Abstract
There are few reports investigating the characterization of methicillin-resistant Staphylococcus pseudintermedius (MRSP) in dogs in Canada and none from Atlantic Canada. The objectives of this study were to strain type MRSP isolates cultured at a regional diagnostic laboratory using direct repeat unit (dru) typing and to describe their antimicrobial resistance profiles. Ninety-four isolates recovered from dogs between 2010 and 2012 had dru typing, cluster analysis, and antimicrobial susceptibility testing done. The majority of isolates belonged to type dt11a (30.9%), dt10h (24.5%), dt9a (18.1%), and dt11af (10.6%) with the remaining 15.9% of isolates distributed among 13 dru types. The predominant dru types identified were similar in Ontario; however, cluster 9a appears to be less common in Atlantic Canada. A significant difference in the distribution of clusters among Atlantic provinces was detected (P = 0.01). Resistance to ≥ 2 non-β-lactam antimicrobials was observed in 71.4% of the isolates. The MRSP isolates from this study were notably less resistant than those reported in the literature. A more comprehensive study of the MRSP dru types could help further elucidate the distribution of this pathogen in Canada.
Résumé
Il y a peu de publications rapportant la caractérisation des isolats de Staphylococcus pseudintermedius résistant à la méticilline (SPRM) chez les chiens au Canada et aucun ne provenant des provinces maritimes. Les objectifs de la présente étude était de typer les isolats de SPRM cultivés à un laboratoire de diagnostic régional en utilisant le typage direct des unités répétées (dur) et de décrire les profils de résistance antimicrobienne. Quatre-vingt-quatorze isolats canins obtenus entre 2010 et 2012 furent soumis au typage du dur, une analyse de regroupement, et un antibiogramme. La majorité des isolats appartenait au type dt11a (30,9 %), dt10h (24,5 %), dt9a (18,1 %), et dt11af (10,6 %) et le 15,9 % des isolats restant étaient distribués parmi 13 types dur. Les types prédominants de dur identifiés étaient similaires à ceux de l’Ontario, Canada, bien que le regroupement 9a semble moins fréquent dans les provinces maritimes. Une différence significative dans la distribution des regroupements entre les provinces maritimes a été détectée (P = 0,01). Une résistance à ≥ 2 antimicrobiens n’appartenant pas à la classe des β-lactames fut observée chez 71,4 % des isolats. Les isolats de SPRM de la présente étude était sensiblement moins résistant que ceux rapportés dans la littérature. Une étude plus complète des types dur des SPRM pourrait aider à élucider un peu plus la distribution de cet agent pathogène au Canada.
(Traduit par Docteur Serge Messier)
Introduction
Over the last decade, methicillin-resistant Staphylococcus pseudintermedius (MRSP) has emerged as a major opportunistic pathogen in dogs, and as the leading cause of skin disease, otitis, surgical site infections, and wound infections (1,2). Methicillin-resistance in MRSP is mediated by the mecA gene, which codes for the expression of a modified penicillin-binding protein, PBP2′, and resistance to most β-lactam antimicrobials (2). Increasing antimicrobial resistance to antimicrobials other than β-lactams, has been reported in MRSP isolates, leaving few therapeutic options available in many instances (1–3). Methicillin-resistant Staphylococcus pseudintermedius has been implicated in hospital-acquired infections within veterinary hospitals, and the potential of hospital-wide outbreaks should not be underestimated (1,4). Not only has MRSP been reported in the canine population, but also there have been several reports of humans infected or colonized with MRSP (5–11). Understanding the diversity and dissemination of MRSP is essential for developing mitigation and control strategies and for future epidemiologic studies. Currently, there is no study characterizing MRSP isolates in Atlantic Canada.
A number of molecular tools have been reported to genetically discriminate among staphylococci. Most of these tools were first developed and standardized for methicillin-resistant Staphylococcus aureus (MRSA), but few have been further refined for discriminating MRSP (1,12–16). While pulsed-field gel electrophoresis (PFGE) remains the gold standard for MRSA outbreak investigations, it has been shown to be less useful for MRSP investigations (12,14,17,18). Historically, multi-locus sequence typing (MLST) has been the primary tool for investigating the population genetic structure of MRSP, and 2 major sequence types (ST) have been identified (1,16,19). Both PFGE and MLST are laborious, costly, and require the use of reference strains, thus making them impractical tools for implementation in smaller diagnostic laboratories.
