Abstract
Introduction:
Idiopathic oligoasthenoteratozoospermia (iOAT) is commonly encountered during the evaluation of men with infertility. Antioxidants have been utilized empirically in the treatment of iOAT based on their ability to reverse oxidative stress (OS)-induced sperm dysfunction often encountered in this patient population.
Methods:
A literature search was performed using MEDLINE/PubMed, focusing on publications of antioxidant therapies for iOAT. The main objective of our review article was to report the rationale and available evidence supporting the use of antioxidants.
Results:
Antioxidants such as glutathione, vitamins E and C, carnitines, coenzyme-Q10, N-acetylcysteine, selenium, zinc, folic acid, and lycopene have been shown to reduce OS-induced sperm damage. While rigorous scientific evidence in the form of double-blind, placebo-controlled clinical trials is limited, recent systematic reviews and meta-analyses have reported a beneficial effect of antioxidants on semen parameters and live birth rates.
Conclusion:
Additional randomized controlled studies are required to confirm the efficacy and safety of antioxidant supplementation in the medical treatment of idiopathic male infertility as well as the dosage required to improve semen parameters, fertilization rates, and pregnancy outcomes in iOAT.
INTRODUCTION
Infertility, the inability to conceive after at least 1 year of regular unprotected intercourse, is a common medical condition prevalent in about 15% of couples worldwide.[1] It is termed “male factor infertility” when an alteration in semen parameters or sexual/ejaculatory dysfunction is detected during evaluation. The male contributes to almost half the cases of infertility among couples[2] and in many circumstances, a cause-directed treatment strategy may be applied. However, in almost 30% of cases,[3] no definite etiology is identified, and hence, male infertility is labeled idiopathic.
In the past few years, progress in diagnostic modalities for assessment of sperm function has refined our understanding of the events leading to abnormal spermatogenesis in idiopathic male infertility. Oxidative stress (OS) has been commonly investigated and found to play a detrimental role on sperm function. Reactive oxygen species (ROS) are oxygen-containing, chemically reactive molecules that are, under normal physiologic conditions, beneficial for optimal sperm functions such as the promotion of sperm capacitation, regulation of sperm maturation, and enhancement of cellular signaling pathways.[4] Nonetheless, at higher levels, ROS have been shown to induce lipid peroxidation (LPO), sperm DNA damage, and abortive apoptosis.[5] To overcome these unwanted events, excessive ROS are naturally stabilized or deactivated by the body's antioxidant system.[6] However, when excessive amounts of ROS are produced, or when the antioxidant system fails, a state of OS develops [Figures 1 and 2]. Spermatozoa are particularly vulnerable to OS as they lack the necessary cytoplasmic-antioxidant repair systems and their membrane is rich in polyunsaturated fatty acids which render them susceptible to OS-induced LPO.
Figure 1.
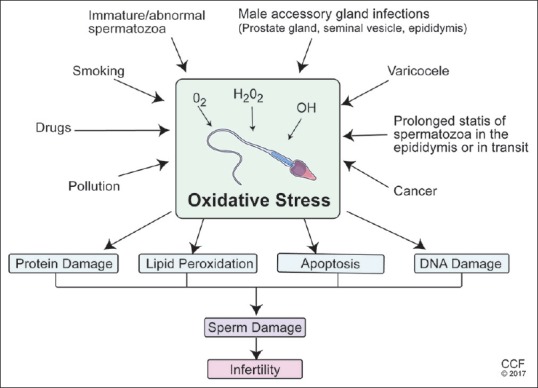
Various etiologies of excessive production of reactive oxygen species resulting in oxidative stress-induced sperm dysfunction and male infertility. Disease states such as varicocele, infections and inflammations of the genital tract, cancer, genetic mutations, chromosomal abnormalities and environmental and habitual exposures have all been identified resulting in DNA damage to the sperm either during spermiation or during its transit through the male reproductive tract
Figure 2.
