Abstract
Introduction:
Oxidative stress (OS) is considered a significant contributor to male infertility. A number of laboratory techniques have been developed to evaluate oxidative stress in the semen. We review these tests and their current use.
Methods:
A literature review was performed utilizing the PubMed search engine for articles studying OS etiology and impact on male fertility, and the laboratory tests used in its assessment.
Results:
The state of OS results from exaggerated production of oxygen-derived free radicals, also known as reactive oxygen species, to an extent overwhelming the body's antioxidant defense mechanisms. Several laboratory tests have been utilized in OS measurement during male fertility evaluation. These tests are classified into direct assays which measure the degree of oxidation within a sperm cell and indirect assays which estimate the detrimental effects of OS. The chemiluminescence assay, flow cytometry, nitroblue tetrazolium assay, and cytochrome c reduction are examples of direct assays while the myeloperoxidase test and measurements of lipid peroxidation, oxidation-reduction potential, and total antioxidant capacity are examples of the indirect assays.
Conclusion:
OS measurement is an important tool that may help in understanding the pathophysiology of male infertility and provide valuable information that would guide treatment decisions and patient follow-up.
INTRODUCTION
The male contributes to almost half the cases of infertility among couples-seeking treatment for failure of conception.[1] Major advancements were witnessed in the past few decades that refined our understanding of the etiologies of infertility in men. Several pretesticular, testicular, and posttesticular causes were identified, and treatment became possible.[2] Oxidative stress (OS) has been recognized as a common denominator through which various etiologies can impair sperm production and function at the molecular level.[3,4,5,6,7,8,9,10] It is essentially defined as the imbalance between oxidants and reductants in any given environment. Like other aerobic cells, sperm oxygen metabolism produces highly reactive oxidizing molecules called reactive oxygen species (ROS), which play an important role in cell signaling and homeostasis. Under normal physiologic circumstances, ROS are necessary for optimal sperm function. They can potentiate sperm fertilizing capabilities such as capacitation, hyperactivation, acrosome reaction, and oocytes fusion.[11,12,13] Excessive levels of ROS can be produced by a variety of environmental toxins or pathologic processes resulting in detrimental impact on health overall and fertility in particular. To minimize the hazardous effects of excessive ROS levels, a number of endogenous enzymatic and nonenzymatic antioxidants exist to scavenge or neutralize excess ROS.
Whenever ROS production exceeds the neutralizing or scavenging capabilities of antioxidants, a state of OS develops. This state can accelerate apoptosis,[14] sperm DNA damage,[15,16] and lipid peroxidation (LPO).[17] Several reports have confirmed the presence of high ROS levels in the semen of infertile men. Negative correlations were detected between ROS levels and sperm morphology, and the presence of high levels of ROS were also associated with an increase in sperm DNA fragmentation.[14,18] This article aims to provide an insight into the factors associated with elevated seminal OS measures, and the various diagnostic modalities used to confirm its presence.
METHODS
A PubMed search of all articles using the terms male infertility, OS, and advanced sperm function tests was performed, and relevant articles were selected for this review. Topics discussing origins of OS, impact of OS on male fertility, and assessment methods for OS were identified for further elaboration.
ORIGINS OF OXIDATIVE STRESS
ROS production can be exaggerated in a number of endogenous and exogenous circumstances placing an individual at risk for OS. A summary of such conditions can be seen in Figure 1.
Figure 1.
