corrected) values varied widely both inter- and intra-patient and there was no statistical correlation of the values at any time point. A limitation to this study is the lack of serum to test these factors before amputation because most dogs were referred for treatment and subsequent enrollment in the study after amputation had been performed. In addition to obtaining pre-treatment serum samples, ideally, all of the tumors would have been reviewed and graded by a single pathologist. This was a pilot study, and as a result, conclusions are inherently limited due to small sample size and lack of a contemporaneous control group. Failure to reliably assay angiogenesis biomarkers in this study show that other measures are needed to determine subclinical implications of anti-angiogenic therapy in dogs.
To our knowledge, this is the first study evaluating the use of single-agent TP (without other concurrent MC drugs such as NSAIDs and/or cyclophosphamide) after amputation and carboplatin chemotherapy in dogs with OSA. Although there was no improvement in outcome compared to historical controls, further work evaluating the use of TP in dogs with OSA should be conducted. Since it has been shown that dogs with gross metastasis from OSA can have stable disease and a clinical benefit from treatment with TP (18), it is likely that using TP in a microscopic disease setting before the onset of visible metastasis may be beneficial.
In conclusion, the combination of carboplatin followed by TP was well-tolerated by dogs with appendicular OSA. There was inter- and intra-patient variability of VEGF and MMP-9 levels at all time points and survival times did not differ from previously published data from dogs treated with amputation and adjuvant chemotherapy. Further larger studies to devise more effective therapeutic options for this disease are warranted.
Acknowledgments
This study was supported, in part, by a grant from Bone Cancer Dogs and provision of drugs and finances from Zoetis. Preliminary results were presented as an abstract at the American College of Veterinary Internal Medicine Annual Forum, Denver, Colorado in June 2016.
References
- 1.Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. 1991;270:159–168. [PubMed] [Google Scholar]
- 2.Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988) J Am Vet Med Assoc. 1992;200:995–999. [PubMed] [Google Scholar]
- 3.Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990) J Am Vet Med Assoc. 1992;200:531–533. [PubMed] [Google Scholar]
- 4.Berg J, Weinstein MJ, Schelling SH, Rand WM. Treatment of dogs with osteosarcoma by administration of cisplatin after amputation or limb-sparing surgery: 22 cases (1987–1990) J Am Vet Med Assoc. 1992;200:2005–2008. [PubMed] [Google Scholar]
- 5.Bergman PJ, MacEwen EG, Kurzman ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993) J Vet Intern Med. 1996;10:76–81. doi: 10.1111/j.1939-1676.1996.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 6.Berg J, Weinstein MJ, Springfield DS, Rand WM. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc. 1995;206:1555–1560. [PubMed] [Google Scholar]
- 7.Berg J, Gebhardt MC, Rand WM. Effect of timing of postoperative chemotherapy on survival of dogs with osteosarcoma. Cancer. 1997;79:1343–1350. [PubMed] [Google Scholar]
- 8.Bailey D, Erb H, Williams L, Ruslander D, Hauck M. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med. 2003;17:199–205. doi: 10.1111/j.1939-1676.2003.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Pirie-Shepherd SR, Coffman KT, Resnick D, et al. The role of angiostatin in the spontaneous bone and prostate cancers of pet dogs. Biochem Biophys Res Commun. 2002;292:886–891. doi: 10.1006/bbrc.2002.6749. [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Brekken R, McMahon G, et al. Matrix metal-loproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 13.Thamm DH, O’Brien MG, Vail DM. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Vet Comp Oncol. 2008;6:126–132. doi: 10.1111/j.1476-5829.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 14.Entz-Werle N, Gaub MP, Lavaux T, et al. KIT gene in pediatric osteosacomas: Could it be a new therapeutic target? Int J Cancer. 2007;120:2510–2516. doi: 10.1002/ijc.22593. [DOI] [PubMed] [Google Scholar]
- 15.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 16.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 17.London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768. [PubMed] [Google Scholar]
- 18.London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate in solid tumors. Vet Comp Oncol. 2012;10:194–205. doi: 10.1111/j.1476-5829.2011.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veterinary co-operative oncology group (VCOG) Veterinary co-operative oncology group — Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 20.Sendur MA, Askoy S, Yaman S, Arik Z, Ozdemir NY, Zengin N. Plasma VEGF levels may not accurately reflect the truth all the time. Med Oncol. 2012;29:1403–1404. doi: 10.1007/s12032-011-9966-0. [DOI] [PubMed] [Google Scholar]
- 21.George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: Clinical evidence of platelet scavenging? Clin Cancer Res. 2000;6:3147–3152. [PubMed] [Google Scholar]
- 22.Bracha S, Walshaw R, Danton T, Holland S, Ruaux C, Obradovich J. Evaluation of toxicities combined metronomic and maximal-tolerated dose chemotherapy in dogs with osteosarcoma. J Small Anim Pract. 2014;55:369–374. doi: 10.1111/jsap.12228. [DOI] [PubMed] [Google Scholar]
- 23.London CA, Gardner HL, Mathie T, et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of appendicular osteosarcoma following amputation and carboplatin chemotherapy: A multi-institutional study. PLoS One. 2015;10:e0124889. doi: 10.1371/journal.pone.0124889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen RM, Kurzman ID, Biller BJ, Guth A, Vail DM. Phase I lead-in and subsequent randomized trial assessing safety and modulation of regulatory T cell numbers following a maximally tolerated dose doxorubicin and metronomic dose cyclophosphamide combination chemotherapy protocol in tumour-bearing dogs. Vet Comp Oncol. 2015:1–10. doi: 10.1111/vco.12179. [DOI] [PubMed] [Google Scholar]
- 25.Burton JH, Mitchell L, Thamm DH, Dow SW, Biller BJ. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J Vet Intern Med. 2011;25:920–926. doi: 10.1111/j.1939-1676.2011.0753.x. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti V, Giorgi M, Fioravanti A, et al. First-line metronomic chemotherapy in a metastatic model of spontaneous canine tumours: A pilot study. Invest New Drugs. 2012;30:1725–1730. doi: 10.1007/s10637-011-9672-y. [DOI] [PubMed] [Google Scholar]