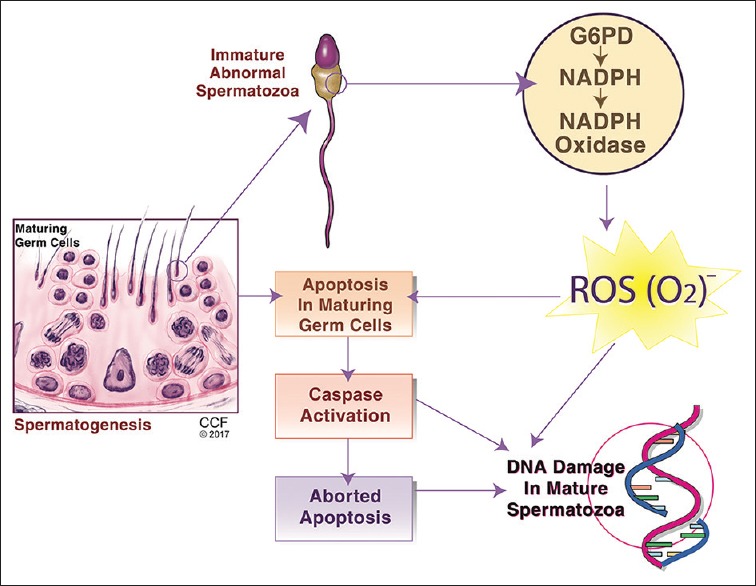
Mechanisms of sperm DNA fragmentation in human spermatozoa. DNA damage is believed to occur secondary to abortive apoptosis, alteration in sperm maturation, or to oxidative stress caused by the excessive production of reactive oxygen species by immature spermatozoa
20%–40% of infertile men have significantly higher levels of ROS in their semen when compared with fertile men.[7] Moreover, significant negative correlations have been detected between OS and semen parameters, fertilization rate, embryonic development, and pregnancy rate.[8,9] Aktan et al. compared 28 men with idiopathic infertility to 14 fertile men and found significantly higher levels of OS measures in the idiopathic infertile men group.[10] Therefore, reversing the state of OS could be considered a potential step in infertility management. While antioxidant supplementation has been proposed as an approach to increase the scavenging capacity of seminal plasma,[11] controversy still surrounds its actual clinical utility. This is mainly because studies examining different antioxidant forms revealed considerable variations in the dosage or combinations used as well as outcome measures. With this review, we aim to investigate the rationale behind using different antioxidant supplements and the evidence surrounding their clinical utility.
METHODS
MEDLINE and PubMed searches were performed using the terms antioxidants and male infertility. Publications were reviewed to assess the use of antioxidants in the management of male infertility with special emphasis given to studies focusing on patients with oligoasthenoteratospermia (iOAT). The rationale behind the use of each antioxidant supplement was explained along with the dosage that is commonly used in clinical practice and the evidence about its impact on fertility outcome.
ANTIOXIDANT USE IN IDIOPATHIC MALE INFERTILITY
Antioxidants are compounds that are acquired through ingesting a balanced diet or from oral supplements. They are often prescribed for the treatment of male subfertility, principally because they are easily accessible as over the counter natural supplements. Antioxidants exist in two forms; enzymatic antioxidants (these include glutathione peroxidase (GPX)/reductase system, superoxide dismutase, and catalase) and nonenzymatic antioxidants which are principally obtained from food supplements. Among all available antioxidants, the most frequently prescribed compounds include vitamins E and C, carnitine, N-acetyl cysteine (NAC), selenium (Se), and zinc [Table 1].
Table 1.
Antioxidants, their recommended daily dose, protective mechanism of action, effect on male fertility, and level of evidence surrounding their beneficial role
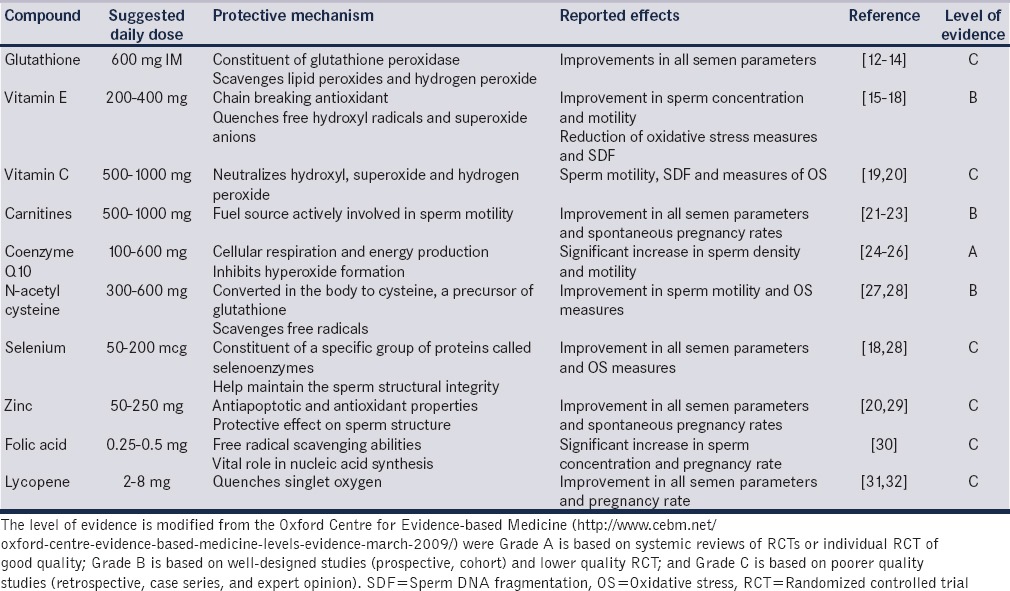
The empiric use of antioxidants for patients with idiopathic iOAT is aimed at improving semen parameters and thereby enhancing the probability for conception. While the plausibility of such a concept has been reported in clinical practice, scientifically acceptable evidence demonstrating benefit from antioxidant therapy in controlled human studies is scarce. Hence, antioxidant use in patients with iOAT has been largely supported by the rationale of protection that each antioxidant delivers against OS. The following antioxidants were selected based on the available evidence for their use, particularly in infertile men with iOAT.
