Abstract
The objective of this study was to evaluate the ability of a long-term antioxidant-supplemented diet to regulate the oxidative stress and general health status of dogs involved in animal-assisted intervention (AAI) programs. Oxidative stress is a consequence of the accumulation of reactive oxygen species (ROS). Exercise-induced oxidative stress can increase muscle fatigue and fiber damage and eventually leads to impairment of the immune system. A randomized, placebo-controlled, crossover clinical evaluation was conducted with 11 healthy therapy dogs: 6 females and 5 males of different breeds and with a mean age of 2.7 ± 0.8 y (mean ± SEM). The dogs were divided into 2 groups, 1 fed a high quality commercial diet without antioxidants (CD) and the other a high quality commercial diet supplemented with antioxidants (SD) for 18 wk. After the first 18 wk, metabolic parameters, reactive oxygen metabolite-derivatives (d-ROMs), and biological antioxidant potential (BAP) levels were monitored and showed a significant reduction of d-ROMs, triglycerides, and creatinine values in the SD group (P < 0.05) and a significant increase in amylase values in the CD group (P < 0.01). At the end of this period, groups were crossed over and fed for another 18 wk. A significant decrease in amylase and glutamate pyruvate transaminase (GPT) values was observed in the CD and SD group, respectively (P < 0.05). In conclusion, a controlled, balanced antioxidant diet may be a valid approach to restoring good cell metabolism and neutralizing excess free radicals in therapy dogs.
Résumé
L’objectif de la présente étude était d’évaluer la capacité d’une diète long-terme supplémentée en antioxydant à réguler le stress oxydatif et l’état de santé général de chiens impliqués dans des programmes d’intervention avec assistance animale (IAA). Le stress oxydatif est une conséquence de l’accumulation d’espèces oxygène réactive (EOR). Le stress oxydatif induit par l’exercice peut augmenter la fatigue musculaire et les dommages aux fibres et éventuellement mener à un mauvais fonctionnement du système immunitaire. Une évaluation clinique croisée, randomisée, et avec groupe témoin-placebo a été menée avec 11 chiens d’assistance en santé : 6 femelles et 5 mâles de races différentes et d’un âge moyen de 2,7 ± 0,8 ans (moyenne ± écart-type). Les chiens ont été divisés en deux groupes, un premier groupe nourri avec une diète commerciale de haute qualité sans antioxydant (DC) et l’autre groupe avec une diète commerciale de haute qualité supplémentée avec des antioxydants (DS) pour 18 semaines. Après les premières 18 semaines, les paramètres métaboliques, les métabolites dérivés d’oxygène réactive (MDOR), et les niveaux de potentiel antioxydant biologique (PAB) ont été surveillés et ont montré une réduction significative des valeurs des MDOR, des triglycérides et de la créatinine dans le groupe DS (P < 0,05) et une augmentation significative des valeurs de l’amylase dans le groupe DC (P < 0,01). À la fin de cette période, les groupes ont été croisés et nourris pour 18 semaines supplémentaires. Une diminution significative des valeurs de l’amylase et de la glutamate pyruvate transaminase (GPT) a été obtenue dans les groupes DC et DS, respectivement (P < 0,05). En conclusion, une diète contrôlée, balancée en antioxydant pourrait être une approche valide pour restaurer un bon métabolisme cellulaire et neutraliser les radicaux libres excédentaires chez les chiens d’assistance.
(Traduit par Docteur Serge Messier)
Introduction
Oxidative stress is a consequence of the accumulation of reactive oxygen species (ROS), reactive nitrogen species (RNS), such as hydroxyl radicals (OH−), superoxide anion radicals (O2), lipid peroxyl radicals (LOO−), and nitrate radicals (NO−) (1). Factors involved in the production of free radicals and reactive metabolites include physical exercise, characterized by an increase in the body’s oxygen consumption and correlated to an increase in ROS production, which causes oxidative stress (2–4). It has been shown that exercise-induced oxidative stress contributes to increased muscle fatigue, muscle fiber damage (5), and eventually leads to immune system impairment (6). Both muscles and blood are endowed with antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and non-enzymatic antioxidants, such as vitamins A, C, and E, to counteract damage caused by free radicals (2,7,8).
Oxidative damage induced by physical exercise has been reported in horses (9–11) and dogs (12,13). One study investigated the oxidative status of 9 dogs after 20 min and 4 h of aerobic exercise, after 30 d of rest (14). Results showed a significant oxidative status due to the high level of reactive oxygen metabolite-derivatives (d-ROMs) and the low level of biological antioxidant potential (BAP) in relation to the values before starting the exercises (P < 0.01) (14).
Ethane exhaled in the breath may be considered a marker of oxidative stress as it is a product of free radical-mediated oxidation of cell membrane lipids (15). The authors evaluated oxidative stress in terms of exhaled ethane in 8 human, 12 dog, and 11 equine athletes after a physical performance (15). A significant increase in exhaled ethane was observed in all species after training and was associated with oxidative stress.