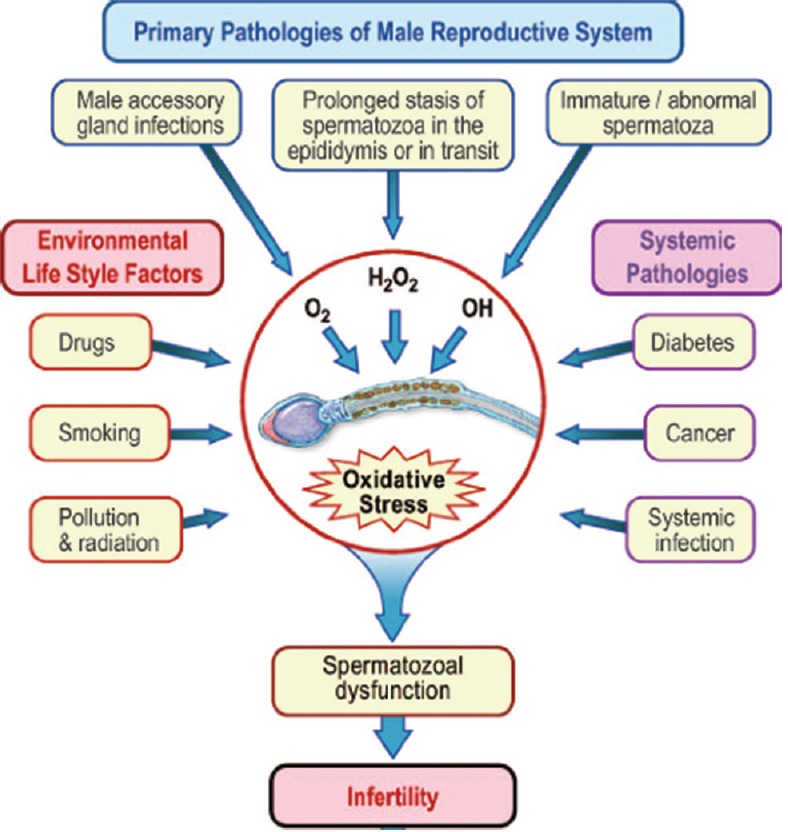
Etiology of oxidative stress
Endogenous sources of reactive oxygen species
Varicocele
It is the leading cause of male factor infertility, prevalent in 40% and 80% of patients with primary and secondary infertility, respectively.[19] Many mechanisms have been postulated in the pathophysiology of varicocele. Testicular hyperthermia and hypoxia are the most commonly accepted theories resulting in OS-induced testicular dysfunction.[9,20] One meta-analysis confirmed the presence of significantly higher OS parameters such as ROS and LPO in semen samples from infertile patients with varicocele compared with normal fertile donors.[20] Furthermore, seminal ROS levels were found to have a positive correlation with varicocele grade, in which significantly higher levels of seminal ROS were seen in men with grades 2 and 3 varicocele versus men with grade 1 varicocele.[21] Mostafa et al. demonstrated a significant reduction in seminal plasma ROS levels and an elevation of antioxidant concentrations after varicocele surgery in infertile men.[22]
Leukocytospermia
Peroxidase-positive leukocytes have been recognized as the most significant source of ROS in seminal plasma.[23] These leukocytes mainly originate from the prostate and seminal vesicles, and their ROS excretion can be increased by 100-folds when activated by infection or inflammation.[24] Leukocytospermia, a condition characterized by abnormally high levels of peroxidase-positive leukocytes in seminal fluid, was detected in up to 20% of infertile men and associated with significant elevations in seminal ROS levels and sperm DNA damage.[24] A recent study by Lobascio et al.[25] demonstrated the presence of a positive correlation between the number of seminal leukocytes and ROS levels (P < 0.001; n = 125). Moreover, they confirmed the negative implication of ROS on sperm parameters and function by identifying negative correlations between ROS with sperm concentration (P = 0.01) and motility (P = 0.02), and a positive correlation with sperm DNA fragmentation (P = 0.08).[25] Further studies have confirmed a link between chronic infectious or inflammatory genitourinary conditions such as chronic prostatitis and accessory sex gland infections and male infertility mediated by OS.
Immature spermatozoa
The sperm cell is capable of producing ROS which may be exaggerated in immature forms. Excessive residual cytoplasm (cytoplasmic droplets) is a typical finding in immature sperm and has been linked with increased ROS generation.[26] Alani and El Yaseen[27] have found a significant positive correlation between creatine kinase activity, a biomarker of cytoplasmic space, and malondialdehyde (MDA), a marker for LPO, in sperm fractions from infertile men. Mitochondria are another major source of ROS which can be amplified in spermatozoa of infertile men with mitochondrial dysfunctions.[28]
Exogenous sources of reactive oxygen species
Various habitual and environmental exposures have been linked with OS-induced infertility in men.