Glutathione
GSH forms an integral part of enzyme GPX. A naturally occurring antioxidant, GPX provides the main endogenous antioxidant protection against LPO in the epididymis and testes.[33] Through scavenging lipid peroxides and hydrogen peroxide (H2O2), these enzymes confer protection for lipid constituents, thus preserving sperm viability and motility.[34] Studies have confirmed the presence of significantly reduced levels of GPX in infertile men as compared to fertile controls.[35]
A few studies to date that have investigated the role of GSH on male infertility and have demonstrated a generally positive effect on semen parameters.[12,13,14] In a placebo-controlled, double-blind, cross-over trial, 600 mg of GSH administered intramuscularly every other day for 2 months led to an improvement in sperm motility and morphology in 20 men with varicocele or male germ-free genital tract inflammation.[13] In vitro studies have confirmed the protective effect of glutathione reductase on tail-beat frequency, LPO reduction, and improvement of sperm membrane characteristics.[36] GSH is poorly absorbed from the gastrointestinal tract making its preferred parenteral method of administration a major drawback to its widespread use.
Vitamin E
Vitamin E (α-tocopherol) is an organic fat soluble compound located mainly in cell membranes. It is a powerful chain-breaking antioxidant capable of quenching free hydroxyl radicals and superoxide anions thereby reducing LPO initiated by ROS at the level of plasma membranes. Therefore, this antioxidant shields sperm membrane components from OS damage. A direct relationship has been detected between the levels of Vitamin E in seminal plasma and the percentage of motile spermatozoa in semen.[37] Furthermore, lower levels of Vitamin E were observed in the semen of infertile men.[38]
In contrast to earlier studies demonstrating no benefit from vitamin E supplementation in the treatment of male infertility,[39,40] recent placebo-controlled studies have suggested a favorable outcome. Suleiman et al. investigated the oral use of 300 mg of daily vitamin E in infertile men, revealing significant improvement in sperm motility concomitant with reduction of OS measures in the treatment group. Moreover, they reported a 21% spontaneous pregnancy rate in the treatment group compared to 0% in the placebo group.[15] Studies investigating vitamin E in combination with other vitamins have specifically revealed a significant improvement in sperm concentration[16,17] together with a decrease in sperm DNA fragmentation (SDF)[16] and seminal ROS.[17] Keskes-Ammar et al. randomized 54 infertile men into two groups; one receiving 400 mg Vitamin E + 225 μg Se while the other receiving 4–5 g Vitamin B daily for 3 months.[18] The authors reported significant reductions in malondialdehyde level, a marker for LPO, together with significant improvement of sperm motility and viability in the Vitamin E/Se group compared with the other group.[18] However, the small sample size is a limitation of this study.
Vitamin C
Vitamin C is a water-soluble compound that exists in seminal plasma at a concentration 10 times higher than in blood serum.[41] The rationale behind its use stems from its ability to neutralize hydroxyl, superoxide, and hydrogen peroxide radicals, thus providing protection against endogenous oxidative damage.[42] Lower Vitamin C levels and higher ROS levels were observed in the seminal plasma of men with asthenozoospermia.[43] Moreover, Vitamin C had a significant positive correlation, in a dose-dependent manner, with sperm motility[44] and percentage of normal sperm morphology.[45]
Vitamin C was mainly investigated in combination with other vitamins and minerals.[19,46] Of particular interest was the combination of Vitamins C and E as the hydrophilicity of the former and lipophilicity of the latter is believed to have a synergistic effect in reducing peroxidative damage on spermatozoa.[46] Such a hypothesis was not confirmed in a randomized controlled double-blind study conducted on men with iOAT, where Rolf et al. failed to show an improvement in semen parameters, sperm survival, or pregnancy rates after the administration of high-doses of Vitamin C and E for 56 days.[40] Despite that, more recent studies have conveyed a beneficial effect for antioxidant regimens-containing Vitamin C in different clinical scenarios. The combination of Vitamin C and E significantly improved SDF, clinical pregnancy, and implantation rates in men undergoing intracytoplasmic sperm injection.[19] Similarly, Omu et al. revealed that a combination treatment with zinc, Vitamin E, and C improved sperm motility, SDF, and measures of OS in men with asthenozoospermia.[20] Additional prospective randomized clinical trials examining the influence of Vitamin C in patients with iOAT is required.