Evaluation of oxidative status with specific parameters could become an interesting and practical method of monitoring the activity of working dogs. Indeed, it is difficult to quantify ROS in practice because of their very short half-life and it requires complex techniques over a long period of time. Due to their high reactivity, ROS react with practically every organic molecule they meet, producing reactive oxygen metabolites (ROMs), which are more stable than the ROS and are therefore easier to quantify. Conversely, BAP matches the total antioxidant capability of plasma and includes either exogenous (ascorbate, tocopherols, carotenoids) or endogenous (protein, GPx, SOD, CAT) components that can oppose the oxidant action of reactive species (16).
In conditions of oxidative stress, a controlled, balanced antioxidant diet may be a valid approach to restoring good cell metabolism and neutralizing excess free radicals. Two studies in dogs and rabbits demonstrated an increase in tissue oxidative stability by supplementing with a diet enriched with different antioxidants (17,13). The effect on exercise-induced oxidative stress of supplementing with oral vitamin E (11 to 14 mg) for 10 wk was studied in 8 dogs and induced a significant reduction in paroxonase-1 activity, erythrocyte membrane fluidity, and vitamin E and a significant increase in plasma malondialdehyde levels (18). After 10 d of vitamin E supplementation, these modifications were significant in the placebo group, but not in the supplemented group.
The objective of this study was to ascertain the ability of a long-term antioxidant-supplemented diet to regulate the oxidative stress and general health status of working dogs involved in animal-assisted intervention (AAI) programs.
Materials and methods
Operative procedures and animal care were carried out in compliance with national and international regulations (Italian regulation D.L. vo 116/1992 and European Union regulation 86/609/EC). The recommendations of the Consolidated Standards of Reporting (CONSORT) 2010 statement on randomized controlled trials were also consulted and considered (19).
Subjects
Eleven therapy dogs (6 females and 5 males) of different breeds (1 Cirneco dell’Etna, 6 Labrador retrievers, 3 mixed breed, and 1 pitbull), aged 2.7 ± 0.8 y and with a body weight of 22.12 ± 8.43 kg (mean ± SEM), were enrolled in this clinical evaluation. Before the recruitment, laboratory examinations (hemogram, biochemical profile, urine analysis, and serum protein electrophoresis) and serological tests (Ehrlichia canis, Rickettsia spp., Leishmania infantum, Anaplasma phagocytophilum, Bartonella spp., Toxoplasma gondii, Neospora caninum, and Borrelia burgdorferi) were conducted on all dogs to confirm their good health status. Owner consent was obtained for each dog.
All dogs were involved in 5 weekly (30 min each) animal-assisted intervention (AAI) sessions in the rehabilitation or social care of humans. All sessions were held in the same place and at similar temperature and humidity conditions. Patients involved in AAI sessions consisted of 55 children with pervasive developmental disorders. Each 30-minute session consisted of 15 min of active playing, such as ball throwing or running, 10 min of collaborative activities, such as the child and the dog looking for a hidden object together, and 5 min of dog-care activities, such as feeding and brushing the dog.
Diets
The dogs were fed 2 varieties of dry dog food: Natural Trainer Adult Medium and Personal Trainer Long Life Adult Medium, which is supplemented with antioxidants (both from Nova Foods, Vicenza, Italy). The Natural Trainer Adult Medium diet consisted of fresh chicken and turkey meat, corn, rice, dehydrated chicken and turkey meat, lard, dehydrated pork meat, flax seed, beet, corn oil, dehydrated fish flour, chicory extract, fructooligosaccharides, brewer’s yeast, pea fiber, Spirulina algae (Arthrospira platensis), ribonucleotides extract from yeast, green-lipped mussels (Perna canaliculus), apple dried extract, black raspberry extract, salt, chloride-choline, methionine, vitamin E, vitamin A (18 000 UI/kg), vitamin D3 (1.350 UI/kg), vitamin E (265 mg/kg), and copper (20 mg/kg).
The Personal Trainer Long Life Adult Medium dog food consisted of corn, dehydrated chicken and turkey meat, fresh chicken and turkey meat, rice, corn oil, lard, hydrolyzed proteins of chicken, beet, corn gluten, flax seeds, dehydrated fish flour, brewer’s yeast (0.50%), sodium chloride, pea fiber, wood cellulose, calcium carbonate, fruit oligosaccharides, broccoli dried extract, green-lipped mussels (Perna canaliculus) (0.05%), Vitaberry Plus (grape seed extract, quercetin, blueberry dried extract, resveratrol, and strawberry and blackberry dried extracts), and silicon dioxide (0.025%).
Experimental design
Dogs were randomly divided into 2 groups using a dedicated software (Research Randomizer) (20). The first group (n = 5) received a high quality commercial control diet (CD) without antioxidant (Natural Trainer Adult Medium, Nova Foods), while the second group (n = 6) received a high quality commercial diet (SD) supplemented with antioxidants (Personal Trainer Long Life Adult Medium, Nova Foods) for 18 wk. At the end of this period, a crossover design of the same duration was conducted.
Sample collection
Blood samples were collected from each dog at the beginning of the evaluation and at the end of the first experimental period after 18 wk. The same procedure was carried out before and at the end of the crossover period. Blood samples were collected from the cephalic vein and stored in 2 tubes, one with heparin and the other without anticoagulant. Heparinized plasma samples and serum samples were obtained by blood centrifugation at 6000 × g for 1.5 min at 37°C.
