Abstract
Purpose:
Tumor genomic profiling (TGP) can reveal secondary findings about inherited disease risks in a patient with cancer. Little is known about how patients with advanced cancer, currently the primary users of TGP, perceive the benefits and harms of secondary germline findings.
Methods:
We conducted semistructured interviews with 40 patients with advanced breast, bladder, colorectal, or lung cancer who had TGP. Qualitative interview data were evaluated by using a thematic content analysis approach.
Results:
Most participants expressed interest in the prospect of learning their secondary germline findings (57%), although a minority was equivocal (29%) or disinterested (14%). Reasons for these preferences varied but were influenced by participants’ perceptions of diverse benefits and harms of this information, which they regarded as relevant to themselves; their families; and other patients with cancer, medical science, and society. These attitudes were uniquely shaped by participants’ personal disease experiences and health status.
Conclusion:
Many patients with advanced cancer are interested in learning secondary germline findings and hold optimistic and perhaps unrealistic beliefs about the potential health benefits. Patients also have important concerns about clinical and emotional implications of this information. These perceptions are necessary to address to ensure that patients make informed decisions about learning secondary germline findings.
INTRODUCTION
Tumor genomic profiling (TGP) can identify genetic variants within a patient’s tumor that may make the cells susceptible to targeted medications. Currently, TGP is used primarily among patients with advanced cancer to identify eligibility for clinical trials of novel therapeutics.1,2 Through TGP, germline variants indicative of inherited disease risks may be identified in tumor DNA. Alternatively, the patient’s normal DNA may be directly sequenced for comparison with tumor sequence, which potentially reveals germline variants that indicate inherited risks for health conditions with differing severity, treatability, likelihood of development, and relevance for the patient’s health.3-5 These germline findings are considered secondary when actively sought (or incidental when not) because they arise outside the original purpose of TGP.6,7 Experts are debating how to ethically and practically manage such germline findings,8-11 with the American College of Medical Genetics and Genomics currently recommending that patients be allowed to opt out of receiving secondary germline findings (SGFs).12-14 Research is needed to examine patients’ perspectives about the discovery, disclosure, and management of SGFs to ensure that their preferences are addressed adequately.7,15
Several studies have demonstrated that patients with varying stages of cancer are interested in the hypothetical16-18 and real19 prospect of learning this risk information gained from TGP. Little is currently known, however, about patient attitudes that contribute to this interest. This issue is particularly critical for patients with advanced cancer who generally face poor prognoses and are thus likely to receive limited clinical benefits from learning SGFs about their future disease risks. The few existing qualitative studies with patients and family members identified an enhanced ability to plan for the future20 and family obligations21 as important reasons to receive germline results, yet patients were concerned about the burden of this information.20,21 There is a need to better understand how patients with advanced cancer perceive the benefits and harms of SGF, as this could allow health care providers to optimally support patients as they make decisions about whether to learn this information or not.
To address this gap in knowledge, we used qualitative semistructured interviews to collect in-depth narratives from patients with advanced cancer who had TGP at our institution. These individuals were informed about the possible incidental discovery of germline variants through the TGP consent process, but at the time of this study, our institution did not routinely conduct secondary analyses. Therefore, these patients had direct experience with TGP and awareness of the issue of potential germline variants but had not made a definitive decision about learning SGFs. We assessed their perceptions of the benefits and harms of learning SGFs as well as of how these attitudes shaped their personal interest in receiving this risk information.
METHODS
Recruitment and Participants
At the time of this study, patients with late-stage solid tumors could undergo TGP with the MSK-IMPACT (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets) test,22,23 a sequencing panel that detects somatic variants in 410 cancer-related genes with analysis of a matched normal sample and germline subtraction, through an institutional research protocol; study participants were recruited from those enrolled in this protocol. Eligible patients were those with breast, colorectal, bladder, or lung cancer; age 18 years or older; New York metropolitan area residents (two were recruited outside this area to meet our target distribution of sex and cancer type); and healthy enough to be approached about the study as determined by their physicians. The MSK Cancer Center institutional review board approved this study.