Carnitines
Carnitine is another water-soluble antioxidant involved in sperm metabolism and considered as a fuel source actively involved in sperm motility. In vitro studies have confirmed this effect where sperm cultured in media containing carnitine demonstrated higher motility and viability in comparison to controls.[47,48] Men with OAT were found to have significantly lower levels of carnitine in their semen.[49] The main forms used in the treatment of male subfertility are L-carnitine (LC) and L-acetyl carnitine (LAC).
In vivo studies examining the effect of carnitines on semen parameters have echoed similar improvements in sperm motility though not consistently. Sigman et al. in a small sized (n = 21) prospective randomized double-blinded placebo-controlled trial of patients with iOAT failed to report a significant improvement in sperm parameters after treatment with LC and LAC.[50] Conversely, Cavallini et al. investigated patients with low-grade varicocele and idiopathic infertility and demonstrated a significant improvement in all semen parameters in addition to higher spontaneous pregnancy rates among patients treated with LC and LAC in comparison to placebo (21.8% vs. 1.7%, respectively).[21] Despite the significant improvement, the inclusion of patients with varicocele in the study population may make it difficult to correctly interpret the effect of LC and LAC in men with idiopathic infertility. Further double-blind placebo-controlled trials investigating LC and LAC in idiopathic asthenozoospermia conveyed significant improvement in sperm motility and pregnancy rate.[22,23,51]
Coenzyme-Q10
Coenzyme-Q10 (CoQ-10) is an essential antioxidant, ubiquitous to almost all body tissues and available at high concentrations in sperm mitochondria playing an integral role in energy production.[52] Its involvement in cellular respiration and energy production justifies its use as a pro-motility and antioxidant molecule. CoQ-10 levels in seminal plasma were found to have a linear correlation with sperm count and motility. Furthermore, the in vitro addition of Co-Q10 to semen samples of men with asthenozoospermia resulted in an improvement in sperm motility.[53] Co-Q10 may also convey protection against OS due to its ability to inhibit hyperoxide formation. Alleva et al. reported a significant negative correlation between Co-Q10 levels and H2O2.[54]
Several studies have assessed the impact of CoQ-10 on semen parameters in patients with iOAT. Safarinejad randomly assigned 212 infertile men with iOAT to receive 300 mg Co-Q10 orally or placebo for a period 26 weeks. He demonstrated a statistically significant increase in sperm density and motility with CoQ-10 therapy (P = 0.01).[24] In a different study, the same author further reported a beneficial effect on spontaneous pregnancy rate in men receiving CoQ-10.[25] In a meta-analysis of three randomized controlled trials on Co-Q10 use in iOAT, Lafuente et al. reported significant improvement in sperm motility and concentration in men receiving Co-Q10 without a significant increase in pregnancy rates.[26]
N-Acetyl cysteine
NAC is an amino acid that exhibits antioxidant properties after being converted in the body to cysteine which is a precursor of glutathione. It is also capable of directly reducing OS through scavenging free radicals.[55] In vitro studies have demonstrated a beneficial role for NAC on germ cell survival as a result of its antioxidant properties.[56] The incubation of semen samples with NAC for 20 min significantly reduced ROS levels and resulted in an improvement of sperm motility.[57]
In a randomized placebo-controlled study of 120 patients with idiopathic infertility, treatment with 600 mg of NAC daily resulted in a significant improvement in sperm motility in comparison to placebo.[27] In addition, authors evaluated OS measures and reported higher serum total antioxidant capacity and lower total peroxide and OS index in the NAC-treated group compared with the placebo group. Another double-blind, placebo-controlled trial randomized 468 infertile men with iOAT into four groups; 200μg Se, 600 mg NAC, 200 μg Se + 600 mg NAC, and placebo.[28] Authors reported a significant improvements in all semen parameters in the Se and NAC groups with an additive effect in the combination group. Furthermore, a dose-dependent positive correlation was detected between the sum of Se/NAC concentrations, and mean sperm concentration (r = 0.67, P = 0.01), motility (r = 0.64, P = 0.01), and percent of normal morphology (r = 0.66, P = 0.01).[28] While the above-mentioned studies advocate the usage of NAC in men with infertility to improve semen quality, it failed to investigate the degree of improvement in pregnancy rate.