Smoking
Cigarette smoking is perhaps most influential; containing more than 7000 chemical compounds including alkaloids, nitrosamines, and inorganic molecules[29] which can adversely affect fertility in a number of ways. Many chemicals were shown to incite an imbalance between ROS and antioxidants in the semen of smokers.[30] Studies have confirmed that smoking has a detrimental effect on conventional semen parameters,[31] sperm fertilizing capacity,[32] and fertility potential.[33] A 48% increase in leukocyte concentrations and a 107% increase in ROS levels have been detected in semen samples of smokers.[34]
Alcohol consumption
Alcohol is another habitual substance that has been recognized as a promoter of ROS production interfering with the body's antioxidant defense mechanism, especially in the liver. Acetaldehyde, a by-product of ethanol metabolism, is particularly involved in ROS production causing molecular damage to proteins, lipids, and DNA. Saalu evaluated 46 alcoholic men of reproductive age and reported a significant increase in serum LPO by-products and a decrease in antioxidants, suggesting a relationship between alcohol consumption and OS within the testes.[35]
Environmental exposures
Environmental toxins such as those found in structural materials or industrial products can accumulate in the body and increase ROS production in the testes which can negatively impact sperm production.[36] Plastic compounds, such as phthalates, that are commonly used for domestic and industrial purposes have been found to impair spermatogenesis and induce sperm DNA damage.[37,38] Moreover, industrial workers regularly exposed to toxins such as cadmium, chromium, lead, manganese, and mercury were more likely to have decreased sperm quality, count, volume, and density[39,40]
Radiation
Radiation exposure has significant clinical effects on humans. Several studies have connected radiation emitted from mobile phones to an increase in seminal ROS production with concurrent impairment in semen quality.[41,42] In vitro studies demonstrated a dose-dependent electromagnetic radiation-induced ROS production and DNA damage in human spermatozoa resulting in an impairment in sperm concentration, motility, and vitality.[43] The International Agency for Research on Cancer classified mobile phone radiation as category 2B with possibly carcinogenic effects on the human body.[44]
The detrimental effects of chemotherapeutic medications on fertility potential are well established. Medications such as cyclosporine and cyclophosphamide were linked with increased ROS levels and decreased antioxidant levels, thus impairing semen parameters.[45]
Iatrogenic
OS during assisted reproductive techniques has been recognized as a potential reason for suboptimal pregnancy outcomes following such procedures. Factors contributing to the accumulation of ROS in vitro originate from various sperm manipulations that are often undertaken in an environment susceptible to OS and lacking endogenous antioxidant defense mechanisms. Centrifugation, leukocyte contamination, oxygen partial pressure, light, culture media, cryopreservation, and thawing have all been implicated.[46]
IMPLICATIONS OF OXIDATIVE STRESS
As previously stated, OS-induced male infertility is believed to result from exaggerated apoptosis, LPO, and sperm DNA damage. Apoptosis or programmed cell death is essential for normal development of spermatozoa as well as the adjustment of the number of sperm cells that are produced by the tests.[47] While apoptosis may be triggered by several intrinsic and extrinsic factors, ROS levels appears to be directly correlated with the extent of sperm cell death. The presence of higher levels of ROS was significantly correlated with a higher percentage of apoptosis in the seminal plasma of infertile men compared to healthy volunteers.[14]
LPO is perhaps the most extensively studied manifestation of ROS in biology. It is generally defined as oxidative damage of fatty acids containing more than two carbon double bonds, also known as polyunsaturated fatty acids (PUFA).[48] Spermatozoa are predominantly vulnerable to the damage caused by excessive ROS because their plasma membranes contain extraordinary large amounts of PUFA increasing their susceptibility for LPO.[49]
Sperm DNA is uniquely structured to keep its nuclear chromatin compact and protected against injury. ROS can alter DNA integrity through the modification of nucleic bases resulting from deletions, cross-links, or even chromosomal rearrangements.[50,51,52] The relationship between OS and DNA damage has been proven in numerous reports. Iommiello et al. investigated semen samples from 56 infertile men and revealed a significant positive correlation between ROS levels and the degree of sperm DNA fragmentation (P = 0.037).[53] In another study, semen collected from 63 patients attending an IVF showed a strong positive correlation between intrinsic ROS levels and sperm DNA fragmentation measurements.[54]
ASSESSMENT OF SEMINAL OXIDATIVE STRESS
Screening for OS is increasingly gaining attention in the evaluation of infertile men as current evidence has confirmed its implications in various disease entities.[55] OS has been linked with unexplained[56] and idiopathic male infertility.[55] Clinical conditions such as varicocele,[4,57] infection, inflammation,[58] and spinal cord injury[59] have also been linked with OS-induced male subfertility highlighting the significance of OS testing in these clinical scenarios to aid in fertility evaluation. Moreover, assessing seminal OS levels over time would help in monitoring antioxidant therapies and define effective doses and durations. Despite a growing body of evidence, routine testing of OS is currently not indicated in the evaluation of infertile men. Reasons are principally related to test availability, complexity, cost-effectiveness, and more importantly, lack of a universally accepted analysis method. Currently, more than thirty different assays have been described to measure the presence of seminal OS. They are classified into direct or indirect ROS measurement assays [Table 1]. Direct assays measure the degree of oxidation within the sperm cell membrane.[60] Despite their ability to provide accurate measures of OS, their routine use is hindered by the difficulties experienced while quantifying the short-lived ROS and also by their expensive nature.[61] On the other hand, indirect assays estimate the detrimental effects of OS, such as DNA damage or LPO levels.[60] Their relatively simple nature is perhaps what makes them favored over the direct assays; however, they assess an end state which could occur secondary to other unknown pathologic processes. The most commonly utilized methods for the detection of ROS levels include as follows.
Table 1.
