Abstract
The Centers for Medicare & Medicaid Services developed the Oncology Care Model as an episode-based payment model to encourage participating practitioners to provide higher-quality, better-coordinated care at a lower cost to the nearly three-quarter million fee-for-service Medicare beneficiaries with cancer who receive chemotherapy each year. Episode payment models can be complex. They combine into a single benchmark price all payments for services during an episode of illness, many of which may be delivered at different times by different providers in different locations. Policy and technical decisions include the definition of the episode, including its initiation, duration, and included services; the identification of beneficiaries included in the model; and beneficiary attribution to practitioners with overall responsibility for managing their care. In addition, the calculation and risk adjustment of benchmark episode prices for the bundle of services must reflect geographic cost variations and diverse patient populations, including varying disease subtypes, medical comorbidities, changes in standards of care over time, the adoption of expensive new drugs (especially in oncology), as well as diverse practice patterns. Other steps include timely monitoring and intervention as needed to avoid shifting the attribution of beneficiaries on the basis of their expected episode expenditures as well as to ensure the provision of necessary medical services and the development of a meaningful link to quality measurement and improvement through the episode-based payment methodology. The complex and diverse nature of oncology business relationships and the specific rules and requirements of Medicare payment systems for different types of providers intensify these issues. The Centers for Medicare & Medicaid Services believes that by sharing its approach to addressing these decisions and challenges, it may facilitate greater understanding of the model within the oncology community and provide insight to others considering the development of episode-based payment models in the commercial or government sectors.
INTRODUCTION
The United States spent 17.5% of its gross domestic product on health care in 2014,1 almost double the Organisation for Economic Co-operation and Development average of 9% of gross domestic product that year.2 From 1990 to 2010, medical costs increased at an annual rate of 6.6%, despite the widespread adoption of managed care programs whose stated goal was the limitation of these increases.3
Bundled payment (including episode-based payment) and global capitation models, which include all expenditures for an episode of illness or during a defined chronological period, respectively, offer alternative payment approaches that align financial incentives between and among payers and providers to encourage high-value care and improve efficiency. It is believed that moving these incentives from the payer level to the provider level will result in the more broad-based adoption of efficient, high-value care that can be individualized to specific practice and patient situations.
For episode payment models (EPMs) to function effectively, it is necessary to align payment accurately with relative costliness to minimize the opportunity for practitioners to benefit financially by withholding needed care or by providing care only to those beneficiaries with low expected expenditures. It is also necessary to reward efforts to redesign care in ways that improve the efficiency and coordination of care for the full population of beneficiaries who resemble the patient population on which the episode prices are based.4
The Centers for Medicare & Medicaid Services (CMS) developed the Oncology Care Model (OCM) as an EPM to encourage practitioners in physician group practices (PGPs) to provide higher-quality, better-coordinated oncology care at a lower cost. To achieve these aims, the model requires participating PGPs to sign an agreement with CMS requiring the PGP to provide a set of enhanced services, centered on care coordination, to OCM beneficiaries receiving chemotherapy during 6-month episodes of care.
OCM includes a per-beneficiary-per-month payment of $160 for the provision of enhanced services, which is termed a monthly enhanced oncology services (MEOS) payment. The $160 MEOS payment was calculated based on the estimated additional staffing required to provide the enhanced services. CMS used Bureau of Labor Statistics values to estimate the cost for this additional staff and spread the costs across the average number of episodes per practice. OCM also includes an opportunity for the participating PGP to receive performance-based payments (PBPs) on the basis of reductions in actual expenditures for the practice’s beneficiaries compared against risk-adjusted episode target prices and the practice’s performance on quality measures.5 Other alternatives considered included an oncology care model similar to the CMS Physician Value-Based Payment Modifier, which would have modified physician payments on the basis of the outcome of selected, oncology-specific quality metrics. The model also includes the participation of commercial payers that are partnering with participating PGPs in alignment with general OCM parameters to leverage opportunities for comprehensive practice transformation.5,6
DESIGN DECISIONS FOR EPMS
Episode Definition: Initiation and Duration
Episodes have both temporal and clinical dimensions that must be defined for an EPM that is based on use and expenditures for beneficiaries. First, an EPM must encompass either a clearly defined clinical episode or a clearly defined interval that is observable from claims data. For OCM, CMS will use 6-month episodes that begin with the receipt of outpatient nontopical chemotherapy for cancer. Oral chemotherapies are included in the list of initiating therapies, although, because the vast majority of orals are covered by Part D (there are a limited number of Part B oral medications), these will only initiate an episode if a beneficiary has Part D coverage.
This 6-month episode length is based on analysis of CMS claims data that showed that expenditures for Medicare beneficiaries treated with chemotherapy for cancer peaked in the first 2 months after chemotherapy initiation before stabilizing after 4 to 6 months.7 These observations suggested that 6-month episodes were most likely to capture discrete treatment courses across a range of cancer types. Beneficiaries who receive further chemotherapy after the completion of a 6-month episode are eligible to begin additional OCM episodes. In analyzing spending patterns over time, CMS considered terminating episodes on the basis of observed gaps in chemotherapy receipt but chose not to use this strategy for several reasons. These included the absence of a gap in some patients as well the complexity of developing a comprehensive risk adjustment methodology that allowed for variable episode duration across and within cancer types.
OCM was developed as a physician-focused specialty model centered on the provision of chemotherapy for cancer by oncology practices, which CMS views as the primary focal point and manager of care for cancer patients receiving chemotherapy. CMS considered other events that occur earlier in the cancer treatment process, such as the diagnostic surgery, as potential episode initiators. However, these earlier clinical events introduced greater expenditure variability into the model and offered limited opportunities for quality improvement in the process of care, because they often occurred before the involvement of the medical oncologist. In addition, beginning episodes before the initiation of chemotherapy would also have the disadvantage of dividing primary accountability for the patient’s episode between the surgeon and the oncologist. CMS is aware that chemotherapy-based episode triggers may create a financial disincentive to perform surgery after the initiation of chemotherapy (neoadjuvant therapy) even when clinically indicated. To address this concern, benchmark prices are adjusted upward for beneficiaries undergoing selected surgeries during an episode.