Selenium
Se is an essential trace element involved in spermatogenesis. It provides protection for sperm DNA against OS damage in a mechanism that is not very well-established. It is believed that Se augments the function of biologic glutathione since it is a major constituent of a specific group of proteins called selenoenzymes. More than 25 selenoproteins exist, such as phospholipid hydroperoxide GPX[58] and sperm capsular selenoprotein GPX,[59] which help maintain the sperm structural integrity.[60] Morphologic sperm midpiece abnormalities and impairment of sperm motility are among the most common sperm abnormalities associated with Se deficiency.[61]
Se has mainly been studied in combination with other vitamins, specifically Vitamin E as it is well known to work in synergy with Se as an antiperoxidant.[62] As previously noted in the randomized controlled trial by Keskes-Ammar et al., the combination of Vitamin E and Se had protective effects on sperm motility and measures of LPO.[18] Safarinejad et al.[28] reported a statistically significant improvement in all semen parameters in men receiving 200ug of Se alone or in combination with NAC for 26 weeks.[28] In a recent literature review, Se was found to have a favorable influence on viability of spermatozoa providing protection against ROS.[63]
Zinc
Zinc is an essential mineral involved in the metabolism of DNA and RNA. It has antiapoptotic and antioxidant properties with a potential positive effect on spermatogenesis. Zinc concentrations of seminal plasma were found to be significantly higher in fertile men in comparison to subfertile men.[64] The antioxidant properties of zinc, however, were first utilized in antiaging supplements and immune boosters. The protective effect zinc has on sperm structure may probably be the most important advocate for male fertility. Sperm flagellar abnormalities, such as hypertrophy and hyperplasia of the fibrous sheath, axonemal disruption, defects of the inner microtubular dynein arms, and abnormal or absent midpiece have all be associated with zinc deficiency.[20]
Studies have shown a beneficial effect for zinc supplementation in patients with iOAT demonstrated by improvements in sperm concentration, progressive motility, sperm integrity, and pregnancy rates.[29,65] Omu et al. randomized 45 men with asthenozoospermia into 4 groups; zinc only, zinc + Vitamin E, zinc + Vitamins E and C, and no therapy. After a period 3 months, zinc therapy alone or in combination with Vitamin E or Vitamin E + C was associated with significant improvement in sperm parameters and reduction in OS, sperm apoptosis, and SDF.[20] The results of this study are limited due to small sample size.
Folic acid
Folic acid is a B Vitamin (B9) that plays a vital role in nucleic acid synthesis and amino acid metabolism. It has free radical scavenging abilities which provoked its use as a potential antioxidant for the treatment of male subfertility. Folic acid intake has been linked with lower frequency of SDF. One study demonstrated that daily consumption of 700 μg of folic acid was associated with a 30% lower risk of disomy X and 21, sex nullisomy, and DNA aneuploidy.[66]
No robust evidence exists to support the use of folic acid for the treatment of men with idiopathic infertility. Murphy et al.[67] examined the association between idiopathic infertility with variants in folic acid-related genes and measures in blood. Despite finding significant correlations between folic acid related gene (phosphatidylethanolamine N-methyltransferase M175V) and serum folic acid levels with male infertility, no significant relationship was detected with semen parameters. In a double-blind, placebo-controlled interventional study, 108 fertile men and 103 subfertile men were randomized into four groups; folic acid only, zinc only, a combination, and placebo.[30] After 26 weeks of treatment, a statistically significant increase in sperm concentration was noted among subfertile men receiving combination therapy. Another study of similar design by Ebisch et al.[68] compared daily treatment with folic acid (5 mg/day) + zinc sulfate (66 mg/day) to placebo on 47 fertile and 40 subfertile men for a period of 26 weeks and reported significant improvement in sperm concentration in the treatment group only.
Lycopene
Lycopene is a naturally synthesized carotenoid present in fruits and vegetables. It plays a major role in the human redox defense system as it has the highest quenching ability against singlet oxygen, a high-energy form of oxygen.[69] Lycopene is detected at high concentrations in human testes and seminal plasma with levels that tend to be lower in men suffering from infertility.[70]
Gupta and Kumar investigated oral lycopene therapy in 30 men with iOAT, who were given 2 mg of lycopene twice daily for 3 months.[31] The authors reported statistically significant improvements in sperm concentration and motility in 66% and 53% of patients, respectively. However, the improvements were only significant in patients who had baseline sperm concentration more than 5 million/ml. Mohanty et al. treated fifty patients with iOAT with 8 mg of lycopene daily until an improvement in semen parameters was detected or pregnancy was achieved. After a follow-up period of 1-year duration, sperm concentration, motility, and morphology improved in 70%, 54%, and 38% of patients, respectively, while pregnancy was achieved in 36% of patients.[32]
SUMMARY OF ANTIOXIDANTS USE FOR MALE INFERTILITY
While the majority of studies investigated the effect of antioxidants on semen parameters, few addressed their influence on pregnancy or live birth rate. The available systemic reviews perhaps provide a summary of the effect of antioxidant therapy for male infertility. A Cochrane review of 48 randomized controlled clinical trials including 4179 subfertile men reported considerable variability in the antioxidant effect on semen parameters, however, with a statistically significant improvement in live birth rate (odds ratio [OR] 4.21, 95% confidence interval [CI] 2.08–8.51, P < 0.0001) and clinical pregnancy rate (OR 3.43, 95% CI 1.92–6.11, P < 0.0001).[71] Ross et al.[72] analyzed 17 randomized trials, including a total of 1665 infertile men looking for changes in semen parameters or reported pregnancy rates. Statistically significant improvements in semen parameters were detected in 14 out of 17 trials. Pregnancy rate was reported in seven trials, six of them showed a significant improvement after antioxidant therapy. The authors concluded that the use of oral antioxidants in infertile men may have a beneficial effect on sperm quality and pregnancy rates. The impact of oral antioxidants on measures of sperm OS and DNA damage was investigated by Gharagozloo and Aitken.[73] They selected 20 trials assessing such an association and reported a significant reduction of OS or DNA damage after treatment with antioxidants in 19 out of the 20 studies selected. Moreover, an improvement in sperm motility, particularly in asthenospermic patients was significantly observed.[73] Although the published studies generally demonstrate a favorable effect of antioxidants on infertile men, the optimal type and dose of antioxidants together with their exact mechanism of action is still unknown. Based on the available grade of evidence and our clinical experience in this field, we propose the utility of combinations of Vitamins E and C, carnitines, CoQ-10, NAC together with minerals such as zinc and Se for the treatment of patients with idiopathic infertility.