Various direct and indirect assays to measure oxidative stress in human semen

Measurement of reactive oxygen species
The chemiluminescence assay is the most commonly utilized technique for seminal ROS measurement among andrology laboratories. This assay quantifies both intracellular and extracellular ROS using sensitive probes[62] that react with oxidative end products forming an electrical signal which can be measured as counted photons per minute with a luminometer [Figure 2].[63] Two probes or reagents are available, namely, luminol (5-amino-2,3-dihydro-1,4-phthalazinedione and 3-aminophthalic hydrazide) and lucigenin (N, N’-dimethyl-9,9’-biacridinium dinitrate). Luminol initiates its signal through a one-electron oxidative event that is mediated by hydrogen peroxide.[64] While lucigenin works through a one-electron reduction reaction forming a radical which gives up its electron to oxygen to create superoxide.[64] Luminol is the better of the two reagents[65,66] as (1) it has the ability to react with different ROS, including superoxide anion, hydroxyl radical, and hydrogen peroxide; (2) it measures both intra and extracellular free radicals; and (3) conducts a fast reaction allowing rapid measurement.[67] To ensure accurate readings, semen samples should be analyzed within the first hour of collection and must contain a sperm concentration of at least 1 × 106/mL. Measurement of ROS using chemiluminescence is relatively sensitive and has well-established ranges in infertile and fertile population.[63] Despite its clinical applicability, the widespread use of the chemiluminescence assay has been hampered by equipment expenses and the presence of assay confounders such as incubation time, leukocyte, and seminal plasma contamination.[62] A variety of luminometers are available for the measurement of the light intensity emitted by the chemiluminescence reaction. Single- and double-tube luminometers are sensitive and inexpensive but can measure only one or two samples at a given time, which are suitable for small research laboratories. On the other hand, multiple tube or plate luminometers are more expensive since they can measure multiple samples at the same time and are suitable for centers that are engaged in regular research work on chemiluminescence.[68]
Figure 2.
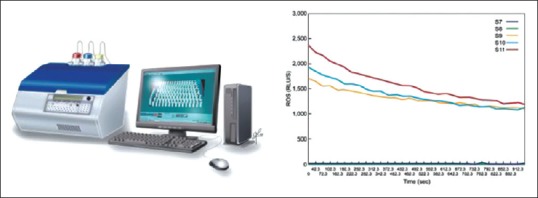
Reactive oxygen species measurement by chemiluminescence assay: AutoLumat 953 Plus Luminometer connected to a computer with a sample result graph
Seminal antioxidant levels can also be measured through the enhanced chemiluminescence assay or through a colorimetric assay. Following the addition of a known concentration of ROS to the semen, the emitted chemiluminescence signal or color change is measured representing residual ROS left after antioxidant depletion. Thus, the intensity of the produced signal is inversely correlated with the total antioxidant capacity of the specimen.[69]
Flow cytometry is alternative method that can also be used to measure intracellular sperm ROS.[70] It quantifies the amount of fluorescence per cell. Excited by a light source, cells emit light that is passed through optical filters before it reaches the optical detectors. Optical filters allow light of specific wavelengths to pass, thereby producing waves of specific colors. Flow cytometry is also an expensive tool that is not practical for widespread clinical use.
The nitroblue tetrazolium (NBT) assay is another cost-effective and user-friendly method that can accurately predict ROS levels and provide insight on the potential sources of OS (such as spermatozoa or leukocytes) with the use of a light microscope. The basis for this test is based on the conversion of NBT into a blue pigment called diformazam after interacting with superoxide released from spermatozoa or leukocytes. The concentration of diformazam can then be measured and correlated with intracellular ROS concentration.[55,71]
Lipid peroxidation markers
Markers of LPO have been widely used as tools for OS measurement. Accumulation of lipid peroxides in the spermatozoa generates a variety of decay end-products such as MDA, hydroxynonenal, 2-propenal (acrolein), and isoprostanes, all which can be measured and act as indicators of OS.[72]
The most commonly used method is MDA level measurement through the thiobarbituric acid (TBA) assay. In this assay, MDA forms a 1:2 adduct with TBA producing a colored substance that can be measured by fluorometry or spectrophotometry. Sensitive high-pressure liquid chromatography equipment[73,74] or spectrofluorometric measurement of iron-based promoters[75] may be utilized for the detection of the low sperm MDA concentrations. On the other hand, seminal plasma MDA levels are 5–10-fold higher than sperm, making measurement with standard spectrophotometers possible.[76] The clinical relevance of MDA measurement emerges from its significant positive correlation with seminal ROS levels in men with infertility, compared with fertile controls or normozoospermic individuals[76,77] Furthermore, in vitro studies have linked ROS-induced abnormalities in motility, sperm DNA integrity, and sperm-oocyte fusion with an increase in MDA concentration[75,78]
Other assays of sperm membrane LPO such as measurement of the isoprostane 8-Iso-PGF2α[79] and the c11-BODIPY assay[80] are promising but are not widely used in clinical practice at this time.