Metabolic profile evaluation
Ten milliliters of blood were collected from the cephalic vein for the oxidative stress assessment. After blood centrifugation in heparinized test tubes, the plasma was separated from the serum and analyzed. Oxidative stress was measured with a spectrophotometric point-of-care assay (Free Radical Analytical System FRAS 4; Evolvo, Langhirano, Italy). The dROMs test and the BAP test were completed. In the dROMs test, reactive oxygen metabolites (primarily hydroperoxides) of the sample generated alkoxyl and peroxyl radicals, in the presence of iron released from plasma proteins by an acidic buffer, according to the Fenton reaction. Such radicals then oxidized an alkyl-substituted aromatic amine (N,N-dietylparaphenylendiamine), thus producing a pink-colored derivative, which is photometrically quantified at 505 nm. The concentration of the dROMs is directly proportional to the color intensity and is expressed as U CARR (Carratelli units). One U CARR corresponds to 0.08 mg/dL hydrogen peroxide. In normal dogs, dROM values range from 50 to 90 U CARR.
In the BAP test, the plasma samples were mixed with a colored solution obtained by mixing a ferric chloride solution with a thiocyanate derivative solution that causes a discoloration, the intensity of which is measured photometrically at 505 nm and is proportional to the ability of the plasma to reduce ferric ion (16). The results are expressed as μMol/L of reduced ferric ions. In normal dogs, BAP values range from 2400 to 2200 μMol/L. The reference values of dROMs and BAP test were validated for canine species (14,21) and are summarized in Table I.
Table I.
Reference values of dROMs (1 U Carr = 0.08 mg H2O2/dL) and BAP test
| dROMs reference values | BAP reference values | ||
|---|---|---|---|
| Normal values | 50 to 90 U CARR | Optimal values | 2400 to 2200 μmol/L |
| Threshold borderline | 92 to 95 U CARR | Threshold borderline | 2200 to 2000 μmol/L |
| Mild oxidative stress | 100 to 120 U CARR | Slight deficiency | 2000 to 1800 μmol/L |
| Oxidative stress | 140 to 200 U CARR | Deficiency | 1800 to 1600 μmol/L |
| Strong oxidative stress | 220 to 300 U CARR | Strong deficiency | 1600 to 1400 μmol/L |
| Strong oxidative stress | Over 300 U CARR | Very strong deficiency | < 1400 μmol/L |
dROMs — reactive oxygen metabolites-derivatives; U Carr — Carratelli units; BAP — biological antioxidant potential.
A metabolic profile was also investigated for each dog, including albumin, alkaline phosphatase (ALP), total bilirubin, calcium, cholesterol, creatine phosphokinase (CPK), creatinine, phosphorus, gamma-glutamyl transferase (GGT), glucose, aspartate aminotransferase (GOT), glutamate pyruvate transaminase (GPT), total proteins, triglycerides, and urea (Dimension RxL Max Integrated Chemistry System; Siemens Healthcare Diagnostics, Milan, Italy).
Statistical analysis
Data were analyzed using GraphPad Prism 6 software (GraphPad Software, La Jolla, California, USA). All data are presented as the means ± SEM and were first checked for normality using the D’Agostino-Pearson normality test. Differences in dROMs, BAP, and metabolic profile parameters between the 2 diets before (T0) and at the end (T1) of the first evaluation and crossover were analyzed using a 2-way analysis of variance (ANOVA), followed by Sidak’s multiple comparisons test.
Results
Both diets were similar in chemical composition and fatty acid profile. The predominant fatty acids of the lipid fraction in both diets were palmitic acid (C16:0) and oleic acid (C18:1 c9), which were present in similar proportions (22% for palmitic acid and 35% for oleic acid). The amount of linolenic acid (C18:3 n3) was higher (+28%) in the experimental than in the control diet.