The use of a single-locus marker for isolate discrimination has also been explored for MRSA, and provides a less expensive and less laborious approach to strain typing (20,21). Sequence analysis of the staphylococcal protein A region (spa typing) has been shown to have greater discriminatory power than MLST and PFGE for MRSA, but it is less successful for strain typing all MRSP isolates (14,20–22). The mec-associated direct repeat unit (dru) typing was first demonstrated to be useful in discriminating highly-clonal MRSA isolates in Scotland (13), and has more recently been successfully applied to MRSP isolates from Canada, the United States (US), Europe, and Australia (22–24). In a previous study, significant associations between dru cluster and MLST were established, in which cluster 9a was associated with ST71 (the “International clone”) and cluster 11a was associated with ST68 (the “North American clone”) (1,22). This single-locus sequence-based method is rapid, standardized, and cost-effective, making it an ideal candidate for use in small-scale laboratories (22,23).
In a previous study, significant differences have been reported in the distribution of the 2 main dru clusters, 9a and 11a, between Canada, the US, and Europe (22). The report also inferred that variation within a country may exist, as some dru types were detected in some US states, but not the others included in the study. To date, there has been only one study investigating dru types in Canada, which was limited to Ontario; therefore, genetic information on MRSP isolates from Canadian regions other than Ontario is needed. Thus, the primary objective of this current study was to explore the strain type diversity of MRSP isolates from dogs using the dru typing method from submissions to a regional diagnostic laboratory in Atlantic Canada. A secondary objective was to describe the antimicrobial resistance (AMR) pattern of these MRSP isolates.
Materials and methods
Isolate screening and collection
Isolates were recovered from canine specimens submitted to the Atlantic Veterinary College (AVC) Diagnostic Services Bacteriology Laboratory for routine culture and susceptibility testing. Staphylococci were identified by colony morphology, including hemolysis, a positive tube coagulase test and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Antimicrobial susceptibilities to the following drugs were determined using the Kirby-Bauer disk diffusion method, following Clinical Laboratory Standards Institute (CLSI) standard M31-A3 (25): ampicillin (10 μg), amikacin (30 μg), amoxicillin-clavulanic acid (30 μg), cephalexin (30 μg), cefovecin (30 μg), chloramphenicol (30 μg), clindamycin (30 μg), doxycycline (30 μg), enrofloxacin (5 μg), erythromycin (15 μg), fusidic acid (10 μg), gentamicin (10 μg), penicillin (10 μg), and trimethoprim-sulfamethoxazole (SXT; 25 μg). Methicillin resistance was detected by oxacillin (1 μg) disk diffusion, and confirmed by mecA expression using the PBP2′ latex agglutination test (PBP2′ Latex Agglutination Test; Oxoid Company, Nepean, Ontario). All isolates identified presumptively as MRSP were placed in a medium containing 15% glycerol and frozen at −80°C.
Patient province of residence, submitting clinic, anatomical source/site of specimen, and antimicrobial susceptibility data were extracted from the laboratory data management system and descriptive statistics were computed. Only 1 isolate from each patient was included for analysis in most patients. If multiple MRSP isolates on repeated lab submissions were recovered from a patient but had the same dru type, only the first isolate was included for analysis. If different dru types were isolated from the same patient on different lab submissions, then both isolates were retained for analysis.
Molecular identification and typing
Genomic material was extracted using a resin matrix (InstaGene Matrix; Bio-Rad Laboratories, Montreal, Quebec). Manufacturer’s guidelines were followed except a large loopful of bacterial colonies were suspended in 1.0 mL of polymerase chain reaction (PCR) water, and the incubation time at 56°C was increased from 30 min to 1 h. Isolated DNA was frozen at −20°C until use. A multiplex PCR reaction was used to identify coagulase-positive staphylococci to the species level based on partial amplification of the nuc gene locus (26). Staphylococcus pseudintermedius isolates were characterized using the dru typing method, as previously described (17). A 40-μL reaction was prepared containing 4 μL of template DNA, 0.8 U of DNA polymerase, buffer mixture containing 1.5 mM MgCl2 and 200 μM of each dNTP (KAPA2G Fast Hot Start DNA polymerase; KAPA Biosystems, Boston, Massachusetts, USA), additional 0.3 mM MgCl2, and 0.8 μM of the forward primer druGF (5′-GTTAGCATATTACCTCTCCTTGC-3′) and the reverse primer druGR (5′-GCCGATTGTGCTTGATGAG-3′). Reaction mixtures were thermally cycled for an initial denaturation step of 94°C for 2 min followed by 30 cycles at 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min (17). The PCR products were purified using a kit (QIAquick PCR Purification Kit; Qiagen, Mississauga, Ontario) prior to sequencing.