Measurement of oxidation-reduction potential
Oxidation-reduction potential (ORP), also known as the redox potential, is a measure of the potential for electrons to move from one chemical species to another.[81] ORP is a measure of this relationship between oxidants and antioxidants, providing a comprehensive measure of OS. Recently, a novel technology based on a galvanostatic measure of electrons has been developed (MiOXSYS, Aytu BioScience, USA) and utilized to evaluate changes in OS in trauma patients and as a function of extreme exercise[82,83,84] The MiOXSYS is a simple, rapid, and inexpensive system composed of the analyzer and disposable test sensor [Figure 3]. It measures the electron transfer from reductants (antioxidants) to oxidants under a steady low voltage reducing current. Thus, it provides an aggregate measure of all current oxidant activity and antioxidant activity in a sample. Higher ORP values (millivolts, mV) indicate a higher oxidant activity relative to the antioxidant activity, and therefore, a state of higher OS. Recent studies have confirmed the reliability of the MiOXSYS system in measuring ORP levels in semen and seminal plasma demonstrating the presence of significant negative correlations between ORP results and abnormalities of semen parameters.[85] A further attempt to utilize ORP measurement as a surrogate marker for male subfertility has stemmed from its ability to accurately predict abnormal semen parameters. Agarwal et al. investigated the measures of ORP in a group of infertile men (n = 106) comparing them to healthy controls (n = 51).[86] They confirmed the presence of significantly higher levels of ORP among the infertile group and demonstrated, using receiver operator curve analysis, that a cut-off level of 1.36 mv//106 sperm/ml was associated with a sensitivity of 69.6%, specificity of 83.1%, and overall accuracy of 75.2% (area under the curve = 0.770).[86]
Figure 3.
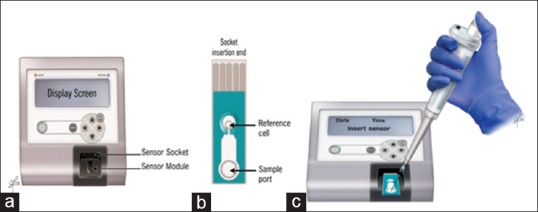
The MiOXSYS system comprises an (a) analyzer, (b) a disposable sensor, (c) application of the sample
Predictors of oxidative stress in semen studies
A number of routine laboratory tests have been suggested as possible predictors for the presence of OS in the semen.[87] While any abnormality in routine semen parameters (count, motility, and morphology) may be associated with OS, asthenozoospermia is probably the best surrogate marker for OS in a routine semen analysis[88,89] The presence of an exaggerated number of round cells in the semen may represent leukocytes and hence possible OS.[90] These cells, however, may represent immature germ cells and hence need to be tested with ancillary tests such as peroxidase test, CD45 staining, or measurement of seminal elastase activity to determine if they are leukocytes.[91,92] Poor sperm viability detected by the hypoosmotic swelling test or dye exclusion assays has also been linked with the presence of sperm OS.[93] In addition, macroscopic semen parameters have also been considered. Hyperviscosity of the seminal plasma, an observation that is commonly seen with infection, has been linked to increased levels of seminal plasma MDA[94] and reduced seminal plasma antioxidant status.[95]
CONCLUSION
OS has been acknowledged as a common pathway through which many etiologies and exposures impair sperm production. Much of our understanding of the pathophysiologic processes involved in male infertility stems from recent developments in OS testing. While several methods for testing have been described, their widespread use in clinical practice is hindered by their complexity and lack of standardization across laboratories. In this regard, ORP measurement appears to be a promising technique that is capable of overcoming obstacles faced with standard testing methods. Further research is still required to determine the most cost-effective, simple, and accurate technique that can be routinely used when evaluating male factor infertility.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 2.Kolettis PN, Lipshultz LR, McClure RD, Nangia AK, Naughton CK, Prins GS, et al. The optimal evaluation of the infertile male: AUA best practice statement. In: Jrrow J, Sigman M, editors. American Urological Association Education and Research. Linthicum, Maryland: American Urological Association, Inc; 2010. pp. 1–38. [Google Scholar]
- 3.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: Part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 5.Mahfouz RZ, du Plessis SS, Aziz N, Sharma R, Sabanegh E, Agarwal A. Sperm viability, apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. Fertil Steril. 2010;93:814–21. doi: 10.1016/j.fertnstert.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 6.du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H(2)O(2) exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42:206–10. doi: 10.1111/j.1439-0272.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 7.Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril. 2010;94:1531–4. doi: 10.1016/j.fertnstert.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh S, Riyaz AM, Shamsi MB, Kumar R, Gupta NP, Mittal S, et al. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia. 2009;41:251–6. doi: 10.1111/j.1439-0272.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 9.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 10.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 11.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–94. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- 12.Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108(Pt 5):2017–25. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- 13.de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med. 1995;18:487–95. doi: 10.1016/0891-5849(94)00169-k. [DOI] [PubMed] [Google Scholar]