Episode Definition: Included Services in the Episode (Total Cost of Care Model)
Oncology care is complex and multifactorial, with many direct and indirect patient outcomes affected by treatment decisions. As a result, individual cases may be highly idiosyncratic, and it is often difficult to separate services for oncology-related complications and care management from those that are unrelated, because of the broad effects on patient health of oncology treatment. In addition, CMS anticipates that nearly 200,000 episodes will be initiated at participating PGPs during each performance year of OCM; this volume makes case-by-case determination of related services impractical. For these reasons, CMS decided that the most feasible and appropriate methodology to bundle payment for services included in the OCM episode and to set episode prices was to include expenditures for all Medicare Parts A and B services and, for beneficiaries with Part D, those Part D services not paid on a capitated basis (the Low-Income Subsidy and 80% of the Gross Drug Cost Above Catastrophic Threshold). Although some CMS models exclude the cost of certain medical conditions not believed to be related to the primary diagnosis, these exclusions engender their own set of complexities and were not believed to be feasible given the breadth and complexity of cancer care. For this reason, a total cost of care model was selected.
Total cost of care means that some acute events out of the oncologist’s control, such as motor vehicle–related injuries or trauma or adverse health events from comorbid conditions, may lead to costs that accrue to an episode’s expenditures. The cost of these services could increase episode expenditures but, to the extent that these rare, high-cost events also randomly occurred in the historical baseline period, they have been incorporated into the risk-adjusted benchmark episode price methodology (see Practice Expenditure Variation, Risk Adjustment, and Benchmark Episode Prices). In addition, the model includes other key features designed to mitigate participant financial risk (see Pricing Features that Mitigate Participant Financial Risk).
Attribution of Patients
An EPM must accurately attribute beneficiaries to the participating PGP that primarily manages the beneficiaries’ care to assure appropriate alignment of financial incentives. CMS primarily considered two main approaches to claims-based attribution methodologies when designing OCM. First, CMS explored a prospective methodology that would attribute a beneficiary to the participating PGP (as identified by a taxpayer identification number [TIN]) whose practitioners billed for an initial chemotherapy administration claim or were listed as the prescriber on an initial Part B or Part D chemotherapy drug claim. Second, CMS explored a plurality methodology that would attribute the beneficiary to the TIN whose practitioners furnished the plurality of outpatient cancer-related evaluation and management (E&M) services (identified by lines on a claim that contain both an E&M service code and a qualifying cancer diagnosis). Using Medicare claims data and the OCM episode definition to identify what would have been chemotherapy episodes initiated from mid-2009 to mid-2013, CMS found that the two methodologies attributed 81% of episodes to the same TIN.
Three main drawbacks were identified for the prospective attribution method. First, it did not correctly attribute beneficiaries who switched providers soon after diagnosis. Second, certain beneficiaries may have their chemotherapy and outpatient E&M claims billed under different TINs. This generally occurs when the E&M services are provided in a physician’s office and the chemotherapy is provided in a hospital outpatient department. In those cases, the prospective methodology would not necessarily appropriately attribute beneficiaries to the TIN responsible for managing patient care (as opposed to the TIN administering chemotherapy). In the plurality attribution method, chemotherapy delivered in a hospital outpatient department not associated with the participating PGP will not influence how the episode is attributed, but the costs (as with all other expenditures previously described) will be attributed to the episode expenditures for the beneficiary. Third, the prospective methodology would require differential treatment of episodes triggered by Part B and Part D claims, because the prescribing physician’s National Provider Identification number and TIN do not consistently appear on Part D claims.
The main advantages of the plurality attribution method are that it is more likely to correctly identify the physician group (eg, TIN) caring for the patient over time and that it works across a wide variety of clinical and business settings. Its main disadvantage is its retrospective nature. This prevents practices from knowing which beneficiaries are attributed to them until after the completion of the episode and delays episode-specific feedback to the practices until the care episode is complete. After weighing the advantages and disadvantages of both methods, CMS decided to use the plurality attribution method.
Because of the large number of beneficiaries in OCM and because it, like other Center for Medicare & Medicaid Innovation models tested under Section 1115A of the Social Security Act, is evaluated for possible national expansion if it meets defined statutory criteria, other attribution alternatives, such as individual contact with beneficiaries to see who they identify as their primary oncologist, were deemed infeasible. Such an approach may not yield consistent attribution if beneficiaries switch oncologists during the course of treatment, and incomplete beneficiary response rates would create challenges. In addition, it would not be possible to implement such an approach to attribute historical episodes. CMS would consider opportunities to refine the OCM attribution approach in the future if additional administrative data on the relationship between practices and beneficiaries become available.
Patient Shifting, Multiple Practice Locations, and Complex Business Models
EPMs inherently include beneficiaries for whom expenditures are substantially above or below the benchmark price. These extremes may create a financial incentive for some practitioners to shift beneficiaries expected to be high cost out of the EPM—for example, to an alternate practice location or TIN not participating in the EPM—as well as for the participating PGP to focus their treatment on a greater number of beneficiaries expected to be low cost. EPMs may also create an incentive to prolong treatment over a greater number of episodes to collect additional Per Beneficiaries Per Month (PBPMs) or PBPs. For this reason, all practice locations of a participating PGP must participate in OCM.
Many oncology practices have complex billing arrangements. Some practitioners may bill under different TINs at different locations (such as outreach clinics), even though these may be part of the same practice, whereas different employees at the same site may also bill under different TINs (eg, if some practitioners are employed by a hospital and others are in independent practice). Some practices are completely owned by hospitals, and others have agreements to provide their chemotherapy infusions in hospital outpatient departments. On some occasions, hospitals may provide practices with leased employees who can bill under the TIN of the practice where they work or the hospital that employs them. Hospital-employed physicians often bill under their own TIN, but sometimes they bill under the hospital’s TIN.