The dru repeats and types were assigned using a website (www.dru-typing.org) following the previously described nomenclature (17). Cluster analysis was used to compare the relatedness among dru types, and a minimum spanning tree (MST) was generated. Distance intervals (or similarity values) were created using a bin distance of 1.0%, in which dru types separated by an MST distance of ≤ 2 repeats (> 98.5% similarity) were considered closely related and assigned to the same cluster. Root nodes were assigned to the dru type with the greatest number of isolates. Cluster analysis was completed using the TRST plug-in tool (BioNumerics version 6.6; Applied Maths, Austin, Texas, USA), as described previously for MRSP dru typing (22–24,27).
Statistical analysis
Multi-drug resistant (MDR) MRSP were defined as being resistant to ≥ 2 antimicrobials classes in addition to β-lactams. Dru clusters were used for comparisons to decrease the number of tested groups. Unconditional associations between dru cluster and patient data and dru cluster and resistance to the non-β-lactam antimicrobials were assessed using Chi-squared or Fisher’s exact tests, where appropriate, with significance set at P ≤ 0.05. Subtables were explored for any significant association to determine where the differences were. All statistical computations were done using computer software (Stata/IC 13.1 for Mac; StataCorp, College Station, Texas, USA).
Results
Isolate collection
The diagnostic laboratory collected 129 isolates from 90 dogs between January 2010 and December 2012. Of those, 98 isolates were retained for analysis. Twenty-three patients had multiple submissions. Isolates were collected from various specimens (n = 98): skin (n = 52, 53.1%), ears (n = 18, 18.4%), wounds (n = 8, 8.2%), surgical sites (n = 6, 6.1%), urine (n = 5, 5.1%), abscesses (n = 2, 2.0%), and other (n = 7, 7.1%). Most of the patients were seen by private veterinary clinics in the region (71.4%) compared to being seen by the AVC Veterinary Teaching Hospital (AVC-VTH) (28.6%).
Antimicrobial resistance patterns
Only 8.2% (n = 8) of isolates were susceptible to all tested drugs with the exception of β-lactams (Table I). Another 20.4% (n = 20) of isolates were resistant to 1 non-β-lactam antimicrobial class, while 71.4% (n = 70) were resistant to ≥ 2 non-β-lactam antimicrobial classes. The most common resistance was to SXT (74.5%), followed by erythromycin (68.4%), clindamycin (55.1%), enrofloxacin (46.9%), gentamicin (34.7%), chloramphenicol (23.5%), doxycycline (15.3%), and fusidic acid (3.1%). In isolates that displayed multi-drug resistance (n = 70), 68.6% of isolates were resistant to SXT, erythromycin, and clindamycin, while 55.7% (n = 70) of those were also resistant to enrofloxacin. Amikacin resistance was not detected in any of the isolates.
Table I.
Number (and percent) of antimicrobial resistant methicillin-resistant Staphylococcus pseudintermedius (MRSP) isolates overall and by dru cluster, following Clinical Laboratory Standards Institute (CLSI) guidelines. Multidrug resistance (MDR) in each cluster is also reported
| Antimicrobial | Overall (n = 94) | 9a (n = 18) | 10h (n = 27) | 11a (n = 45) | No cluster (n = 4) |
|---|---|---|---|---|---|
| Amikacin | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0%) |
| Chloramphenicol | 23 (24.5%) | 3 (16.7%) | 0 (0%) | 19 (42.2%) | 1 (25%) |
| Clindamycin | 54 (57.5%) | 16 (88.9%) | 7 (25.9%) | 29 (64.4%) | 2 (50%) |
| Doxycycline | 14 (14.9%) | 1 (5.6%) | 7 (25.9%) | 4 (8.9%) | 2 (50%) |
| Erythromycin | 63 (67.0%) | 17 (94.4%) | 11 (40.7%) | 32 (71.1%) | 3 (75%) |
| Enrofloxacin | 46 (48.9%) | 16 (88.9%) | 0 (0%) | 29 (64.4%) | 1 (25%) |
| Fusidic acid | 2 (2.1%) | 1 (5.6%) | 1 (3.7%) | 0 (0%) | 0 (0%) |
| Gentamicin | 34 (36.2%) | 10 (55.6%) | 2 (7.4%) | 20 (44.4%) | 2 (50%) |
| SXT | 70 (74.5%) | 18 (100%) | 23 (85.2%) | 27 (60.0%) | 2 (50%) |
| MDR | 66 (70.2%) | 17 (94.4%) | 15 (55.6%) | 31 (68.9%) | 3 (75%) |
SXT — trimethoprim-sulfamethoxazole.