To obtain a breadth of participant perspectives and remain consistent with recommended qualitative research guidelines for thematic saturation,24 we aimed to recruit 40 patients—10 female patients with breast cancer, 10 patients with colorectal cancer (five female, five male), 10 patients with bladder cancer (five female, five male), and 10 patients with lung cancer (five female, five male). A research assistant approached 66 eligible patients during a clinic visit or mailed a recruitment letter with a subsequent telephone follow-up; of these patients, 18 actively or passively declined participation, and eight could not be contacted by telephone.
Data Collection and Analysis
We used qualitative methodology, which is well suited for exploratory and evaluative research in understudied areas and generates findings that can be investigated further in quantitative studies.25,26 Specifically, we conducted individual semistructured interviews.27-30 Our multidisciplinary research team developed an interview guide consisting of exploratory questions designed to elicit participant perspectives. Two team members conducted interviews in person or by telephone on the basis of participant preference. All participants provided informed consent before the interview. Interviews lasted approximately 45 minutes and were audio recorded and transcribed. Limited demographic data were collected in the interview and abstracted from participants’ medical records. Participants received a $25 gift card in appreciation for their contribution.
Transcripts were analyzed through thematic content analysis, an inductive qualitative data analysis method that identifies and interprets recurring conceptual patterns directly from the data through intensive reading, coding, and interpretation.28,29,31-33 This gold standard analytic approach27,29 included the use of four coders to achieve analyst triangulation34 and iterative rounds of consensus analysis to ensure trustworthiness of the findings.35 ATLAS.ti software was used to facilitate analysis.36 We selected illustrative participant quotes from the interview data to support our findings and computed descriptive statistics for demographic data.
RESULTS
Participant Characteristics
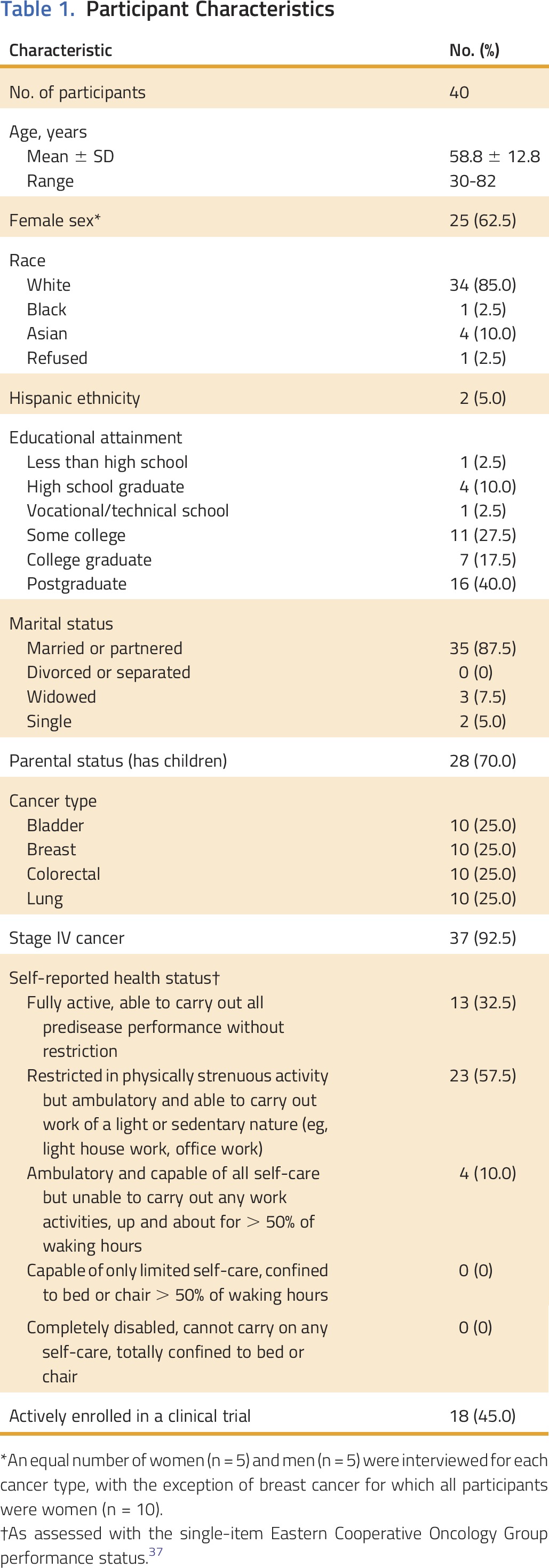
Participants’ characteristics are listed in Table 1. The majority had stage IV disease (92.5%), and one third (32.5%) self-reported as being fully active and capable of all predisease physical activities. Participants were predominantly white (85%), were married or partnered (87.5%), and had children (70%). Participants did not differ from decliners (n = 26) in age, cancer, race, or clinical trial status; however, women were more likely to participate (P = .005).