CONCLUSION
Antioxidants are under active investigation in the treatment of male infertility. However, collection of high-quality evidence, such as that from double-blind placebo-controlled trials, on the efficacy of these compounds is a slow and arduous process. While a number of studies have confirmed a beneficial effect for antioxidants in reversing OS-induced sperm dysfunction specifically in patients with idiopathic male infertility, there are few controlled or randomized clinical trials, thus limiting strong evidence in support of their use. Nonetheless, an individualized treatment approach is preferred with adjustments in dose and type of antioxidant made according to the clinical presentation and/or the level of seminal OS.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(Suppl 1):33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 2.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–82. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 3.Sabanegh E, Agarwal A. Male infertility. In: Campbell MF, Walsh PC, Wein AJ, editors. Campbell-Walsh Urology. Philadelphia: Saunders Elsevier; 2012. pp. 616–47. [Google Scholar]
- 4.Ford CE, Jones KW, Miller OJ, Mittwoch U, Penrose LS, Ridler M, et al. The chromosomes in a patient showing both mongolism and the Klinefelter syndrome. Lancet. 1959;1:709–10. doi: 10.1016/s0140-6736(59)91891-4. [DOI] [PubMed] [Google Scholar]
- 5.Brooker RJ. Genetics: Analysis and Principles. 4th ed. Ohio, USA: McGraw-Hill Higher Education; 2011. [Google Scholar]
- 6.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–97. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 8.Tremellen K. Oxidative stress and male infertility – A clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 10.Aktan G, Dogru-Abbasoglu S, Küçükgergin C, Kadioglu A, Ozdemirler-Erata G, Koçak-Toker N. Mystery of idiopathic male infertility: Is oxidative stress an actual risk? Fertil Steril. 2013;99:1211–5. doi: 10.1016/j.fertnstert.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Majzoub A. Role of antioxidants in male infertility. BJUI Knowledge. 2016 DOI 10.18591/BJUIK.0510. [Google Scholar]
- 12.Lenzi A, Lombardo F, Gandini L, Culasso F, Dondero F. Glutathione therapy for male infertility. Arch Androl. 1992;29:65–8. doi: 10.3109/01485019208987710. [DOI] [PubMed] [Google Scholar]
- 13.Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F. Placebo-controlled, double-blind, cross-over trial of glutathione therapy in male infertility. Hum Reprod. 1993;8:1657–62. doi: 10.1093/oxfordjournals.humrep.a137909. [DOI] [PubMed] [Google Scholar]
- 14.Lenzi A, Picardo M, Gandini L, Lombardo F, Terminali O, Passi S, et al. Glutathione treatment of dyspermia: Effect on the lipoperoxidation process. Hum Reprod. 1994;9:2044–50. doi: 10.1093/oxfordjournals.humrep.a138391. [DOI] [PubMed] [Google Scholar]
- 15.Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: Protective role of Vitamin E. J Androl. 1996;17:530–7. [PubMed] [Google Scholar]
- 16.Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68:519–24. doi: 10.1016/s0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 17.Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000;63:159–65. doi: 10.1054/plef.2000.0174. [DOI] [PubMed] [Google Scholar]
- 18.Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral Vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 19.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–53. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 20.Omu AE, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract. 2008;17:108–16. doi: 10.1159/000112963. [DOI] [PubMed] [Google Scholar]
- 21.Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl. 2004;25:761–70. doi: 10.1002/j.1939-4640.2004.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 22.Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, et al. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004;81:1578–84. doi: 10.1016/j.fertnstert.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005;84:662–71. doi: 10.1016/j.fertnstert.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 24.Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237–48. doi: 10.1016/j.juro.2009.02.121. [DOI] [PubMed] [Google Scholar]
- 25.Safarinejad MR. The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: An open-label prospective study. Int Urol Nephrol. 2012;44:689–700. doi: 10.1007/s11255-011-0081-0. [DOI] [PubMed] [Google Scholar]