- 14.Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, Jr, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19:129–38. doi: 10.1093/humrep/deh024. [DOI] [PubMed] [Google Scholar]
- 15.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79(Suppl 3):1597–605. doi: 10.1016/s0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Sharma RK, Sikka SC, Thomas AJ, Jr, Falcone T, Agarwal A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003;80:531–5. doi: 10.1016/s0015-0282(03)00756-8. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 18.Aziz N, Agarwal A, Lewis-Jones I, Sharma RK, Thomas AJ., Jr Novel associations between specific sperm morphological defects and leukocytospermia. Fertil Steril. 2004;82:621–7. doi: 10.1016/j.fertnstert.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 19.Kolettis P, Lipshultz L, McClure D, Nangia A, Naughton K, Prins G, et al. The optimal evaluation of the infertile male: AUA best practice statement. In: Jrrow J, Sigman M, editors. American Urological Association Education and Research. Linthicum, Maryland: American Urological Association, Inc; 2010. pp. 1–38. [Google Scholar]
- 20.Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: A meta-analysis. Reprod Biomed Online. 2006;12:630–3. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 21.Allamaneni SS, Naughton CK, Sharma RK, Thomas AJ, Jr, Agarwal A. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril. 2004;82:1684–6. doi: 10.1016/j.fertnstert.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 22.Mostafa T, Anis TH, El-Nashar A, Imam H, Othman IA. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl. 2001;24:261–5. doi: 10.1046/j.1365-2605.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 23.Sandoval JS, Raburn D, Mausher S. Leukocytospermia: Overview of diagnosis, implications, and management of a controversial finding. Middle East Fertil Soc J. 2013;18:129–34. [Google Scholar]
- 24.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78:1215–24. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 25.Lobascio AM, De Felici M, Anibaldi M, Greco P, Minasi MG, Greco E. Involvement of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology. 2015;3:265–70. doi: 10.1111/andr.302. [DOI] [PubMed] [Google Scholar]
- 26.Tanphaichitr N, Kongmanas K, Kruevaisayawan H, Saewu A, Sugeng C, Fernandes J, et al. Remodeling of the plasma membrane in preparation for sperm-egg recognition: Roles of acrosomal proteins. Asian J Androl. 2015;17:574–82. doi: 10.4103/1008-682X.152817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alani GT, El Yaseen HD. Creatine kinase activity and malondialdehyde in the seminal plasma of normospermic infertile males. Fac Med Baghdad. 2009;51:336–40. [Google Scholar]
- 28.Schatten H, Sun QY, Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol. 2014;12:111. doi: 10.1186/1477-7827-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ASH Fact Sheet on Smoking Statistics – Illness and Death. 2016. [Last accessed on 2017 May 12]. Available from: http://www.ash.org.uk/files/documents/ASH_107.pdf .
- 30.Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: A common end for numerous pathways. Reprod Toxicol. 2012;34:298–307. doi: 10.1016/j.reprotox.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Esteves SC, Agarwal A, Sharma R, Harlev A. Reply to Eugenio Ventimiglia, Montorsi Francesco, and Andrea Salonia's letter to the editor re: Reecha Sharma, Avi Harlev, Ashok Agarwal, Sandro C. Esteves. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol. 2016;70:635–45. Eur Urol 2017;71:e21-2. [Google Scholar]
- 32.Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol. 2008;20:281–91. doi: 10.1097/GCO.0b013e3282fc9c1e. [DOI] [PubMed] [Google Scholar]
- 33.Yang F, Li L, Chen JP, Liu XQ, Zhong CL, Yang Y, et al. Couple's infertility in relation to male smoking in a Chinese rural area. Asian J Androl. 2017;19:311–5. doi: 10.4103/1008-682X.168685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ., Jr Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil Steril. 2002;78:491–9. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 35.Saalu LC. The incriminating role of reactive oxygen species in idiopathic male infertility: An evidence based evaluation. Pak J Biol Sci. 2010;13:413–22. doi: 10.3923/pjbs.2010.413.422. [DOI] [PubMed] [Google Scholar]