Table 1.
Participant Characteristics
Interest in SGFs
When asked to imagine that SGFs arising from their use of TGP were now available, the majority of participants (57%) expressed interest in this information. However, many were equivocal about their current interest (29%), and a minority expressed disinterest (14%). Reasons for these preferences varied but were influenced by participants’ perceptions of the benefits and harms of this information as well as their experiences associated with their present health status. These attitudes are described in the next sections.
Perceived Benefits and Harms
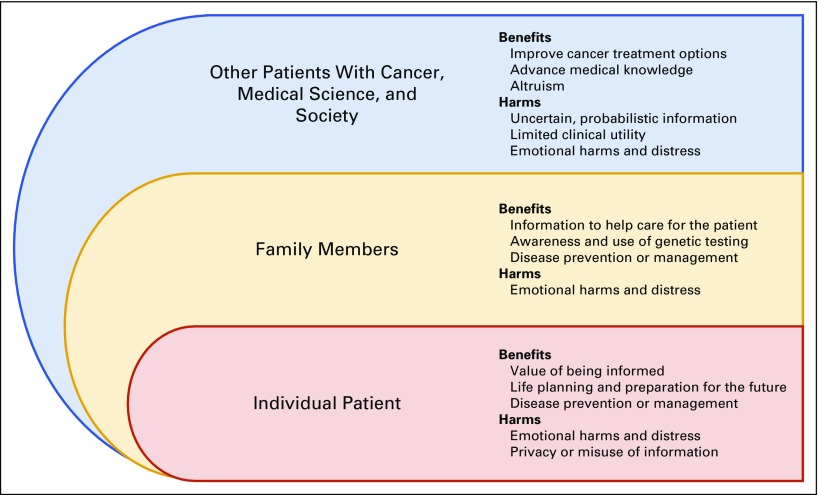
Participants articulated a number of potential benefits and harms associated with learning their SGFs. Participants perceived that three different groups may experience these outcomes: the individual patient; family members; and other patients with cancer, medical science, and society (Appendix Fig A1, online only). We describe the various benefits and harms that participants identified as relevant for each group (key themes indicated by italicized text; illustrative participant quotes listed in Table 2).
Table 2.
Perceived Benefits and Harms of Learning Secondary Germline Findings* and Illustrative Participant Quotes

Individual patient
Participants identified three primary benefits of SGFs for themselves. First, many placed a high value on being informed and noted that “knowledge is power.” Many believed that the ability to learn any information relevant to their present or future health is an obvious benefit. A second benefit involved the opportunity for life planning and preparation for the future. Participants primarily associated this benefit with nonactionable SGFs (ie, indicating risk for a disease without effective treatment, one’s status as a healthy carrier of a disease-causing mutation). They believed that such risk information could inform life decisions about reproduction or help them to prepare for the possibility of serious, incurable conditions (eg, Alzheimer’s disease). A related benefit was disease prevention or management; although several participants anticipated that this information could improve their current cancer treatment, many more described the possible identification of treatment options for other diseases for which they may be at risk, early detection of future diseases, and improved motivation for adopting healthy behaviors.
Approximately one half of participants (53%) did not anticipate that they would directly experience any harms upon learning their SGFs. However, some believed that learning this information would cause them emotional harms and distress. For example, participants believed that they could experience stress about whether their current lifestyle behaviors would increase their disease risks, and anxiety upon learning that family and future generations could be at risk. A few participants also anticipated harms associated with privacy or the misuse of information. These individuals noted that information to be gained from SGFs is private, and there are risks to how institutions or groups (eg, insurance companies, employers, government) may mishandle it.