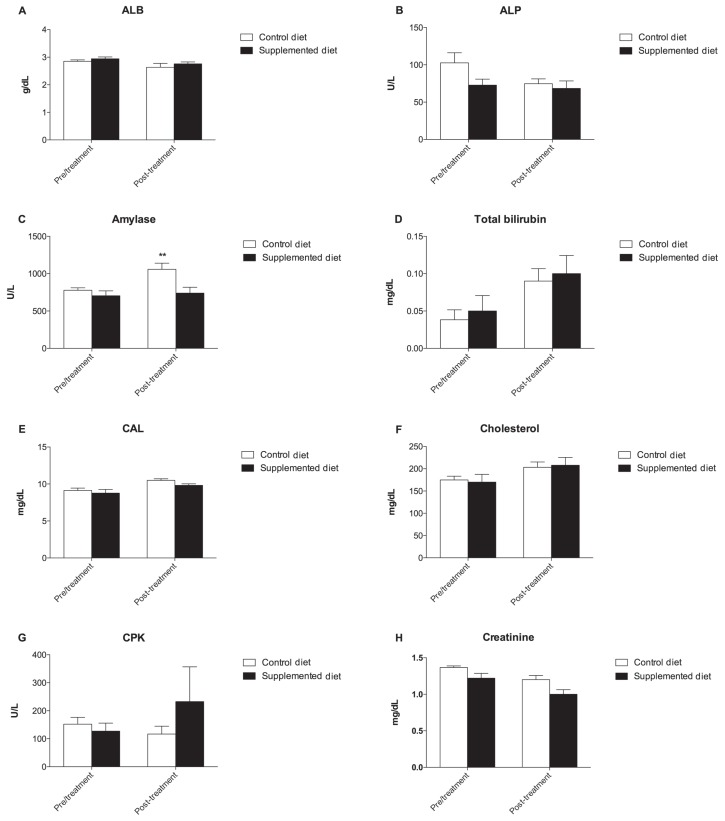
The metabolic profile trend of the CD and SD group during the evaluation period is shown in Figure 1. Amylase concentration significantly increased from a T0 value of 778 ± 32.10 U/L to 1059 ± 82.75 U/L at T1 in the CD group (P < 0.01) (Figure 1C). Conversely, creatinine and triglycerides decreased significantly in the SD group from a T0 value of 1.22 ± 0.06 mg/dL to 1.00 ± 0.06 mg/dL at T1 and from a T0 value of 164.6 ± 55.73 mg/dL to 60.20 ± 9.51 mg/dL at T1, respectively (P < 0.05) (Figures 1H to P).
Figure 1.
The metabolic profile trend of the commercial diet (CD) and supplemental diet (SD) groups during the evaluation period
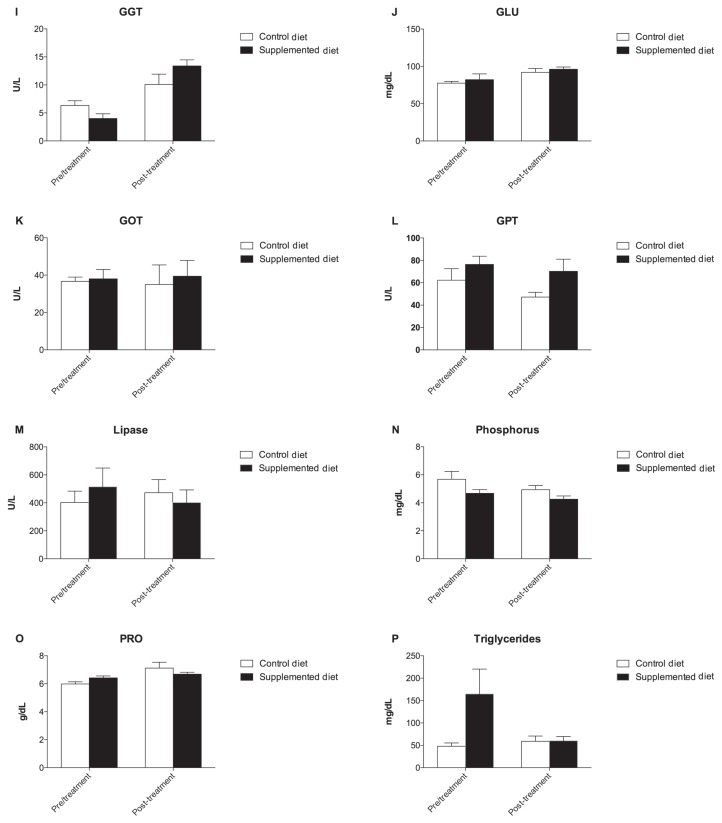
The concentration of dROMs and BAP decreased significantly from a T0 value of 142.6 ± 13.86 U CARR to 111.4 ± 7.97 U CARR at T1 in the SD group and from a T0 value of 3332 ± 177.5 μMol/L to 2954 ± 58.14 μMol/L at T1 in the CD group, respectively (P < 0.05) (Figure 2).
Figure 2.
Graphical representation of oxidative stress parameters concentration of dogs. A significant decrease in dROMs and BAP concentration was observed after 18 weeks of supplementation with SD and CD diet, respectively (*P < 0.05). A — dROMs and B — BAP values before (T0) and after 18 weeks (T1) of diet supplementation.