- 36.Esfandiari N, Saleh RA, Blaut AP, Sharma RK, Nelson DR, Thomas AJ, Jr, et al. Effects of temperature on sperm motion characteristics and reactive oxygen species. Int J Fertil Womens Med. 2002;47:227–33. [PubMed] [Google Scholar]
- 37.Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, et al. Role of oxidative stress in germ cell apoptosis induced by di (2-ethylhexyl) phthalate. Biochem J. 2002;365(Pt 3):849–56. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latini G, Del Vecchio A, Massaro M, Verrotti A, De Felice C. Phthalate exposure and male infertility. Toxicology. 2006;226:90–8. doi: 10.1016/j.tox.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Jurasovic J, Cvitkovic P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals. 2004;17:735–43. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 40.Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res Int. 2014;21:11066–74. doi: 10.1007/s11356-014-2986-5. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: An observational study. Fertil Steril. 2008;89:124–8. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 42.Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int J Androl. 2005;28:171–9. doi: 10.1111/j.1365-2605.2005.00531.x. [DOI] [PubMed] [Google Scholar]
- 43.De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. 2009;4:e6446. doi: 10.1371/journal.pone.0006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frei P, Poulsen AH, Johansen C, Olsen JH, Steding-Jessen M, Schüz J. Use of mobile phones and risk of brain tumours: Update of Danish cohort study. BMJ. 2011;343:d6387. doi: 10.1136/bmj.d6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrader M, Müller M, Straub B, Miller K. The impact of chemotherapy on male fertility: A survey of the biologic basis and clinical aspects. Reprod Toxicol. 2001;15:611–7. doi: 10.1016/s0890-6238(01)00182-4. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86:503–12. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 47.Print CG, Loveland KL. Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–30. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–46. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 50.Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:1200–7. doi: 10.1016/s0015-0282(00)01591-0. [DOI] [PubMed] [Google Scholar]
- 51.Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6:139–48. [PubMed] [Google Scholar]
- 52.Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–70. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 53.Iommiello VM, Albani E, Di Rosa A, Marras A, Menduni F, Morreale G, et al. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int J Endocrinol 2015. 2015:321901. doi: 10.1155/2015/321901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: An update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 56.Doshi SD, Sharma R, Agarwal A. Oxidative stress in unexplained male infertility. In: Schattman GL, Esteves SC, Agarwal A, editors. Unexplained Infertility. New York: Springer Science+Business Media, LLC; 2015. [Google Scholar]
- 57.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: Part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 58.Potts JM, Pasqualotto FF. Seminal oxidative stress in patients with chronic prostatitis. Andrologia. 2003;35:304–8. [PubMed] [Google Scholar]
- 59.Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed (Yazd) 2016;14:231–40. [PMC free article] [PubMed] [Google Scholar]
- 60.Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 2014;12:45. doi: 10.1186/1477-7827-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cocuzza M, Sikka SC, Athayde KS, Agarwal A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int Braz J Urol. 2007;33:603–21. doi: 10.1590/s1677-55382007000500002. [DOI] [PubMed] [Google Scholar]
- 62.Aitken RJ, Baker MA, O’Bryan M. Shedding light on chemiluminescence: The application of chemiluminescence in diagnostic andrology. J Androl. 2004;25:455–65. doi: 10.1002/j.1939-4640.2004.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 63.Athayde KS, Cocuzza M, Agarwal A, Krajcir N, Lucon AM, Srougi M, et al. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl. 2007;28:613–20. doi: 10.2164/jandrol.106.001966. [DOI] [PubMed] [Google Scholar]
- 64.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2. Free Radic Biol Med. 1993;15:447–51. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Stansbury KH, Zhu H, Trush MA. Biochemical characterization of lucigenin (Bis-N-methylacridinium) as a chemiluminescent probe for detecting intramitochondrial superoxide anion radical production. Biochem Biophys Res Commun. 1999;262:80–7. doi: 10.1006/bbrc.1999.1174. [DOI] [PubMed] [Google Scholar]
- 66.McKinney KA, Lewis SE, Thompson W. Reactive oxygen species generation in human sperm: Luminol and lucigenin chemiluminescence probes. Arch Androl. 1996;36:119–25. doi: 10.3109/01485019608987087. [DOI] [PubMed] [Google Scholar]
- 67.Aitken RJ, Buckingham DW, West KM. Reactive oxygen species and human spermatozoa: Analysis of the cellular mechanisms involved in luminol- and lucigenin-dependent chemiluminescence. J Cell Physiol. 1992;151:466–77. doi: 10.1002/jcp.1041510305. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal A, Allamaneni SS, Said TM. Chemiluminescence technique for measuring reactive oxygen species. Reprod Biomed Online. 2004;9:466–8. doi: 10.1016/s1472-6483(10)61284-9. [DOI] [PubMed] [Google Scholar]