Family members
Participants identified benefits of their SGFs for nonbiologic and biologic family. For nonbiologic family members (predominantly spouses and partners), knowledge of this information would help them to care for the patient; for example, it could enhance understanding of the patient’s health and enable family to optimally support the patient. Participants also anticipated that by becoming aware of the utility of TGP and their SGFs, both nonbiologic and biologic family members could benefit from an increased awareness and use of genetic testing. Finally, a majority of participants expected that for biologic family members (primarily children and siblings), their SGFs could prompt disease prevention or management. Biologic family members could make more-informed decisions about their current and future health, such as engagement in preventive or surveillance behaviors.
Emotional harms and distress was the primary harm that participants identified for their families. Some anticipated that this risk information could generate intense fear, anxiety, and perhaps fatalistic thinking about the likelihood of developing disease. A few participants believed that this information could be particularly emotionally burdensome for their parents.
Other patients with cancer, medical science, and society
Participants described several benefits of SGFs most relevant to individuals separate from themselves and their families. Some believed that their SGFs could ultimately improve cancer treatment options for other patients. They anticipated that this information could help to identify cures and treatments, enhance quality of life, and extend patients’ lives. In addition, participants anticipated that their SGFs could advance medical knowledge by contributing to valuable scientific research. Finally, some described benefits that reflect a sense of altruism. These participants believed that their SGFs could lead to general societal benefits associated with “paying it forward” to future generations in terms of identifying improved health and disease management options, despite a belief that they themselves would not accrue such benefits from this information.
Participants identified harms that they believed were most applicable to other patients with cancer who may receive SGFs. Several participants acknowledged that SGFs represent uncertain, probabilistic information that may be inaccurate and complicate others’ decision making about how to best manage their health. Consequently, other patients could overreact to this information and engage in overtreatment or interventions that would negatively affect their quality of life (eg, prophylactic surgeries, pregnancy terminations). Similarly, a few participants described how the uncertainty of SGFs could lead to limited clinical utility for other patients. For instance, upon learning their SGFs, patients with cancer may pay for unnecessary testing and interventions that may not be personally useful, which would lead to inappropriate health care utilization and costs.
The prospect of emotional harms and distress was most often described as relevant for other patients with cancer. Participants believed that if other patients received SGFs, they may experience anxiety and attribute a higher degree of certainty to the risk information than is appropriate or realistic. Participants identified characteristics that might place one at greater risk for experiencing emotional distress, including being younger, being prone to anxiety, and having limited effective coping strategies.
Unique Perspectives as Patients With Advanced Cancer
Participants considered their present medical and personal context, which included their diagnosis of advanced cancer, age, and life stage, when assessing the prospect of learning SGFs (Table 3). These factors appeared to directly inform participants’ attitudes about the value of health risk information. For some, receipt of an unexpected cancer diagnosis and the challenges of treatment consequently led them to place great importance on the possibility of being prepared for other diseases. Some participants articulated a link between their cancer diagnosis and their belief that any knowledge is worthwhile, strongly valuing the receipt of their SGFs regardless of whether this information had any negative implications. Yet a number of participants, on the basis of their disease severity and older age, also recognized that they may not derive significant direct benefits from learning their SGFs (eg, “It’s too late for me”). However, many expressed a desire to help others by acknowledging the value of this information for family and society.
Table 3.