Any model with financial risk for services automatically creates some incentive to shift high-cost beneficiaries to other providers. CMS observed that complex billing arrangements might create the potential for PGPs to shift high-cost beneficiaries to affiliated nonparticipating TINs. As previously stated, to reduce this possibility, CMS decided that any participating PGP is required to include in its OCM participation all practitioners who furnish chemotherapy services and all locations at which such services are furnished. A PGP in which practitioners bill for the professional component of chemotherapy-related E&M services under multiple TINs is required to either have practitioners reassign their billing rights to the applicant TIN or apply (and participate) jointly with these other TINs. Furthermore, PGPs’ providers cannot routinely treat beneficiaries at excluded entities, such as selected cancer hospitals exempt from payment by the Inpatient Prospective Payment System, Critical Access Hospitals, Rural Health Clinics, Federally Qualified Health Centers, and entities with locations in Maryland, although beneficiaries may receive care from other providers in these settings (see Medicare FFS Payment System Constraints).
Practice Expenditure Variation, Risk Adjustment, and Benchmark Episode Prices
In establishing benchmark prices, CMS sought to determine a price that reflects the cost of caring for a beneficiary with defined risk factors (identified from claims) in a particular practice. These episode prices can be established in several ways. For a given disease, they can reflect national, regional, or individual practice historical expenditures or a hybrid of these variations. In other models, we have used historical claims information for a hospital (for example) to set an episode price with a policy for using regional claims for low-volume hospitals. However, in OCM we were challenged by the broad diversity of patients, diseases, treatments, and business practices. We considered alternative approaches that relied solely on practice-specific data to construct prices but found that for most practices there were insufficient historical episodes in the analysis period to implement a comprehensive and reliable risk-adjustment methodology. CMS ultimately used a regression-based approach to develop practice-specific OCM benchmark episode prices from a combination of national, regional, and practice-specific data.
The process of refining the regression-based risk-adjustment methodology involved running regressions of standardized episode-level expenditures (that account for geographic variation and inflation) for chemotherapy episodes that occurred nationally from 2009 to 2013 (the analysis period used for initial model design; the historical baseline period used to construct baseline prices for OCM practices is for episodes beginning from 2012 to 2014) on myriad permutations of covariates and risk-adjustment factors that are currently available in Medicare enrollment and claims data. These included age; sex; site of cancer; comorbidities identified by hierarchical condition categories8; Part D enrollment; Medicare-Medicaid dual eligibility; receipt of radiation therapy, surgery, and/or hematopoietic stem cell transplantation during the episode; regional expenditure variations; practice-specific variables; time since prior chemotherapy; and other factors.
One iteration included practice-specific variables in the regression model to calculate practice-specific benchmark episode prices. In another, we used a two-step process that excluded practice-specific variables from the risk-adjustment regression model. A practice-specific adjustment ratio was then calculated by comparing all actual expenditures for patients in a practice during the analysis period with the predicted expenditures on the basis of the risk-adjustment regression model. For example, practices whose aggregate expenditures are greater than the predicted values have an adjustment ratio > 1, whereas those whose expenditures are less have an adjustment ratio < 1. Because average expenditures for individual practices tend to move toward average expenditures over time, known as regression toward the mean, we could not apply the practice-specific adjustment factor in its entirety. In considering this method, we assessed that giving practice-level adjustments a weight of 50% would move predicted expenditures toward the national average while leaving some variation in the predicted expenditures to be addressed through model-induced adjustments. We labeled this approach a practice adjustment factor.
Although both yielded similar results in the historical analyses, the practice adjustment factor methodology was selected as the method for determining practice-specific benchmark episode prices in the final model design. In particular, the adjustment factor methodology is more adaptable to changing practice dynamics, such as practice mergers. Specifically, including practice-specific covariates in the regression would require rerunning the entire risk-adjustment model to account for a practice merger or acquisition, which would result in altered target prices not just for the practices that merged but for all other practices. The adjustment factor methodology overcomes this by using the adjustment factor to account for changes in practice structure rather than the regression model.
The forward trend factor is an adjustment to the baseline price that is intended to reflect the changing costs of care nationally. It is based on the characteristics of each practice’s performance period episodes and will be calculated using regression analysis that incorporates the same risk factors as the primary regression model.
Table 1 shows the covariates included in the final OCM Prediction Model. We specify the model as a generalized linear model with a log link and γ distribution. This type of model is commonly used in predicting health care expenditures and yields only positive predicted values. More detailed information on the risk adjustment model is available.9
Table 1.
Oncology Care Model Prediction Model Variables
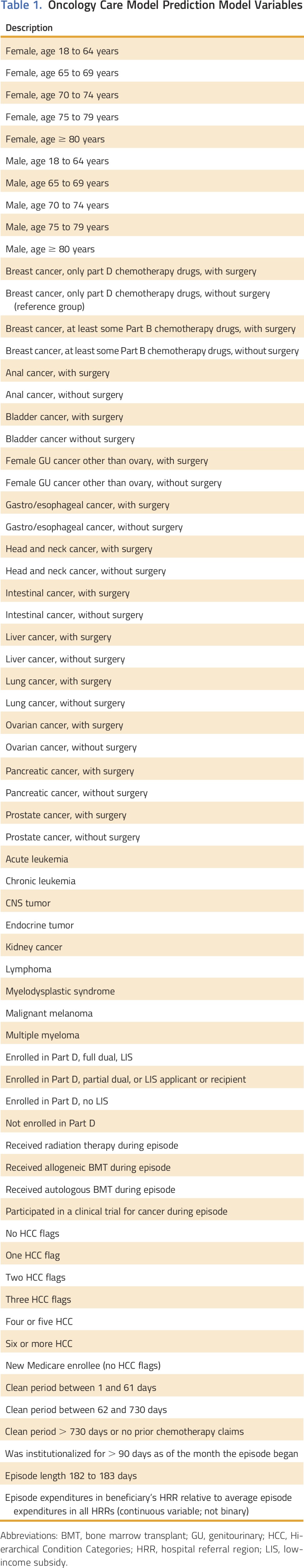
In the performance period, we will calculate the benchmark episode price as the product of the predicted risk-adjusted episode expenditures from the regression model, the practice-specific adjustment factor, the practice-specific forward trend factor that is risk adjusted based on the practice’s performance period episodes on the basis of national expenditures at non-OCM practices, and the novel therapies adjustment (see Novel Therapies and Increasing Expenditures on Health Care).