Direct repeat unit typing
The dru types were determined for 94/98 isolates, because 4 isolates were not available for typing. From the 94 isolates, 18 dru types were recovered with 3 predominant dru types contributing more than 70% of the distribution: dt11a (30.9%), dt10h (24.5%), and dt9a (18.1%). The frequency of each dru type can be found in Table II. Nine of the dru types were previously unidentified at the time of analysis and are represented by one isolate each: dt5k, dt6t, dt8ag, dt9bd, dt10cc, dt10cj, dt11ca, and dt11cm.
Table II.
Frequency of methicillin-resistant Staphylococcus pseudintermedius dru types from 94 isolates collected at a regional diagnostic laboratory between 2010 and 2012
| dru type | Frequency (n = 94) | Cluster |
|---|---|---|
| dt11a | 29 (30.9%) | 11a |
| dt10h | 23 (24.5%) | 10h |
| dt9a | 17 (18.1%) | 9a |
| dt11af | 10 (10.6%) | 11a |
| dt10as | 3 (3.2%) | 10h |
| dt10cca | 1 (1.1%) | 10h |
| dt10cja | 1 (1.1%) | 11a |
| dt11bn | 1 (1.1%) | 11a |
| dt11caa | 1 (1.1%) | 11a |
| dt11v | 1 (1.1%) | 11a |
| dt11y | 1 (1.1%) | 11a |
| dt5ka | 1 (1.1%) | — |
| dt6ta | 1 (1.1%) | — |
| dt8aga | 1 (1.1%) | — |
| dt9baa | 1 (1.1%) | 9a |
| dt9bda | 1 (1.1%) | — |
| dt11cma | 1 (1.1%) | 11a |
Novel dru types identified in this study.
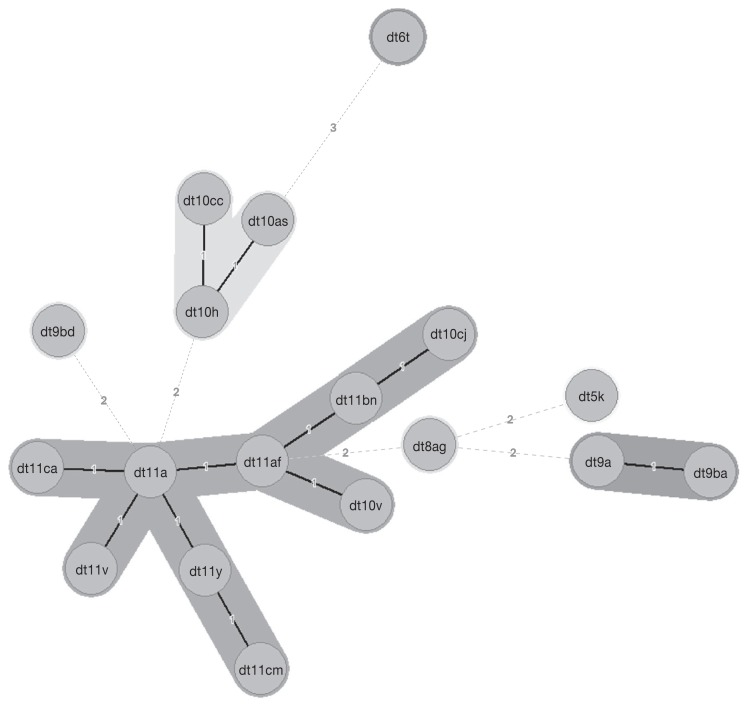
A minimum spanning tree (MST) was constructed to identify relatedness among dru types, and to determine the predominate clusters (Figure 1). The cluster analysis identified 3 main dru clusters that included 90/94 typed isolates (Figure 1). Cluster 11a contained the most diversity, comprising 9 dru types and 47.9% of the typed isolates. Cluster 10h contained 3 dru types and 28.7% of the isolates, while cluster 9a contained 2 dru types and 19.2% of the isolates. Table II contains the dru type and cluster association.