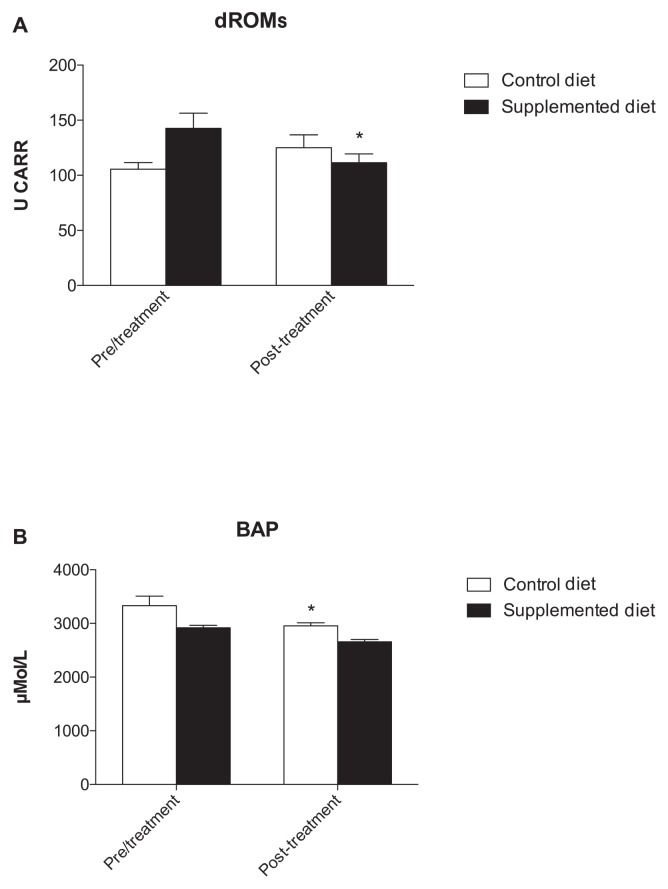
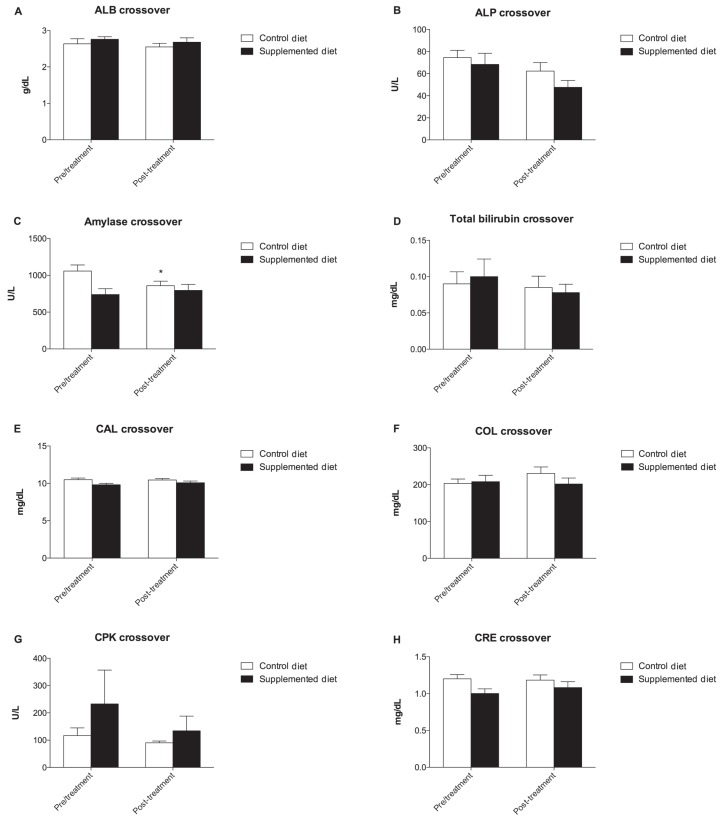
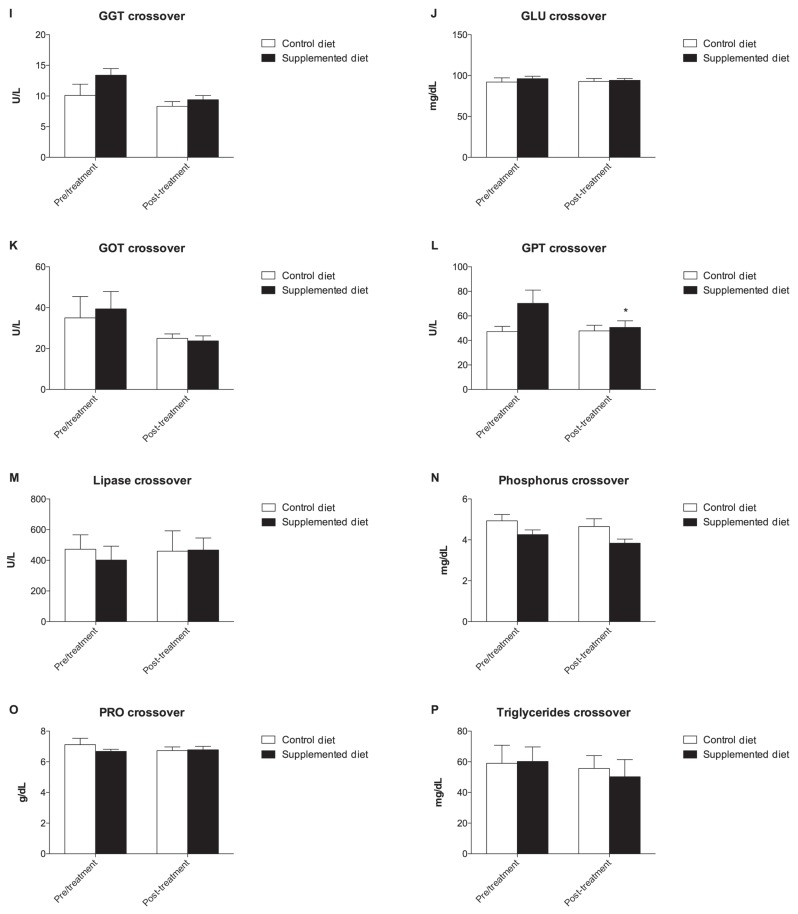
At the end of this period, groups were crossed over and fed for another 18 wk and the metabolic profile was reassessed (Figure 3). Amylase and GPT concentration significantly decreased from a T0 value of 1059 ± 82.75 U/L to 859.2 ± 61.02 U/L at T1 in the CD group and from a T0 value of 70.20 ± 10.83 U/L to 50.60 ± 5.38 U/L at T1 in the SD group, respectively (P < 0.05) (Figures 3C to L). Although no significant variation of dROMs was observed, a decrease in the CD group and an increase in the SD group was observed at the end of the crossover period, probably due to the antioxidant activity of the diet (Figure 4A). Similar to what was observed in the first evaluation, a decrease in BAP concentration was observed in the CD group (Figure 4B).