Once the benchmark episode price has been calculated, we will then calculate the target price for an episode by applying to the benchmark episode price the appropriate CMS discount percentage, which differs between the one-sided (no potential for financial loss) and two-sided (potential for financial loss) risk arrangements (4% v 2.75%, respectively). The lower discount in the two-sided risk arrangement may provide a financial incentive for OCM practices to opt for that risk arrangement.
For instance, an episode may have a baseline price of $25,000. If the forward trend factor for that practice in a specific performance period showed an increase of 10%, then the benchmark price would be $27,500. Applying the 4% discount for a one-sided model to that benchmark price would yield a target price of $26,400. An example of a summary PBP calculation for a practice is shown in Table 2. Additional details are available in the OCM Performance-Based Payment Methodology document available on the OCM Web site.10
Table 2.
Performance-Based Payment Calculation for an Example Participating Group Practice10
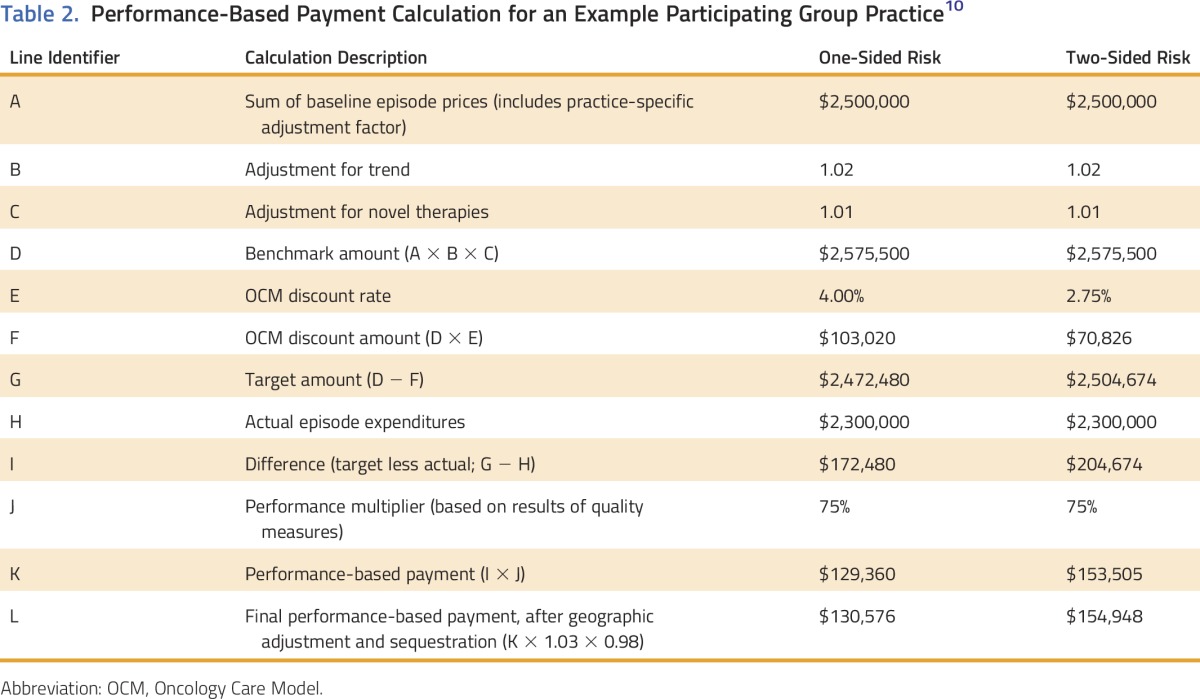
Some external stakeholders have maintained that this method penalizes current highly efficient practices and argued instead for regional or national benchmark episode prices. However, benchmark episode prices, limited to regional or national data, would financially reward already efficient practices that made limited improvements while making it difficult for less-efficient practices to improve enough to achieve the episode savings necessary for a PBP. Thus, CMS believes that partially individualizing benchmark episode prices at the practice level will provide each participating PGP with attainable goals to improve quality and efficiency of care.
Disease staging and benchmark episode prices
Although the risk-adjustment methodology described captures several key drivers of episode expenditures, CMS is limited by the fact that many important cancer-specific clinical factors are not captured on claims. Notably, Medicare claims currently include data on disease type (eg, lung cancer, colon cancer, breast cancer), but CMS does not collect more specific data on anatomical cancer stage, histology, biomarkers, or molecular mutations (eg, hormone receptor status, epidermal growth factor receptor–activating mutations, and anaplastic lymphoma kinase [ALK] mutation status), which can dramatically affect the cost of treatment.
Therefore, the model’s initial benchmarking risk-adjustment methodology, which is limited to data collected in CMS claims, cannot incorporate such factors. Under this current methodology, benchmark episode prices will likely accurately account for aggregate OCM episode expenditures if those practices serve large numbers of beneficiaries. This methodology also protects small practices. However, because of statistical variation in smaller patient populations, smaller practices may be more likely to experience substantial fluctuation in episode spending because of random variation than larger practices and could be adversely affected by the initial benchmarking risk-adjustment methodology if they see more high-cost beneficiaries than statistically expected (or positively affected if they see fewer high-cost beneficiaries than expected). It is important to note, however, that practices will continue to receive Medicare Fee for Service (FFS) and MEOS payments for all patient care, and in a one-sided risk model, the adverse effect would be limited to a decreased or absent PBP and mitigated by Winsorization (see Pricing Features that Mitigate Participant Financial Risk).