Influence of Participants’ Experiences With Advanced Cancer Upon Their Attitudes About Secondary Germline Findings and Illustrative Quotes
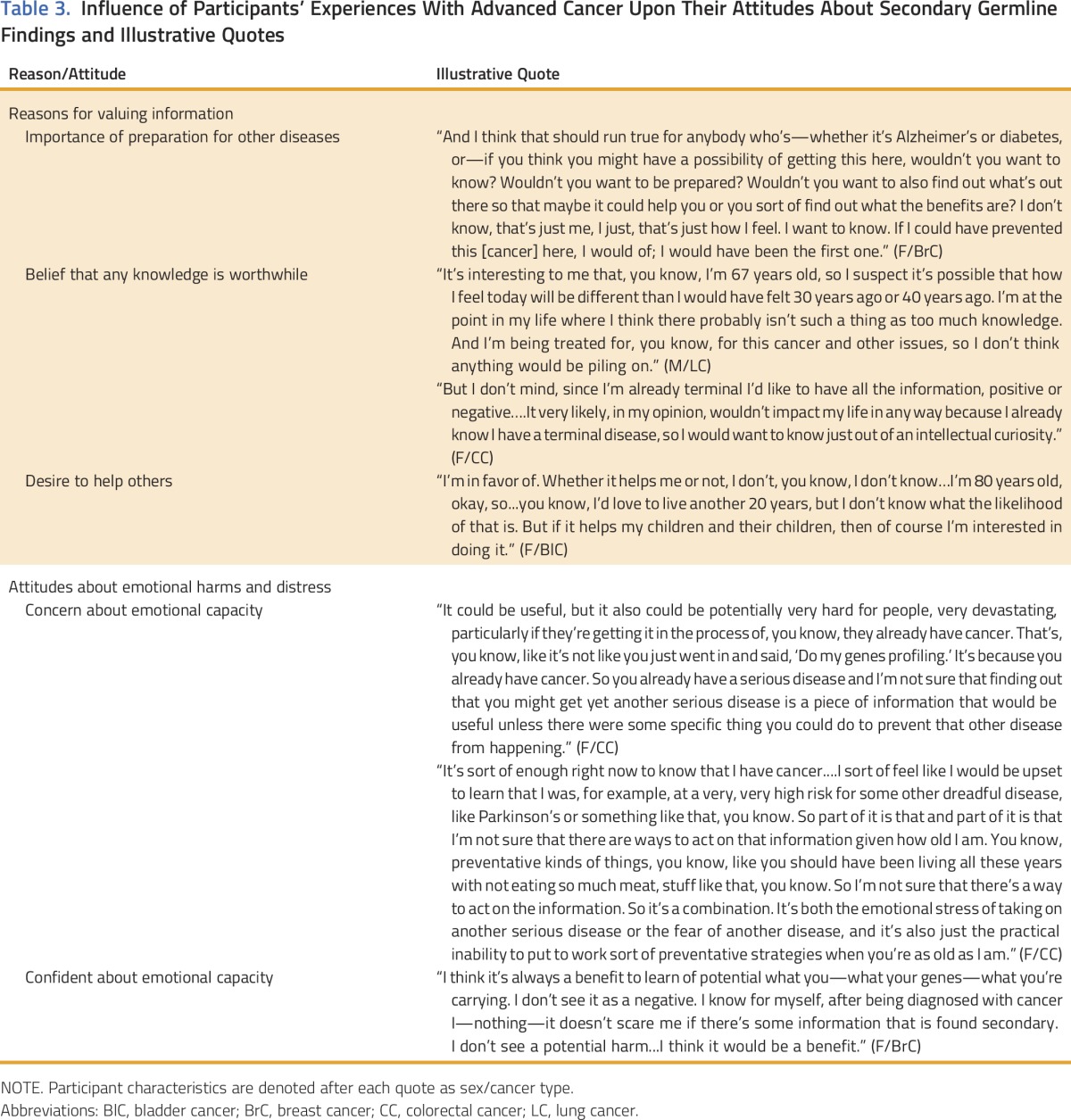
Similarly, participants’ experiences as patients with advanced cancer influenced their attitudes about the emotional harms that could arise from learning their SGFs. Two distinct perspectives emerged with regard to their own emotional capacity. First, some participants believed that they had already met their own threshold for learning negative health information and expressed concern about their emotional capacity. As such, learning SGFs could be “too much” to manage after having contended with cancer. However, others expressed confidence about their emotional capacity because they already possessed the fortitude to manage distress associated with a terminal cancer diagnosis. These individuals expressed confidence in their ability to manage any distress that might come from learning their SGFs, which may pale in comparison with that associated with their cancer diagnosis.