Figure 3.
Graphical representation of metabolic profile of dogs. A significant decrease in GPT concentration was observed after SD diet supplementation (3L, *P < 0.05). (A) albumin, (B) alkaline phosphatase, (E) calcium, (G) creatine phosphokinase, (I) Gamma-glutamyl transpeptidase, (J) glucose, (K) Glutamic-oxaloacetic transaminase, (L) Glutamic — pyruvic transaminase, (O) total protein.
Figure 4.
Graphical representation of oxidative stress parameters concentration of dogs. A — dROMs values and B — BAP before (T0) and after 18 weeks (T1) of diets supplementation.
Discussion
The objective of this study was to evaluate the potential ability of a long-term antioxidant-supplemented diet to control the oxidative stress and general health status of therapy dogs involved in AAI programs. Environmental stress, such as temperature, working, and dog competitions may induce psychological and physical stress, which could also influence the food intake of dogs, whether it is excessive or not enough.
An unbalanced diet lacking in essential nutrients weighs on the general health status of dogs and may represent a risk factor for metabolic and degenerative diseases. Among the mechanisms that can influence health status, the balance of oxidative stress plays a relevant role. Metabolic oxidative reactions constantly take place in animals in order to balance the production of free radicals with antioxidant molecules.
The significant reduction (P < 0.05) of dROMs and BAP values observed in dogs fed the SD diet during the first experimental period clearly demonstrated the ability of the diet to reduce oxidative stress, thus indicating a positive trend towards the normal values observed for healthy dogs (14). Conversely, the significant reduction observed in the CD-fed group confirmed the lack of any antioxidant effect related to the diet. It is worth noting that, while a long period of washout was not observed, the overall trend of BAP and dROMs improved after SD supplementation, although slightly influenced by the previous diet assumption.
On the other hand, the reduction of the amylase enzyme after the crossover could be related to the inhibitory activity of antioxidant molecules on key enzymes linked to type-2 diabetes (α-amylase and α-glycosidase) as previously observed in in-vitro studies by using flavonoid, anthocyanin, and other polyphenol compounds (22–25). The decrease in GPT level after the crossover suggests that the SD diet is endowed with liver-protective properties as reported in studies where different polyphenol extracts were tested in laboratory animals (26,27). As a result, the beneficial effects of antioxidant supplementation on liver and pancreatic metabolism were demonstrated.
The antioxidant formulation used in this study was based on grape seed extract, quercetin, blueberry, resveratrol, and strawberry and blackberry dried extracts (Vitaberry Plus). All these compounds contain anthocyanins and polyphenols, which have antioxidant effects. The total proanthocyanidin content of grape seed extract also included catechins and epicatechins and possesses antioxidant properties and protects the vascular system (28). The present study clearly showed a decrease in dROM species after the administration of a specific diet enriched with antioxidants. This scavenger activity was in agreement with other studies about the antioxidant effects of single active ingredients included in antioxidant supplements. For example, it has been demonstrated that supplementing with grape seed extract decreased the activity of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs (29).
In a similar study, grape seed extract significantly reduced (by 60%) the formation of azoxymethane-induced aberrant crypt foci, probably due to its activity in reducing the levels of cyclin D1, COX-2, and iNOS involved in the foci genesis (30). While grape seed and blueberry extract are endowed with antioxidant activity, quercetin supplementation is known to prevent age-related diseases in dogs (31). Moreover, the anthocyanins and polyphenols in blueberry extract have been shown to exert antioxidant effects in some pathological conditions, such as hypobaric hypoxia, due to the inhibition of lipid peroxidation by interfering with glutathione activity (32,33). The antioxidant levels of sled dogs supplemented with blueberries were studied and blood creatine kinase levels and oxidative status were evaluated 48 h after exercise (34). Although the exercise protocol did not influence concentrations of creatine kinase, supplementation with blueberries significantly increased the total antioxidant status after exercise. In addition to being an antioxidant, blueberry extract has also demonstrated genoprotective and behavioral effects (35). Specifically, 30 mice received a supplementation of blueberry extract for 30 d. After that period, all treated animals had a higher significant memory retention in all test sessions after 7 and 30 d of supplementation, while in-vitro examination revealed significantly reduced DNA damage in hippocampal tissues than in the control group.