- 26.Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, et al. Coenzyme Q10 and male infertility: A meta-analysis. J Assist Reprod Genet. 2013;30:1147–56. doi: 10.1007/s10815-013-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009;74:73–6. doi: 10.1016/j.urology.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: A double-blind, placebo controlled, randomized study. J Urol. 2009;181:741–51. doi: 10.1016/j.juro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Tikkiwal M, Ajmera RL, Mathur NK. Effect of zinc administration on seminal zinc and fertility of oligospermic males. Indian J Physiol Pharmacol. 1987;31:30–4. [PubMed] [Google Scholar]
- 30.Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: A double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77:491–8. doi: 10.1016/s0015-0282(01)03229-0. [DOI] [PubMed] [Google Scholar]
- 31.Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility – A preliminary report. Int Urol Nephrol. 2002;34:369–72. doi: 10.1023/a:1024483520560. [DOI] [PubMed] [Google Scholar]
- 32.Mohanty NK, Kumar S, Jha AK, Arora RP. Management of idiopathic oligoasthenospermia with lycopene. Indian J Urol. 2001;18:57–61. [Google Scholar]
- 33.Mora-Esteves C, Shin D. Nutrient supplementation: Improving male fertility fourfold. Semin Reprod Med. 2013;31:293–300. doi: 10.1055/s-0033-1345277. [DOI] [PubMed] [Google Scholar]
- 34.Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009;19:638–59. doi: 10.1016/j.rbmo.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Garrido N, Meseguer M, Alvarez J, Simón C, Pellicer A, Remohí J. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil Steril. 2004;82(Suppl 3):1059–66. doi: 10.1016/j.fertnstert.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Griveau JF, Le Lannou D. Effects of antioxidants on human sperm preparation techniques. Int J Androl. 1994;17:225–31. doi: 10.1111/j.1365-2605.1994.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 37.Thérond P, Auger J, Legrand A, Jouannet P. alpha-Tocopherol in human spermatozoa and seminal plasma: Relationships with motility, antioxidant enzymes and leukocytes. Mol Hum Reprod. 1996;2:739–44. doi: 10.1093/molehr/2.10.739. [DOI] [PubMed] [Google Scholar]
- 38.Omu AE, Fatinikun T, Mannazhath N, Abraham S. Significance of simultaneous determination of serum and seminal plasma alpha-tocopherol and retinol in infertile men by high-performance liquid chromatography. Andrologia. 1999;31:347–54. doi: 10.1046/j.1439-0272.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 39.Giovenco P, Amodei M, Barbieri C, Fasani R, Carosi M, Dondero F. Effects of kallikrein on the male reproductive system and its use in the treatment of idiopathic oligozoospermia with impaired motility. Andrologia. 1987;19:238–41. doi: 10.1111/j.1439-0272.1987.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 40.Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose Vitamin C and Vitamin E: A randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–33. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- 41.Jacob RA, Pianalto FS, Agee RE. Cellular ascorbate depletion in healthy men. J Nutr. 1992;122:1111–8. doi: 10.1093/jn/122.5.1111. [DOI] [PubMed] [Google Scholar]
- 42.Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88:11003–6. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: Recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27:325–37. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Dawson EB, Harris WA, Teter MC, Powell LC. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril. 1992;58:1034–9. [PubMed] [Google Scholar]
- 45.Thiele JJ, Friesleben HJ, Fuchs J, Ochsendorf FR. Ascorbic acid and urate in human seminal plasma: Determination and interrelationships with chemiluminescence in washed semen. Hum Reprod. 1995;10:110–5. doi: 10.1093/humrep/10.1.110. [DOI] [PubMed] [Google Scholar]
- 46.Baker HW, Brindle J, Irvine DS, Aitken RJ. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65:411–9. doi: 10.1016/s0015-0282(16)58109-6. [DOI] [PubMed] [Google Scholar]
- 47.Shi JZ, Zhang SS, Zhang Z, Liang Q, Shi Y, Hua JL, et al. Expressions of sperm-specific genes in carnitine-cultured testis sperm of obstructive azoospermia patients. Zhonghua Nan Ke Xue. 2010;16:504–9. [PubMed] [Google Scholar]
- 48.Banihani S, Agarwal A, Sharma R, Bayachou M. Cryoprotective effect of L-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia. 2014;46:637–41. doi: 10.1111/and.12130. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed SD, Karira KA, Jagdesh, Ahsan S. Role of L-carnitine in male infertility. J Pak Med Assoc. 2011;61:732–6. [PubMed] [Google Scholar]
- 50.Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the treatment of idiopathic asthenospermia: A randomized, double-blind, placebo-controlled trial. Fertil Steril. 2006;85:1409–14. doi: 10.1016/j.fertnstert.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 51.Lenzi A, Lombardo F, Sgrò P, Salacone P, Caponecchia L, Dondero F, et al. Use of carnitine therapy in selected cases of male factor infertility: A double-blind crossover trial. Fertil Steril. 2003;79:292–300. doi: 10.1016/s0015-0282(02)04679-4. [DOI] [PubMed] [Google Scholar]