Figure 1.
Minimum spanning tree (MST) of methicillin-resistant Staphylococcus pseudintermedius in Atlantic Canada. Numerical values on the branches indicate the similarity (MST distance) among different dru types. The dru types separated by an MST distance of ≤ 2 (≥ 98.5% similar) were considered closely related and assigned to the same cluster.
Associations among dru clusters, patient demographics, and AMR
Significant differences were determined among the distributions of dru clusters in the 4 Canadian Atlantic provinces (P = 0.01; Table III). In Newfoundland and Labrador (NL), cluster 9a was over-represented with 5/7 strains belonging to dt9a. Cluster 10h was present in Nova Scotia (NS; 15/52) and New Brunswick (NB; 5/23), but not NL, and was the predominant cluster from Prince Edward Island (PEI; 7/12) isolates. Both mainland provinces, NB and NS, shared the same predominant cluster, 11a, which comprised over 50% of the isolates from each province.
Table III.
Number (and percent) of methicillin-resistant Staphylococcus pseudintermedius (MRSP) isolates in each province overall and by dru cluster
| dru cluster | NB | NL | NS | PEI |
|---|---|---|---|---|
| Overall (n = 94) | 23 (24.5%) | 7 (7.4%) | 52 (55.3%) | 12 (12.8%) |
| 9a (n = 18) | 6 (33.3%) | 5 (27.8%) | 6 (33.3%) | 1 (5.6%) |
| 10h (n = 27) | 5 (18.5%) | 0 (0.0%) | 15 (55.6%) | 7 (25.9%) |
| 11a (n = 45) | 12 (26.7%) | 2 (4.4%) | 28 (62.2%) | 3 (6.7%) |
| No cluster (n = 4) | 0 (0.0%) | 0 (0.0%) | 3 (75.0%) | 1 (25.0%) |
NB — New Brunswick; NL — Newfoundland and Labrador; NS — Nova Scotia; PEI — Prince Edward Island.
The dru cluster was not significantly different among the AVC-VTH and private practice (P = 0.18) or specimen types (P = 0.91). Significant associations among AMR and dru clusters were detected for all antimicrobials except fusidic acid (P = 0.31; Table I). For clindamycin, erythromycin, enrofloxacin, and SXT, cluster 9a had a significantly higher proportion of resistant isolates (P ≤ 0.01). Cluster 10h had significantly higher proportion of isolates resistant to doxycycline (P = 0.04), while cluster 11a had a significantly higher proportion of chloramphenicol resistance (P < 0.001). The proportion of MDR isolates was significantly different among dru clusters (P = 0.03), with cluster 9a having the highest proportion of MDR (94.4%), followed by 10h (55.6%) and 11a (68.8%).
Discussion
The majority of isolates (73.5%) collected as part of this convenience sampling at the AVC Diagnostic Services Bacteriology Laboratory were represented by dru types dt11a, dt10h, and dt9a, which is similar to the findings in a previous multi-national study (22). However, in the current study, clusters 11a and 10h comprised more than 75% of the isolates collected, whereas in the Ontario study, cluster 11a (including dt10h) and 9a were evenly distributed (22). The distribution of MRSP dru clusters in Atlantic Canada was more similar to what has been reported in California, Illinois, North Carolina, Tennessee, and Texas (22). Differences among geographic regions within the US were also noted in the previous study (22).
Significant differences in the diversity of strains were detected among provinces of Atlantic Canada (Table III). It should be noted that this study was not designed for prevalence estimation because of the convenience sampling approach. Although it may appear that the incidence of MRSP is higher in certain provinces, these numbers are reflective of the sample submission demographics from these regions (i.e., the laboratory receives approximately 45% of their canine samples from NS, but only 5% are from NL). These provincial differences in the distribution of dru types could be attributed to geographic separation, since differences among Newfoundland and Labrador and the Maritime Provinces (NB, NS, and PEI) exist, as well as population density, since provinces with larger populations have greater dru type diversity. The small sample size of isolates from Newfoundland and Labrador may bias its estimate. It is possible that the true diversity of the isolates from some provinces is underestimated, since each province except PEI has its own animal health microbiology laboratory, decreasing submissions from these provinces to the AVC diagnostic laboratory.