Resveratrol (3,5,4′-trihydroxystilbene), a polyphenol naturally occurring in grapes, berries, peanuts, and other vegetables, has also been shown to have antioxidant, anti-inflammatory, and anti-proliferative properties (36,37). Although there is a lack of scientific studies on resveratrol supplementation in pets, it has been demonstrated that resveratrol was able to protect from oxidative stress in hepatic steatosis conditions, significantly increasing GSH and GPx levels in liver tissues and significantly decreasing ROS levels in 30 mice (38).
Antioxidant and anti-inflammatory activity have been ascribed to strawberry extract due to its high content of flavonoids (39). For example, a daily intake of 500 g of strawberries for 9 d increased non-urate plasma 2,2-diphenyl-1-picrylhydrazyl radical decomposition, thus possibly decreasing the risk of systemic oxidants over activity (excessive activity) (40).
Antioxidant and anti-inflammatory activities were also investigated for a polyphenolic blackberry extract by means of the oxygen radical absorbance capacity assay (ORAC) (41). The ORAC value of the blackberry extract was higher than the ORAC of quercetin and ellagic acid. Moreover, the blackberry extract inhibited superoxide production by NADPH oxidase (nicotinamide adenine dinucleotide phosphate-oxidase) in THP-1 cells and nitrite production in J774A.1 cells after 4 h of incubation, showing a scavenger activity, while a reduction of nitrites was observed after 24 h of incubation.
A dog’s diet may be qualitatively and quantitatively appropriate to the type and intensity of the activity as well as to its age, breed, and gender. Balanced nutrition could therefore be crucial to restoring good overall health status. This study confirms that long-term supplementation with a specific diet enriched with antioxidants is able to regulate the antioxidant status and general health status of working dogs involved in animal-assisted intervention (AAI) programs and prevent damage due to oxidative stress before and after their work.
Acknowledgment
This study was supported by a grant from Nova Foods in Vicenza, Italy.
Footnotes
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Mantovani G, Maccio A, Madeddu C, et al. Quantitative evaluation of oxidative stress, chronic inflammatory indices and leptin in cancer patients: Correlation with stage and performance status. Int J Cancer. 2002;98:84–91. doi: 10.1002/ijc.10143. [DOI] [PubMed] [Google Scholar]
- 2.Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc. 2000;32:1576–1581. doi: 10.1097/00005768-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 4.Watson TA, MacDonald-Wicks LK, Garg ML. Oxidative stress and antioxidants in athletes undertaking regular exercise training. Int J Sport Nutr Exerc Metab. 2005;15:131–146. doi: 10.1123/ijsnem.15.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985) 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan SD, Anuradha CV. Exercise, depletion of antioxidants and antioxidant manipulation. Cell Biochem Funct. 1998;16:269–275. doi: 10.1002/(SICI)1099-0844(1998120)16:4<269::AID-CBF797>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Leaf DA, Kleinman MT, Hamilton M, Deitrick RW. The exercise-induced oxidative stress paradox: The effects of physical exercise training. Am J Med Sci. 1999;317:295–300. doi: 10.1097/00000441-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hargreaves BJ, Kronfeld DS, Waldron JN, et al. Antioxidant status and muscle cell leakage during endurance exercise. Equine Vet J Suppl. 2002:116–121. doi: 10.1111/j.2042-3306.2002.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirschvink N, Art T, de Moffarts B, et al. Relationship between markers of blood oxidant status and physiological variables in healthy and heaves-affected horses after exercise. Equine Vet J Suppl. 2002:159–164. doi: 10.1111/j.2042-3306.2002.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 11.White A, Estrada M, Walker K, et al. Role of exercise and ascorbate on plasma antioxidant capacity in thoroughbred race horses. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:99–104. doi: 10.1016/s1095-6433(00)00286-5. [DOI] [PubMed] [Google Scholar]
- 12.Baskin CR, Hinchcliff KW, DiSilvestro RA, et al. Effects of dietary antioxidant supplementation on oxidative damage and resistance to oxidative damage during prolonged exercise in sled dogs. Am J Vet Res. 2000;61:886–891. doi: 10.2460/ajvr.2000.61.886. [DOI] [PubMed] [Google Scholar]
- 13.Piercy RJ, Hinchcliff KW, DiSilvestro RA, et al. Effect of dietary supplements containing antioxidants on attenuation of muscle damage in exercising sled dogs. Am J Vet Res. 2000;61:1438–1445. doi: 10.2460/ajvr.2000.61.1438. [DOI] [PubMed] [Google Scholar]
- 14.Pasquini A, Luchetti E, Cardini G. Evaluation of oxidative stress in hunting dogs during exercise. Res Vet Sci. 2010;89:120–123. doi: 10.1016/j.rvsc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Wyse C, Cathcart A, Sutherland R, et al. Effect of maximal dynamic exercise on exhaled ethane and carbon monoxide levels in human, equine, and canine athletes. Comp Biochem Physiol A Mol Integr Physiol. 2005;141:239–246. doi: 10.1016/j.cbpb.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power:” The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Bote CJ, Rey AI, Sanz M, Gray JI, Buckley DJ. Dietary vegetable oils and alpha-tocopherol reduce lipid oxidation in rabbit muscle. J Nutr. 1997;127:1176–1182. doi: 10.1093/jn/127.6.1176. [DOI] [PubMed] [Google Scholar]