During the model test, CMS is collecting anatomical staging and relevant histology, biomarker, and molecular mutation data through a new data registry. If the data show that these variations significantly affect episode expenditures, CMS will explore incorporating more granular risk-adjustment methodologies in later performance periods of the model.
Novel therapies and increasing expenditures on health care
Expenditures for health care in the United States continue to increase. In some clinical areas, such as oncology, expenditures have increased faster than the overall expenditures for health care.11 Retrospective payment analysis of historical episodes used to set disease-specific benchmark episode prices must include a methodology for adjusting these benchmark episode prices over time so that they accurately reflect current expenditures for patient care, while maintaining the incentive to provide high-value care. As described above, CMS plans to analyze expenditure data from Medicare FFS beneficiaries outside of OCM to create annual forward trend factors that will be applied to the risk-adjusted benchmark episode prices to reflect current Medicare expenditures for beneficiaries with cancer types and other characteristics similar to those beneficiaries treated by each OCM practice. By necessity, these practice-specific forward trend factors will be constrained by the information available in administrative claims data.
With rapid increases in the understanding of oncogenesis and the subsequent creation of drugs targeting specific molecular mutations, the expenditures for a select group of patients within a disease subset might increase dramatically during the 5 years of the model. This could occur if a new standard of care dictates the use of one or more newly developed and expensive medications (or other novel therapies) for patients with that disease subtype. Because CMS does not currently collect national data on disease subtypes (eg, ALK-positive lung cancer), we lack the data necessary to generate subtype-specific trend factors or to risk adjust by subtype when new drugs are approved for a subset of patients within a larger disease category (eg, lung cancer). Although these substantially greater expenditures for a subset of patients will ultimately be reflected in a larger disease-specific trend factor over time as the new drugs are adopted broadly and mitigated by Winsorization or trimming of outliers (see Pricing Features that Mitigate Participant Financial Risk), participating PGPs that serve a disproportionate share of these expensive patients or are early adopters of novel therapies may incur significant financial losses in a two-sided model or a considerably decreased PBP in a one-sided model, because of the more expensive treatments necessary for their patients, a circumstance in which the practice has limited opportunity to increase its efficiency. Thus, we decided that providing a further price adjustment to the risk-adjusted episode target price beyond the forward trend factors—to account for the higher cost of certain new drugs—would be appropriate so practices treating patients who need these treatments would not shoulder the entire cost of new technologies.
Therefore, for the first 2 years after new drug approval, CMS will calculate the proportion of spending on new oncology drugs for Food and Drug Administration–approved indications in both participating and nonparticipating practices. When the proportion of qualified spending on novel therapies is greater in a given OCM practice compared with non-OCM practices, CMS will include an upward adjustment in the calculation of the PGP’s benchmark episode prices. This adjustment will be calculated at 80% of the cost difference in the proportion of episode spending on new drugs for the purposes of PBP calculations. Subsequent methodologies may more fully link compensation for new oncology drugs to effectiveness in treating different cancer types.
CMS considered other methodologies to incorporate the cost of new drugs and novel therapies in its benchmark prices. One option would have passed through the cost of new drugs by not including them in the total cost of care calculation that determined PBPs. Another option would have made no provision for the cost of these new therapies, leaving the forward trend factor to account for the uptake of new drugs. CMS determined that the first option would have removed the incentive for high-value care in OCM by removing the cost of these new drugs from the PBP calculation. In addition, it would have created an incentive to use new drugs in cases where similar, equally efficacious drugs were already available. CMS chose not to accept the second option because of concerns about restricting beneficiary’s access to new cancer therapies.
New technologies, such as biosimilars, have the potential to decrease health care spending and may do so in some cases. Because biosimilars reflect existing biologics that have already been on the market, and whose cost has already been factored into historical benchmark prices, these new biosimilars will not be considered new for the purposes of the novel therapy adjustment. The OCM total cost of care model will encourage the rapid adoption of these higher-value drugs.
Pricing Features that Mitigate Participant Financial Risk
It is important to note that most high-cost oncology and nononcology services are accounted for in the calculation of benchmark episode prices, assuming that these events occur in similar frequency in the baseline and performance periods. Even so, CMS has incorporated additional features to mitigate participant risk, including one-sided risk, stop-loss provisions under the two-sided risk arrangement, and Winsorization.
One-sided risk and stop-loss provisions
Because risk adjustment may not fully account for the significant variability in oncology expenditures, OCM participants have the option to minimize financial risk by choosing one-sided risk for the duration of the model. Under the one-sided risk arrangement, benchmark episode prices will be subject to a larger CMS discount percentage than under the two-sided risk arrangement (4% compared with 2.75%), but participants will not be required to pay CMS in the event that aggregate actual episode expenditures for all episodes attributed to their practice in a performance period exceed the sum of the target prices for these episodes.
Participating PGPs also have the option to elect two-sided risk beginning in 2017. The two-sided risk arrangement of OCM is an Advanced Alternative Payment Model under the Quality Payment Program, and practices selecting the two-sided risk arrangement may qualify for incentive payments under the Quality Payment Program for sufficient participation in OCM.12 Under the two-sided arrangement, participants are required to pay CMS if aggregate actual episode expenditures for episodes attributed to their practice in a performance period exceed the sum of the target prices for those episodes. Under the two-sided risk arrangement, a stop-loss provision limits the amount that any participant will be required to pay back to CMS within a given performance period to 20% of the sum of the benchmark episode prices for all episodes in the performance period. Regardless of the risk arrangements, CMS will cap a participant’s PBP for reducing expenditures below the target at 20% of the sum of the benchmark episode prices for all episodes in a performance period, which is consistent with stop-loss limits in other CMS EPMs. In addition, participating PGPs in the one-sided risk track that do not achieve a performance-based payment by the initial reconciliation of the fourth performance period must elect to either enter the two-sided risk track or to discontinue participation in OCM.