This study detected 9 dru types that had not been previously identified (Table II), and required entry into the dru database. These new dru types are results of a random single-nucleotide polymorphism in one of the 40bp dru repeat sequences, subsequently creating a new dru repeat and thus new dru type. Novel dru types have also been identified in a previous study (22). Although there were no significant differences in the distribution of the new dru types, most of the novel dru types were isolated from Nova Scotia (5/9), and from skin samples (5/9).
More than 70% of the isolates recovered in this study were resistant to ≥ 2 non-β-lactam antimicrobial classes, highlighting the clinical concerns regarding management of MRSP infections. Multi-drug resistance has been frequently reported in MRSP isolated from canine specimens (1,28). The isolates recovered in this study were from submissions to a regional diagnostic bacteriology laboratory that also is the diagnostic laboratory for the AVC-VTH, thus a selection bias is possible, based on submission of specimens from complicated referral cases from the AVC-VTH. However, most samples in this study were from primary care practices. Information about prior antimicrobial exposure was not always available, but submissions to a diagnostic laboratory for bacterial culture and susceptibility testing can be from cases proving difficult to treat, with the primary reason being non-response to antimicrobial therapy. It is likely that diagnostic laboratory submissions are biased to be more resistant.
Interestingly, cluster 9a isolates had the highest proportion of MDR at 94.4%, and also had a significantly higher proportion of resistant isolates for each antimicrobial except fusidic acid, chloramphenicol, and doxycycline. Strong associations between cluster 9a and ST71 have been shown (22), and reports have also shown ST71 to have an increased antimicrobial resistance compared with other STs (1,29). MLST was not done on the isolates in this study, but the cluster 9a isolates in this study likely belong to ST71. Although this study was not designed for prevalence estimation, the ST71 clone associated dru cluster 9a seems to have not disseminated into Atlantic Canada (18.1%), with the possible exception of Newfoundland, to the degree that it has in Europe (90%), the US (66%), and Ontario, Canada (47%) (22).
Resistance of the MRSP isolates to the non-β-lactam antimicrobials in this study was lower than in previous reports of multi-drug resistance in MRSP, which could be explained by the low prevalence of cluster 9a isolates. However, caution should be taken when comparing studies because of differences in testing methodology. Specifically, it was reported that 62% (n = 107) of study isolates were resistant to doxycycline in Ontario (24), while in our study doxycycline resistance of isolates was 15%. One study in Australia and another in the United Kingdom (UK) found similarly high tetracycline resistance at over 50% and 35%, respectively (23,30). Since the time of this study, canine specific doxycycline breakpoints for S. pseudintermedius were proposed and implemented by CLSI (31,32). This change in zone diameter breakpoints would likely increase the number of doxycycline resistant isolates in both this study and the Ontario study. Similar trends can be observed where the proportion of resistant isolates in Atlantic Canada is much lower than those reported in Australia and the UK. A recent study completed at a Texas (USA) Veterinary Medical Teaching hospital reported amikacin resistance in 36% of their MRSP isolates from dogs, whereas in our study amikacin resistance was not detected (33). A systematic literature review of antimicrobial resistance in MRSP isolates reported individual antimicrobial resistance ranging between 0% and 100%, with most studies reporting AMR estimates > 50% for most antimicrobials, except for chloramphenicol and amikacin (28). Thus, it can be inferred that the MRSP isolates in Atlantic Canada are typically less resistant, specifically to doxycycline and amikacin, than their counterparts in some other regions, even though multi-drug resistance is still common. A possible explanation for this low-level resistance could be the low population density of the region as a whole, when compared with larger, more densely populated regions, which could mean less exposure to AMR organisms and less total antimicrobial use.
The overall picture of MRSP in Atlantic Canada is similar to reports elsewhere in Canada and the world. The distribution of the dru types reported in this study is similar to other reports from North America; however, the distribution is more similar to what was observed in the US versus Ontario, Canada. The international MRSP clone, ST71, which is disseminated throughout Europe and made up almost half the isolates in Ontario, Canada, was less common in Atlantic Canada. This confirms that dru type distributions can vary significantly across the same country, and a larger, more comprehensive study of the dru types in Canada could help further clarify the dissemination of this pathogen. Multi-drug resistance in these isolates is common, especially within cluster 9a isolates, but resistance to the non-β-lactam antimicrobials is still considerably lower than has been previously reported.