- 18.Motta S, Letellier C, Ropert M, Motta C, Thiebault JJ. Protecting effect of vitamin E supplementation on submaximal exercise-induced oxidative stress in sedentary dogs as assessed by erythrocyte membrane fluidity and paraoxonase-1 activity. Vet J. 2009;181:288–295. doi: 10.1016/j.tvjl.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Bian ZX, Shang HC. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2011;154:290–291. doi: 10.7326/0003-4819-154-4-201102150-00016. [DOI] [PubMed] [Google Scholar]
- 20.Urbaniak GC, Plous S. Research Analyzer 1997–2016. [Last accessed April 25, 2017]. [page on Internet]. Available from: https://www.randomizer.org/
- 21.Sechi S, Chiavolelli F, Spissu N, et al. An antioxidant dietary supplement improves brain-derived neurotrophic factor levels in serum of aged dogs: Preliminary results. J Vet Med. 2015;2015:412501. doi: 10.1155/2015/412501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Xu P, Wang Y, Wang Y, Hochstetter D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against alpha-amylase and alpha-glucosidase in vitro. Molecules. 2013;18:11614–11623. doi: 10.3390/molecules180911614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64:2458–2461. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- 24.Oboh G, Ademosun AO, Ademiluyi AO, Omojokun OS, Nwanna EE, Longe KO. In vitro studies on the antioxidant property and inhibition of alpha-amylase, alpha-glucosidase, and angiotensin I-converting enzyme by polyphenol-rich extracts from cocoa (Theobroma cacao) bean. Patholog Res Int. 2014;2014:549287. doi: 10.1155/2014/549287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006;52:149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 26.Nwanna EE, Oboh G. Antioxidant and hepatoprotective properties of polyphenol extracts from Telfairia occidentalis (fluted pumpkin) leaves on acetaminophen induced liver damage. Pak J Biol Sci. 2007;10:2682–2687. doi: 10.3923/pjbs.2007.2682.2687. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda H, Tanaka J, Kikuchi M, et al. Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine: Hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut. J Agric Food Chem. 2008;56:4444–4449. doi: 10.1021/jf8002174. [DOI] [PubMed] [Google Scholar]
- 28.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14:226–246. [PubMed] [Google Scholar]
- 29.Gessner DK, Fiesel A, Most E, et al. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet Scand. 2013;55:18. doi: 10.1186/1751-0147-55-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010;49:641–652. doi: 10.1002/mc.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinboth M, Wolffram S, Abraham G, Ungemach FR, Cermak R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br J Nutr. 2010;104:198–203. doi: 10.1017/S000711451000053X. [DOI] [PubMed] [Google Scholar]
- 32.Moskaug JO, Carlsen H, Myhrstad MC, Blomhoff R. Polyphenols and glutathione synthesis regulation. Am J Clin Nutr. 2005;81:277S–283S. doi: 10.1093/ajcn/81.1.277S. [DOI] [PubMed] [Google Scholar]
- 33.Zepeda A, Aguayo LG, Fuentealba J, et al. Blueberry extracts protect testis from hypobaric hypoxia induced oxidative stress in rats. Oxid Med Cell Longev. 2012;2012:975870. doi: 10.1155/2012/975870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlap KL, Reynolds AJ, Duffy LK. Total antioxidant power in sled dogs supplemented with blueberries and the comparison of blood parameters associated with exercise. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:429–434. doi: 10.1016/j.cbpa.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Barros D, Amaral OB, Izquierdo I, et al. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol Biochem Behav. 2006;84:229–234. doi: 10.1016/j.pbb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Frombaum M, Le Clanche S, Bonnefont-Rousselot D, Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and *NO bioavailability: Potential benefits to cardiovascular diseases. Biochimie. 2012;94:269–276. doi: 10.1016/j.biochi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Yu W, Fu YC, Wang W. Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem. 2012;113:752–759. doi: 10.1002/jcb.23431. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W, Chen S, Li Z, et al. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr Metab (Lond) 2014;11:35. doi: 10.1186/1743-7075-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu A, Wilkinson M, Penugonda K, Simmons B, Betts NM, Lyons TJ. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr J. 2009;8:43. doi: 10.1186/1475-2891-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prymont-Przyminska A, Zwolinska A, Sarniak A, et al. Consumption of strawberries on a daily basis increases the non-urate 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of fasting plasma in healthy subjects. J Clin Biochem Nutr. 2014;55:48–55. doi: 10.3164/jcbn.13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azofeifa G, Quesada S, Boudard F, et al. Antioxidant and anti-inflammatory in vitro activities of phenolic compounds from tropical highland blackberry (Rubus adenotrichos) J Agric Food Chem. 2013;61:5798–5804. doi: 10.1021/jf400781m. [DOI] [PubMed] [Google Scholar]