Winsorization
Winsorization,13 also referred to as truncation or trimming, limits calculated OCM episode expenditures on both the low and high ends. This statistical method substitutes extreme outlier values in expenditure calculations with values that are more consistent with the spread and variance of the remaining values. For all episode-level calculations (namely, setting benchmark episode prices and calculating actual performance period episode expenditures), CMS will truncate expenditures at the 5th and 95th percentiles of the cancer-specific distribution of national episode expenditures. This limits participants’ financial exposure related to rare and costly events.
Investments and Safeguards to Ensure High-Quality Care
In an FFS payment system, where payment is made separately for each service, there can be a financial incentive to provide additional care, some of which may be unnecessary and wasteful. In an EPM, there can be a financial incentive to provide less care, some of which may be necessary and helpful. Thus, an EPM must have robust quality and clinical outcome measures to identify poorly performing practices and use pay-for-performance measures to encourage high-quality care in real time.
In OCM, PBPs will be affected by the performance of participating PGPs on a dozen quality measures related to outcomes, patient experiences, and oncology-specific clinical processes (Table 3).10 Eight of these measures are practice-reported quality measures that will start as pay-for-reporting and transition to pay-for-performance during the model. The reporting and performance on the quality measures is linked to PBPs to allow OCM to align the financial incentive to reduce costs with the goal of providing high-quality care. In addition, CMS monitors for quality of care and model integrity through a number of data sources, such as claims data, practice-reported data, and site visits to the practices. Administrative and claims data, as well as information from the data registry, provide information on changes in clinical practice patterns, such as the number of episodes triggered for a specific cancer diagnosis; changes in use, such as shifting of services inside or outside of an episode; and clinical outcome measures, such as hospitalizations and progression-free and overall survival. Practice-reported data show how resources are used to implement the model. Site visits provide an opportunity to verify information and monitor activities that cannot be measured through other data sources, such as accuracy of data submission and implementation of OCM Practice Redesign Activities. A participating PGP may be subject to increased scrutiny or termination from the model if CMS notes significant negative differences in performance on these measures, either as compared with other PGPs or disease-specific norms or as compared with the practice’s own previous practice patterns. CMS expects to see changes in clinical practice spurred by OCM; it will encourage change that improves value and patient care, while monitoring for changes that do not.
Table 3.
Quality Measure Components of the Performance-Based Payment Calculation
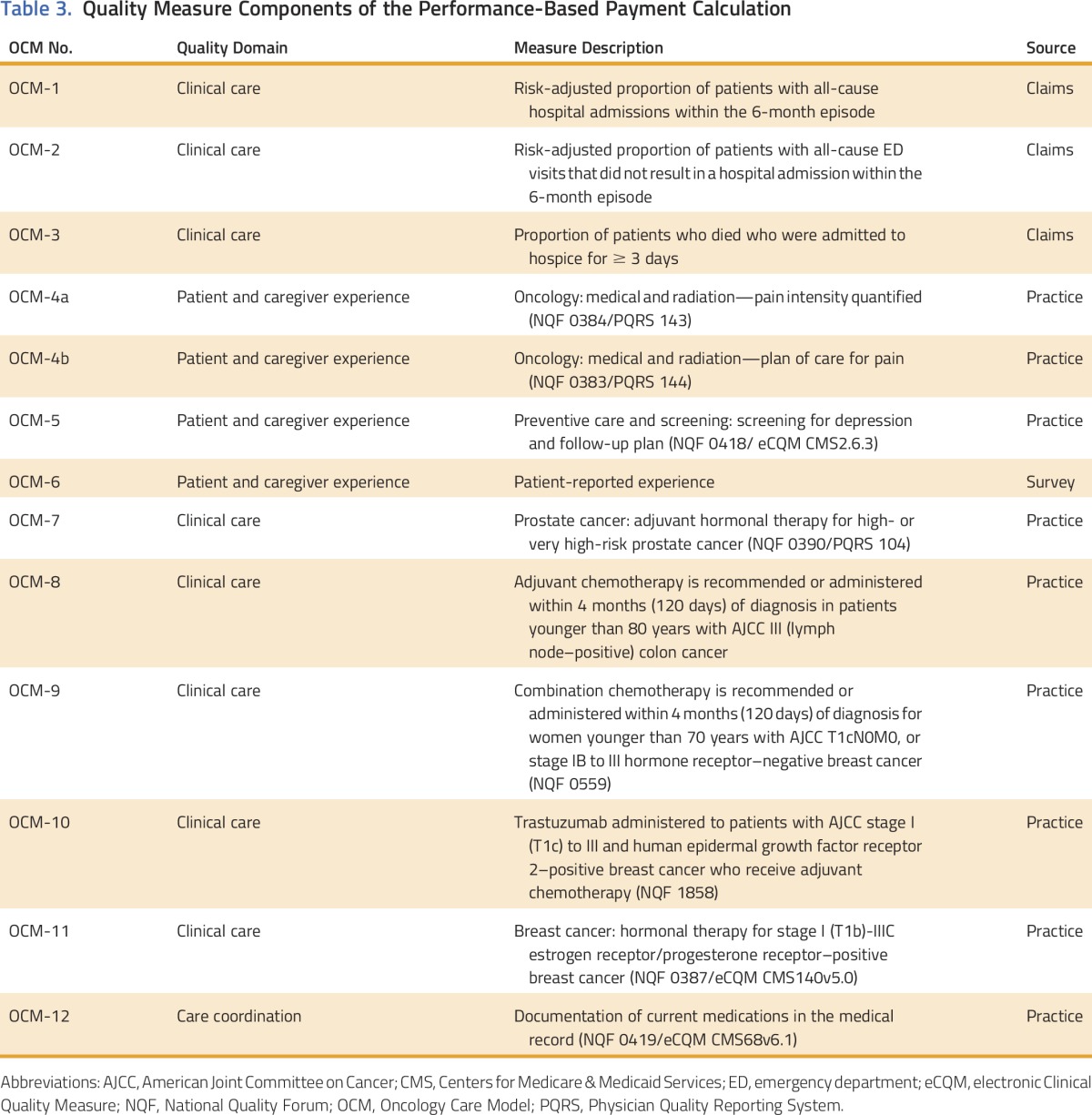
CMS also views the monthly MEOS payment of $160 as an investment in practice transformation that supports high-quality cancer care. Physicians have long objected to the lack of payment for additional services provided to patients with cancer. The MEOS payment is an effort by CMS to support the enhanced services necessary for excellent care.