DISCUSSION
Patients with advanced cancer are presently the principal users of TGP,1,2 and a sizeable minority is likely to harbor pathogenic germline variants that could be detected as SGFs; for example, presumed pathogenic germline variants were observed in 15.7% of patients tested with the MSK-IMPACT test.38 Exploration of the unique perspectives of these individuals is a critical step toward determining how to best support patients while they decide whether to learn SGFs. Consistent with studies conducted with patients with cancer in various settings,16-19 we found that a majority of participants with advanced cancer who had previously undergone TGP were interested in the prospect of learning their SGFs. Yet many felt unsure or disinterested in this information. Furthermore, participants considered both the potential benefits and the potential harms when assessing the value of SGFs, which suggests that these perceptions strongly influence patients’ interest in receiving this risk information.
We observed that participants held generally optimistic views of the benefits of SGFs for themselves, including the benefits of being informed and able to prevent and prepare for future health challenges. However, it is important to recognize that some of these anticipated benefits may be unrealistic for many patients given their advanced disease status and poor prognosis. To ensure that patients with advanced cancer make fully informed decisions about the receipt of SGFs, health care providers must devote attention to helping these patients to accurately understand their prognosis and the limited likelihood of deriving direct health benefits.
Participants anticipated little harm for themselves in response to SGFs. Their greatest concerns were related to emotional distress, although this was perceived as being most applicable to others, including family and other patients with cancer. Research has confirmed that genetic testing for cancer predisposition generally has a limited impact on emotional distress,39,40 but little is known about how patients with cancer who are facing the end of life may respond to this information. Participants noted two potential options reflecting either emotional resilience or emotional overload. Such anticipated responses not only support the importance of allowing patients to opt out of the return of SGFs12 but also highlight the need for future research to examine the diverse ways in which patients with advanced cancer may emotionally respond to and cope with inherited risks revealed by TGP.
Most participants anticipated a number of highly valued potential health benefits of learning their SGFs for individuals beyond themselves, including biologic and nonbiologic family. Yet, participants expressed concerns about their families experiencing distress and were particularly concerned about the disclosure of information to their parents, which may be a result of the older age of this sample (median, 59 years). Many expressed a strong desire to help other patients, medical science, and society, with a belief that learning their SGFs would allow them to contribute to scientific knowledge. However, this belief may reflect an important misperception: it is unlikely that patients would be required to agree to receive their SGFs for their biospecimens to be used in medical research. Educational interventions may be needed to ensure that patients understand such distinctions when they consent to both TGP and the receipt of SGFs.
This study has several notable strengths. The qualitative study design allowed for an in-depth analysis of the perspectives of a small sample of patients with advanced cancer that is diverse in cancer type, sex, education, and health status. However, this sample was racially and ethnically homogenous and obtained from a single institution. Thus, these findings may not be generalizable to the broader population of patients with advanced cancer treated in other care settings who are grappling with the decision to receive SGFs. Future work should examine decision-making processes as patients are increasingly presented with the option of learning SGFs and should explore how patients in other settings and with different cancer stages engage with TGP. All participants had direct experience with TGP, although their consideration of the receipt of SGFs was hypothetical. Furthermore, interviews focused on the possibility of learning SGFs that are pathogenic (reflecting both actionable and nonactionable risk information). However, estimates suggest that most patients (approximately 84%) will not receive meaningful germline information from TGP.38 How patients may respond cognitively, emotionally, or behaviorally in this situation is unclear. We also did not explicitly assess participants’ attitudes about the possibility of SGFs reflecting variants of uncertain clinical significance, although many expressed concerns about the uncertain, probabilistic nature of genetic risk information in general. Future studies should explore patients’ experiences with these types of SGFs as TGP is increasingly adopted.
In conclusion, these results demonstrate that many, but certainly not all, patients with advanced cancer are interested in learning their SGFs from TGP. Patients anticipate diverse benefits for themselves and their families as well as for other patients with cancer and society, yet many have serious concerns about the emotional and clinical implications of this information. Furthermore, these patients have unique needs and expectations given their disease experiences, prognosis, age, and life stage, which appear to be important in shaping their perspectives. These results have direct relevance for clinicians and researchers involved in precision medicine and cancer care because they highlight the core values and potential misperceptions that need to be addressed with educational and decision support interventions for this patient population. By addressing these issues, patients with advanced cancer could make truly informed decisions about learning SGFs.