- 52.Lewin A, Lavon H. The effect of coenzyme Q10 on sperm motility and function. Mol Aspects Med. 1997;18(Suppl 1):S213–9. doi: 10.1016/s0098-2997(97)00036-8. [DOI] [PubMed] [Google Scholar]
- 53.Mancini A, Conte B, De Marinis L, Hallgass ME, Pozza D, Oradei A, et al. Coenzyme Q10 levels in human seminal fluid: Diagnostic and clinical implications. Mol Aspects Med. 1994;15(Suppl 1):s249–55. doi: 10.1016/0098-2997(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 54.Alleva R, Scararmucci A, Mantero F, Bompadre S, Leoni L, Littarru GP. The protective role of ubiquinol-10 against formation of lipid hydroperoxides in human seminal fluid. Mol Aspects Med. 1997;18(Suppl 1):S221–8. doi: 10.1016/s0098-2997(97)00040-x. [DOI] [PubMed] [Google Scholar]
- 55.Gressier B, Cabanis A, Lebegue S, Brunet C, Dine T, Luyckx M, et al. Decrease of hypochlorous acid and hydroxyl radical generated by stimulated human neutrophils: Comparison in vitro of some thiol-containing drugs. Methods Find Exp Clin Pharmacol. 1994;16:9–13. [PubMed] [Google Scholar]
- 56.Erkkilä K, Hirvonen V, Wuokko E, Parvinen M, Dunkel L. N-acetyl-L-cysteine inhibits apoptosis in human male germ cells in vitro . J Clin Endocrinol Metab. 1998;83:2523–31. doi: 10.1210/jcem.83.7.4949. [DOI] [PubMed] [Google Scholar]
- 57.Oeda T, Henkel R, Ohmori H, Schill WB. Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: A possible therapeutic modality for male factor infertility? Andrologia. 1997;29:125–31. doi: 10.1111/j.1439-0272.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 58.Roveri A, Casasco A, Maiorino M, Dalan P, Calligaro A, Ursini F. Phospholipid hydroperoxide glutathione peroxidase of rat testis. Gonadotropin dependence and immunocytochemical identification. J Biol Chem. 1992;267:6142–6. [PubMed] [Google Scholar]
- 59.Alvarez JG, Storey BT. Lipid peroxidation and the reactions of superoxide and hydrogen peroxide in mouse spermatozoa. Biol Reprod. 1984;30:833–41. doi: 10.1095/biolreprod30.4.833. [DOI] [PubMed] [Google Scholar]
- 60.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–6. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 61.Noack-Füller G, De Beer C, Seibert H. Cadmium, lead, selenium, and zinc in semen of occupationally unexposed men. Andrologia. 1993;25:7–12. doi: 10.1111/j.1439-0272.1993.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 62.Burton GW, Traber MG. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. 1990;10:357–82. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 63.Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz MH, et al. Role of selenium in male reproduction – A review. Anim Reprod Sci. 2014;146:55–62. doi: 10.1016/j.anireprosci.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Chia SE, Ong CN, Chua LH, Ho LM, Tay SK. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J Androl. 2000;21:53–7. [PubMed] [Google Scholar]
- 65.Omu AE, Al-Qattan F, Al-Abdul-Hadi FM, Fatinikun MT, Fernandes S. Seminal immune response in infertile men with leukocytospermia: Effect on antioxidant activity. Eur J Obstet Gynecol Reprod Biol. 1999;86:195–202. doi: 10.1016/s0301-2115(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 66.Young SS, Eskenazi B, Marchetti FM, Block G, Wyrobek AJ. The association of folate, zinc and antioxidant intake with sperm aneuploidy in healthy non-smoking men. Hum Reprod. 2008;23:1014–22. doi: 10.1093/humrep/den036. [DOI] [PubMed] [Google Scholar]
- 67.Murphy LE, Mills JL, Molloy AM, Qian C, Carter TC, Strevens H, et al. Folate and Vitamin B12 in idiopathic male infertility. Asian J Androl. 2011;13:856–61. doi: 10.1038/aja.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebisch IM, Pierik FH, DE Jong FH, Thomas CM, Steegers-Theunissen RP. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int J Androl. 2006;29:339–45. doi: 10.1111/j.1365-2605.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 69.Kelkel M, Schumacher M, Dicato M, Diederich M. Antioxidant and anti-proliferative properties of lycopene. Free Radic Res. 2011;45:925–40. doi: 10.3109/10715762.2011.564168. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal A, Sekhon LH. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: Is it justified? Indian J Urol. 2011;27:74–85. doi: 10.4103/0970-1591.78437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No.: CD007411. doi: 10.1002/14651858.CD007411.pub3. DOI: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 72.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–23. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]