MEDICARE FFS PAYMENT SYSTEM CONSTRAINTS
OCM relies on being able to calculate all episode expenditures for a patient and compare those to expected expenditures, absent the model. For certain providers and services, it is not possible to calculate actual Medicare payments on a timely basis because of additional payments that are made to these entities, sometimes months to years after the service was provided, and sometimes not linked to a specific patient or service. This is because, for a variety of public policy reasons, certain health care entities are paid in indirect or supplemental ways. These complex cost accounting systems change the direct relationship between the provision of medical care and payment for that care, complicating the financial alignment of payers and providers necessary for the effective implementation of an EPM. For these reasons, practices that are part of or have a formal or written agreement with one of the excluded entities for the routine provision of outpatient chemotherapy to Medicare beneficiaries with cancer are excluded from the model.
The excluded entities are prospective payment system–exempt cancer hospitals,14 critical access hospitals,15 rural health clinics,15 federally qualified health centers16 and entities with locations in Maryland.17 CMS will continue to explore ways to include excluded entities that do not operate under the traditional Medicare FFS into models.
Overlap With Other CMS Models and Programs
CMS has launched a number of alternative payment models since the passage of the Affordable Care Act in 2010. Most of these models use reductions in growth in health care expenditures and performance on quality metrics as bases for performance or shared savings payments. When two models both assume responsibility for a beneficiary for an overlapping period of time, savings or excess spending must be allocated between the participants in the overlapping models. Examples include a beneficiary aligned to an Accountable Care Organization (ACO) who receives cancer care at an OCM practice and an OCM beneficiary, either admitted to a hospital participating in the Bundled Payments for Care Improvement Initiative for a clinical condition that resulted in hospital admission, or receiving lower extremity joint replacement surgery in a hospital participating in the Comprehensive Care for Joint Replacement (CJR) model.
In the ACO/OCM example, CMS decided that when a beneficiary is aligned to both an ACO and an OCM practice, the savings (or excess spending) that the OCM practice or ACO generates for that beneficiary would accrue exclusively to the OCM participant and that savings allocated to Medicare for the OCM discount are not counted as savings by an ACO. This is analogous to the arrangement between ACOs and other voluntary CMS bundled payment models, including the Bundled Payments for Care Improvement Initiative. In the event that a participating PGP is also part of an ACO and the ACO achieves shared savings in the ACO reconciliation, CMS would recoup from the OCM participant a portion of the OCM Medicare discount amount that was paid to the ACO as shared savings for beneficiaries who are also aligned to the ACO.
We also note that CMS plans to test different overlap arrangements in other models. For example, we recently put forth an alternative overlap policy for CJR and the Acute Myocardial Infarction, Coronary Artery Bypass Graft, and Surgical Hip/Femur Fracture Treatment models in the EPM final rule published January 3, 2017, and currently scheduled to take effect on May 20, 2017. Although the models will overlap with some initiatives like OCM, beneficiaries prospectively assigned to certain other models with downside financial risk, like Next-Generation ACOs, will be excluded from CJR and the Acute Myocardial Infarction, Coronary Artery Bypass Graft, and Surgical Hip/Femur Fracture Treatment models.
Financial Arrangements and Beneficiary Incentives
Participating PGPs may wish to enter into financial arrangements under which they will share episode savings or downside risk (or both) with the health care providers or other entities whose collaboration might help the participating PGP earn PBPs. In addition, participating PGPs may wish to promote beneficiary engagement in their health care by providing certain in-kind items and services, such as devices to monitor and transmit medical indications and symptoms or transportation to attend medical appointments. Such arrangements may facilitate the financial alignment of health care providers, encourage the provision of higher-quality care at reduced cost, and increase beneficiary engagement in their health care. However, these arrangements may implicate certain federal fraud and abuse laws, including the physician self-referral (Stark) law, the antikickback statute, and the beneficiary inducements civil monetary penalty law. Pursuant to the law that established the Innovation Center,18 the Secretary of Health and Human Services issued waivers of certain fraud and abuse laws for certain financial arrangements or beneficiary incentives that are part of OCM.19
SUMMARY
EPMs have shown promise in improving quality and reducing spending in a variety of clinical settings.20 By moving financial and quality accountability for an episode of care from the managerial level to the level of a provider who cares for and directly manages the care of the patient throughout the episode, the EPM provides a flexible methodology to encourage and then reward improvements in quality and efficiency of care in the context of a spectrum of clinical scenarios and individual patient needs. CMS has seen interest from physicians and other clinicians in EPMs being tested by CMS, and OCM represents a significant step in testing this method of payment in the oncology setting.20 These models test the belief that improvement in quality and efficiency are possible in discrete episodes of care. These models also align with patient-centered goals of better outcomes over an entire episode of care.
Despite their promise, however, EPMs have limitations and challenges inherent in the complexity of the diverse and fragmented US health care system. The medical complexity of oncology care, which encompasses a wide diversity of diseases, spans a broad spectrum of care, and involves a large number of medical specialties, adds further challenges to the design of a model. Finally, the complex business arrangements among oncology providers, with similar services sometimes provided under multiple TINs depending on the site of care or the specific provider, require comprehensive eligibility rules and requirements to ensure that high-cost beneficiaries are not excluded from the EPM and that they continue to have access to high-quality care, with fair and appropriate payment for services.