Acknowledgments
The authors thank AVC Diagnostic Services Bacteriology Laboratory for their valuable work with sample and isolate collection; Joyce Rousseau, Ontario Veterinary College, for assistance with molecular techniques; Spencer Greenwood, AVC Lobster Science Centre, for use of equipment; Henrik Stryhn, Atlantic Veterinary College, for assistance with statistical analyses; and Robert Page, University of Prince Edward Island, for laboratory data extraction.
This study was financially supported by the Atlantic Veterinary College Companion Animal Trust Fund and the Sir James Dunn Animal Welfare Centre.
References
- 1.Perreten V, Kadlec K, Schwarz S, et al. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. J Antimicrob Chemother. 2010;65:1145–1154. doi: 10.1093/jac/dkq078. [DOI] [PubMed] [Google Scholar]
- 2.Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol. 2010;140:418–429. doi: 10.1016/j.vetmic.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 3.van Duijkeren E, Catry B, Greko C, et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. 2011;66:2705–2714. doi: 10.1093/jac/dkr367. [DOI] [PubMed] [Google Scholar]
- 4.Boerlin P, Eugster S, Gaschen F, Straub R, Schawalder P. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol. 2001;82:347–359. doi: 10.1016/s0378-1135(01)00396-0. [DOI] [PubMed] [Google Scholar]
- 5.Boost MV, So SYC, Perreten V. Low rate of methicillin-resistant coagulase-positive staphylococcal colonization of veterinary personnel in Hong Kong. Zoonoses Public Hlth. 2011;58:36–40. doi: 10.1111/j.1863-2378.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 6.Gerstadt K, Daly JS, Mitchell M, Wessolossky M, Cheeseman SH. Methicillin-resistant Staphylococcus intermedius pneumonia following coronary artery bypass grafting. Clin Infect Dis. 1999;29:218–219. doi: 10.1086/520168. [DOI] [PubMed] [Google Scholar]
- 7.Morris DO, Boston RC, O’Shea K, Rankin SC. The prevalence of carriage of methicillin-resistant staphylococci by veterinary dermatology practice staff and their respective pets. Vet Dermatol. 2010;21:400–407. doi: 10.1111/j.1365-3164.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 8.Paul NC, Moodley A, Ghibaudo G, Guardabassi L. Carriage of methicillin-resistant Staphylococcus pseudintermedius in small animal veterinarians: Indirect evidence of zoonotic transmission. Zoonoses Public Hlth. 2011;58:533–539. doi: 10.1111/j.1863-2378.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. Methicillin-resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. J Clin Microbiol. 2007;45:1118–1125. doi: 10.1128/JCM.02193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starlander G, Börjesson S, Grönlund-Andersson U, Tellgren-Roth C, Melhus Å. Cluster of infections caused by methicillin-resistant Staphylococcus pseudintermedius in a human hospital. J Clin Microbiol. 2014;52:3118–3120. doi: 10.1128/JCM.00703-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stegmann R, Burnens A, Maranta CA, Perreten V. Human infection associated with methicillin-resistant Staphylococcus pseudintermedius ST71. J Antimicrob Chemother. 2010;65:2047–2048. doi: 10.1093/jac/dkq241. [DOI] [PubMed] [Google Scholar]
- 12.Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet Dermatol. 2012;23:253–266. e51–52. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 13.Black CC, Solyman SM, Eberlein LC, Bemis DA, Woron AM, Kania SA. Identification of a predominant multilocus sequence type, pulsed-field gel electrophoresis cluster, and novel staphylococcal chromosomal cassette in clinical isolates of mecA-containing, methicillin-resistant Staphylococcus pseudintermedius. Vet Microbiol. 2009;139:333–338. doi: 10.1016/j.vetmic.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Moodley A, Stegger M, Ben Zakour NL, Fitzgerald JR, Guardabassi L. Tandem repeat sequence analysis of staphylococcal protein A (spa) gene in methicillin-resistant Staphylococcus pseudintermedius. Vet Microbiol. 2009;135:320–326. doi: 10.1016/j.vetmic.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 15.Murchan S, Kaufmann ME, Deplano A, et al. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: A single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J Clin Microbiol. 2003;41:1574–1585. doi: 10.1128/JCM.41.4.1574-1585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solyman SM, Black CC, Duim B, et al. Multilocus sequence typing for characterization of Staphylococcus pseudintermedius. J Clin Microbiol. 