ACKNOWLEDGMENT
Supported by the MSKCC Survivorship, Outcomes, and Risk Developmental Funds Award (J.G.H. and M.E.R. principal investigators); National Cancer Institute grant P30 CA008748; the Robert and Kate Niehaus Center for Inherited Cancer Genomics; and the Andrew Sabine Family Foundation. J.G.H was also supported by a Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-16-020-01-CPPB) from the American Cancer Society. Presented at the American Society of Human Genetics Annual Meeting, Baltimore, MD, October 6-10, 2015. We are grateful to all participating patients.
Appendix
Fig A1.
Study results about perceived benefits and harms of learning secondary germline findings from tumor genomic profiling of patients with advanced cancer as categorized into three groups that may experience these outcomes.
AUTHOR CONTRIBUTIONS
Conception and design: Jada G. Hamilton, Elyse Shuk, Jennifer L. Hay, Kenneth Offit, Mark E. Robson
Collection and assembly of data: Jada G. Hamilton, Elyse Shuk
Data analysis and interpretation: Jada G. Hamilton, Elyse Shuk, Margaux C. Genoff, Vivian M. Rodríguez, Jennifer L. Hay, Mark E. Robson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Interest and Attitudes of Patients With Advanced Cancer With Regard to Secondary Germline Findings From Tumor Genomic Profiling
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Jada G. Hamilton
No relationship to disclose
Elyse Shuk
No relationship to disclose
Margaux C. Genoff
No relationship to disclose
Vivian M. Rodríguez
No relationship to disclose
Jennifer L. Hay
No relationship to disclose
Kenneth Offit
No relationship to disclose
Mark E. Robson
Honoraria: AstraZeneca
Consulting or Advisory Role: McKesson, AstraZeneca
Research Funding: AstraZeneca (Inst), AbbVie (Inst), Myriad Genetics (Inst), Medivation (Inst)
Travel, Accommodations, Expenses: AstraZeneca
REFERENCES
- 1.Tripathy D, Harnden K, Blackwell K, et al. : Next generation sequencing and tumor mutation profiling: Are we ready for routine use in the oncology clinic? BMC Med 12:140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons DW, Roy A, Plon SE, et al. : Clinical tumor sequencing: An incidental casualty of the American College of Medical Genetics and Genomics recommendations for reporting of incidental findings. J Clin Oncol 32:2203-2205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassa CA, Savage SK, Taylor PL, et al. : Disclosing pathogenic genetic variants to research participants: Quantifying an emerging ethical responsibility. Genome Res 22:421-428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewey FE, Grove ME, Pan C, et al. : Clinical interpretation and implications of whole-genome sequencing. JAMA 311:1035-1045, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg JS, Khoury MJ, Evans JP: Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genet Med 13:499-504, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Wolf SM, Lawrenz FP, Nelson CA, et al: Managing incidental findings in human subjects research: Analysis and recommendations. J Law Med Ethics 36:219-248, 2008. [DOI] [PMC free article] [PubMed]
- 7. Presidential Commission for the Study of Bioethical Issues: Anticipate and Communicate: Ethical Management of Incidental and Secondary Findings in the Clinical, Research and Direct-to-Consumer Contexts. Washington, DC, Bioethics Commission, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Burke W, Antommaria AH, Bennett R, et al. : Recommendations for returning genomic incidental findings? We need to talk! Genet Med 15:854-859, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross LF, Rothstein MA, Clayton EW: Mandatory extended searches in all genome sequencing: “Incidental findings,” patient autonomy, and shared decision making. JAMA 310:367-368, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Green RC, Lupski JR, Biesecker LG: Reporting genomic sequencing results to ordering clinicians: Incidental, but not exceptional. JAMA 310:365-366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klitzman R, Appelbaum PS, Chung W: Return of secondary genomic findings vs patient autonomy: Implications for medical care. JAMA 310:369-370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Medical Genetics and Genomics Board of Directors : ACMG policy statement: Updated recommendations regarding analysis and reporting of secondary findings in clinical genome-scale sequencing. Genet Med 17:68-69, 2015 [DOI] [PubMed] [Google Scholar]