- 69.Said TM, Kattal N, Sharma RK, Sikka SC, Thomas AJ, Jr, Mascha E, et al. Enhanced chemiluminescence assay vs. colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma. J Androl. 2003;24:676–80. doi: 10.1002/j.1939-4640.2003.tb02726.x. [DOI] [PubMed] [Google Scholar]
- 70.Guthrie HD, Welch GR. Using fluorescence-activated flow cytometry to determine reactive oxygen species formation and membrane lipid peroxidation in viable boar spermatozoa. Methods Mol Biol. 2010;594:163–71. doi: 10.1007/978-1-60761-411-1_12. [DOI] [PubMed] [Google Scholar]
- 71.Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. Int J Urol. 2009;16:449–57. doi: 10.1111/j.1442-2042.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 72.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 73.Shang XJ, Li K, Ye ZQ, Chen YG, Yu X, Huang YF. Analysis of lipid peroxidative levels in seminal plasma of infertile men by high-performance liquid chromatography. Arch Androl. 2004;50:411–6. doi: 10.1080/01485010490484138. [DOI] [PubMed] [Google Scholar]
- 74.Li K, Shang X, Chen Y. High-performance liquid chromatographic detection of lipid peroxidation in human seminal plasma and its application to male infertility. Clin Chim Acta. 2004;346:199–203. doi: 10.1016/j.cccn.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil. 1993;98:257–65. doi: 10.1530/jrf.0.0980257. [DOI] [PubMed] [Google Scholar]
- 76.Tavilani H, Doosti M, Saeidi H. Malondialdehyde levels in sperm and seminal plasma of asthenozoospermic and its relationship with semen parameters. Clin Chim Acta. 2005;356:199–203. doi: 10.1016/j.cccn.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 77.Hsieh YY, Chang CC, Lin CS. Seminal malondialdehyde concentration but not glutathione peroxidase activity is negatively correlated with seminal concentration and motility. Int J Biol Sci. 2006;2:23–9. doi: 10.7150/ijbs.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–97. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 79.Khosrowbeygi A, Zarghami N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol. 2007;7:6. doi: 10.1186/1472-6890-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol Hum Reprod. 2007;13:203–11. doi: 10.1093/molehr/gal119. [DOI] [PubMed] [Google Scholar]
- 81.McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–9. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 82.Rael LT, Bar-Or R, Mains CW, Slone DS, Levy AS, Bar-Or D. Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J Neurotrauma. 2009;26:1203–11. doi: 10.1089/neu.2008.0816. [DOI] [PubMed] [Google Scholar]
- 83.Rael LT, Bar-Or R, Aumann RM, Slone DS, Mains CW, Bar-Or D. Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem Biophys Res Commun. 2007;361:561–5. doi: 10.1016/j.bbrc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 84.Stagos D, Goutzourelas N, Bar-Or D, Ntontou AM, Bella E, Becker AT, et al. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep. 2015;20:154–62. doi: 10.1179/1351000214Y.0000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: A novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016;106:566–73.e10. doi: 10.1016/j.fertnstert.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 86.Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Tremellen K. Oxidative stress and male infertility – A clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 88.Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral Vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 89.Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008;89:1183–90. doi: 10.1016/j.fertnstert.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 90.Sharma RK, Pasqualotto AE, Nelson DR, Thomas AJ, Jr, Agarwal A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl. 2001;22:575–83. [PubMed] [Google Scholar]
- 91.Zorn B, Sesek-Briski A, Osredkar J, Meden-Vrtovec H. Semen polymorphonuclear neutrophil leukocyte elastase as a diagnostic and prognostic marker of genital tract inflammation – A review. Clin Chem Lab Med. 2003;41:2–12. doi: 10.1515/CCLM.2003.002. [DOI] [PubMed] [Google Scholar]
- 92.Kopa Z, Wenzel J, Papp GK, Haidl G. Role of granulocyte elastase and interleukin-6 in the diagnosis of male genital tract inflammation. Andrologia. 2005;37:188–94. doi: 10.1111/j.1439-0272.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 93.Dandekar SP, Nadkarni GD, Kulkarni VS, Punekar S. Lipid peroxidation and antioxidant enzymes in male infertility. J Postgrad Med. 2002;48:186–89. [PubMed] [Google Scholar]
- 94.Aydemir B, Onaran I, Kiziler AR, Alici B, Akyolcu MC. The influence of oxidative damage on viscosity of seminal fluid in infertile men. J Androl. 2008;29:41–6. doi: 10.2164/jandrol.107.003046. [DOI] [PubMed] [Google Scholar]
- 95.Siciliano L, Tarantino P, Longobardi F, Rago V, De Stefano C, Carpino A. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oligoasthenozoospermia. J Androl. 2001;22:798–803. [PubMed] [Google Scholar]