2013;51:306–310. doi: 10.1128/JCM.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goering RV, Morrison D, Al-Doori Z, Edwards GFS, Gemmell CG. Usefulness of mec-associated direct repeat unit (dru) typing in the epidemiological analysis of highly clonal methicillinresistant Staphylococcus aureus in Scotland. Clin Microbiol Infect. 2008;14:964–969. doi: 10.1111/j.1469-0691.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 18.Strandén A, Frei R, Widmer AF. Molecular typing of methicillinresistant Staphylococcus aureus: Can PCR replace pulsed-field gel electrophoresis? J Clin Microbiol. 2003;41:3181–3186. doi: 10.1128/JCM.41.7.3181-3186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannoehr J, Ben Zakour NL, Waller AS, et al. Population genetic structure of the Staphylococcus intermedius group: Insights into agr diversification and the emergence of methicillin-resistant strains. J Bacteriol. 2007;189:8685–8692. doi: 10.1128/JB.01150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koreen L, Ramaswamy SV, Graviss EA, Naidich S, Musser JM, Kreiswirth BN. Spa typing method for discriminating among Staphylococcus aureus isolates: Implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42:792–799. doi: 10.1128/JCM.42.2.792-799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shopsin B, Gomez M, Montgomery SO, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadlec K, Schwarz S, Goering RV, Weese JS. Direct repeat unit (dru) typing of methicillin-resistant Staphylococcus pseudintermedius from dogs and cats. J Clin Microbiol. 2015;53:3760–3765. doi: 10.1128/JCM.01850-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siak M, Burrows AK, Coombs GW, et al. Characterization of methicillin-resistant and methicillin-susceptible isolates of Staphylococcus pseudintermedius from cases of canine pyoderma in Australia. J Med Microbiol. 2014;63:1228–1233. doi: 10.1099/jmm.0.076117-0. [DOI] [PubMed] [Google Scholar]
- 24.Weese JS, Sweetman K, Edson H, Rousseau J. Evaluation of minocycline susceptibility of methicillin-resistant Staphylococcus pseudintermedius. Vet Microbiol. 2012;162:968–971. doi: 10.1016/j.vetmic.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Laboratory Standards Institute (CLSI) Performance standards for antimicrobial disk diffusion and dilution susceptibility tests for bacteria isolated from animals; approved guideline. Wayne, Pennsylvania: CLSI; 2008. CLSI Document No.: M31-A3. [Google Scholar]
- 26.Sasaki T, Tsubakishita S, Tanaka Y, et al. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J Clin Microbiol. 2010;48:765–769. doi: 10.1128/JCM.01232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels MD, Boye K, Oliveira DC, Worning P, Goering R, Westh H. Associations between dru types and SCCmec cassettes. PLoS ONE. 2013;8:e61860. doi: 10.1371/journal.pone.0061860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moodley A, Damborg P, Nielsen SS. Antimicrobial resistance in methicillin susceptible and methicillin resistant Staphylococcus pseudintermedius of canine origin: Literature review from 1980 to 2013. Vet Microbiol. 2014;171:337–341. doi: 10.1016/j.vetmic.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Osland AM, Vestby LK, Fanuelsen H, Slettemeås JS, Sunde M. Clonal diversity and biofilm-forming ability of methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. 2012;67:841–848. doi: 10.1093/jac/dkr576. [DOI] [PubMed] [Google Scholar]
- 30.Maluping RP, Paul NC, Moodley A. Antimicrobial susceptibility of methicillin-resistant Staphylococcus pseudintermedius isolated from veterinary clinical cases in the UK. Br J Biomed Sci. 2014;71:55–57. doi: 10.1080/09674845.2014.11669965. [DOI] [PubMed] [Google Scholar]
- 31.Maaland MG, Papich MG, Turnidge J, Guardabassi L. Pharmaco-dynamics of doxycycline and tetracycline against Staphylococcus pseudintermedius: Proposal of canine-specific breakpoints for doxycycline. J Clin Microbiol. 2013;51:3547–3554. doi: 10.1128/JCM.01498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical Laboratory Standards Institute (CLSI) Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved guideline. 3rd Edition. Wayne, Pennsylvania: CLSI; 2015. CLSI Document No.: VET01S. [Google Scholar]
- 33.Gold RM, Cohen ND, Lawhon SD. Amikacin resistance in Staphylococcus pseudintermedius isolated from dogs. J Clin Microbiol. 2014;52:3641–3646. doi: 10.1128/JCM.01253-14. [DOI] [PMC free article] [PubMed] [Google Scholar]