CMS has considered and attempted to address these numerous challenges in designing OCM. Currently, 190 practices and 16 commercial payers are participating in OCM, which is expected to include nearly 200,000 episodes per year for an estimated $6 billion per year in medical spending and Medicare beneficiaries receiving chemotherapy for cancer. Through OCM and the innovative work of its participating PGPs, CMS will determine whether an episode-based payment model for oncology episodes of care can deliver better patient care and spend health care dollars more wisely. The model is an exciting step forward for oncology care in the United States.
ACKNOWLEDGMENT
The views expressed in the manuscript represent the authors’ and not necessarily the views or policies of the Centers for Medicare & Medicaid Services. We thank Matthew J. Press and Nevin Laib for their helpful reviews of the manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Ronald M. Kline, L. Daniel Muldoon, Larisa M. Strawbridge, Andrew W. York, Laura K. Mortimer, Alison F. Falb, Katherine J. Cox, Ellen W. Lukens, Mary C. Kapp, Rahul Rajkumar
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Design Challenges of an Episode-Based Payment Model in Oncology: The Centers for Medicare & Medicaid Services Oncology Care Model
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Ronald M. Kline
Stock or Other Ownership: Las Vegas CyberKnife (I)
Other Relationship: Centers for Medicare & Medicaid Services
L. Daniel Muldoon
Other Relationship: Centers for Medicare & Medicaid Services
Heidi K. Schumacher
Other Relationship: Centers for Medicare & Medicaid Services
Larisa M. Strawbridge
Other Relationship: Centers for Medicare & Medicaid Services
Andrew W. York
Other Relationship: Centers for Medicare & Medicaid Services
Laura K. Mortimer
Other Relationship: Centers for Medicare & Medicaid Services
Alison F. Falb
Other Relationship: Centers for Medicare & Medicaid Services
Katherine J. Cox
Other Relationship: Centers for Medicare & Medicaid Services
Carol Bazell
Other Relationship: Centers for Medicare & Medicaid Services
Ellen W. Lukens
Employment: Avalere Health
Other Relationship: Centers for Medicare & Medicaid Services
Mary C. Kapp
Stock or Other Ownership: Medtronic (I)
Other Relationship: Centers for Medicare & Medicaid Services
Rahul Rajkumar
Leadership: CareFirst Health BlueCross BlueShield
Travel, Accommodations, Expenses: CareMore
Other Relationship: Centers for Medicare & Medicaid Services
Amy Bassano
Other Relationship: Centers for Medicare & Medicaid Services
Patrick H. Conway
Other Relationship: Centers for Medicare & Medicaid Services
REFERENCES
- 1.Martin AB, Hartman M, Benson J, et al. : National health spending in 2014: Faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood) 35:150-160, 2016 [DOI] [PubMed] [Google Scholar]
- 2. Organisation for Economic Co-operation and Development: Health expenditure and financing. http://stats.oecd.org/Index.aspx?DataSetCode=SHA.
- 3. Centers for Medicare & Medicaid Services: National health expenditure data. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/Tables.zip.
- 4.Press MJ, Rajkumar R, Conway PH: Medicare’s new bundled payments: Design, strategy, and evolution. JAMA 315:131-132, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Kline RM, Bazell C, Smith E, et al. : Centers for medicare and medicaid services: Using an episode-based payment model to improve oncology care. J Oncol Pract 11:114-116, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar R, Conway PH, Tavenner M: CMS--engaging multiple payers in payment reform. JAMA 311:1967-1968, 2014 [DOI] [PubMed] [Google Scholar]
- 7. Huckfeldt P, Chan C, Hirshman S, et al: Specialty payment model opportunities and assessment: Oncology model design report. http://www.rand.org/content/dam/rand/pubs/research_reports/RR700/RR763/RAND_RR763.pdf. [PMC free article] [PubMed]
- 8. Centers for Medicare & Medicaid Services: Risk adjustment. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors.html.
- 9. RTI International, Actuarial Research Corporation: OCM prediction model. https://innovation.cms.gov/Files/x/ocm-predictionmodel.pdf.
- 10. RTI International, Actuarial Research Corporation: OCM performance-based payment methodology. https://innovation.cms.gov/Files/x/ocm-methodology.pdf.
- 11. Institute of Medicine: Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC, National Academies Press, 2013, p 2. [PubMed]
- 12. Federal Register: Medicare program: Merit-Based Incentive Payment System (MIPS) and Alternative Payment Model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. https://www.federalregister.gov/documents/2016/11/04/2016-25240/medicare-program-merit-based-incentive-payment-system-mips-and-alternative-payment-model-apm. [PubMed]
- 13. Tukey JW: The future of data analysis. Ann Math Statist 33:17-19, 1962.
- 14. US Government Accountability Office: GAO Report 15-199. Medicare: Payment methods for certain cancer hospitals should be revised to promote efficiency. Washington, DC, 2015.
- 15. Centers for Medicare & Medicaid Services, Department of Health and Human Services: Critical Access Hospital, Rural Health Fact Sheet Series. Baltimore, MD, 2016.
- 16. Centers for Medicare & Medicaid Services: Medicare benefit policy manual, publication 100-02, Chapter 13. Baltimore, MD.
- 17.Rajkumar R, Patel A, Murphy K, et al. : Maryland’s all-payer approach to delivery-system reform. N Engl J Med 370:493-495, 2014 [DOI] [PubMed] [Google Scholar]
- 18. 42 USC. §1315a. Section 1115A(d)(1)
- 19.Centers for Medicare & Medicaid Services: Fraud and abuse waivers. https://www.cms.gov/Medicare/Fraud-and-Abuse/PhysicianSelfReferral/Fraud-and-Abuse-Waivers.html.
- 20.Shih T, Chen LM, Nallamothu BK: Will bundled payments change health care? Examining the evidence thus far in cardiovascular care. Circulation 131:2151-2158, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]