- 13.American College of Medical Genetics and Genomics : Incidental findings in clinical genomics: A clarification. Genet Med 15:664-666, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Green RC, Berg JS, Grody WW, et al. : ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 15:565-574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robson ME, Bradbury AR, Arun B, et al. : American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol 33:3660-3667, 2015 [DOI] [PubMed] [Google Scholar]
- 16. Gray SW, Hicks-Courant K, Lathan CS, et al: Attitudes of patients with cancer about personalized medicine and somatic genetic testing. J Oncol Pract 8:329-335, 2012. [DOI] [PMC free article] [PubMed]
- 17.Yushak ML, Han G, Bouberhan S, et al. : Patient preferences regarding incidental genomic findings discovered during tumor profiling. Cancer 122:1588-1597, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Yusuf RA, Rogith D, Hovick SR, et al. : Attitudes toward molecular testing for personalized cancer therapy. Cancer 121:243-250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray SW, Park ER, Najita J, et al. : Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: Results from the CanSeq study. Genet Med 18:1011-1019, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clift KE, Halverson CM, Fiksdal AS, et al. : Patients’ views on incidental findings from clinical exome sequencing. Appl Transl Genomics 4:38-43, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller FA, Hayeems RZ, Bytautas JP, et al. : Testing personalized medicine: Patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur J Hum Genet 22:391-395, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.3791/50710. Won HH, Scott SN, Brannon AR, et al: Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. J Vis Exp(80):e50710, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guest G, Bruce A, Johnson L: How many interviews are enough? An experiment with data saturation and variabilty. Field Methods 18:59-82, 2006 [Google Scholar]
- 25. Crabtree BF, Miller WL: Doing Qualitative Research (ed 2). Thousand Oaks, CA, Sage, 1999. [Google Scholar]
- 26. Office of Behavioral and Social Sciences Research: Qualitative Methods in Health Research: Opportunities and Considerations in Application and Review. Bethesda, MD, National Institutes of Health, 2001.
- 27. Brinkman S, Kvale S. InterViews: Learning the Craft of Qualitative Research Interviewing (ed 3). Thousand Oaks, CA, Sage, 2015. [Google Scholar]
- 28. Green J, Thorogood N. Qualitative Methods for Health Research (ed 3). London, UK, Sage, 2014. [Google Scholar]
- 29. Patton MQ. Qualitative Evaluation and Research Methods (ed 3). Thousand Oaks, CA, Sage, 2002. [Google Scholar]
- 30. Rubin HJ, Rubin IS. Qualitative Interviewing: The Art of Hearing Data (ed 3). Thousand Oaks, CA, Sage, 2012. [Google Scholar]
- 31. Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development (ed 5). Thousand Oaks, CA, Sage, 2009. [Google Scholar]
- 32. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. Thousand Oaks, CA, Sage, 2014. [Google Scholar]
- 33. Saldana J. The Coding Manual for Qualitative Researchers (ed 2). London, UK, Sage, 2013. [Google Scholar]
- 34. Denzin NK. The Research Act: A Theoretical Introduction to Sociological Methods (ed 5). New Brunswick, NJ, Aldine Transaction, 2009. [Google Scholar]
- 35.Morse JM, Barrett M, Mayan M, et al. : Verification strategies for establishing reliability and validity in qualitative research. Int J Qual Methods 1:1-19, 2002 [Google Scholar]
- 36. Friese S. Qualitative Data Analysis With ATLAS.ti (ed 2). London, UK, Sage, 2014. [Google Scholar]
- 37.Oken MM, Creech RH, Tormey DC, et al. : Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-655, 1982 [PubMed] [Google Scholar]
- 38. doi: 10.1001/jamaoncol.2015.5208. Schrader KA, Cheng DT, Joseph V, et al: Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol 2:104-111, 2016 [Erratum: JAMA Oncol 2:279, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton JG, Lobel M, Moyer A: Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol 28:510-518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heshka JT, Palleschi C, Howley H, et al. : A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med 10:19-32, 2008 [DOI] [PubMed] [Google Scholar]